Abstract
In fetal mammals, serum levels of both total and ionized calcium significantly exceed those in the adult. This relative fetal hypercalcaemia is crucial for skeletal development and is maintained irrespectively of maternal serum calcium levels. Elegant studies by Kovacs and Kronenberg have previously addressed the role of the CaSR in creating and maintaining this relative fetal hypercalcaemia, through the regulation of parathyroid hormone-related peptide secretion. More recently we have shown that the CaSR is widely distributed throughout the developing fetus, where the receptor plays major, unexpected roles in ensuring growth and maturation of several organs. In this article, we present evidence for a role of the CaSR in the control of skeletal development, and how fetal hypercalcaemia, acting through the CaSR, regulates lung development.
Keywords: calcium-sensing receptor, fetal hypercalcemia, skeletal development, fetal lung development, growth plate cartilage, osteoblasts, RANK ligand
Calcium, the CaSR and fetal calcium homeostasis
Extracellular Ca2+ (Ca2+ o) homeostasis is organized and regulated quite differently in the fetus when compared to the adult and enables the fetus to meet the demands of intrauterine skeletal growth. In humans, up to 30 g of Ca2+ is actively transported from the mother to the fetus through the placenta, mostly during the last trimester [1] and requires a short-term reset of maternal calcium metabolism and its regulatory hormones with no long-term consequences for maternal skeletal integrity [1]. Feto-maternal adaptations are intrinsically difficult to investigate in humans, and our knowledge is limited to analyses of cord blood samples and skeletal mineral content of aborted fetuses [1]. Several mammalian models have been developed and have focused on rodents (mice and rats) or sheep. However, differences in litter size, placental anatomy and gestational period have led to divergent results [1].
Species differences notwithstanding, fetal serum total and ionized Ca2+ levels are higher than those set and maintained by the calcium-sensing receptor (CaSR) in the adult [1]. Active transport of Ca2+ to the fetus and fetal hypercalcemia are retained even in the context of maternal Ca2+ deficiency [2] [3]. Maternal serum PTHrP levels are substantially increased due to production in the placenta [4]. Murine models of targeted gene deletion have demonstrated that the CaSR negatively regulates fetal PTH secretion while promoting fetal PTHrP production, and that CaSR ablation exacerbates fetal hypercalcemia and promotes bone loss, increased serum PTH and increased 1,25-dihydroxyvitamin D, decreased placental Ca2+ transport and increased urinary Ca2+ excretion [5]. Evidence gathered over the last five years suggests that, in addition to regulating fetal PTH and PTHrP secretion, the CaSR plays major roles in developing bone and cartilage, and promotes optimal development of the lung, sympathetic nervous system and various other organs and tissues. Finally, it should be noted that relative fetal hypercalcemia should increase the activity of the CaSR. Previous studies show that, in the fetus, CaSR regulation occurs at the level of receptor expression, rather than through physiological changes in Ca2+ o concentration, as those seen in the adult. In this manner, spatio-temporally restricted CaSR expression allows for receptor regulation of events occurring at well-defined time points [6, 7]. This is the case for the developing lung in which, in the context of hypercalcemia, CaSR expression delays the process of branching morphogenesis and promotes distension arising from luminal fluid secretion [6], and in the sympathetic nervous system, in which upregulated CaSR expression during the perinatal period promotes neurite outgrowth and target tissue innervation by sympathetic neurons [7]. For brevity, this chapter focuses on the CaSR in the developing skeleton and lung.
CaSR in the Developing Skeletal Tissue
The CaSR and Growth Plate Development
Skeletal development in vertebrates begins in the early stages of embryogenesis and continues postnatally until peak bone mass is achieved in adulthood. Longitudinal bone growth is mediated by endochondral bone formation, which is initiated by condensation of mesenchymal progenitors in embryos and their commitment to the chondrocytic lineage to form cartilaginous anlagen [8]. Chondrocytes in this structure proliferate and produce extracellular matrix to form the growth plate.
In the growth plate (GP), chondrocytes proliferate, mature, hypertrophy, and reach terminal differentiation in a highly organized manner and then deposit Ca2+/phosphate-containing mineral in the surrounding matrix (Figure 1A). Within this mineralized matrix, growth plate chondrocytes (GPCs) release growth factors to induce vascular invasion and to guide the differentiation of incoming osteoblast (OB) and osteoclast (OC) progenitors whose respective bone forming and bone resorbing activities replace cartilage with bone at the chondro-osseous junction in the metaphysis [8] . GPs continue to support longitudinal bone growth through cycles of the steps described above until they eventually close with resultant cessation of growth in adulthood.
