Abstract
Aim:
In this study, it was aimed to investigate which method was superior by applying selective head cooling or whole body cooling therapy in newborns diagnosed with moderate or severe hypoxic ischemic encephalopathy.
Materials and Method:
Newborns above the 35th gestational age diagnosed with moderate or severe hypoxic ischemic encephalopathy were included in the study and selective head cooling or whole body cooling therapy was performed randomly. The newborns who were treated by both methods were compared in terms of adverse effects in the early stage and in terms of short-term results. Ethics committee approval was obtained for the study (06.01.2010/35).
Results:
Fifty three babies diagnosed with hypoxic ischemic encephalopathy were studied. Selective head cooling was applied to 17 babies and whole body cooling was applied to 12 babies. There was no significant difference in terms of adverse effects related to cooling therapy between the two groups. When the short-term results were examined, it was found that the hospitalization time was 34 (7–65) days in the selective head cooling group and 18 (7–57) days in the whole body cooling group and there was no significant difference between the two groups (p=0.097). Four patients in the selective head cooling group and two patients in the whole body cooling group were discharged with tracheostomy because of the need for prolonged mechanical ventilation and there was no difference between the groups in terms of discharge with tracheostomy (p=0.528). Five patients in the selective head cooling group and three patients in the whole body cooling group were discharged with a gastrostomy tube because they could not be fed orally and there was no difference between the groups in terms of discharge with a gastrostomy tube (p=0.586). One patient who was applied selective head cooling and one patient who was applied whole body cooling died during hospitalization and there was no difference between the groups in terms of mortality (p=0.665).
Conclusions:
There is no difference between the methods of selective head cooling and whole body cooling in terms of adverse effects and short-term results.
Keywords: Hypoxic ischemic encephalopathy, selective head cooling, whole body cooling
Introduction
Hypoxic ischemic encephalopathy (HIE) is one of the leading causes of neonatal mortality and disability in the whole world (1–3). Despite developments in prenatal and perinatal follow-up methods, the incidence of HIE in developed countries is reported to range between 1/1000 and 2/1000 live births (3–4). Ten-15% of the babies diagnosed with HIE die in the neonatal period, 10–15% develop cerebral palsy and 40% develop significant disabilities including cognitive disorders, neuromotor developmental delay, seizures, hearing defects and blindness (5). In the studies performed in recent years, it has been reported that the mortality rates decrease and positive neurodevelopmental outcomes are obtained with cooling therapy applied in babies with mild or severe HIE (1–2, 6–11). Cooling therapy is applied as selective head cooling (SHC) in some centers and as whole body cooling (WBC) in other centers. In whole body cooling therapy, the rectal temperature is kept at 33–34°C and a moderate systemic hypothermia is created (12–14). In selective head cooling therapy, cooling of the head and thus of the brain is targeted and the rectal temperature is kept 1°C higher (34–35°C) compared to whole body cooling to minimize the potential side effects of hypothermia (2, 4, 15). Despite this important difference between the two methods, it is not known which method is more efficient and safer. The aim of this study was to apply SHC and TBC methods to babies diagnosed with moderate or severe HIE and to investigate if these two methods are superior to each other.
Material and Methods
The study was conducted in Mersin University, Medical Faculty, Neonatal Intensive Care Unit. Approval was obtained from the ethics committee for the study (06.01.2010/35) and informed consent was obtained from the families. Before the patients were admitted, a contact meeting about the method of the study was held together with neonatologists and pediatricians who were working in the other hospitals around and could refer patients to our hospital. Passive cooling was applied to the babies referred from the other hospitals with a prediagnosis of HIE during transportation by turning off the incubators and it was targeted to keep the axillary temperature between 34 and 35°C by taking measurements with 30-minute intervals during transportation. The diagnostic criteria recommended by the American Association of Gynecology and Obstetrics for hypoxic ischemic encephalopathy were used (16). The Modified Sarnat Score was used to determine the severity of hypoxic ischemic encephalopathy (17). The babies who reached the center where the study was conducted later than six hours, whose gestational age was below the 36th week and who had severe congenital defect or severe intrauterine growth retardation were not included in the study. Among the other babies who were diagnosed with HIE, the ones who had an APGAR score of 5 or lower in the 10th minute or who were applied positive pressure ventilation until the 10th minute or who had a pH value of <7.0 or base excess of ≤−16 in blood gases tested in the first one hour were evaluated further. In the patients who were evaluated further, amplitude integrated electroencephalogram (aEEG) (Brainz, Natus medical, San Carlos, CA, USA) was recorded if one of the findings including lack of sucking, hypotonia, abnormal reflex (abnormal pupillary response to light) or seizure was present in addition to lethargy, stupor or coma which are signs of moderate or severe encephalopathy. If moderate or severe defect or seizure was found on encephalogram, the baby was included in the study. aEEG was considered severely defective in presence of continuous low voltage, burst suppression and flat tracing on the background design of the encephalogram and it was considered moderately defective in presence of discontinuous normal voltage (18–21). Since there was only one aEEG device in the unit, the device was put on another patient who needed EEG only after an at least 30-minute recording was made. If no baby with HIE presented to the unit or if there was no need of the aEEG device for another baby, aEEG recording of the same baby was continued for three days and even until the seizures were controlled if the seizures were persisting.
