Abstract
Standard fractionated radiotherapy for the treatment of cancer consists of daily irradiation of 2-Gy X-rays, 5 days a week for 5–8 weeks. To understand the characteristics of radioresistant cancer cells and to develop more effective radiotherapy, we established a series of novel, clinically relevant radioresistant (CRR) cells that continue to proliferate with 2-Gy X-ray exposure every 24 h for more than 30 days in vitro. We studied three human and one murine cell line, and their CRR derivatives. Guanine nucleotide-binding protein 1 (GBP1) gene expression was higher in all CRR cells than their corresponding parental cells. GBP1 knockdown by siRNA cancelled radioresistance of CRR cells in vitro and in xenotransplanted tumor tissues in nude mice. The clinical relevance of GBP1 was immunohistochemically assessed in 45 cases of head and neck cancer tissues. Patients with GBP1-positive cancer tended to show poorer response to radiotherapy. We recently reported that low dose long-term fractionated radiation concentrates cancer stem cells (CSCs). Immunofluorescence staining of GBP1 was stronger in CRR cells than in corresponding parental cells. The frequency of Oct4-positive CSCs was higher in CRR cells than in parental cells, however, was not as common as GBP1-positive cells. GBP1-positive cells were radioresistant, but radioresistant cells were not necessarily CSCs. We concluded that GBP1 overexpression is necessary for the radioresistant phenotype in CRR cells, and that targeting GBP1-positive cancer cells is a more efficient method in conquering cancer than targeting CSCs.
Keywords: GTP-binding proteins, head and neck neoplasms, neoplasms, radiation, radiation oncology
Radiotherapy is one of the major therapeutic modalities for eradicating malignant tumors. The existence of radioresistant cells remains one of the major obstacles in radiotherapy and chemoradiotherapy. Ordinary radiotherapy for cancers is composed of fractionated radiation (FR), with approximately 2 Gy of X-ray irradiation once a day, 5 days a week, over a 5–8-week period.1 In order to develop more effective tumor radiotherapies, we established three human and one murine clinically relevant radioresistant (CRR) cell lines independently. These cells continue to proliferate under exposure to 2 Gy/day for more than 30 days in vitro.2,3 The total dose to these CRR cells over the whole process added up to more than 1500 Gy. We carried out cDNA microarray analyses of differential gene expression in association with the CRR phenotype. We found that the Guanine nucleotide-binding protein 1 (GBP1) gene was overexpressed in all of the CRR cells examined.
GBP1 is a member of the large GTPase family.4 The human large GTPase family consists of seven members, encoded by a gene cluster located on chromosome 1.5 GBP1 is one of the genes most strongly induced by interferons.6 GBP1 is highly expressed in endothelial cells, where it inhibits the proliferation and invasion of endothelial cells in response to γ-interferon and is activated by inflammatory cytokines in vitro and in vivo.7–9 Downregulation of GBP1 by siRNA resulted in higher levels of hepatitis C virus replication in a human hepatoma cell line, Huh-7.10 In addition to the GTPase activity and its involvement in viral infections, GBP1 overexpression also contributes to cell survival by inhibiting apoptosis in human umbilical vein endothelial cells after growth factor and serum depletion.11
Ovarian cancer cases with GBP1 protein overexpression are resistant to paclitaxel, leading to poor prognoses.12 GBP1 overexpression is directly associated with moderate levels of paclitaxel resistance in ovarian cancer cell lines.13 Higher GBP1 levels are associated with higher pathological stages, positive perineural invasion, and poorer prognosis of patients with oral squamous cell carcinoma.14 In this study we found that GBP1 is necessary but not sufficient for cellular radioresistance in vitro. We carried out immunohistochemical studies on clinicopathological specimens from head and neck cancers (HNC) to confirm the relevance of GBP1 in cancer treatment. This study revealed that GBP1 is one of the key molecules contributing to radioresistance.
Materials and Methods
Cell culture and drugs
Human cancer cell lines SAS, HepG2, and KB and a mouse breast cancer cell line, MM102, were obtained from the Cell Resource Center for Biomedical Research (IDAC, Tohoku University, Sendai, Japan). We established CRR cell lines SAS-R1, HepG2-8960-R, and KB-R by exposing these parental cells to FR of X-rays for more than 5 years.3 For the maintenance of the CRR phenotype, FR at 2 Gy was carried out every 24 h. All cells used in this study were maintained in RPMI-1640 medium (Nacalai Tesque, Kyoto, Japan) and supplemented with 5% FBS (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere at 37°C in air with 5% CO2. Acute exposure experiments were carried out with cells in the exponential growth phase, 24 h after the last maintenance irradiation. We introduced pIRES GBP1 expression vectors13 into CRR cells by Lipofectamine 2000 (Invitrogen) and selected colonies resistant to 100 μg/mL G418 (Geneticin, Grand Island, NY, USA).
Irradiation
X-ray irradiation was carried out in a 150-KVp X-ray generator (MBR-1520R; Hitachi, Tokyo, Japan) with a total filtration of 0.5 mm aluminum plus 0.1 mm copper filter, at a dose rate of 1.0 Gy/min.
Cell survival assay after irradiation
Cell survival was determined by the modified high density survival (MHDS) assay.15
Microarray analysis
Genome-wide expression arrays (Illumina, San Diego, CA, USA) were used for the analysis of SAS (human WG6, version 3, 48 803 genes) and HepG2 (human WG6, version 1, 47 296 genes). Data were analyzed by TransGenic (Kumamoto, Japan). For MM102, a 3D-Gene mouse Oligo chip 24k (23 522 genes; Toray Industries, Tokyo, Japan) was used and the data were analyzed by Toray Industries using GeneSpring GX10 (Agilent Technologies, Santa Clara, CA, USA). HepG2 versus HepG2 cancer stem cell (CSC) analysis was carried out by MOGERA-Array self (Tohoku Chemical, Iwate, Japan).