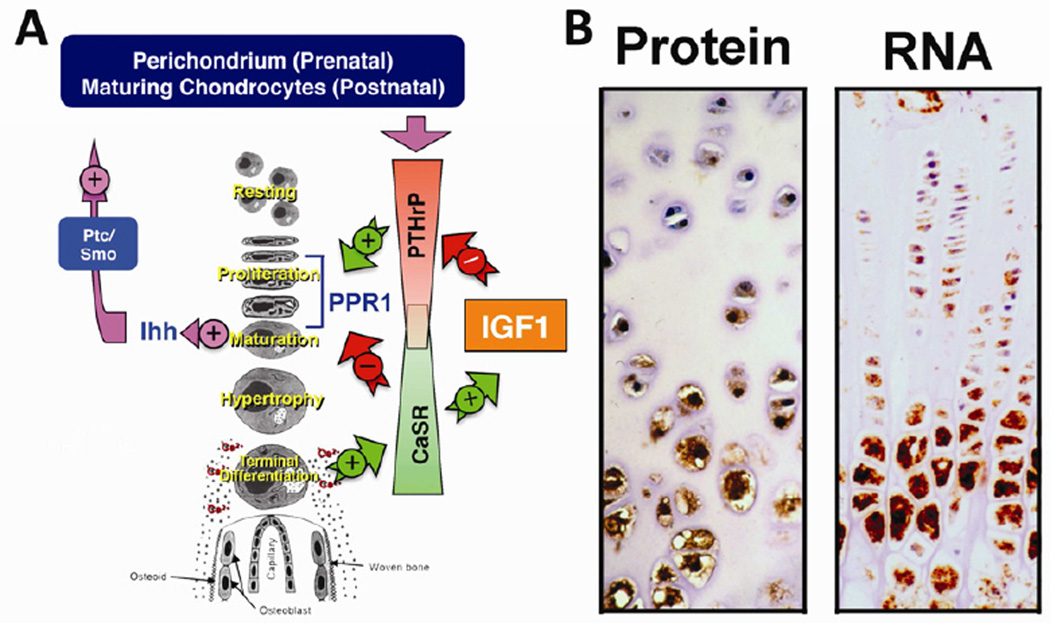
Figure 1. Roles of the CaSR in skeletal development.
(A) A scheme for growth plate chondrocyte differentiation and its regulation by PTHrP/Ihh, IGF1/IGF1R, and Ca2+/CaSR signaling pathways. (B) Expression of CaSR protein and RNA in growth plate chondrocytes assessed by immunohistochemistry and in situ hybridization. See the main text for detailed descriptions.
Accelerated or delayed chondrocyte differentiation leads to disorganized GPs and retarded bone growth. Various endocrine and paracrine factors control the pace of chondrocyte differentiation and GP development. Among them, a PTHrP/Indian Hedgehog (PTHrP/Ihh) feedback loop is a well-established autocrine/paracrine pathway that prevents aberrant acceleration of chondrocyte differentiation and early closure of the GP [8] (Figure 1A). PTHrP is synthesized by cells of the perichondrium in the fetal skeleton and by maturing/prehypertrophic chondrocytes during postnatal growth and diffuses into the proliferative zone to activate type 1 PTH/PTHrP receptors (PPR1s) in proliferating chondrocytes to sustain cellular growth and delay maturation [9] (Figure 1A). As chondrocytes mature they release Ihh. Ihh acts via receptors known as ‘Patched’ (ptch) in neighboring cells to increase PTHrP production and thus slow cell differentiation [10]. Mice with knockouts (KO) of either PPR1, PTHrP, or Ihh genes exhibit accelerated chondrocyte differentiation and early closure of the GP [8]. On the other hand, in mature chondrocytes, insulin-like growth factor 1 (IGF1) and IGF1 receptor (IGF1R) constitute a mechanism that counteracts PTHrP/PPR1/Ihh actions to promote hypertrophic chondrocyte differentiation and matrix mineralization [11]. Recent studies suggest that Ca2+ and the CaSR could be key modulators of the mechanism.
Ca2+ o critically supports terminal differentiation of chondrocytes and normal GP development. Ca2+ o deficiency produces childhood rickets [12] as a result of delayed chondrocyte differentiation and reduced matrix synthesis and mineralization in their GPs, and these defects could be reversed with dietary calcium supplements [13]. Similarly, the severe rickets seen in vitamin D receptor (VDR) and Cyp27b1 KO mice was healed by a high Ca2+ diet [14, 15], suggesting that Ca2+ availability may be important in normal GP development. In addition, in vitro studies on chondrogenic cell lines or primary GP chondrocytes demonstrated the existence of Ca2+ o-stimulated G protein-mediated signaling responses that promoted terminal differentiation [16–20].
In vitro and in vivo studies have concluded that the CaSR mediates extracellular Ca2+-sensing in chondrocytes [16–20]. In the GP, the CaSR has been detected in maturing chondrocytes and its expression increases as the cells hypertrophy ([17]; Figure 1B). This expression pattern suggests a role for the CaSR in mediating terminal differentiation. In support of this concept, knockdown of the CaSR by RNAi impaired high Ca2+ o-induced cell differentiation and matrix mineralization in cultured chondrocytes [18]. Furthermore, mice with chondrocyte-specific ablation of the CaSR gene developed a shorter, undermineralized skeleton due to delayed differentiation of hypertrophic chondrocytes [16]. In addition, the expressions of IGF1 and IGF1R were profoundly reduced in hypertrophic chondrocytes from homozygous knockout mice [16], suggesting that Ca2+/CaSR promotes chondrocyte differentiation, at least in part, by enhancing IGF1 production and/or signaling. Consistent with this regulatory scheme, ablating IGF1R genes in cultured chondrocytes inhibited (by ≈50%) the ability of high Ca2+ o to enhance terminal differentiation and matrix mineralization. This study also demonstrated IGF1R-independent actions of Ca2+ o in promoting chondrocyte differentiation.
Do the Ca2+/CaSR and IGF1/IGF1R signaling mechanisms interact with the PTHrP/Ihh feedback loop? In cultured chondrocytes, raising Ca2+ o profoundly inhibited PTHrP and PPR1 expression and impaired PTHrP-induced suppression of cell differentiation and matrix mineralization [[19] and unpublished]. Interestingly, however, ablation of the IGF1R gene inhibited expression of PTHrP but not PPR1 [11]. These observations suggest a novel regulatory scheme in which Ca2+/CaSR signaling promotes chondrocyte differentiation and GP development by suppressing (i) PTHrP expression via an IGF1/IGF1R-dependent pathway and (ii) PPR1 expression independent of IGF1/IGF1R (Figure 1A).