SHC or WBC was applied to the babies included in the study by sealed tender randomly. While selective head cooling was applied by a manually controlled device (Olympic Medical Cool Care System, Olympic Medical, Seattle, WA, USA), WBC was applied by cooling the environment. Whole body cooling was applied by cooling the environment where the baby was found using a room air conditioner. Continuous temperature monitoring was done by placing sensors in the rectum and abdominal right upper quadrant in the babies who were applied whole body cooling and in the rectum, abdominal right upper quadrant and scalp in the babies who were applied SHC and the temperatures were recorded with 30-minute intervals. It was targeted to keep the rectal temperature between 33 and 34°C in the WBC method and between 34 and 35°C in the SBC method for 72 hours. At the end of 72 hours, the rectal temperatures of the babies who were applied cooling therapy with both methods were increased at most 0.5°C hourly and brought to 36.5°C. It was planned to remove the patient from the study, if the rectal temperature remained outside the range desired for longer than one hour during the cooling therapy. Blood samples were obtained from the patients included in the study before the process of grouping and at the 24th and 72nd hours of cooling for laboratory investigations. When examining the side effects which might develop in relation with cooling therapy, a mean arterial blood pressure below 40 mmHg was considered hypotension, an heart rate below 80/min was considered bradycardia, a 24-hour urine output below 0.5 mL/kg or a serum creatinine level above 1.5 mg/dL was considered renal dysfunction, a serum sodium level below 130 mEq/L was considered hyponatremia, a serum potassium level below 3.5 mEq/L was considered hypokalemia, a platelet count below 100 000/µL was considered significant thrombocytopenia, a serum calcium level below 7 mg/dL or a serum ionized calcium level below 1mmol/l was considered hypocalcemia, a blood glucose level below 47 mg/dL was considered hypoglycemia, more than 20% increase in the hematocrit level was considered hemoconcentration, growth of microorganisms in hemoculture combined with clinical findings was considered sepsis and an aspartate aminotransaminase (AST) value of >200U/L or an alanine aminotransaminase value of >100U/L was considered increased liver enzymes. Echocardiography was performed in all patients. The diagnosis of persistent pulmonary hypertension was made by echocardiography. A gastrostomy tube was placed to maintain feeding at home in the babies who were planned to be discharged, but who could not be fed orally. A tracheostomy was performed in the babies whose requirement for invasive mechanical ventilation continued.
Statistical analysis
The continuous variables were expressed as mean ±standard deviation and the categorical variables were expressed as frequency and percentage (summarized in the table). Mann-Whitney U test was used in comparison of the cooling groups in terms of continuous variables. In examination of the relations between the categorical variables, Pearson Chi-sqare test was used in cases where the expected value assumptions were realized in cross tables and Fisher’s exact test was used in cases where the expected value assumptions were not realized. Statistical analyses were performed using PASW v.18 package program and a p value of <0.05 was considered statistically significant.
