Abstract
Both ligand-dependent and ligand-independent activation of estrogen receptor (ER)α is modulated by receptor phosphorylation and results in activation of the ERα-dependent pathways that are involved in endometrioid endometrial cancer (EEC) pathogenesis. It is also known that the mammalian target of rapamycin (mTOR)/p70 S6 kinase 1 (S6K1) and MAPK/p90 ribosomal S6 kinase (RSK) signaling pathways coordinately regulate phosphorylated-ERα at Ser167 (p-Ser167-ERα). However, the expression of p-Ser167-ERα in EEC and its prognostic role in ECC is largely unexplored. The purpose of the present study was to investigate the expression of p-Ser167-ERα in ECC and its relationship with prognosis. Immunohistochemical staining of primary EEC surgical specimens (n = 103) was carried out using antibodies specific for p-Ser167-ERα and for p-mTOR/p-S6K1 and p-MAPK/p-RSK. The correlation of p-Ser167-ERα expression with clinicopathological features and survival of ECC was studied. Patients that were positive for nuclear p-Ser167-ERα had significantly shorter relapse-free survival, and although the result was not significant, levels of nuclear p-Ser167-ERα tended to be higher in advanced-stage ECC patients. Nuclear p-Ser167-ERα was significantly positively correlated with p-MAPK and p-S6K1, and with significantly shorter relapse-free survival in EEC.
Keywords: Endometrial cancer, location, outcome, p-S6K1, p-Ser167-ERα
Endometrial cancer (EC) is the most common gynecological cancer and is thought to be estrogen-related. The estrogen receptor (ER) is a biological target for EC that has attracted considerable attention over the years. For many EC patients, particularly those with endometrial endometrioid cancer (EEC), which are tumors that express high levels of the ER, the observed response rates to hormonal agents such as progestins, antiestrogens, and aromatase inhibitors have not been satisfactory; indeed, many patients with advanced or refractory disease eventually develop resistance to this type of therapy and median survival is short at 7–12 month.1,2
Both ligand-dependent and ligand-independent activation of the ERα is modulated by receptor phosphorylation, and receptor phosphorylation is enhanced by ligand binding.3 The major phosphorylation sites of ERα reside in the N-terminal domain at serines 104, 105, 118, and 167. Phosphorylation at Ser167 was shown to be important in receptor binding to DNA.3 In breast cancer, which is also an estrogen-related tumor, one mechanism by which resistance to hormone therapy develops is through phosphorylation of ERα at Ser167 (p-Ser167-ERα), a modification that allows the receptor to function in an estrogen-independent manner.4 It has also been reported that two signaling pathways, mammalian target rapamycin (mTOR)/p70 S6 kinase 1 (S6K1) and MAPK/p90 ribosomal S6 kinase (RSK), coordinately regulate p-Ser167-ERα and the development of resistance, which can serve as a prognostic marker for breast cancer.5,6 Previously published in vivo data suggest that Akt is phosphorylated leading to active p-Ser167-ERα and resulting in activation of ERα-dependent pathways involved in EEC pathogenesis.7 However, to the best of our knowledge, there are no published reports regarding the influence of p-Ser167-ERα and mTOR/S6K1 and MAPK/RSK activity on outcomes in EEC patients.
The current study was designed to investigate correlations between p-Ser167-ERα levels in EEC with clinicopathological features, disease outcomes, and levels of phosphorylated mTOR/S6K1 and phosphorylated MAPK/RSK (p-mTOR/p-S6K1 and p-MAPK/ p-p90RSK, respectively), as determined by examination of medical records and by immunohistochemical analysis.
Materials and Methods
Patients
The study group comprised 103 EEC patients who underwent total abdominal or radical hysterectomy plus bilateral salpingo-oophorectomy with or without lymphadenectomy during a 5-year period at the University of Fukui Hospital (Fukui, Japan) (Table1). Clinicopathological characteristics and follow-up data were obtained from the subjects' medical records. Staging, histology, and grading criteria were based on the 2009 International Federation of Gynecology and Obstetrics surgical staging classification. Definitive diagnosis was determined by postoperative histopathology and all specimens were evaluated by subsequent immunohistochemical analysis. Patients with deep myometrial invasion, cervical involvement, special histology (such as undifferentiated adenocarcinoma), or lymph-node metastasis were treated with four to six rounds of postoperative adjuvant chemotherapy consisting of 180 mg/m2 paclitaxel and carboplatin, according to Chatelut's formula (area under the curve = 5 mg/mL/min). No patient was treated with hormone therapy, whether past or current. All patients were evaluated for disease recurrence for at least 2 years by annual physical examination and pap smear of the vaginal vault. In addition, diagnostic imaging (including ultrasonography, computed tomography, and/or MRI) was carried out every 3–6 month along with analysis of tumor markers. This study was approved by the institutional review board of the University of Fukui Hospital and written informed consent was obtained from all patients.