The CaSR and Bone Development
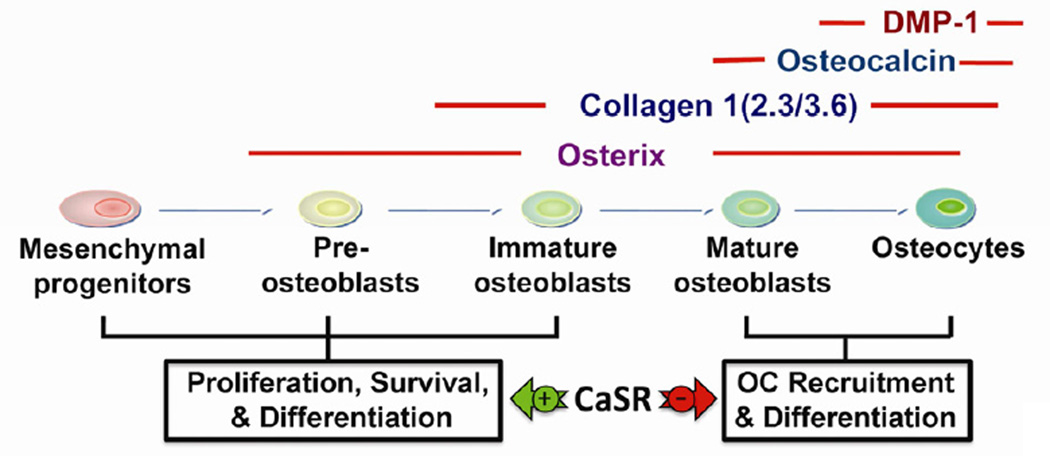
At the end of chondrogenesis, OCs are recruited to the chondro-osseous junction to resorb mineralized cartilage matrix and release cytokines to recruit mesenchymal progenitors and induce their differentiation into cells of the osteoblastic lineage. Different stages of OB differentiation are indexed by the expression of specific marker proteins (Figure 2). For example, osterix (Osx), type I collagen [Col(I)], osteocalcin (Ocn), and dentin matrix protein 1 (DMP1) can be used as markers of pre-OB, immature OB, mature OB, and osteocytes, respectively (Figure 2).
Figure 2. A scheme for the progression of osteoblast differentiation and expression of marker genes and their regulation by CaSR signaling pathways.
See the main text for detailed descriptions. OC: osteoclast. DMP-1: dentin matrix protein 1.
Various local and systemic factors, including Ca2+ o, modulate OB differentiation. The physiological significance of Ca2+ in bone development is accentuated by the manifestation of osteomalacia in patients with Ca2+ and/or vitamin D deficiency and in VDR- and Cyp27b1-null mice and by the ability of calcium supplements to heal these conditions [15]. Although, the impact of Ca2+ o on bone can be indirect through parathyroid hormone (PTH) or other endocrine factors [21], studies using cultured osteoblastic cell lines, bone marrow stromal cells, and bone-derived OBs and osteocytes have demonstrated direct actions of Ca2+ o to stimulate acute signaling responses and induce cell migration, proliferation, survival, differentiation and mineralization (see Reviews, [22–24]).
Although several studies have concluded that the CaSR mediates Ca2+ o-sensing in OBs, its role in bone development in vivo has been controversial. This was due to the lack of apparent skeletal defects in global CaSR−/− mice [25] that were rescued from severe hyperparathyroidism and hypercalcemia by concurrent deletion of the Gcm2 gene which specifies parathyroid development, or of the PreProPTH gene [26, 27]. It was later found that CaSR−/− mice produced a truncated CaSR with a deletion of 77 amino acids (encoded by the exon 5) in the extracellular domain. This alternatively spliced CaSR appears to compensate, at least in part, for the loss of the full-length CaSR in skin, bone, and lung [24].
A different gene targeting strategy was subsequently implemented to completely ablate CaSR functions in OBs in vivo by deleting exon 7 of the CaSR gene, which encodes the entire seven transmembrane domain and C-terminal tail of the receptor that are essential for its expression and function. A floxed-CaSR mouse, which carries lox-P sequences flanking exon 7 of the CaSR [16], was bred with transgenic mice expressing bacterial P1-Cre recombinase under the control of Osx, Col(I) (2.3 and 3.6 kb), Ocn, or Dmp1 promoters to generate OsxCaSR-KO, 2.3ColCaSR-KO, 3.6ColCaSR-KO, OcnCaSR-KO, and DmpCaSR-KO mice, which had CaSR genes knocked out in their preOBs, immature OBs, mature OBs, and osteocytes, respectively [16, 28–30]. Studies of these animals not only confirmed the biological significance of the CaSR in bone development, but also revealed its functions at specific stages of OB differentiation.
Deleting the CaSR in early OBs in OsxCaSR-KO, 2.3ColCaSR-KO, and 3.6ColCaSR-KO mice severely retarded post-natal bone development. These mice fail to thrive and died before 3–4 weeks of age in association with multiple skeletal fractures, inactivity and inadequate feeding [16, 29]. Although all skeletal elements were present in these mice, the bones were soft and the internal matrices were severely disorganized and undermineralized with large amounts of immature osteoid [16, 29]. These bone defects were associated with decreased OB number, aberrant mineralizing activities, and increased OC number and activity [16, 29]. In addition the OBs manifested enhanced apoptosis and reduced mineralization capability [16]. These results indicate that the CaSR is essential for the proliferation, survival, and maturation of pre-OB, consistent with findings from in vitro studies with cultured OBs and observations of osteomalacia in calcium- and vitamin D-deficient patients.