Results
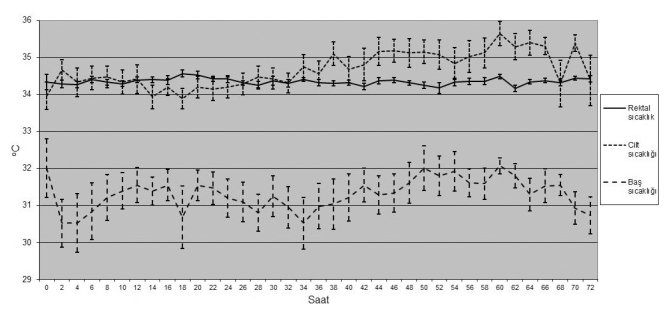
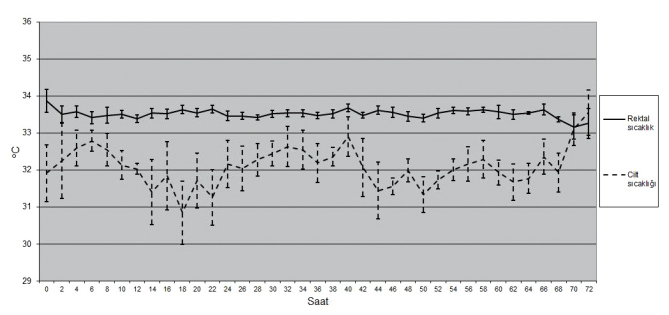
Fifty tree babies were diagnosed with HIE in the study period. Among the babies who were diagnosed with hypoxic ischemic encephalopathy, 13 were excluded because of absence of cooling criteria, 7 were excluded because they were older than 6 hours at the time of grouping, one was excluded because of presence of congenital defect, 2 were excluded because their general states were poor and required recurrent resuscitation. SHC was started in 17 of the remaining 30 babies and WBC was started in 13 (Figure 1). The cooling therapy was finalized at the 48th hour and the patient was removed from the study if treatment-resistant hypotension developed in the whole body cooling group. In all patients included in the study, the rectal temperatures could be kept in the desired range for 72 hours (Figure 2, 3). There was no significant difference between the babies in whom selective head cooling was applied and the babies in whom WBC was applied in terms of gestational age, birth weight, head circumference, gender, being born in the center where the cooling therapy was performed, mode of delivery, Apgar scores at the 5th and 10th minutes, blood gases tested in the first one hour, need for invasive mechanical ventilation, Sarnat stage, presence of clinical seizure, aEEG findings, age at the time of enrollment and the rectal temperature at the time of enrollment (Table 1). The rectal temperatures were between 33 and 35°C at the time of grouping in all babies included in the study except for three patients. The baseline rectal temperature was 35.5°C in a baby included in the selective head cooling group and 35.2 and 32.3°C in two babies included in the whole body cooling group.
Figure 1.
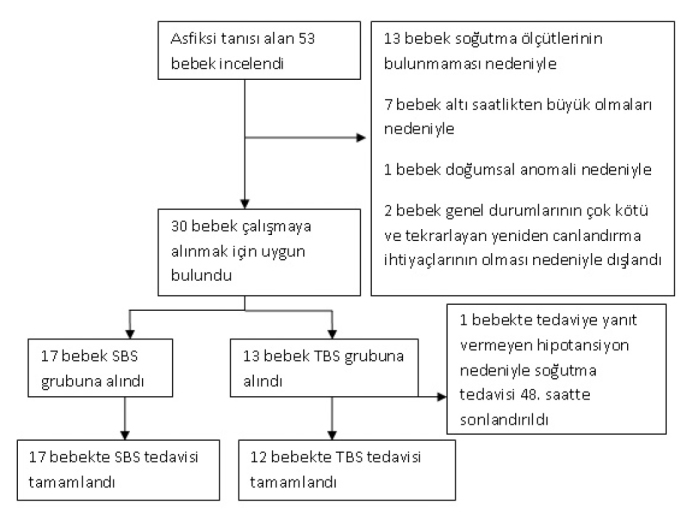
Flowchart showing enrollment of the patients and randomization into groups
(SHC. Selective head cooling; WBC: whole body cooling)
Figure 2.
Variation of the rectal, skin and head temperature by time in the newborns who were applied selective head cooling
Figure 3.
Variation of the rectal and skin temperature by time in the newborns who were applied whole body cooling
Table 1.