Table 1.
Clinicopathological features of 103 endometrioid endometrial cancers
| No. (n = 103) | % | |
|---|---|---|
| Patient age, years | ||
| Median | 59.19 ± 11.1 | NA |
| Range | 38–92 | NA |
| Clinical stage | ||
| I | 71 | 69 |
| II | 12 | 12 |
| III | 13 | 12 |
| IV | 7 | 7 |
| Histological grade | ||
| Grade 1 | 64 | 62 |
| Grade 2 | 25 | 24 |
| Grade 3 and undifferentiated | 14 | 14 |
| Myometrial invasion | ||
| <50% | 78 | 64 |
| >50% | 25 | 36 |
| LVSI | ||
| Positive | 21 | 20 |
| Negative | 77 | 75 |
| Miss | 5 | 5 |
| LN metastases | ||
| Positive | 6 | 6 |
| Negative | 80 | 77 |
| Miss | 17 | 17 |
| Recurrence | ||
| No recurrence | 89 | 86 |
| Recurrence | 14 | 14 |
LN, lymph node; LVSI, lymphovascular space invasion; Miss, missing data; NA, not applicable.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue was immunohistochemically stained using the avidin–biotin–peroxidase complex technique with an LSAB kit (Dako, Glostrup, Denmark).8 Sections (2.5-μm thick) were dewaxed in xylene for 15 min three times, dehydrated in alcohol, and subjected to antigen retrieval in a pressure cooker for 15 min in 10 mM sodium citrate buffer (pH 6.0). After cooling, sections were washed three times in PBS (pH 7.2). Endogenous peroxidase activity was blocked by immersion in 3% hydrogen peroxide for 5 min. Non-specific binding of primary antibodies was blocked by incubating sections with diluted (Dako Protein Block Serum-Free) for 10 min at room temperature. Samples were then incubated overnight with primary antibodies to the following proteins, diluted in PBS: ERα (Ser167) (p-Ser167-ERα) (rabbit polyclonal, 1:100; Abcam, Cambridge, UK); p-MAPK (Thr202/Thy204) (rabbit monoclonal, 1:300); p-p90RSK (Thr359/Ser363) (rabbit polyclonal, 1:250); p-mTOR (Ser2448) (rabbit monoclonal, 49F9, 1:50) and p70S6 (p-S6K1) (rabbit monoclonal, 49D7, 1:50) (all from Cell Signaling Technology, Beverly, MA, USA). After washing with PBS, sections were incubated for 10 min with diluted biotinylated goat anti-mouse immunoglobulins (Dako LSAB kit, Bottle 1) as the secondary antibody. After incubation with the avidin–biotin–peroxidase complex (Dako LSAB kit, Bottle 2) for 10 min and washing with PBS, the signal was visualized with substrate and 3,3′-diaminobenzidine in chromogen solution (Dako EnVision+ kit). Sections were then counterstained with Mayer's acidic hematoxylin and washed multiple times in alcohol (70–100%). After xylene treatment, sections were covered until used.
Sections from human colon and breast cancers were used as positive controls and, for negative controls, incubation with the primary antibody was omitted. The intensity and distribution of p-Ser167-ERα, p-MAPK, p-p90RSK, p-mTOR, and p-S6K1 staining was evaluated using a semiquantitative method (IRS score) as previously described,8 and was calculated as follows: IRS = ΣSI × PP, where SI is the optical stain intensity (graded 0, no; 1, weak; 2, moderate; 3, strong staining) and PP is the degree of positively stained cells (defined as 0, no staining; 1, <10%; 2, 11–50%; 3, 51–80%; 4, >81%). Those IRS scores greater than 6 (IRS ≥6) were defined as “positive”, and less than 5 (IRS 0–5) as “negative”. Immunostaining was scored by two independent observers (T. K. and A. S.) who are specialists in gynecological pathology. Discrepancies of more than two points in either optimal stain intensities or in the degree of positively stained cells were rare. However, if such discrepancies occurred, these slides were again evaluated by both observers. If the observers could not reach agreement on evaluation of immunostainings, these cases were excluded from this analysis. Ultimately, staining data for p-Ser167-ERα, p-MAPK, p-p90RSK, and p-mTOR were available for 103 patients and staining data for p-S6K1 were available for 98 patients.