On the other hand, OcnCaSR-KO and DmpCaSR-KO mice were viable and developed into adulthood. While most osteoblastic parameters were not impacted in these KO mice, their bones were severely osteoporotic [16, 28, 30] associated with increased OC number and activity together with increased expression of receptor activator of nuclear factor kappa-B ligand (RANKL) and decreased expression of osteoprotegerin (OPG) (manuscript in preparation). These observations support a role for the CaSR in mature OBs in the negative modulation of OC recruitment and differentiation via RANKL/OPG signaling. We propose that the CaSR exerts bone anabolism in conditions of calcium abundance by promoting commitment, survival, and differentiation of early OBs, and by suppressing local RANKL-dependent control of osteoclastogenesis (Figure 2).
Therapeutic Potential for The CaSR in Cartilage and Bone
The demonstration that the CaSR has important anabolic actions in bone and cartilage raise the question of whether specific compounds can be devised to target this receptor for direct skeletal treatment without off-target effects on other CaSR-expressing tissues. Based on their cDNA sequences, the CaSRs in OBs and chondrocytes are identical to that expressed in parathyroid glands [17, 20]. However, immunoblotting demonstrated distinct glycosylation patterns in chondrocytes and OBs compared to those in parathyroid cells and HEK-293 cells expressing CaSR cDNA heterologously [17]. This difference in post-translational modification may contribute to differential pharmacological profiles of the receptor at different anatomical sites, but this concept has not been formally addressed. Furthermore, the CaSR forms heteromeric complexes with type B γ-aminobutyric acid receptor (GABA-B-R) 1 and 2 in OBs and chondrocytes [31], and unpublished data]. In cultured chondrocytes, deleting the GABA-B-R1 gene significantly reduced Ca2+ o-stimulated acute signaling responses [31]. GABA-B-R1 and R2 are co-expressed with the CaSR in many tissues at various levels [31, 32]. It is conceivable that different stoichiometric interactions among these receptors, and perhaps with other members of family C GPCRs, could produce receptor complexes with distinct pharmacological characteristics. For example, CaSR RNA is expressed at levels about 100-fold higher than the level of GABA-B-R1 RNA in parathyroid glands (manuscript in preparation), favoring CaSR homomeric complexes. In contrast, GABA-B-R1 RNA levels are 10-fold higher than CaSR RNA levels in GPCs, potentially supporting the formation of CaSR/GABA-B-R1 heteromeric complexes. Thus differences in the formation of receptor complexes may provide opportunities for the design of tissue-specific compounds to enhance skeletal anabolism.
CaSR in the developing Lung
The Role of the CaSR in Fetal Lung Development
During embryonic life, the lungs are unnecessary as breathing organs; however they must be sufficiently developed to begin functioning immediately after birth. Hence, optimal postnatal lung function is reliant on a tightly controlled embryonic lung development program. The program has been divided into five stages [33, 34]: embryonic; pseudo-glandular; canalicular; terminal saccular; and alveolar.
The embryonic stage begins at embryonic day (E)9.5 in the mouse (~ week 3 of gestation in humans), with the formation of the trachea from the foregut endoderm, which is followed quickly by two primordial lung buds [35]. During the pseudoglandular stage (E9.5 – 16.5 in mice, weeks 5 – 17 in humans) the lung undergoes much of its budding and branching morphogenesis, and this is followed by the canalicular phase (E16.5 – 17.5 in mice, weeks 16 – 25 in humans), during which growth of the lung is accompanied by organisation of the vasculature along the airway. In the terminal saccular stage (E17.5 – postnatal (P)5 in mice, week 24 to term in humans) thinning of the airway walls starts, and the peripheral airways and gas-exchange surface areas enlarge to enable air exchange. Finally, in the alveolar stage (P5 – P30 in mice, term to 18 months postnatally in humans) the alveoli form, to provide the major gas exchange surface [33, 34].
Branching morphogenesis, which occurs mainly in the pseudoglandular stage, is a crucial part of lung development. Despite the apparent complexities of lung branching, the budding patterns at early stages take place through only three simple genetically-encoded modes of branching termed domain-branching, planar bifurcation, and orthogonal bifurcation [33, 35, 36], which are continuously repeated to form various branching compositions. These processes are modulated by interactions between intrinsic signals, receptor proteins, intracellular signaling pathways, and transcription factors (reviewed in depth in Ref [37]), and disruptions to these interactions have a negative impact on lung development and function [38].
Several studies have identified a role for Ca2+ in lung development. Roman [39] demonstrated that the L-type calcium channel blocker, nifedipine, impaired branching morphogenesis and abolished spontaneous airway contractions, indicating that Ca2+ influx is necessary for normal lung development. Further work by Jesudason et al. [40] confirmed these observations and demonstrated that nifedipine abolishes both peristaltic smooth muscle contractions and lung growth mediated by Fibroblast Growth Factor-10 (FGF-10), a critical intrinsic factor in mammalian lung development [41].
Peristaltic waves initiated by pacemaker areas within the proximal airway [40] produce pulses of fluid directed to the distal lung bud and control intraluminal pressure through rhythmic phases of stretching and relaxation [42]. The frequency of these waves is positively associated with lung growth rates [40]. Measurements of intracellular Ca2+ (Ca2+ i) using Ca2+-sensitive fluorescent dyes have revealed that contractions are induced by regenerative Ca2+ i waves in airway smooth muscle cells that are dependent on both extra- and intracellular Ca2+ stores [43]. Interestingly, Ca2+ i waves are impaired in experimental models of lung hypoplasia [44].