General properties of the subjects included in the study by groups
| Selective head cooling (n=17) | Whole body cooling (n=12) | p | |
|---|---|---|---|
| Gestational agea | 38.6 weeks±1.2 | 39.3 weeks±0.9 | 0.140 |
| Birth weighta | 3166 g±426 | 3144 g±495 | 0.948 |
| Head circumferencea | 34.8 cm±0.8 | 34.6 cm±0.8 | 0.586 |
| Female | 12 (70%) | 9 (75%) | 0.568 |
| Delivery by cesarean sectio | 3 (18%) | 5 (42%) | 0.158 |
| Being born in the same center | 1 (5%) | 0 (0%) | 0.586 |
| Age at the time of groupinga | 5.2 hours±1.4 | 5.8 hours±0.3 | 0.527 |
| Rectal temperature at the time of groupinga | 34.1°C±0.7 | 34.2°C±1 | 0.647 |
| Presence of clinical seizure | 15 (88%) | 10 (83%) | 0.556 |
| Apgar score at the 5th minute (n=26) | |||
| 0–3 | 8 (57%) | 4 (33%) | 0.225 |
| 4–6 | 6 (43%) | 8 (67%) | 0.225 |
| 7–10 | |||
| Apgar score at the 10th minute (n=25) | |||
| 0–3 | 3 (23%) | 3 (25%) | 0.637 |
| 4–6 (%) | 10 (77%) | 8 (67%) | 0.450 |
| 7–10 | 0 | 1 | 0.480 |
| Blood gases measured in the first hour | |||
| pH | 6.89±0.2 | 6.92±0.1 | 0.999 |
| bas excess, mmol/L | −18±5 | −19±2 | 0.673 |
| aEEG before grouping | |||
| moderately abnormal | 3 (18%) | 2 (17%) | 0.671 |
| severely abnormal | 14 (82%) | 10 (83%) | 0.671 |
| Sarnat stage | |||
| Stage II | 8 (47%) | 4 (33%) | 0.363 |
| Stage III | 9 (53%) | 8 (77%) | 0.363 |
mean±SD
No clinically significant adverse effect was observed in the selective head cooling and whole body cooling groups. There was no significant difference between the two groups in terms of hypotension, bradycardia, thrombocytopenia, abnormality in the coagulation tests, renal dysfunction, electrolyte imbalance, increased liver enzymes, culture positive sepsis, persistent pulmonary hypertension, pulmonary air leak, need for mechanical ventilation and ability to perform extubation during cooling (Table 2). Necrotizing enterocolitis, hypoglycemia or hemoconcentration was not observed in any baby in the SHC and WBC groups. One of the babies who were applied SHC and one of the babies who were applied WBC (8%) died in the hospital. There was no significant difference between the two groups in terms of in-hospital mortality (p=0.665). The baby in the SHC group who was died in the hospital had severe HIE. This patient could never be separated from mechanical ventilator support and was lost following hypoxemia which could not be corrected on the 22th day despite high-frequency oscillatory ventilatory support. The baby in the WBC group who was lost in the hospital had persistent pulmonary hypertension in combination with severe HIE and was lost on the 7th day of life despite high-frequency oscillatory ventilation, inhaled nitric oxide and inotropic support therapies. The hospitalization period was 34 (7–65) days in the SHC group and 18 (7–57) days in the WBC group and there was no significant difference between the two groups (p=0.097). Four patients in the SHC group and two patients in the WBC group were discharged with tracheostomy. Five patients in the SHC group and three patients in the WBC group were discharged with gastrostomy tube (Table 3).
Table 2.
Comparison of the selective head cooling group and the whole body cooling group in terms of adverse effects
| Findings | Selective head cooling (n=17)
|
Whole body cooling (n=12)
|
p | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Hypotension | 12 | 70 | 7 | 58 | 0.385 | ||
| Bradycardia | 1 | 0 | 0.586 | ||||
| Platalet count <100 000/µL | 10 | 59 | 3 | 25 | 0.071 | ||
| Abnormal coagulation tests | 7 | 41 | 4 | 33 | 0.486 | ||
| Renal dysfunction | 5 | 29 | 4 | 33 | 0.568 | ||
| Hyponatremia | 9 | 53 | 5 | 42 | 0.550 | ||
| Hypokalemia | 5 | 29 | 3 | 25 | 0.568 | ||
| Increased liver enzymes | 11 | 65 | 6 | 50 | 0.341 | ||
| Culture positive sepsis | 2 | 1 | 0.633 | ||||
| Edema in the scalp | 1 | 0 | 0.586 | ||||
| Hypocalcemia | 10 | 59 | 9 | 75 | 0.309 | ||
| Persistent pulmonary hypertension | 0 | 1 | 0.414 | ||||
| Lung air leak | 0 | 1 | 0.414 | ||||
| Requirement for invasive mechanical ventilation during the period of cooling | 16 | 94 | 11 | 92 | 0.665 | ||
| Ability to perform extubation during the cooling period | 1 | 1 | 0.665 | ||||
Table 3.