Statistical analysis
The chi-square-test was used to test possible associations between clinicopathological factors and p-Ser167-ERα, p-MAPK, p-p90RSK, p-mTOR, or p-S6K1. This test was also used to assess correlations between p-Ser167-ERα and p-MAPK, p-p90RSK, p-mTOR, or p-S6K1 levels. Kaplan–Meier curves were plotted to assess the effects of p-Ser167-ERα level on relapse-free survival (RFS). Survival curves were compared using the log–rank test. P-values of 0.05 or less were considered statistically significant. Multivariate α proportional Cox models were used to assess the prognostic significance of p-Ser167-ERα, p-MAPK, p-p90RSK, p-mTOR, and p-S6K1 levels, and their relationship to several clinicopathological factors. Statistical analysis was carried out using spss for Windows 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Phosphorylated Ser167-ERα was observed in the nuclei and cytoplasm of EEC cells (in 10.7% and 12.6% of the cells, respectively) (Fig.1), as was p-mTOR (in 21.4% and 51.5% of the cells, respectively). Phosphorylated MAPK, p-p90RSK, and p-S6K1 were observed only in the cytoplasm (in 16.5%, 44.7%, and 55.3% of the cells, respectively).
Figure 1.
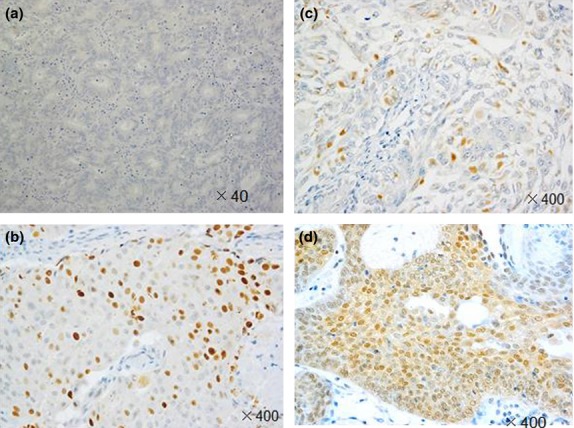
Immunostaining for phosphorylated estrogen receptor α at Ser167 (p-Ser167-ERα) in representative endometrioid endometrial cancer specimens. (a) Negative for cytoplasmic and nuclear p-Ser167-ERα (40×). (b) Positive for cytoplasmic p-Ser167-ERα only. Positive for nuclear p-Ser167-ERα (c) and positive for cytoplasmic and nuclear p-Ser167-ERα (d) (400×).
There was a positive correlation between nuclear and cytoplasmic levels of p-Ser167-ERα (P = 0.001) and p-mTOR (P = 0.024). Nuclear p-Ser167-ERα was positively correlated with p-MAPK and p-S6K1, and cytoplasmic p-Ser167-ERα was positively correlated with p-MAPK; in all cases correlations were significant. Cytoplasmic p-Ser167-ERα was marginally correlated with cytoplasmic p-mTOR (P = 0.05). There was significant correlation between p-S6K1 and p-p90RSK, and between cytoplasmic p-mTOR and p-p90RSK (Table2).
Table 2.
