Abstract
Urogenital myiasis results when flies lay their eggs near the exit of the urethra and the larvae proceed upward along the urogenital tract. In this case report, a 10 year-old female patient diagnosed with urogenital myiasis was reported. The patient presented with complaints including painful and frequent urination, genital pruritus and moving larvae in urine. The patient had received Enterobius vermicularis treatment previously for two times. A 24-hour urine sample was collected and two black larvae were found in the urine. It was found that these larvae were fourth-stage larvae of Psychoda albipennis. Although there was no risk factor, the patient was affected with this rare parasitological disease. This case was presented to draw attention to myiasis in children. Myiasis may be observed in individuals with a favourable hygiene status and a high socioeconomical level. If a detailed history is not taken and appropriate laboratory tests are not performed, the diagnosis may be missed.
Keywords: Myiasis, Psychoda albipennis, urogenital myiasis
Introduction
The word myiasis is drived from the greek word “myia” which means fly. Myiasis is infestation of the tissues of humans and other vertebrates caused by eggs and larvae of flies belonging to the Diptera species. Although it occurs rarely in humans, it is observed more frequently in tropical and subtropical countries in crowded places where hygienic conditions are poor. The clinical properties of this zoonotic parasitosis depends on the body part involved and the degree of tissue damage. Myiasis in different parts of the body can be divided into seven types because of different outcomes: eye, nose, external ear, skin, gastrointestinal system, urogenital and traumatic myiasis. Urogenital myiasis occurs when the fly larvae are localized in the urogenital canal. Although many species maybe the cause for this condition, almost all of the cases of urogenital myiasis reported from our country are caused by Psychoda albipennis which belongs to the Psychodidae family and which lives especially in damp bathrooms of houses. Psychoda albipennis larvae are slightly cylindrical and have a grey-white color. They have short pale hair on the surface. The fly has four larva stages. It is possible to observe the fourth stage larvae (4–8 mm) in urine of infested humans (1–3).
In this article, a case of urogenital myiasis in a 10-year old girl whose complaints of pruritus and dysuria did not improve with Enterobius vermicularis treatment was reported. Her complaints were ceased by water and urinary antiseptic medication.
Case
A 10-year old female patient who had complaints of mild dysuria and itching for about 25–26 days and moving worms in urine in recent days (the worms were white in the beginning, then they became black subsequently) presented to 2 different healthcare institutions with an interval of 12 days. Complete urinalysis and stool microscopic examination were found to be normal and albendazole and pyrantel pamoate treatments were given with a diagnosis of Enterobius vermicularis.
The patient’s complaints continued and she presented to our Pediatrics Outpatient clinic. At presentation, physical examination, complete urinalysis, complete bloood count and biochemical values were found to be normal. Urinary system ultrasonography was found to be normal. Leukocytes, erythrocytes, parasites or parasite eggs were not found on microscopic examination of stool. The patient was asked to collect all her urine as 24-hour urine samples until the larvae were seen. After two days two black larvae were seen in urine. A diagnosis of myiasis was made on examination in our microbiology clinic. The diagnosis of urogenital myiasis was confirmed by observing the larvae again when the patient was kept under supervision for 48 hours to exclude a false diagnosisof myiasis. The types of the larvae were determined in our parasitology laboratory. The larvae were incubated in transparent glass bottles containing 30% potassium hydroxide until they became transparent. Subsequently, the parts which would demonstrate the morphological properties determining the type and stage of transparent larvae were dissected under stereo microscope and placed on glass slides with C-M medium (4, 5). As a result of microscopic examination of the preparations, the larvae were found to be 4th stage larvae of Psychoda albipennis. Typical mouth skeleton (Figure 1) and syphon structure (Figure 2) of the larvae were visualized. The patient was recommended to drink plenty of water and use urinary tract antiseptic medication. On the follow-up visit, after one week, patient’s complaints of itching and dysuria improved.
Figure 1.

Oral skelton of the fourth stage larva of P. albipennis
Figure 2.