Comparison of the selective head cooling group and the whole body cooling group in terms of adverse effects
| Early outcomes | Selective head cooling (n=17) | Whole body cooling (n=12) | p |
|---|---|---|---|
| Mortality | 1/17 (6%) | 1/12 (8%) | 0.665 |
| Hospitalization time, median (the least-the highest) | 34 days (7–65) | 18 days (7–57) | 0.097 |
| Discharged with tracheostomy | 4/16 (25%) | 2/11 (18%) | 0.528 |
| Discharged by feeding with gastrostomy tube | 5/16 (31%) | 3/11 (27%) | 0.586 |
Discussion
In recent years, cooling therapy has become a widely used treatment method in babies with moderate or severe HIE (1, 3, 22–26). It is thought that the neuron protecting effects of cooling are related with reduction of brain metabolism rate, stimulating amino acid release, apoptosis, nitric oxide production, lipid peroxidation and limitation of free radical damage (27). For cooling therapy to create the desired effect it is recommended that cooling be performed as soon as possible following hypoxic ischemic event and initiated in at least 6 hours (4, 28). However, cooling therapy is not performed in many centers where babies with HIE are delivered and thus babies with HIE have to be referred for cooling therapy. If initiation of cooling is postponed until the baby reaches the center where cooling will be applied, the cooling therapy may be delayed and its efficiency may decrease. Therefore, active or passive cooling is performed during transportation in babies who are referred for cooling therapy (29). All patients included in our study except for one were admitted from other centers and passive cooling was applied during transportation by turning off the incubator heaters. Thus, the rectal temperatures were within the desired range in all babies when they reached our center except for three. The rectal temperature was above 35°C in two babies whose rectal temperatures were not in the desired range and one baby had a rectal temperature of 32.2°C. The baby who developed deep hypothermia though active cooling was not performed was transported in a cold winter day and the babies whose rectal temperatures were above 35°C were transported on hot summer days. Therefore, we think that seasonal conditions and environmental temperature should also be considered in babies who are transported by applying passive cooling. It may be questioned why active cooling was not performed during transportation in the babies whose rectal temperatures were above 35° at the time of admission in our center in our study. The reason for this was concern about the possibility of development of deep hypothermia related with active cooling and active cooling during transportation is only recommended in cases where the rectal temperature can be monitorized continuously (30). However, continuous rectal temperature monitorization could not be performed during transportation because of technical causes in our study and therefore active cooling was not applied. Use of sensors which are placed on the skin is not recommended for monitorization of the temperature during transportation of the babies with HIE, because the skin temperature is easily affected by vasoconstriction and environmental temperature and thus may show considerably different values compared to the rectal temperature values (30). Therefore, the ideal action during transportation is continuous rectal temperature monitorization. However it has been reported that axillary temperature monitorization may also be used if rectal temperature monitorization cannot be performed (30). In this study, temperature monitorization of the babies during transportation was performed by the axillary route.
Cooling therapy may be applied using specially produced devices or by contacting some cooled substances to the head or body, by cooling the environment or it may be applied passively (3, 31, 32). Actually, the important point is to keep the rectal temperature in the desired range rather than the route of cooling in cooling therapy. In this study, SHC therapy was applied using a device produced for cooling therapy and WBC was applied by cooling the environment. The rectal temperatures of the babies were kept in the targeted range for 72 hours with both methods. Although no special device was used while applying WBC, no significant technical problem was experienced and no deviations in the targeted rectal temperatures were observed. Therefore, we think that centers which cannot purchase the devices produced for cooling therapy can apply WBC therapy by way of cooling the environment with the condition of continuous monitorization of the rectal temperatures and close follow-up of patients.
Adverse effects including bradycardia, hypotension, thrombocytopenia and abnormalities in coagulation tests may develop with cooling therapy (2). Currently, cooling therapy is generally applied in babies at and above the 35th gestational week because of adverse effects (2, 4, 12–14, 28). In this study, SHC or WBC therapy was applied only in the newborns above the 35th gestational week. Creation of a milder systemic hypothermia in the SHC method compared to the WBC method brings forth the expectation that side effects will also be observed with a lower rate (4, 15, 33, 34). However, recent short-term studies did not meet this expectation (27, 31, 35, 36). In our study, no difference was found between the SHC group and the WBC group in terms of adverse side effects. Sarkar et al. (35) examined organ function disorders in the 72-hour cooling period in babies with HIE who were applied WBC (n:28) or SHC (n:31) (36). In this study, no difference was found between the WBC group and the SHC group in terms of need for mechanical ventilation during cooling, ventilator variables in the babies who needed mechanical ventilation and the ability to perform extubation during cooling (35). Similarly, no difference was found between the groups in terms of mechanical ventilation requirement and the ability to perform extubation during cooling in our study. Again, Sarkar et al. (27) found no significant difference between the WBC and SHC groups in terms of persistent pulmonary hypertension, bleeding disorder requiring fresh frozen plasma, thromocytopenia and hypotension requiring vasopressor support, oliguria (<0.5 mL/kg/h), increase in serum creatinine level (>0.9 mg/dL) and electrolyte disorder. In our study, the WBC and SHC methods were compared in terms of similar adverse effects and no significant difference was found.
