Abstract
Joint instability and cartilage trauma have been previously studied and identified as key mediators in the development of posttraumatic osteoarthritis (PTOA). The purpose of this study was to use an in vivo model to compare the effect of joint instability, caused by the rupture of the anterior cruciate ligament (ACL), versus cartilage compression. In this study, mice were subjected to cyclical axial loads of twelve Newtons (N) for 240 cycles or until the ACL ruptured. One and eight weeks after this procedure, knees were sectioned coronally and evaluated for osteoarthritis by histology. Using a scoring scale established by Pritzker et al. 2006, the articular cartilage across each surface was scored and combined to produce a total degeneration score. The ACL-ruptured group had a significantly greater total degeneration score than either control or compression treated joints at one and eight weeks. Additionally, only sections from ACL-ruptured knees consistently showed synovitis after one week and osteophyte formation after eight weeks. Thus, it appears using that ACL rupture consistently creates a severe osteoarthritis phenotype, while axial cartilage compression alone does not appear to be an appropriate method of inducing PTOA in vivo.
Introduction
Posttraumatic osteoarthritis (PTOA) is defined as cartilage degeneration caused by a defined injury to the ligaments, cartilage, and/or bone of a joint1–4. In particular, the incidence of PTOA is especially prevalent in highly active populations, for example war veterans and “elite” level athletes5,6. In addition, PTOA affects patients at a younger age compared to idiopathic osteoarthritis. Joint replacements which are effective treatments for end stage osteoarthritis, unfortunately have poorer long term results in the younger population7.
Etiologically, PTOA is the result of multiple processes working both independently and in conjunction with each other to lead to joint degeneration 1,2,4,8. These factors include biomechanical instability, joint incongruity, inflammation, and trauma related factors that interact with each other throughout the progression of the disease2,4,9–12. Although the exact pathophysiology of PTOA is not well understood, several mechanisms that contribute to the degeneration of articular cartilage following trauma to the joint have been well studied. Because PTOA develops following an injury to the joint, understanding the underlying pathological mechanisms of disease can help clinicians delay or even prevent the onset or progression PTOA4,8,11,13.
In humans, injury to the soft-tissues has shown to be a significant risk factor for developing PTOA1,2,4–6,12,14. To model this, transection of the anterior cruciate ligament (ACL) or by meniscectomy has been developed in a variety of small animals to study the mechanisms of and possible treatments for PTOA15–18. However, surgically inducing injury may not be the most appropriate to understanding the complete pathophysiology of disease. Clinically, surgeons have the ability to mechanically address some of these factors through surgical reconstruction of ligaments and reduction and fixation of fractures. The restoration of functional stability by ACL reconstruction, does not seem to reduce the risk of patients developing osteoarthritis 19–22.
These ligamentous models do not address PTOA as a result of articular fracture or chondral insult. Cartilage impaction or injurious compression has been shown to lead to chondrocyte death and other deleterious changes in cartilage quality in several in vitro models23–26. In the setting of trauma, the cartilage is subjected to these loading forces in addition to potential joint destabilization in the form of ligament or bony injury. Therefore, it may be more appropriate to induce PTOA in these animal models by subjecting them to these forces.
Closed compression has previously been used to study changes to cortical and trabecular bone 27,28. More recently, this compression model has been used to evaluate the articular cartilage following articular fracture, ACL rupture, and repeated cartilage trauma 28–30. However, no single study using a small animal model has directly compared the histological phenotypes of in vivo injury by ACL rupture and cartilage trauma using similar loading parameters. We hypothesized that the ACL rupture combined with cartilage injury would create a more severe PTOA phenotype than cartilage injury alone when compared to uninjured knees.
Materials and Methods
Specimens
In this study, 3-month old FVB strain mice (Jackson Laboratories; Bar Harbor, Maine) were subject to axial compression to both knees. Mice were weighed to control for mass, and only males were used to control for estrogen-dependent factors. In total, there were 13 control joints, 11 joints with ACL rupture, and 18 joints that experienced the full 240-cycle compression. Of the control joints, 7 were harvested at one week and 6 were harvested at eight weeks. The ACL deficient joints were divided into groups of 6 at one week and 5 at eight weeks. Finally, the cyclical axial compression-only treated joints were divided into a group of 8 at one week and 10 at eight weeks. All animals were handled using protocols approved by an institutional animal care veterinarian (IACUC # 11-037).