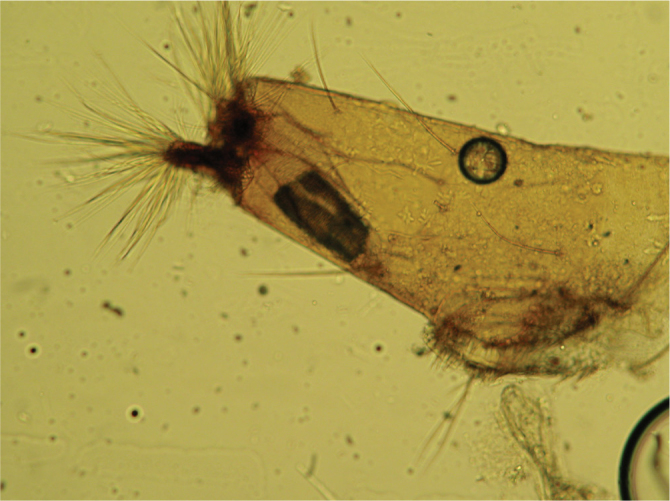
Syphon of the fourth stage larva of P. albipennis
Discussion
The picture which occurs when different fly species lay their eggs or larvae in body parts where nutrition for larvae is appropriate and in pyogenic or wounded tissues without need for a host is called facultative myiasis. Urogenital myiasis is also facultative. In the literature, cases of urogenital myiasis caused by Sarcophaga spp., Megaselia scalaris, Thyrsocnema incisilobata, Lucilia sericata, Eristalis tenax, Dermatobia hominis, Chrysomya bezziana, Fannia canilicularis and Psychoda albipennis (similar to our case) have been reported (3, 6–9). Psychoda albipennis which is responsible for most of the cases of myiasis reported from Turkey is a species which can be observed in Europe with a mild climate and which is known to be common in different provinces of Turkey including mainly Ankara, Edirne, Tekirdağ, İstanbul and Bursa (10). The larvae of Psychoda albipennis are found especially in damp and dirty areas, in vegetables and fruit going bad, in piles of rabbish and in places where plants are irrigated with canalization. It is possible to see 4th stage larvae in urine of infested humans. The fourth stage larvae are whitish gray slightly flat worms with a diameter of 3–5 mm. They are covered with short hair or scales and sometimes they have denticles on the edges. The number of plaques localized in the back of the body is variable and 7–8 rings are generally noted towards the back. Its syphon thins from the base to the tip and there is a double knob covered with long hair at the end (3, 4, 11).
In our country, three different cases caused by three different species including scrotal myiasis due to Dermatobia hominis in a 21-year old patient (12), urogenital myiasis due to Eristalis tenax in a 58-year old woman (13) and urogenital myiasis due to Lucilia sericata in another patient (8) were reported. However, P. Alfrom bipennis is the causative agent in the other cases of myiasis reported from Turkey including a 29-year old man from Trabzon (4), a 15-year old man from Diyarbakır (2), a 50-year old woman from Eskişehir (3) and a 29-year old man from Kırşehir (14). It is notable that the cases reported from Turkey constitute a significant portion of the cases of urogenital myiasis reported in the literature. In our patient, the isolated causative agent was the 4th stage larvae of P. albipennis. Although rural life, poor hygiene conditions, limitation of mobility, urinary obstruction, ulcerative lesions, sleeping without blanket in summer months, low socioeconomical level are considered to be predisposing factors, a significant portion of the cases have been reported form developed countries or in individuals who have good hygiene conditions without risk factors similar to our patient. Although this does not reflect the actual frequency in developing countries, it shows that it should be kept in mind that myiasis may be observed in developed countries or in people living under good hygiene conditions (15).
In patients with urogenital myiasis, vomiting, side pain, dysuria, pollakiuria may be observed, though some patients may have worms in urine as the only complaint (1–3, 7–14). Urogenital myiasis maybe misdiagnosed as an ureter stone by leading to obstruction in the ureter. In our case, the patient had complaints of dysuria, pollakiuria and worms in urine. Since our patient was young and Enterobius vermicularis infection is observed frequently in this age group, it was thought that the worms observed could be E. vermicularis passed from the anus to the urogenital region. Thus albendazole and praziquantel treatments were administered to the patient in two different centers. The patient was brought to our clinic with persisting complaints and with a prediagnosis of resistant E. vermicularis infection. This diagnosis was excluded when the worms were investigated. In one study (16), 54 cases of myiasis were reviewed and 72% of the patients were found to be in the childhood age group. However, all patients reported from our country are adults except for one patient aged 15 years old. This suggests that cases of pediatric myiasis might be falsely diagnosed with E. vermicularis infection or might be missed in our country which has a rich fly fauna and appropriae climate.
Treatment in urogenital myiasis varies according to the localization of infestation and the severity of the symptoms. Mechanical removal of larvae if they can be reached, benefiting form the washing effect of urine by drinking plenty of fluid, urinary tract antiseptic medication and use of antibiotics if the symptoms are severe are among these therapeutic options (2, 3, 7, 14). In our patients, the complaints regressed as a result of plenty of fluid intake and use of urinary tract antiseptic medication. At the follow-up visit 10 days later, it was learned that 3–4 nonmoving worms were seen in urine, but the patient had no complaints in the last few weeks.
Conclusively, myiasis should be considered in the differential diagnosis especially in pediatric patients in presence of persistent findings related with the urogenital system even if they live in cities under good environmental and socioeconomical conditions considering climate conditions and the richness of the fly fauna. It should be kept in mind that the frequency of myiasis will increase further in rural areas where animals are found, in unhealthy places with poor environmental and socioeconomical conditions and in places where piles of rubbish are found or places near trash damps. (Especially in cases which do not respond to E. vermicularis treatment and worms are reported to be observed in the genital area and in urine, examination of these worms parasitologically would be an appropriate approach.) Especially in conditions when there is no response to E.Vermicularis treatment and if worms are seen on genital area and in urine, parasitological investigations of these worms should be performed.