In one study performed by Hoque et al. (31), the effects of different cooling methods on temperature and hemodynamic stability were examined in newborns with HIE. Seventy tree newborns with HIE were included in this study and SHC was applied in 20 of these newborns by a manually controlled device, WBC was applied in 23 by a manually controlled device, WBC was applied in 28 by an autoregulatory device and WBC was applied in 2 by gloves filled with water. In this study, the rectal temperatures could be kept in the targeted narrow range by each of the cooling methods, but it was found that excessive cooling occurred with a higher rate in the beginning and the rectal temperature variability occurred with a higher rate with manually controlled methods compared to autoregulatory methods. In our study, SHC was applied by a manually controlled device and WBC was applied by cooling the environment; the rectal temperatures of the babies could be kept in the targeted range and no significant variation in the rectal temperature was observed with both methods. We think that this outcome is related with the fact that babies in whom cooling therapy is applied in our clinic are followed up very closely and rectal temperature changes are intervened in the early period. Hoque et al. (31) compared the mean arterial blood pressure values and heart rates of the babies in whom different cooling methods were applied and found no significant difference between the groups. In our study, the babies who were applied SHC or WBC were compared in terms of frequency of clinically significant hypotension or bradycardia and no significant difference was found between the groups. However, severe hypotension which did not respond to fluid and inotropic support developed in one baby in our study and the patient was removed from the study following discontinuation of cooling therapy. However, it is difficult to state that this was directly related with cooling therapy, because hypotension was not observed in multi-center randomized studies in which WBC was applied in a large number of babies and in which these patients were compared with patients who were not applied cooling (2, 12–14). Conclusively, the findings of our study and other studies showed that there was no difference between the SHC and WBC methods in terms of adverse effects. Therefore, if one of the two methods is to be preferred, this will be determined by the efficiency of the treatment method rather than the adverse effects. It is considerably difficult to compare the efficiencies of selective head cooling and WBC methods with current data. In selective head cooling method, the head is cooled actively and it is hoped that the brain will be protected more efficiently in this way (4). Animal studies have shown that the basal ganglion temperature can be reduced 5°C below the rectal temperature with SHC (13). Again, animal studies have shown that a difference of approximately 6°C occurs between the superficial parts and the deep parts of the brain with SHC and this difference has been found to be below 0.6°C with WBC (15). Therefore, it has been proposed that SHC would protect the brain cortex more efficiently compared to WBC (15). Rutherfold et al. (33) supported this hypothesis in a study. In this study, severe cortex lesions on brain magnetic resonance imaging were observed with a significantly lower rate in babies who were applied SHC compared to the WBC method. However, one recent study conducted by Sarkar et al. (37) did not support this finding and in contrast it was reported that brain damages were more frequent and more severe in the babies who were applied SHC compared to the WBC method. In the medical literature, the results obtained in studies which compare the efficiencies of SHC and C methods are controversial. In addition, comparison of the long-term outcomes of both cooling methods will contribute to achievement of more definite results. However, such a study has not been reported in the medical literature yet. In our study, the SHC and WBC methods were compared in terms of short-term results and no significant difference was found between the groups in terms of hospitalization time, discharge with tracheostomy or gastrostomy tube and mortality.
The most important limitation of our study was the low number of patients. More patients are needed to compare the efficiencies of the two cooling methods more strongly. Therefore, it was planned to continue enrollment of patients into the study and to compare the efficiencies of the two cooling methods again with larger numbers of patients and longer follow-up times.
Conclusively, no difference was found between the SHC and WBC methods in terms of adverse effects in our study. In addition, it was understood that there was also no difference between the babies with HIE who were applied SHC and WBC in terms of short-term results. Further studies with larger numbers of patients and longer follow-up times are needed to compare the efficiencies of the two cooling methods.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Written informed consent was obtained from the parent of the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.A., Y.Ç.; Design - A.A., Y.Ç.; Supervision - A.A. Y.Ç.; Kaynaklar - A.A, Y.Ç, S.G., A.H.T.; Materials - A.A., Y.Ç., S.G., A.H.T., Ç.O., M.A.S.; Data Collection and/or Processing - A.A. Y.Ç., S.G., A.H.T, Ç.O.; Analysis and/or Interpretation - A.A., Y.Ç, S.G., A.H.T., Ç.O.ß, M.A.S.; Literature Review - A.A., Y.Ç, S.G., A.H.T., Ç.O., M.A.S.; Writer - A.A. Y.Ç.; Critical Review - A.A., Y.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was funded by Mersin University Scientific Research Projects (Protocol no: BAP-TF (AHT) 2009-9).