Loading Protocol
Mice were induced and kept under general anesthesia with continuous isoflurane (Forane®, Baxter Healthcare Corporation; Deerfield, IL) using a nose cone. To manage pain, each mouse was given a single subcutaneous injection of buprenorphine at a dose of 0.1mg/kg (Buprenex®, Reckitt Benckiser Pharmaceuticals; Richmond, Virginia). Compressions were performed using a Bose Electroforce® 3200 (Framingham, Massachusetts) biomechanical testing device. The lower extremity was locked in place at approximately ninety degrees of flexion, with a jig molded for the knee and a jig to hold the foot. A sensor, tracking displacement and force, was placed at the bottom of the system (Figure 1). Twelve Newton (N) loads were delivered to the knee at a velocity of 5mm/second. This force was chosen based upon prior experiments showing that 12N was a threshold force in these mice where ACL rupture would occur randomly. ACL rupture was identified through the waveform as a disruption of normal loading protocol coupled with an audible “popping” sound, and later confirmed by histology. Once the sensor detected a load of 12N, the position was held for two seconds to allow fluid within the joint to settle before returning to the rest position at 1N of load. The treatment animals were subjected to 240 cycles of load or until the ACL ruptured. Compressions were administered on both legs, creating a bilateral injury to avoid introducing the variable of histology from an overloaded uninjured knee compensating for the contralateral injured limb. Control mice were placed under isoflurane anesthesia, given the single dose of buprenorphine, and were not subjected to any compression. Following injury, mice were allowed ad-lib behavior until the time of their sacrifice. Animals were sacrificed with isoflurane induction followed by cervical dislocation. Time points of one and eight weeks were used to track the progression of injury.
Figure 1.
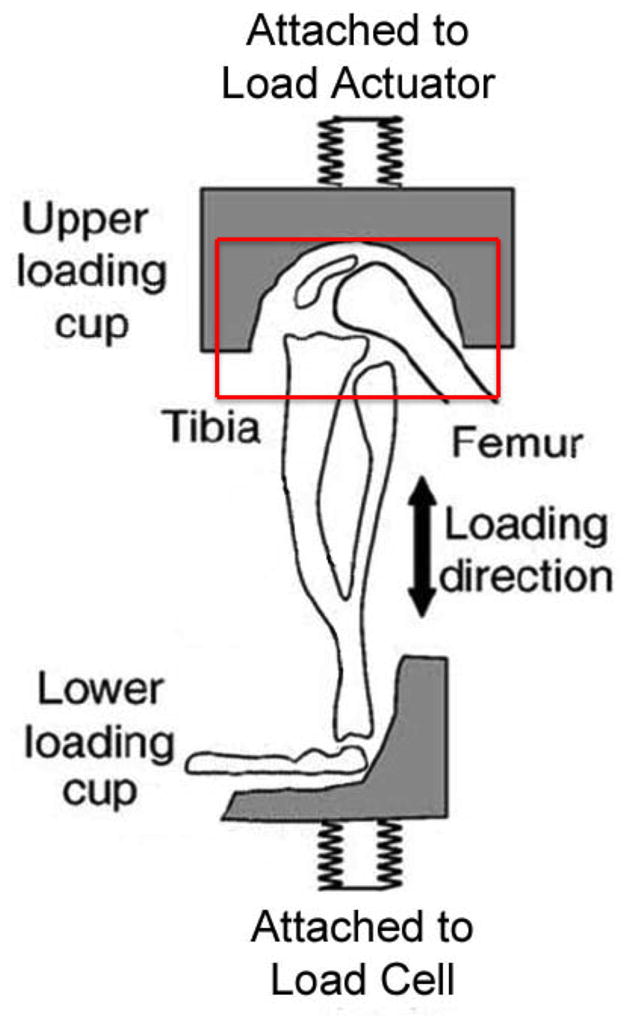
Sagittal cut-away view of mouse in limb in axial loading mechanism. Mouse knee is in flexion of about 90°. The 12 N loads were distributed at a 5mm/sec velocity for 240 cycles or until the ACL ruptured.