The mouse lung culture model has been widely used for studies of embryonic lung development in vitro [45–47]. Lungs are dissected from E11.5 – E13.5 mouse embryos (i.e., at the pseudoglandular stage) and cultured for up to 96 h using chemically-defined, serum-free conditions. Interestingly, Ca2+ o in the culture media employed in studies of this type has varied widely in the range 1.05 – 2.50 mM, and has frequently differed substantially from the fetal serum ionized Ca2+ level (~ 1.60 mM) [1]. However, until recently, little was known regarding the role of Ca2+ o as a signal in lung development, or whether the CaSR, a key mediator of the actions of Ca2+ o, was expressed in the fetal lung. Initial observations revealed that the CaSR is, in fact, widely expressed in developing mouse and human fetal lungs, particularly in the pseudoglandular stage [6, 24]. Thus, we set out to determine whether changes in ambient Ca2+ o affect the lung development program focusing on branching morphogenesis, lung growth and vasculogenesis, and whether the CaSR might mediate its effects.
Using mouse E12.5 lung explant cultures [45, 46], we demonstrated that lung branching is sensitive to Ca2+ o over the range 0.5 – 2.0 mM [6]. Interestingly, higher Ca2+ o levels, similar to those seen in fetal blood (~ 1.70 mM), had a suppressive effect on branching over 48 h [6]. Furthermore, the effect was mimicked in lungs exposed to the calcimimetic NPS R-568 in the presence of 1.05 mM Ca2+ o, demonstrating that CaSR activation negatively modulates branching morphogenesis [6].
Similar to effects observed in other CaSR-expressing cell types [48, 49], exposure of isolated E12.5 lung buds to increased Ca2+ o or NPS R-568 induced elevations in Ca2+ i arising from release of internal calcium stores [6]. In addition, the PLC inhibitor U73122 blocked the suppressive effect of high Ca2+ o (1.70 mM) on branching morphogenesis, restoring branching to levels seen at low Ca2+ o (1.05 mM). A role for phosphoinositide 3-kinase (PI3K) was also identified in the CaSR-mediated pathway using the selective inhibitor LY294002 [6], however, inhibitors of several MAP kinases had no effect [6].
How does the CaSR suppress branching morphogenesis? Previous studies have shown that the CaSR negatively controls proliferation of several cell types including parathyroid cells [50, 51] and colonic epithelial cells [52]. Similarly, culturing E12.5 lung explants at 1.70 mM Ca2+ o approximately halved the number of cells that were positive for phospho-histone H3 [6], used as a marker of mitosis [53]. Thus, CaSR-mediated suppression of the lung branching program arises from negative control of cellular proliferation and requires PLC-dependent Ca2+ i release and activation of PI3K.
Fetal lung growth is also reliant on fluid secretion into the developing lumen [54] and defective or excessive fluid secretion results in hypo- or hyperplastic lungs, respectively [33]. Fluid secretion is driven by secondary active Cl− transport [55], with basolateral entry of Cloccurring via Na+-K+-2Cl− cotransporter (NKCC1) [56, 57]. Cl− influx raises its intracellular concentration above equilibrium to facilitate its luminal exit through apical Cl− channels [58], followed by iso-osmotic water transport [55]. Although several Cl− channels are expressed in lung epithelial cells including the cystic fibrosis conductance regulator (CFTR) [59] and several members of the Chloride Channel (CLC) family [60], the identities of the channels are not yet clear.
Secretion of Cl− into the lumen supports a negative transepithelial potential difference (PD) that is proportional to the rate of fluid secretion [6]. E12.5 mouse lungs cultured in medium containing 1.7 mM Ca2+ o, or 1 mM Ca2+ o plus NPS R-568, exhibited increased luminal fluid volume and greater distension when compared to lungs cultured in medium containing 1.0 mM Ca2+ o [6] together with a significantly more negative PD. These results suggest that CaSR activation stimulates transepithelial fluid transport either via NKCC1 or an apical Clchannel. It has been previously suggested that fluid accumulation leading to lung distension, may act to block airway branching [61]. However, lungs that were cultured for 24 h in 1.70 mM Ca2+ o and then switched to low Ca2+ o, responded with enhanced branching with no change in transepithelial PD [6]. Thus, branching morphogenesis and fluid secretion are controlled by distinct programs, both of which respond to CaSR activation.
Vasculogenesis is another essential requisite of functional lungs, developing in symmetry with branching morphogenesis, so that optimal gaseous exchange can take place postnatally. Interestingly, our recent results show that, in E12.5 mouse lung explants, vasculogenesis during the pseudoglandular stage is also Ca2+ o-dependent, with expression of Flk-1 (the vascular endothelial growth factor (VEFG) cognate receptor) being significantly increased in lung explants exposed to medium containing 1.7 mM [Ca2+]o compared to that seen in those cultured in medium containing 1.05 mM [Ca2+]o. However, calcimimetics were unable to mimic the effects of the higher [Ca2+]o on vasculogenesis, while still inhibiting branching morphogenesis, suggesting that these effects are CaSR-independent (Brennan et al, unpublished observations).
Therapeutic Potential and Clinical Significance of the CaSR in the Developing Lung
As detailed above, Ca2+ o, acting via the CaSR, acts as a key factor in the control of lung branching morphogenesis [6], and stimulates fluid secretion. In this way, branching morphogenesis is matched by appropriate luminal distension and Ca2+ o, independent of the CaSR, promotes new vessel formation to ensure optimal lung development. Pharmacological modulators of the CaSR may be effective in the treatment of defective lung development, particularly under conditions where the lungs are hypoplastic due to delayed branching morphogenesis. The results of ‘rescue’ experiments in which fetal lung buds were incubated for 24 h in either low or high Ca2+ o then for a further 24 h in high or low Ca2+ o indicate that, once the lung branching program has been switched on (e.g., by exposure to low Ca2+ o), it may not be arrested. It may be possible, however, to force under-developed lungs to start branching in response to a calcilytic that suppresses CaSR-mediated signalling in lung epithelial progenitor cells.