References
- 1.Gardiner J, Wagh D, McMichael J, Hakeem M, Rao S. Outcomes of hypoxic ischaemic encephalopathy treated with therapeutic hypothermia using cool gel packs experience from Western Australia. Eur J Paediatr Neurol. 2014:391–8. doi: 10.1016/j.ejpn.2014.02.003. http://dx.doi.org/10.1016/j.ejpn.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;(1):CD003311. doi: 10.1002/14651858. Art No: CD003311. Pub3. http://dx.doi.org/10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takenouchi T, Iwata O, Nabetani M, Tamura M. Therapeutic hypothermia for neonatal encephalopathy: JSPNM & MHLW Japan working group practice guidelines consensus statement from the working group on therapeutic hypothermia for neonatal encephalopathy, Ministry of Health, Labor and Welfare (MHLW), Japan, and Japan Society for Perinatal and Neonatal Medicine (JSPNM) Brain Dev. 2012;34:165–70. doi: 10.1016/j.braindev.2011.06.009. http://dx.doi.org/10.1016/j.braindev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. http://dx.doi.org/10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 5.Al-Macki N, Miller SP, Hall N, Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr Neurol. 2009;41:399–405. doi: 10.1016/j.pediatrneurol.2009.06.001. http://dx.doi.org/10.1016/j.pediatrneurol.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Kracer B, Hintz SR, Van Meurs KP, Lee HC. Hypothermia therapy for neonatal hypoxic ischemic encephalopathy in the State of California. J Pediatr. 2014;165:267–73. doi: 10.1016/j.jpeds.2014.04.052. http://dx.doi.org/10.1016/j.jpeds.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen SL, Dejonge M, Kline A, et al. Optimizing therapeutic hypothermia for neonatal encephalopathy. Pediatrics. 2013;131:e591–603. doi: 10.1542/peds.2012-0891. http://dx.doi.org/10.1542/peds.2012-0891. [DOI] [PubMed] [Google Scholar]
- 8.Kasdorf E, Perlman JM. Strategies to prevent reperfusion injury to the brain following intrapartum hypoxia-ischemia. Semin Fetal Neonatal Med. 2013;18:379–84. doi: 10.1016/j.siny.2013.08.004. http://dx.doi.org/10.1016/j.siny.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. http://dx.doi.org/10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010:340–63. doi: 10.1136/bmj.c363. http://dx.doi.org/10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson DJ. Cool heads: ethical issues associated with therapeutic hypothermia for newborns. Acta Paediatr. 2009;98:217–20. doi: 10.1111/j.1651-2227.2008.01127.x. http://dx.doi.org/10.1111/j.1651-2227.2008.01127.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-Ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. http://dx.doi.org/10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 13.Azzopardi D, Brocklehurst P, Edwards D, et al. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. http://dx.doi.org/10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. http://dx.doi.org/10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 15.Gunn AJ, Gluckman PD. Head cooling for neonatal encephalopathy: the state of the art. Clin Obstet Gynecol. 2007;50:636–51. doi: 10.1097/GRF.0b013e31811ebe68. http://dx.doi.org/10.1097/GRF.0b013e31811ebe68. [DOI] [PubMed] [Google Scholar]
- 16.American Collge of Obstetricians and Gynecologists (ACOG) Neonatal encephalopathy and cerebral palsy: executive summary. Obstet Gynecol. 2004;103:780–1. doi: 10.1097/01.AOG.0000120142.83093.30. [DOI] [PubMed] [Google Scholar]
- 17.Levene MI, de Vries L. Hypoxic-ischemic ensephalopathy. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Neonatal-perinatal medicine. 8th ed. Philadelphia: Elsevier; 2006. pp. 938–56. [Google Scholar]
- 18.Azzopardi D, TOBY study group Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:80–2. doi: 10.1136/archdischild-2013-303710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn AR, Swingler GH, Myer L, Linley LL, Chandrasekaran M, Robertson NJ. Early clinical signs in neonates with hypoxic ischemic encephalopathy predict an abnormal amplitude-integrated electroencephalogram at age 6 hours. BMC Pediatr. 2013;13:52. doi: 10.1186/1471-2431-13-52. http://dx.doi.org/10.1186/1471-2431-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoresen M, Hellström-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. http://dx.doi.org/10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 21.Hellstrom-Westas L, de Vries LS, Rosen I. Atlas of amplitude-integrated EEGs in the newborn. 2nd ed. London, UK: Informa Healthcare; 2008. http://dx.doi.org/10.3109/9781439813898 [Google Scholar]