Tissue Preparation
After one and eight weeks, knees were dissected with the majority of soft tissue removed. Joints were fixed in zinc buffered formalin (Anantech LTD; Battle Creek, MI) at 4°C overnight. They were decalcified in a 0.45M ethylenediamine tetraacetic acid solution (EDTA) solution for one week, changing the solution once every three days. Following decalcification, knees were dehydrated and embedded in paraffin (Richard-Allan Scientific; Kalamazoo, MO) with approximately thirty degrees of flexion for sectioning.
Sectioning and Staining
Knees were embedded front side down, with the tibia to parallel to the bottom of the molds. Seven micron-thick coronal (frontal) sections were taken using a Microm® HM 325 Microtome. Sections between samples were standardized to location based upon the depth from the start of the anterior joint surface and by the shape of the medial and lateral meniscus. Sections were stained using Safranin-O (Sigma; St. Louis, MO)/Fast Green (Sigma; St. Louis, MO) to evaluate proteoglycan content (PG) and cartilage morphology. The knee was divided into four quadrants: lateral tibial plateau, medial tibial plateau, lateral femoral condyle, and medial femoral condyle.
Statistical Analysis
The articular cartilage at each quadrant was assessed using the grading scale established by Pritzker et al. 200631. Two blinded observers independently scored the specimens. After grading, the score by each observer was averaged providing a combined score between graders. Scores at each region were combined together to provide a total degeneration score, the lowest being 0 (normal) and highest being 24 (severe osteoarthritis). Non-parametric statistical analysis was performed using Kruskal-Wallis test, followed by Mann-Whitney U-Test for pair-wise differences. Resulting p-values were compared to a Bonferroni corrected value (p=0.017), calculated based upon the number of pair-wise comparisons made. All statistical analysis was conducted using the computer software R (The R Foundation for Statistical Computing).
Results
Histological Scoring
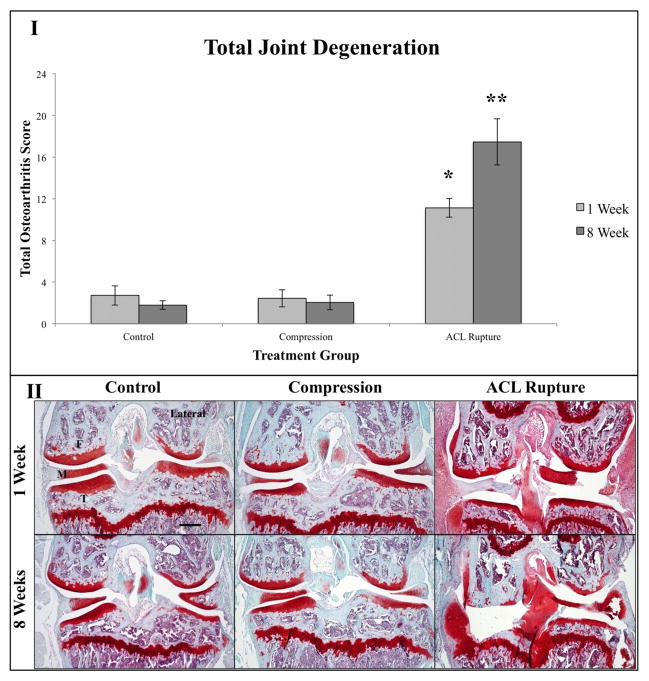
Total degeneration scores for each treatment group at one and eight weeks are presented as a mean±sem. To compare the two independent ratings on the condition of the cartilage, we performed a Pearson’s correlation test for significance. The test indicated that the two ratings are correlated by 92%, with an explained variance between the two ratings of 83%, and that they agree with significance (p<0.001) at a 95% confidence interval. After one week, the total degeneration score was 2.71±0.92 for controls, 2.44±0.82 for 240-cycle compression knees, and 11.1±0.90 for ACL ruptured knees. The Kruskal-Wallis test revealed a statistically significant difference among various treatment groups at this time point (p=0.002). ACL ruptured knees had a statistically significantly higher total degeneration score than both controls and compression treated knees (p=0.003 and p<0.001, respectively). There are no statistically significant differences between control and axial compression-treated knees (p=0.816).