Respiratory problems have been observed previously in patients with CaSR mutations. Thus, inactivating mutations of the CaSR have previously been linked with chronic and interstitial lung disease and a reduction in gas exchange [62–64]. It is possible that in these cases fetal hypercalcemia was unable to control excessive proliferation, leading to pulmonary hyperplasia. Conversely, activating mutations of the CaSR have been linked to ectopic calcification of the mouse lung [65] and recurring upper respiratory tract infections in humans [66]. Further studies are required to properly define the incidence of respiratory pathologies in kindreds with inactivating/activating mutations of the CaSR. An attempt has been made to examine the effect of ablation of the CaSR in the development of the lung, using the exon 5-deleted CaSR knockout mouse model (CaSREx5−/−) [25, 67]. However, as has been previously reported in keratinocytes [68, 69] and growth plate chrondrocytes [20], knocking out CaSR exon-5 induced the expression of a functional exon 5-less spliced variant, which effectively rescued the phenotype [67]. Lung explants from E12.5 CaSREx5−/− mice responded to high [Ca2+]o as well as the calcimimetic NPS R-568 with suppressed branching morphogenesis in a fashion similar to their wild type CaSREx5+/+ littermates [67] and as previously reported for normal mice [6].
Therefore, further studies are required to fully determine the effects of aberrant CaSR function on lung development and its potential post-natal consequences. Nevertheless, the CaSR has been identified as an important regulator of lung development, both directly, by helping to suppress lung branching morphogenesis while stimulating lumen distension, and indirectly, by contributing to the generation and maintenance of relative fetal hypercalcaemia, which in turn promotes new blood vessel formation. The results suggest that pharmacological modulators of the CaSR have potential use as novel therapeutics in the treatment of hypo- or hyper-plastic lungs.
Summary
The CaSR is a master controller of skeletal development, where it exerts direct anabolic actions on both bone and cartilage. Furthermore, by promoting placental secretion of PTHrP, the CaSR resets the fetal “calciostat” to a level, which is above that seen in the adult. Studies using genetic, molecular and pharmacological manipulation of the CaSR have shown that this hypercalcemic fetal environment promotes optimal maturation of organs including the lungs and even neuronal networks including the peripheral nervous system. In addition, CaSR expression is found in the fetal kidney and intestine, as well as the central nervous system and heart (25). Further studies are required to evaluate whether the CaSR mediates Ca2+ o-dependent fetal developmental programs for any other tissues. Thus, new tissue-specific murine models of CaSR deletion may cast new light on additional developmental roles of the CaSR, and provide new avenues for calcimimetic/calcilytic therapy to rescue impaired or premature organ development.
Figure 3. Regulation of fetal lung development by Ca2+ o and the CaSR.
Ca2+ o and the CaSR are both important regulators of fetal lung development. The relatively hypercalcemic environment of the embryo leads to activation of the CaSR which A, suppresses lung branching morphogenesis through the control of cellular proliferation and B, increases Cl− driven fluid secretion, demonstrated by a more negative transepithelial PD (44), possibly through interactions with basolateral and/or apical chloride channels, leading to greater lumen distension after 42 h (44). In addition, C, in vitro experiments have demonstrated that fetal [Ca2+]o also promotes the increased expression of the VEGF receptor Flk-1, as shown by the β-galactosidase staining in Flk-1Lacz+/− mice, indicative of increased vasculogenesis (Brennan et al., unpublished observations). Lung figure is adapted from [70].
Research Agenda.
Development of osteoblast/osteoclast-specific modulators/agonists of the CaSR for the treatment of skeletal conditions
Studies to examine the effect of CaSR mutations on the predisposition to respiratory conditions
The potential for CaSR modulators to be used as medical intervention in cases of lung hypo- or hyper-plasia
Examining the role of CaSR activation in the developmental programme of other organs where the CaSR is expressed (e.g. kidney, intestine and central nervous system)
Acknowledgements
This work was supported by the Marie Curie Initial Training Network Multifaceted CaSR (FP7-264663 to DR), by the UK BBSRC (BB/D01591X to DR), by US NIH grant (RO1-AG21353 to WC) and by the US Department of Veterans Affairs Merit Review grant and Program Project Award (to WC). SCB is a Marie Curie Experienced Researcher.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795–826. doi: 10.1016/j.ecl.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Oberst WF, Plass ED. The Variations in Serum Calcium, Protein, and Inorganic Phosphorus in Early and Late Pregnancy, during Parturition and the Puerperium, and in Non-Pregnant Women. J Clin Invest. 1932;11(1):123–127. doi: 10.1172/JCI100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senior PV, Heath DA, Beck F. Expression of parathyroid hormone-related protein mRNA in the rat before birth: demonstration by hybridization histochemistry. J Mol Endocrinol. 1991;6(3):281–290. doi: 10.1677/jme.0.0060281. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs CS, Ho-Pao CL, Hunzelman JL, Lanske B, Fox J, Seidman JG, Seidman CE, Kronenberg HM. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. Journal of Clinical Investigation. 1998;101(12):2812–2820. doi: 10.1172/JCI2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. The Journal of physiology. 2008;586(Pt 24):6007–6019. doi: 10.1113/jphysiol.2008.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vizard TN, O'Keeffe GW, Gutierrez H, Kos CH, Riccardi D, Davies AM. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat Neurosci. 2008;11(3):285–291. doi: 10.1038/nn2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 9.Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A. 1998;95(22):13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127(3):543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, Clemens TL, Bikle DD, Chang W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011;26(7):1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thacher TD. Calcium-deficiency rickets. Endocr Dev. 2003;6:105–125. doi: 10.1159/000072773. [DOI] [PubMed] [Google Scholar]