- 22.Levene MI. Cool treatment for birth asphyxia, but what’s next? Arch Dis Child Fetal Neonatal Ed. 2010;95:154–7. doi: 10.1136/adc.2009.165738. http://dx.doi.org/10.1136/adc.2009.165738. [DOI] [PubMed] [Google Scholar]
- 23.Azzopardi D, Strohm B, Edwards AD, et al. Steering Group and TOBY Cooling Register participants Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94:260–4. doi: 10.1136/adc.2008.146977. http://dx.doi.org/10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 24.Hagmann CF, Brotschi B, Bernet V, Latal B, Berger TM, Robertson NJ. Hypothermia for perinatal asphyxial encephalopathy. Swiss Med Wkly. 2011;141:w13145. doi: 10.4414/smw.2011.13145. http://dx.doi.org/10.4414/smw.2011.13145. [DOI] [PubMed] [Google Scholar]
- 25.Austin T, Shanmugalingam S, Clarke P. To cool or not to cool? Hypothermia treatment outside trial criteria. Arch Dis Child Fetal Neonatal Ed. 2013;98:451–3. doi: 10.1136/archdischild-2012-302069. http://dx.doi.org/10.1136/archdischild-2012-302069. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar S, Donn SM, Bhagat I, Dechert RE, Barks JD. Esophageal and rectal temperatures as estimates of core temperature during therapeutic whole-body hypothermia. J Pediatr. 2013;162:208–10. doi: 10.1016/j.jpeds.2012.08.039. http://dx.doi.org/10.1016/j.jpeds.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol. 2009;29:558–63. doi: 10.1038/jp.2009.37. http://dx.doi.org/10.1038/jp.2009.37. [DOI] [PubMed] [Google Scholar]
- 28.Peliowski-Davidovich A, Canadian Paediatric Society, Fetus and Newborn Committee Hypothermia for newborns with hypoxic ischemic encephalopathy. Paediatr Child Health. 2012;17:41–6. doi: 10.1093/pch/17.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhary R, Farrer K, Broster S, McRitchie L, Austin T. Active versus passive cooling during neonatal transport. Pediatrics. 2013;132:841–6. doi: 10.1542/peds.2013-1686. http://dx.doi.org/10.1542/peds.2013-1686. [DOI] [PubMed] [Google Scholar]
- 30.Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ, Cooling on Retrieval Study Group Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:408–12. doi: 10.1136/adc.2010.187211. http://dx.doi.org/10.1136/adc.2010.187211. [DOI] [PubMed] [Google Scholar]
- 31.Hoque N, Chakkarapani E, Liu X, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics. 2010;126:e124–30. doi: 10.1542/peds.2009-2995. http://dx.doi.org/10.1542/peds.2009-2995. [DOI] [PubMed] [Google Scholar]
- 32.Daetwyler K, Brotschi B, Berger TM, Wagner BP. Feasibility and safety of passive cooling in a cohort of asphyxiated newborn infants. Swiss Med Wkly. 2013;143:w13767. doi: 10.4414/smw.2013.13767. http://dx.doi.org/10.4414/smw.2013.13767. [DOI] [PubMed] [Google Scholar]
- 33.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–6. doi: 10.1542/peds.2005-0328. http://dx.doi.org/10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 34.Tooley J, Satas S, Eagle R, Silver IA, Thoresen M. Significant selective head cooling can be maintained long-term after global hypoxia ischemia in newborn piglets. Pediatrics. 2002;109:643–9. doi: 10.1542/peds.109.4.643. http://dx.doi.org/10.1542/peds.109.4.643. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar S, Barks JD, Bhagat I, Dechert R, Donn SM. Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns: whole body versus selective head cooling. Am J Perinatol. 2009;26:265–70. doi: 10.1055/s-0028-1103154. http://dx.doi.org/10.1055/s-0028-1103154. [DOI] [PubMed] [Google Scholar]
- 36.Allen KA. Moderate hypothermia: is selective head cooling or whole body cooling better? Adv Neonatal Care. 2014;14:113–8. doi: 10.1097/ANC.0000000000000059. http://dx.doi.org/10.1097/ANC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S, Donn SM, Bapuraj JR, Bhagat I, Barks JD. Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: selective head versus whole body cooling. Arch Dis Child Fetal Neonatal Ed. 2012;97:3. doi: 10.1136/fetalneonatal-2011-300964. http://dx.doi.org/10.1136/fetalneonatal-2011-300964. [DOI] [PubMed] [Google Scholar]