After eight weeks, joints were scored as 1.79±0.41 for controls, 2.05±0.70 for the compressed knees, and 17.5±2.21 for the ACL ruptured knees. The Kruskal-Wallis test revealed that there was a statistically significant difference among the three treatment groups (p=0.004). Once again, the ACL rupture group had a significantly greater total degeneration score than both controls and compression treated knees (p=0.004 and p=0.003, respectively). There was not, however, a significant difference in degeneration score between control and cyclical compression-treated knees (p=0.870).
Between one and eight weeks there were no statistically significant differences between control (p=0.719), compression (p=0.789), and ACL rupture (p=0.083) treatment groups. Figure 2 summarizes the scoring results and shows representative micrographs for each treatment at one and eight weeks.
Figure 2.
Results from histology with Safranin-O/Fast Green staining. I) Graph comparing total degenerative scores for control, 240-cycle compression, and ACL rupture knees. “*” denotes that after one week the ACL ruptured joints were statistically significantly higher in their total degeneration score than the control and 240-cycle impaction group (p=0.003 and p<0.001, respectively). “**” denotes that after eight weeks the ACL ruptured joints were statistically significantly higher in their total degeneration score than the control and 240-cycle impaction group (p=0.004 and p=0.003, respectively). II) Representative pictures of knees at all three conditions at one and eight weeks. Lateral side is marked as reference. Femur (F), meniscus (M), and tibia (T) are labeled. All micrographs were taken at 5x magnification. Scale Bar=300 μm.
Grossly, the ACL rupture group shows severe synovitis and early fibrillation of the articular surfaces at one week. At eight weeks, osteoarthritis has progressed in the ACL rupture group, however not in the other two groups. These scores are characterized by osteophyte formation, complete deterioration of the articular cartilage, and wear of the subchondral bone to the growth plate. Both the control and impaction groups do not share these characteristics with the ACL rupture group at either time point. Figure 3 highlights the synovitis at one week and osteophyte formation at eight weeks at 10x magnification.
Figure 3.
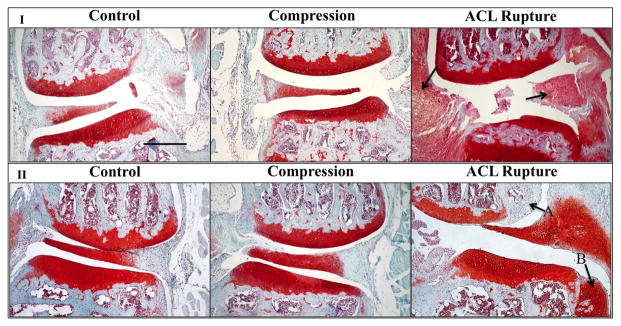
Representative histology pictures highlighting synotivits, inflammation, and osteophyte formation. I) Medial aspect of knees at one week for all treatment conditions. Note the high amount of inflammation in the synovium and ACL in the ACL rupture group. Arrows indicate location of synovium and ACL. II) Lateral aspect of knees at eight weeks for all treatment conditions. Osteophyte formation was seen only in the ACL rupture group at this time point, indicated by arrows. A) Osteophyte at the lateral aspect of the femoral condyle. B) Osteophyte forming at the lateral aspect of the tibial plateau, currently in chondral stage. All micrographs were taken at 10x magnification. Scale Bar=300 μm.
Discussion
Posttraumatic osteoarthritis is a debilitating disease that affects an active patient population. However, because it follows some form of trauma to the joint, PTOA presents physicians with an opportunity for early intervention4,8. The purpose of this study was to assess whether the loading parameters we used with the cyclical axial compression injury model would be appropriate for understanding the progression of PTOA. In particular, we were also interested in how the histology from the mice that withstood the complete 240-cycle regimen compared with mice that endured an ACL rupture using the same 12N loading force.