- 14.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140(11):4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 15.Goltzman D, Miao D, Panda DK, Hendy GN. Effects of calcium and of the Vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol. 2004;89–90(1–5):485–489. doi: 10.1016/j.jsbmb.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calciumsensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1(35):ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA, Miller S, Shoback D. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999;140(12):5883–5893. doi: 10.1210/endo.140.12.7190. [DOI] [PubMed] [Google Scholar]
- 18.Chang W, Tu C, Pratt S, Chen TH, Shoback D. Extracellular Ca(2+)-sensing receptors modulate matrix production and mineralization in chondrogenic RCJ3.1C5.18 cells. Endocrinology. 2002;143(4):1467–1474. doi: 10.1210/endo.143.4.8709. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology. 2005a;146(11):4597–4608. doi: 10.1210/en.2005-0437. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, Chang W. Expression and functional assessment of an alternatively spliced extracellular Ca2+- sensing receptor in growth plate chondrocytes. Endocrinology. 2005b;146(12):5294–5303. doi: 10.1210/en.2005-0256. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, Liang N, Chen TH, Li A, Maria CS, You M, Ho H, Song F, Bikle D, Tu C, Shoback D, Chang W. Sex and age modify biochemical and skeletal manifestations of chronic hyperparathyroidism by altering target organ responses to Ca(2+) and PTH in mice. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010;46(3):571–576. doi: 10.1016/j.bone.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak MM, Riccardi D. Ca2+ as an extracellular signal in bone. Cell Calcium. 2004;35(3):249–255. doi: 10.1016/j.ceca.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 25.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11(4):389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 26.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111(7):1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111(7):1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang W, Cheng Z, Chen TH, You M, Li A, Tu C, Santa Maria C, Shoback D. Conditional Ca2+-sensing receptor knockouts in mature osteoblasts and osteocytes reveal critical roles for this molecule in maintenance of bone mass and facture healing. J Bone Miner Res. 2011;26(Suppl 1) Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=51d4e88b-f79d-47e2-a15b-134f0c57b52e. [Google Scholar]
- 29.Dvorak-Ewell MM, Chen TH, Liang N, Garvey C, Liu B, Tu C, Chang W, Bikle DD, Shoback DM. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26(12):2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang N, Chen TH, Cheng Z, Li A, Santa Maria C, Tu C, Chang W, Shoback D. Calcium-sensing receptors (CaSRs) in mature osteoblasts regulate bone formation and maintenance of bone mass: studies in osteocalcin (OCN) conditional knockout mice. J Bone Miner Res. 2012;27(Suppl 1) Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=51d4e88b-f79d-47e2-a15b-134f0c57b52e. [Google Scholar]
- 31.Cheng Z, Tu C, Rodriguez L, Chen TH, Dvorak MM, Margeta M, Gassmann M, Bettler B, Shoback D, Chang W. Type B gamma-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148(10):4984–4992. doi: 10.1210/en.2007-0653. [DOI] [PubMed] [Google Scholar]
- 32.Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, Bettler B, Margeta M, Jan LY, Shoback D. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282(34):25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 33.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13(Spec No 2):R207–R215. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- 35.Hogan BL. Morphogenesis. Cell. 1999;96(2):225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 36.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453(7196):745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57(5 Pt 2):26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 38.Warburton D, Olver BE. Coordination of genetic, epigenetic, and environmental factors in lung development, injury, and repair. Chest. 1997;111(6 Suppl):119S–122S. doi: 10.1378/chest.111.6_supplement.119s. [DOI] [PubMed] [Google Scholar]
- 39.Roman J. Effects of calcium channel blockade on mammalian lung branching morphogenesis. Exp Lung Res. 1995;21(4):489–502. doi: 10.3109/01902149509031754. [DOI] [PubMed] [Google Scholar]
- 40.Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD. Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am J Respir Cell Mol Biol. 2005;32(2):118–127. doi: 10.1165/rcmb.2004-0304OC. [DOI] [PubMed] [Google Scholar]
- 41.Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol. 1998;201(2):125–134. doi: 10.1006/dbio.1998.8994. [DOI] [PubMed] [Google Scholar]
- 42.Jesudason EC. Airway smooth muscle: an architect of the lung? Thorax. 2009;64(6):541–545. doi: 10.1136/thx.2008.107094. [DOI] [PubMed] [Google Scholar]
- 43.Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol. 2005;33(2):153–160. doi: 10.1165/rcmb.2005-0137OC. [DOI] [PubMed] [Google Scholar]