Relationships between molecular markers phosphorylated estrogen receptor α at Ser167 (p-Ser167-ERα), p-MAPK, p90 ribosomal S6 kinase (p-p90RSK), mammalian target of rapamycin (p-mTOR), and p70 S6 kinase 1 (p-S6K1) in endometrioid endometrial cancers (n = 103)
| p-Ser167-ERα (cytoplasma) | p-MAPK | p-p90RSK | p-mTOR (nucleus) | p-mTOR (cytoplasmic) | p-S6K1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | |
| p-Ser167-ERα (nuclear) | ||||||||||||||||||
| N | 84 | 8 | 0.001* | 81 | 11 | 0.001* | 51 | 41 | 0.955 | 74 | 18 | 0.199 | 45 | 47 | 0.83 | 40 | 1 | 0.019* |
| P | 6 | 5 | 5 | 6 | 6 | 5 | 7 | 4 | 5 | 6 | 47 | 10 | ||||||
| p-Ser167-ERα (cytoplasma) | ||||||||||||||||||
| N | NA | 80 | 10 | 0.001* | 51 | 39 | 0.476 | 73 | 17 | 0.107 | 47 | 43 | 0.05 | 38 | 3 | 0.207 | ||
| P | NA | 6 | 7 | 6 | 7 | 8 | 5 | 3 | 10 | 48 | 9 | |||||||
| p-MAPK | ||||||||||||||||||
| N | NA | NA | 48 | 38 | 0.828 | 69 | 17 | 0.375 | 44 | 42 | 0.23 | 37 | 44 | 0.092 | ||||
| P | NA | NA | 9 | 8 | 12 | 5 | 6 | 11 | 4 | 13 | ||||||||
| p-p90RSK | ||||||||||||||||||
| N | NA | NA | NA | 46 | 11 | 0.57 | 33 | 24 | 0.04* | 30 | 24 | 0.002* | ||||||
| P | NA | NA | NA | 35 | 11 | 17 | 29 | 11 | 33 | |||||||||
| p-mTOR (nucleus) | ||||||||||||||||||
| N | NA | NA | NA | NA | 44 | 6 | 0.02* | 30 | 11 | 0.378 | ||||||||
| P | NA | NA | NA | NA | 37 | 16 | 46 | 11 | ||||||||||
| p-mTOR (cytoplasma) | ||||||||||||||||||
| N | NA | NA | NA | NA | NA | 17 | 24 | 0.207 | ||||||||||
| P | NA | NA | NA | NA | NA | 31 | 26 | |||||||||||
P-values from χ2-tests.
P < 0.05. N, negative; P, positive; NA, not applicable.
Nuclear p-Ser167-ERα levels tended to be higher in patients with advanced disease, but this result was not significant. Cytoplasmic p-Ser167-ERα, cytoplasmic p-mTOR, and p-MAPK were not correlated with any clinicopathological factors (Table3). However, p-p90RSK was positively correlated with stage (P = 0.004) and lymphovascular space invasion (LVSI) (P = 0.017), as was p-S6K1 (P = 0.003 and P = 0.041, respectively). Nuclear p-mTOR was positively correlated with grade (P = 0.002).
Table 3.
Relationships between the molecular markers phosphorylated estrogen receptor α at Ser167 (p-Ser167-ERα), p-MAPK, p90 ribosomal S6 kinase (p-p90RSK), mammalian target of rapamycin (p-mTOR), and p70 S6 kinase 1 (p-S6K1) and clinicopathological factors in endometrioid endometrial cancers (n = 103)
| p-Ser167-ERα (nuclear) | p-Ser167-ERα (cytoplasma) | p-MAPK | p-p90RSK | p-mTOR (nucleus) | p-mTOR (cytoplasmic) | p-S6K1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | N | P | P-value | |
| Stage | |||||||||||||||||||||
| I–II | 79 | 7 | 0.06 | 76 | 10 | 0.495 | 71 | 15 | 0.565 | 46 | 40 | 0.395 | 69 | 17 | 0.375 | 43 | 43 | 0.506 | 29 | 53 | 0.003* |
| III–IV | 13 | 4 | 14 | 3 | 15 | 2 | 11 | 6 | 12 | 5 | 7 | 10 | 12 | 4 | |||||||
| Grade | |||||||||||||||||||||
| I–II | 82 | 8 | 0.122 | 80 | 10 | 0.225 | 75 | 15 | 0.907 | 45 | 45 | 0.004* | 75 | 15 | 0.002* | 44 | 46 | 0.854 | 34 | 51 | 0.346 |
| III | 10 | 3 | 10 | 3 | 11 | 2 | 12 | 1 | 6 | 7 | 6 | 7 | 7 | 6 | |||||||
| Invasion | |||||||||||||||||||||
| >50% | 70 | 8 | 0.806 | 68 | 10 | 0.914 | 64 | 14 | 0.484 | 40 | 38 | 0.143 | 65 | 13 | 0.4 | 36 | 42 | 0.391 | 31 | 42 | 0.829 |
| <50% | 22 | 3 | 22 | 3 | 22 | 3 | 17 | 8 | 16 | 9 | 14 | 11 | 10 | 15 | |||||||
| LVSI | |||||||||||||||||||||
| N | 70 | 7 | 0.735 | 68 | 9 | 0.993 | 63 | 14 | 0.210 | 39 | 38 | 0.017* | 63 | 14 | 0.297 | 37 | 40 | 0.715 | 27 | 45 | 0.041* |
| P | 15 | 2 | 15 | 2 | 16 | 1 | 14 | 3 | 12 | 5 | 9 | 8 | 11 | 6 | |||||||
| Recurrence | |||||||||||||||||||||
| N | 83 | 6 | 0.001* | 80 | 9 | 0.053 | 75 | 14 | 0.593 | 48 | 41 | 0.469 | 74 | 15 | 0.005* | 44 | 45 | 0.647 | 33 | 51 | 0.21 |
| P | 9 | 5 | 10 | 4 | 11 | 3 | 9 | 5 | 7 | 7 | 6 | 8 | 8 | 6 | |||||||
P-values from χ2-tests.