Delivering 12N compressive loads over 240-cycles did not cause osteoarthritis in the mouse knee when the ACL remained intact by the Pritzker grading scale. At one week, there was little evidence of inflammation in the surrounding soft tissues. Furthermore, the cartilage remained healthy and intact, almost identical to that of the negative control groups. There is no progression of disease by eight weeks in the 240-cycle compression group. Again there was no statistically significant difference in osteoarthritis score and no gross observational difference between this group and the control group. Our results differed from those presented by Ko et al., who also used a cyclical compressive load while maintaining normal joint congruity and stability 29. However, Ko et al. used a different loading protocol that was repeated for several weeks and resulted in a mild phenotype 29.
Several in vitro studies have demonstrated that direct impact to the articular cartilage causes deleterious changes that put the tissue at risk for developing osteoarthritis. Jeffrey et al. showed that the viability of chondrocytes in bovine articular cartilage significantly decreases proportionally with increased loading energy after a single load24. This group also demonstrated histologically that there was a correlation between increased loading energy and more severe fibrillation, leading to subsequent degeneration of the superficial layer of the articular cartilage 24. Huser and Davies confirmed these findings in an equine study, comparing impacted articular cartilage explants with non-impacted osteoarthritic explants 23. Physical changes to the cartilage have been associated with biochemical changes in the cartilage following impaction as well23. Similarly, Jones et al. reported changes lubricin expression across the non-calcified portion of the articular cartilage and physical changes superficially 26. There may also be some underlying physiological mechanisms that lead to cartilage degeneration following impaction, beyond the physical damage. Ding et al. has proposed that mitogen activated protein kinases (MAP kinases), found to be expressed higher in chondrocytes in areas of impaction and the periphery, contribute to the degeneration of articular cartilage in vitro 25. In all, these in vitro studies provide evidence to suggest that high-energy compression onto the articular cartilage mediates degenerative processes, which may lead to osteoarthritis.
However, our results suggest that compressive injury alone in a mouse may not be appropriate for studying the effect of repeated compressive trauma to the articular cartilage in vivo. It is difficult to compare the energy experienced by the knees in this in vivo model with the in vitro experiments because we do not know the exact contact pressure area and force distribution of our loads. Furthermore, as pointed out by Little and Hunter in their review, these in vitro experiments do not address the interactions between the various tissues and structures in the joint32. Thus, it is likely that these surrounding tissues are protecting the articular cartilage, and preventing an injury that would lead to the development of PTOA.
By contrast, after rupture of the ACL, mice developed severe PTOA in the injured joint. At one week, the synovium showed increased cellularity, indicating an acute inflammatory response. Additionally, some of the articular cartilage at the various surfaces of the joint began to show signs of deterioration. Although not statistically significant, there is an increase in osteoarthritis score between one and eight weeks. After eight weeks, the articular surfaces at various surfaces had eroded through the articular cartilage and into the subchondral bone. At eight weeks the inflammation in the surrounding soft-tissues had subsided, however osteophytes had developed along the periphery of the femoral condyles and tibial surface. Our results are similar to Christiansen et al., who used a single load to rupture the ACL in a closed axial loading system 28. Thus, it can be concluded that like this group, our model can be used to study biological events associated with PTOA, such as synovitis and osteophyte formation, following ACL rupture 28.
ACL rupture is a traumatic injury that is often followed by PTOA about 10–15 years later 14,20,21. Nelson et al. published a study showing a reduction of articular cartilage health following ACL rupture in patients undergoing ACL reconstruction surgery 14. Joint instability, appears to be one of the chief biomechanical factors and an important predictor of PTOA following ACL rupture 10,14. Instability causes changes to normal gait and biomechanics, changing loading patterns on the joint 3. These changes in load subject areas of cartilage to pathologic compression and shear forces, leading to damage that may lead to osteoarthritis 33.