- 44.Featherstone NC, Connell MG, Fernig DG, Wray S, Burdyga TV, Losty PD, Jesudason EC. Airway smooth muscle dysfunction precedes teratogenic congenital diaphragmatic hernia and may contribute to hypoplastic lung morphogenesis. Am J Respir Cell Mol Biol. 2006;35(5):571–578. doi: 10.1165/rcmb.2006-0079OC. [DOI] [PubMed] [Google Scholar]
- 45.Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290(1):177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 46.De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277(2):316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Jaskoll TF, Don-Wheeler G, Johnson R, Slavkin HC. Embryonic mouse lung morphogenesis and type II cytodifferentiation in serumless, chemically defined medium using prolonged in vitro cultures. Cell Differ. 1988;24(2):105–117. doi: 10.1016/0045-6039(88)90062-0. [DOI] [PubMed] [Google Scholar]
- 48.Brennan SC, Conigrave AD. Regulation of cellular signal transduction pathways by the extracellular calcium-sensing receptor. Curr Pharm Biotechnol. 2009;10(3):270–281. doi: 10.2174/138920109787847484. [DOI] [PubMed] [Google Scholar]
- 49.Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35( 3):217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Miller G, Davis J, Shatzen E, Colloton M, Martin D, Henley CM. Cinacalcet HCl prevents development of parathyroid gland hyperplasia and reverses established parathyroid gland hyperplasia in a rodent model of CKD. Nephrol Dial Transplant. 2012;27(6):2198–2205. doi: 10.1093/ndt/gfr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riccardi D, Martin D. The Role of the Calcium-Sensing Receptor in the Pathophysiology of Secondary Hyperparathyroidism. NDT Plus. 2008;1(suppl 1):i7–i11. doi: 10.1093/ndtplus/sfm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol. 2010;225(1):73–83. doi: 10.1002/jcp.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K. Two mitosisspecific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol. 2006;30(1):83–89. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- 54.Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86(4):1270–1277. doi: 10.1172/JCI114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974;241(2):327–357. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walters DV, Olver RE. Liquids in the lung. Respir Physiol Neurobiol. 2007;159( 3):245–246. doi: 10.1016/j.resp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2- chloride transporter. Am J Respir Cell Mol Biol. 2001;25(1):14–20. doi: 10.1165/ajrcmb.25.1.4500. [DOI] [PubMed] [Google Scholar]
- 58.McCray PB, Jr, Bettencourt JD, Bastacky J. Secretion of lung fluid by the developing fetal rat alveolar epithelium in organ culture. Am J Respir Cell Mol Biol. 1992;6(6):609–616. doi: 10.1165/ajrcmb/6.6.609. [DOI] [PubMed] [Google Scholar]
- 59.McCray PB, Jr, Reenstra WW, Louie E, Johnson J, Bettencourt JD, Bastacky J. Expression of CFTR and presence of cAMP-mediated fluid secretion in human fetal lung. Am J Physiol. 1992;262(4 Pt 1):L472–L481. doi: 10.1152/ajplung.1992.262.4.L472. [DOI] [PubMed] [Google Scholar]
- 60.Blaisdell CJ, Morales MM, Andrade AC, Bamford P, Wasicko M, Welling P. Inhibition of CLC-2 chloride channel expression interrupts expansion of fetal lung cysts. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L420–L426. doi: 10.1152/ajplung.00113.2003. [DOI] [PubMed] [Google Scholar]
- 61.Nogawa H, Hasegawa Y. Sucrose stimulates branching morphogenesis of embryonic mouse lung in vitro: a problem of osmotic balance between lumen fluid and culture medium. Dev Growth Differ. 2002;44(5):383–390. doi: 10.1046/j.1440-169x.2002.00651.x. [DOI] [PubMed] [Google Scholar]
- 62.Auwerx J, Boogaerts M, Ceuppens JL, Demedts M. Defective host defence mechanisms in a family with hypocalciuric hypercalcaemia and coexisting interstitial lung disease. Clinical and experimental immunology. 1985b;62(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- 63.Auwerx J, Demedts M, Bouillon R, Desmet J. Coexistence of hypocalciuric hypercalcaemia and interstitial lung disease in a family: a cross-sectional study. European journal of clinical investigation. 1985a;15(1):6–14. doi: 10.1111/j.1365-2362.1985.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 64.Fox L, Sadowsky J, Pringle KP, Kidd A, Murdoch J, Cole DE, Wiltshire E. Neonatal hyperparathyroidism and pamidronate therapy in an extremely premature infant. Pediatrics. 2007;120(5):e1350–e1354. doi: 10.1542/peds.2006-3209. [DOI] [PubMed] [Google Scholar]
- 65.Hough TA, Polewski M, Johnson K, Cheeseman M, Nolan PM, Vizor L, Rastan S, Boyde A, Pritzker K, Hunter AJ, Fisher EM, Terkeltaub R, Brown SD. Novel mouse model of autosomal semidominant adult hypophosphatasia has a splice site mutation in the tissue nonspecific alkaline phosphatase gene Akp2. J Bone Miner Res. 2007;22(9):1397–1407. doi: 10.1359/jbmr.070515. [DOI] [PubMed] [Google Scholar]
- 66.Hu J, Mora S, Weber G, Zamproni I, Proverbio MC, Spiegel AM. Autosomal dominant hypocalcemia in monozygotic twins caused by a de novo germline mutation near the amino-terminus of the human calcium receptor. J Bone Miner Res. 2004;19(4):578–586. doi: 10.1359/JBMR.040106. [DOI] [PubMed] [Google Scholar]
- 67.Finney B, Wilkinson WJ, Searchfield L, Cole M, Bailey S, Kemp PJ, Riccardi D. An exon 5-less splice variant of the extracellular calcium-sensing receptor rescues absence of the full-length receptor in the developing mouse lung. Exp Lung Res. 2011;37(5):269–278. doi: 10.3109/01902148.2010.545471. [DOI] [PubMed] [Google Scholar]
- 68.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998;273(36):23344–23352. doi: 10.1074/jbc.273.36.23344. [DOI] [PubMed] [Google Scholar]
- 69.Tu CL, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J Invest Dermatol. 1999;113(3):340–345. doi: 10.1046/j.1523-1747.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- 70.Bailey FR, Miller AM. Textbook of Embryology. 4th ed. New York: William Wood and Company; 1921. [Google Scholar]