P < 0.05. LVSI, lymphovascular space invasion; N, negative; P, positive.
Patients positive for nuclear p-Ser167-ERα had significantly shorter RFS (P < 0.01) (Fig.2a). Although not significant, patients positive for cytoplasmic p-Ser167-ERα tended to have shorter RFS (Fig.2b) than those negative for the same (P = 0.261). Patients were classified for RFS analysis into the following four subgroups, according to p-Ser167-ERα level: group A, patients negative for cytoplasmic and nuclear p-Ser167-ERα; group B, patients positive for cytoplasmic p-Ser167-ERα only; group C, patients positive for nuclear p-Ser167-ERα only; and group D, patients positive for cytoplasmic and nuclear p-Ser167-ERα (Fig.3). Of the four groups, RFS was shortest in patients positive for cytoplasmic and nuclear p-Ser167-ERα (group D) (P < 0.01), and was the most favorable for patients negative for both (group A).
Figure 2.
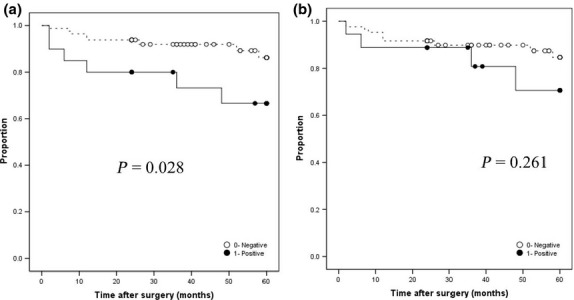
Relapse-free survival of endometrioid endometrial cancer patients with nuclear (a) and cytoplasmic (b) phosphorylated estrogen receptor α at Ser167 (p-Ser167-ERα). Kaplan–Meier curves. ○, Negative p-Ser167-ERα; ●, positive p-Ser167-ERα.
Figure 3.
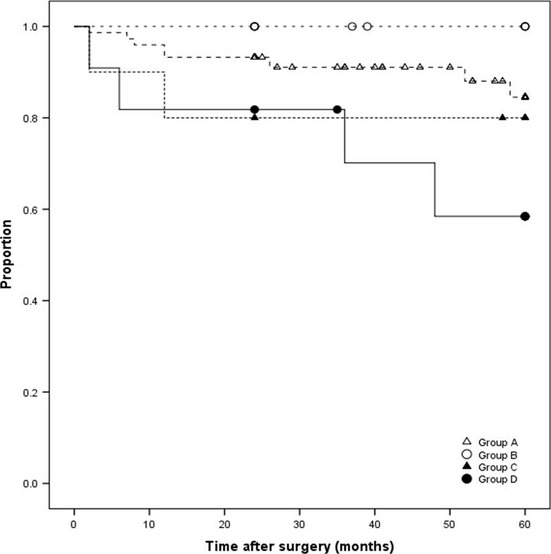
Relapse-free survival of endometrioid endometrial cancer patients. Group A, negative for cytoplasmic and nuclear phosphorylated estrogen receptor α at Ser167 (p-Ser167-ERα); group B, positive for cytoplasmic p-Ser167-ERα only; group C, positive for nuclear p-Ser167-ERα only; group D, positive for cytoplasmic and nuclear phosphorylated mammalian target of rapamycin. Kaplan–Meier curves.