Previously, joint instability and subsequent PTOA has been modeled by surgical transection of the ACL, medial collateral ligament (MCL), and meniscus in small animals. Hayami et al. characterized changes to the articular cartilage, subchondral bone, and formation of osteophytes following ACL transection as well as ACL transection with menisectomy, finding that the combination of surgeries led to more severe osteoarthritis 16. Likewise, Kamekura et al. demonstrated a direct relationship between osteoarthritis severity and instability by transecting different combinations of ligaments in a mouse knee 34. A less severe phenotype has been achieved by menisecal injury as well by Glasson et al15. The results from the ACL ruptured knee are similar to previous surgically induced models. However, because the joint capsule remains intact during our procedure, we can study tissues such as the synovium and avoid iatrogenic injury from the microsurgical procedure. These tissues are important to the overall health of the joint, and studies have shown that changes in the molecular expression of the tissues could contribute to progression of osteoarthritis 11.
There are some limitations to our model that should be considered with our results. First, we could not control whether or not the ACL ruptured during loading using the parameters in this study, but we chose this threshold force of loading to generate a random ACL rupture. Mouse knees that are loaded above 13N will all have ACL tears, those loaded below 10N will not get ACL tears. Furthermore, we cannot offer any comment as to why some specimens experienced ACL rupture and why others did not. Even within the same specimen, ACLs tore in both, or in only one of the knees. To control for this we recorded the weights of animals, and were not able to find a correlation between ACL deficient and intact joints. Nevertheless, the distribution of ACL ruptured and non-ACL ruptured animals makes this protocol useful for ascertaining the differential effects of joint injury, in this case compression alone or compression with instability. Also, in our system we chose to hold the compression at its peak force for two seconds, in order to create a critical level of strain in the cartilage surface, as cartilage strain is associated with magnitude of injury35. This is not a clinically relevant motion, as most injuries prior to PTOA occur instantaneously. For example, ACL rupture is associated with non-contact change of direction or pivot motions 5. However, since there is no significant osteoarthritic phenotype associated with the 240-cycle, it is reasonable to assume that this exaggerated compression does not contribute to any additional trauma at the articular cartilage.
Conclusion
In conclusion, this study compared the histological differences between mice knees that either had an ACL rupture or endured 240 cycles of axial compression. We showed that a closed ACL rupture produces a consistent and severe osteoarthritic phenotype in the mouse knee, in agreement with other findings in the literature. In contrast, mice that experienced 240 cyclical loads of 12N axial compression did not develop osteoarthritis at one or eight weeks. Therefore, in vivo cartilage trauma by axial compression without joint instability does not appear to be sufficient for inducing PTOA at eight weeks in mice. Our study suggests that joint instability, created by injuries such as ACL rupture, may be a vital component to creating osteoarthritis in a small animal model. This is the limitation of translating these small animal models to clinical treatments, as our patients continue to develop PTOA despite surgical stabilization of their joints.
Acknowledgments
The authors would like to thank the following funding sources for their support of this project: UCSF Department of Orthopaedic Surgery and Northern California Institute of Research and Education.
Footnotes
The authors have no financial disclosures that would cause a conflict of interest as it pertains to the data presented in this study.