Footnotes
Informed Consent: Written informed consent was obtained from the parent of the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.I., A.D.D; Design - A.D.D, M.I; Supervision - D.N.S.İ.; Funding - M.I., D.N.S.İ..; Materials - M.I., D.N.S.İ; Data Collection and/or Processing - A.D.D., M.I.; Analysis and/or Interpretation - A.D.D., M.I.; Literature Review - A.D.D., M.I.; Writer - A.D.D.; Critical Review - M.I.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ramalingam S, Nurulhuda A, Bee LH. Urogenital myiasis caused by Chrysomya bezziana (Diptera: Calliphoridae) in peninsular Malaysia. Southeast Asian J Trop Med Public Health. 1980;11:405–7. [PubMed] [Google Scholar]
- 2.Çiçek M, Diker Aİ, İpek DN, Tekin A, Dal T. Psychoda albipennis’in sebep olduğu bir ürogenital myiasis. Türk Parazitol Derg. 2012;36:51–3. doi: 10.5152/tpd.2012.13. http://dx.doi.org/10.5152/tpd.2012.13. [DOI] [PubMed] [Google Scholar]
- 3.Güven E, Kar S, Doğan N, Karaer Z. Bir kadında Psychoda albipennis’in neden olduğu ürogenital myiasis. Türk Parazitol Derg. 2008;32:174–6. [PubMed] [Google Scholar]
- 4.Zumpt F. Myiasis in man and animals in the old world. London: Butterworths; 1965. pp. 1–247. [Google Scholar]
- 5.Clark EW, Morishita F. C-M medium: a mounting medium for small insects, mites and other whole. Mounts Science NY. 1950;112:789. doi: 10.1126/science.112.2922.789. http://dx.doi.org/10.1126/science.112.2922.789. [DOI] [PubMed] [Google Scholar]
- 6.Raposo AA, Schettini AP, Massone C. Concurrent primary and secondary myiasis on basal cell carsinoma. An Bras Dermatol. 2010;87:292–5. doi: 10.1590/s0365-05962012000200016. [DOI] [PubMed] [Google Scholar]
- 7.Kaya S, Arslan M, Karaer Z, Köksal İ. Psychoda albipennisin neden olduğu ürogenital myiasis. Türk Parazitol Derg. 2011;35:172–4. doi: 10.5152/tpd.2011.43. http://dx.doi.org/10.5152/tpd.2011.43. [DOI] [PubMed] [Google Scholar]
- 8.Dinçer Ş, Tanyüksel M, Küçük T. İnsanlarda Psychoda spp. (Diptera: Nematocera) ve Sarcophaga spp. (Diptera: Cyclorrhapha) larvalarının neden olduğu iki myiasis olgusu. Türk Parazitol Derg. 1995;19:402–8. [Google Scholar]
- 9.Taylan-Özkan A, Babür C, Kiliç S, Nalbantoğlu S, Dalkılıç I, Mumcuoğlu KY. Urogenital myiasis caused by Psychoda albipennis (Diptera: Nematocera) in Turkey. Int J Dermatol. 2004;43:904–5. doi: 10.1111/j.1365-4632.2004.02051.x. http://dx.doi.org/10.1111/j.1365-4632.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 10.Merdivenci A. Türkiye parazitleri ve parazitoloji yayınları, Cerrahpaşa Tıp Fakültesi Yayınları, Rektörlük no. 1610, İstanbul. 1970. pp. 322–40. [Google Scholar]
- 11.Dinçer Ş. Özcel MA, Daldal N. Artropod hastalıkları ve vektörler. İzmir: Türk Parazitololoji Derneği Yayını; 1997. İnsan ve hayvanlarda myiasiz; pp. 169–233. İçinde: [Google Scholar]
- 12.Yıldız M, Basar M, Hokelek M, Basar H, Akalin Z. Scrotal myiasis. Br J Urol. 1997;80:493–4. doi: 10.1046/j.1464-410x.1997.00294.x. http://dx.doi.org/10.1046/j.1464-410X.1997.00294.x. [DOI] [PubMed] [Google Scholar]
- 13.Mumcuoglu I, Akarsu GA, Balaban N, Keles I. Eristalis tenax as a cause of urinary myiasis. Scand J Infect Dis. 2005;37:942–3. doi: 10.1080/00365540510043275. http://dx.doi.org/10.1080/00365540510043275. [DOI] [PubMed] [Google Scholar]
- 14.Yenice MG, Demir T, Babür C, Nalbantoğlu S, Kılıç S. Psychoda albipennis’in (Diphtera:Nematocera) neden olduğu ürogenital miyazis olgusu. Mikrobiyol Bul. 2011;45:558–64. [PubMed] [Google Scholar]
- 15.Bletjer J. Tracheostomy wound myiasis in a child: case report and review of the literature. Case Rep Pediatr. 2012;317:862. doi: 10.1155/2012/317862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang C. A collective analysis on 54 cases of human myiasis in China from 1995–2001. Chin Med J. 2002;115:1445–7. [PubMed] [Google Scholar]