The prognostic relevance of levels of p-Ser167-ERα, mTOR, p-MAPK, p-p90RSK, and p-S6K1 was analyzed using a multivariate proportional hazards model adjusted for established clinical prognostic factors; depth of tumor invasion, LVSI, histological grade, and stage (Table4). Histological grade and LVSI were independent prognostic factors for RFS (hazard ratio [HR] = 38.285; 95% confidence interval [CI], 1.882–778.7; P = 0.018; and HR = 6.567; 95% CI, 1.087–39.676; P = 0.040, respectively), but nuclear p-Ser167-ERα level was not independent (HR = 6.707; 95% CI, 0.419–107.406; P = 0.179). In addition, there were no correlations between nuclear p-mTOR and recurrence site.
Table 4.
Prognostic factors for relapse-free survival in endometrioid endometrial cancers (n = 103): Multivariate Cox proportional-hazards regression model analysis
| HR | 95% CI | P-value | ||
|---|---|---|---|---|
| Stage | 0.566 | 0.065 | 4.958 | 0.607 |
| Grade | 38.285 | 1.882 | 778.700 | 0.018* |
| Invasion | 1.650 | 0.146 | 18.637 | 0.686 |
| LVSI | 6.567 | 1.087 | 39.676 | 0.040* |
| p-mTOR (nucleus) | 2.220 | 0.445 | 11.071 | 0.331 |
| p-mTOR (cytoplasma) | 1.592 | 0.346 | 7.323 | 0.550 |
| p-Ser167-ERα (nuclear) | 6.707 | 0.419 | 107.406 | 0.179 |
| p-Ser167-ERα (cytoplasma) | 1.464 | 0.105 | 20.386 | 0.777 |
| p-S6K1 | 0.265 | 0.037 | 1.905 | 0.187 |
| p-MAPK | 1.142 | 0.180 | 7.249 | 0.888 |
| p-p90RSK | 5.882 | 0.553 | 62.595 | 0.142 |
P < 0.05. CI, confidence interval; HR, hazards ratio; LVSI, lymphovascular space invasion; p-mTOR, phosphorylated mammalian target of rapamycin; p-p90RSK, phosphorylated p90 ribosomal S6 kinase; p-S6K1, phosphorylated p70 S6 kinase 1; p-Ser167-ERα, phosphorylated estrogen receptor α at Ser167.
Discussion
In this study we identified that, in EEC, nuclear p-Ser167-ERα is the result of cooperation between mTOR/S6K1 and MAPK/RSK signaling pathways, and indicates development of advanced disease carrying a poor prognosis. Therefore, increased nuclear p-Ser167-ERα may play a pivotal role in the neoplastic process, and may be a marker indicating poor prognosis; this is the opposite of the situation seen in breast cancer patients.9,10
Yamashita et al.9 indicated that patients whose primary breast tumors extensively expressed p-Ser167-ERα responded significantly better to endocrine therapy and had better survival than did other patients. A breast cancer study carried out by Jian et al. also showed that the level of p-Ser167-ERα was strongly associated with p-p90RSK and p-MAPK, and seemed to be indicative of better prognosis. Interestingly, they reported that there was no association between human epidermal growth factor receptor-2 (HER2)-positive status and p-Ser167-ERα, nor were the activities of p-MAPK or p-p90RSK associated with HER2 status. However, HER2 status was associated with phosphorylated AKT (p-AKT), and p-AKT was also associated with p-Ser167-ERα, and with a greater likelihood of relapse and death due to cancer. Although p-AKT was not associated with disease-free survival, p-AKT positivity was associated with a reduction in overall survival. They suggested that p-p90RSK and p-MAPK, rather than p-AKT, may mediate the phosphorylation of p-Ser167-ERα in breast cancer cells, and that p- AKT is instead involved in regulating other cellular processes that lead to reduced patient survival.10 The statement that p-AKT can potentially induce the phosphorylation of p-Ser167-ERα appears to contradict the earlier statement that the authors of the study suggested that it was p-p90RSK and p-MAPK, rather than p-AKT, that may mediate the phosphorylation of p-Ser167-ERα in breast cancer cells.
To our knowledge, there have only been a few reports of studies in which the relationship between p-ERα Ser167 and EEC have been explored. Vilgelm et al.7 indicated that the loss of phosphatase and tensin homologue deleated on chromosome ten (PTEN) and AKT activation results in a Ser167 phosphorylation-dependent enhancement of ERα transcriptional activity that is independent of estrogen and plays a pivotal role in the neoplastic process. Shah et al.3 identified a connection between the ER coactivators of steroid receptor coactivator (Src) kinase and p-ERα Ser167 through activation of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, which, in turn, potentiates tamoxifen agonist action. These data supported the idea that Ser167 phosphorylation of the ER through activation of the PI3K/AKT pathway in the endometrium is an important process.