References
- 1.Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic Osteoarthritis: A First Estimate of Incidence, Prevalence, and Burden of Disease. Journal of Orthopaedic Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Brown TD. Joint Injury, Repair, and Remodeling. Clinical Orthopaedics and Related Research. 2004;423:7–16. [PubMed] [Google Scholar]
- 3.Buckwalter JA, Martin JA. Sports and osteoarthritis. Curr Opin Rheumatol. 2004;16(5):634–639. doi: 10.1097/01.bor.0000132647.55056.a9. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. Journal of Orthopaedic Research. 2011;29(6):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuijt M-TK, Inklaar H, Gouttebarge V, Frings-Dresen MHW. Knee and ankle osteoarthritis in former elite soccer players: A systematic review of the recent literature. Journal of Science and Medicine in Sport. 2012;15(6):480–487. doi: 10.1016/j.jsams.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Rivera JC, Wenke JC, Buckwalter JA, et al. Posttraumatic Osteoarthritis Caused by Battlefield Injuries: The Primary Source of Disability in Warriors. Journal of the American Academy of Orthopaedic Surgeons. 2012;20(suppl):S64–S69. doi: 10.5435/JAAOS-20-08-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeney JA, Eunice S, Pashos G, et al. What is the Evidence for Total Knee Arthroplasty in Young Patients?: A Systematic Review of the Literature. Clinical Orthopaedics and Related Research. 2010;469(2):574–583. doi: 10.1007/s11999-010-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotz MK. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Research & Therapy. 2010;12(3):211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinley TO, Borrelli J, Jr, D’Lima DD, et al. Basic science of intra-articular fractures and posttraumatic osteoarthritis. J Orthop Trauma. 2010;24(9):567–570. doi: 10.1097/BOT.0b013e3181ed298d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinley TO, Rudert MJ, Koos DC, Brown TD. Incongruity versus Instability in the Etiology of Posttraumatic Arthritis. Clinical Orthopaedics and Related Research. 2004;423:44–51. doi: 10.1097/01.blo.0000131639.89143.26. [DOI] [PubMed] [Google Scholar]
- 11.Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4(4):285–298. [PMC free article] [PubMed] [Google Scholar]
- 12.Guilak F, Fermor B, Keefe FJ, et al. The Role of Biomechanics and Inflammation in Cartilage Injury and Repair. Clinical Orthopaedics and Related Research. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 13.Aktas E, Sener E, Gocun PU. Mechanically induced experimental knee osteoarthritis benefits from anti-inflammatory and immunomodulatory properties of simvastatin via inhibition of matrix metalloproteinase-3. Journal of Orthopaedics and Traumatology. 2011;12(3):145–151. doi: 10.1007/s10195-011-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson F, Billinghurst R, Pidoux I, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis and Cartilage. 2006;14(2):114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Hayami T, Pickarski M, Zhuo Y, et al. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Mooney RA, Sampson ER, Lerea J, et al. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Research & Therapy. 2011;13(6):R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L, Fleming BC, Sun X, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. Journal of Orthopaedic Research. 2010;28(7):900–906. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee Osteoarthritis After Anterior Cruciate Ligament Injury: A Systematic Review. The American Journal of Sports Medicine. 2009;37(7):1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 20.Struewer J, Frangen TM, Ishaque B, et al. Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long-term follow-up. International Orthopaedics. 2011;36(1):171–177. doi: 10.1007/s00264-011-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claes S, Hermie L, Verdonk R, et al. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surgery, Sports Traumatology, Arthroscopy. 2012 doi: 10.1007/s00167-012-2251-8. [DOI] [PubMed] [Google Scholar]
- 22.Friel NA, Chu CR. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin Sports Med. 2013;32(1):1–12. doi: 10.1016/j.csm.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huser CAM, Davies ME. Validation of an in vitro single-impact load model of the initiation of osteoarthritis-like changes in articular cartilage. Journal of Orthopaedic Research. 2006;24(4):725–732. doi: 10.1002/jor.20111. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322(1):87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 25.Ding L, Heying E, Nicholson N, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis and Cartilage. 2010;18(11):1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones ARC, Chen S, Chai DH, et al. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis & Rheumatism. 2009;60(1):133–142. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 27.De Souza RL, Matsuura M, Eckstein F, et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: A new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen BA, Anderson MJ, Lee CA, et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis and Cartilage. 2012;20(7):773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Ko FC, Dragomir C, Plumb DA, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis & Rheumatism. 2013;65(6):1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furman BD, Strand J, Hembree WC, et al. Joint degeneration following closed intraarticular fracture in the mouse knee: A model of posttraumatic arthritis. Journal of Orthopaedic Research. 2007;25(5):578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 31.Pritzker K, Gay S, Jimenez S, et al. Osteoarthritis cartilage histopathology: grading and staging 1, 2. Osteoarthritis and Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nature Reviews Rheumatology. 2013 doi: 10.1038/nrrheum.2013.72. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhari AMW, Briant PL, Bevill SL, et al. Knee Kinematics, Cartilage Morphology, and Osteoarthritis after ACL Injury. Medicine & Science in Sports & Exercise. 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 34.Kamekura S, Hoshi K, Shimoaka T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis and Cartilage. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Quinn TM, Allen RG, Schalet BJ, et al. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19(2):242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]