Recent molecular profiling has shown that increased PI3K/AKT/mTOR signaling is associated with aggressive disease and poor prognosis, irrespective of endometrial cancer tumor.11 A major downstream effector of AKT is mTOR complex1 (mTORC1); its downstream targets, such as ribosomal S6K1, control protein synthesis. Another mTOR complex, mTORC2, participates in the activation of AKT. In our previous study of a series of 82 patients with ECC, we reported that nuclear mTORC1 was significantly elevated in poorly differentiated tumors with lymph node involvement and in patients with shorter survival.8 In the present study, immunohistochemical evaluation of the expression of p-S6K1 indicated that it was positively correlated with LVSI. Lymphovascular space invasion includes lymphatic vessel invasion and blood vessel invasion, thought to be the beginnings of lymphogenous and hematogenous metastases, respectively. Koskas et al.12 suggested that LVSI should be considered as an independent risk factor for lymph node metastasis. It is therefore a reasonable finding that overexpression of p-S6K1 was observed in the present LVSI cases.
Signaling of mTORC1 is also involved in cross-talk with MAPK signaling.11 The corresponding effectors of these pathways, S6K1 and RSK respectively, have been shown to converge on a common set of targets, most notably in control of protein translation.13–15
In this study, we identified nuclear p-Ser167-ERα as a recipient of coordinated phosphorylation inputs from MAPK and mTOR and showed that nuclear p-Ser167-ERα might be related to the biological behavior of ECC. These findings are similar to results reported in breast cancer; Yamnik et al.5,6 reported that mTOR/S6K1 and MAPK/RSK coordinately regulate p-Ser167-ERα and the development of resistance, which can serve as a prognostic marker for breast cancer.
In conclusion, we showed that, in EEC, nuclear p-Ser167-ERα was strongly positively correlated with p-MAPK and p-S6K1. The coordinate action of mTOR/S6K1 and MAPK/RSK pathways provide a strong stimulus for EEC tumor growth, and may contribute to the development of advanced stages of cancer with poor prognosis. We suggest that dual inhibition of the mTOR/S6K1 and MAPK/RSK signaling pathways, which lead to ERα activation and stimulation of EEC development, may result in better clinical responses in advanced EEC patients.
Acknowledgments
This study was partly funded by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (21592124).
Disclosure Statement
The authors have no conflict of interest.
References
- Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2010;(8):CD007926. doi: 10.1002/14651858.CD007926.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16:363–80. doi: 10.1677/ERC-08-0266. [DOI] [PubMed] [Google Scholar]
- Shah YM, Rowan BG. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (alpha) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol. 2005;19:732–48. doi: 10.1210/me.2004-0298. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–9. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–8. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgelm A, Lian Z, Wang H, et al. Akt-mediated phosphorylation and activation of estrogen receptor alpha is required for endometrial neoplastic transformation in Pten+/- mice. Cancer Res. 2006;66:3375–80. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Kurokawa T, Horiuchi Y, Sawamura Y, Shinagawa A, Kotsuji F. Localisation of phosphorylated mTOR expression is critical to tumour progression and outcomes in patients with endometrial cancer. Eur J Cancer. 2010;46:3445–52. doi: 10.1016/j.ejca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nishio M, Toyama T, et al. Low phosphorylation of estrogen receptor alpha (ERalpha) serine 118 and high phosphorylation of ERalpha serine 167 improve survival in ER-positive breast cancer. Endocr Relat Cancer. 2008;15:755–63. doi: 10.1677/ERC-08-0078. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sarwar N, Peston D, et al. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clin Cancer Res. 2007;13:5769–76. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–64. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- Koskas M, Bassot K, Graesslin O, et al. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol Oncol. 2013;129:292–7. doi: 10.1016/j.ygyno.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–91. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–64. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y, Yoon SO, Kubota K, Mendoza MC, Gygi SP, Blenis J. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J Biol Chem. 2009;284:14939–48. doi: 10.1074/jbc.M900097200. [DOI] [PMC free article] [PubMed] [Google Scholar]