Antibodies
The primary antibodies used were as follows: anti-β-actin (A5316; Sigma, St. Louis, MO, USA), anti-GBP1 (15303-1-AP; Proteintech Group, Chicago, IL, USA), purified anti-H2AX-phosphorylated (γH2AX) (Ser139; BioLegend, San Diego, CA, USA), anti-Oct4 antibody 7E7 (ab105931; Abcam, Cambridge, MA, USA), CD34 (ab8158, Abcam), anti-GBP2 N1C1 (GTX114426; GeneTex, Irvine, CA, USA), anti-GBP3 C-term (AP18451b; Abgent, San Diego, CA, USA), anti-GBP5 N1N3 (GTX106994; GeneTex), anti-TAP1 53H8 (GTX10356; GeneTex), and interleukin (IL)-15 (sc-1296; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibodies used were as follows: goat anti-rabbit IgG (H1202; Nichirei Bioscience, Tokyo, Japan), mouse anti-rat IgG (H1104; Nichirei Bioscience), Alexa Fluor 488 goat anti-mouse IgG (A11001; Invitrogen), and Alexa Fluor 594 goat anti-rabbit IgG (A11012, Invitrogen).
Western blot analysis
Western blot of whole cell lysates was carried out as previously described.16
Reverse transcription–PCR
Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). cDNA was synthesized by RT using SuperScriptIII Reverse Transcriptase (Invitrogen). Reverse transcription–PCR of GBP1 was carried out using the primer pair 5′-CTGCACAGGCTTCAGCAAAA-3′ and 5′-AAGGCTCTGGTCTTTAGCTT-3′.13 Reverse transcription–PCR of ISG20 was carried out using the primer set 5′-ATCTCTGAGGGTCCCCAAG-3′ and 5′-TTCAGTCTGACACAGCCAGG-3′.17 The RT-PCR was carried out using TB SYBR gPCR Mix (Toyobo, Osaka, Japan). The PCR conditions were: 95°C for 1 min, followed by 60 cycles of 95°C for 15 s, and 60°C for 30 s using the Thermal Cycler Dice Real Time System (Takara, Shiga, Japan).
RNA interference
Lipofectamine 2000 was used for transfection. GBP1 siRNA (Hs_GBP1_8 and Hs_GBP1_9) and AllStars Negative Control siRNA were purchased from Qiagen.
Apoptosis assay
Apoptotic cells were quantified using an annexin V–FITC apoptosis detection kit (BioVision, Mountain View, CA, USA). Cells (5 × 105) were collected 48 h after irradiation and were analyzed by a FACScan (Cytomics FC500; Becton Dickinson, Mountain View, CA, USA).
Immunofluorescence staining of culture cells
Immunofluorescence staining was carried out as previously described.18 Images were randomly captured in a fluorescence microscope (BZ-8000; Keyence, Osaka, Japan). We scored γH2AX foci and Oct4-positive cells by counting 50 cells in total.
Animal experiments
This study was approved by Regulations for Animal Experiments and Related Activities, Tohoku University, and carried out as described previously.16 Atelo Gene (Koken, Tokyo, Japan) was used to deliver siRNA into animal tissues according to the manufacturer's protocol.
Immunohistochemistry
Tumor tissues were fixed in 10% formalin and immunohistochemical staining was carried out as described previously.19
In situ hybridization
GBP1 (NM_002053) gene expression in tumor tissues was visualized using RNAscope and the HybEZ system according to the manufacturer's protocol (Advanced Cell Diagnostics, Hayward, CA, USA).
Demographic analysis
This study was approved by the committees of medical ethics, Shinshu University School of Medicine (No.354; Matsumoto, Japan) and Tohoku University, Graduate School of Medicine (No. 2011-42, 2013-1-1). We carried out immunohistochemical studies on biopsy specimens from HNC before treatment at Shinshu University Hospital and Tohoku University Hospital. The correlation between relative protein expression and clinicopathological data was analyzed using the Behrens–Fisher test.
Statistical analysis
Experiments in vitro were carried out in triplicate and were statistically analyzed using Student's t-test.
Results
Identification of GBP1 overexpression by cDNA array analysis
We carried out a cDNA array analysis to identify genes whose expression was changed 12 h after exposure to 2-Gy X-rays (2-Gy-12 h). We compared the expression profile of CRR cells and their corresponding parental cells. In order to avoid the influence of the maintenance irradiation other than radioresistance, we also analyzed SAS-R1-NOIR cells, which were cultured CRR cells without maintenance exposure. We confirmed that NOIR cells were also radioresistant, as determined by the MHDS assay (Fig. S1a). We first compared SAS, SAS 2-Gy-12 h, SAS-R1 2-Gy-12 h, and SAS-R1 NOIR (Fig.1a). We found 1636 genes whose expression in CRR cells was more than twice that in corresponding parental cells, regardless of irradiation. We also carried out the same analysis among the MM102 series and found 206 genes that were overexpressed in CRR cells compared with parental cells, regardless of irradiation. Compared with the corresponding parental cells, 23 genes were commonly overexpressed in SAS-R1 and MM102-R cells. We finally confirmed that two genes, GBP1 and ISG20, were commonly overexpressed in CRR cells of the HepG2 series (Fig.1a,b).
Figure 1.
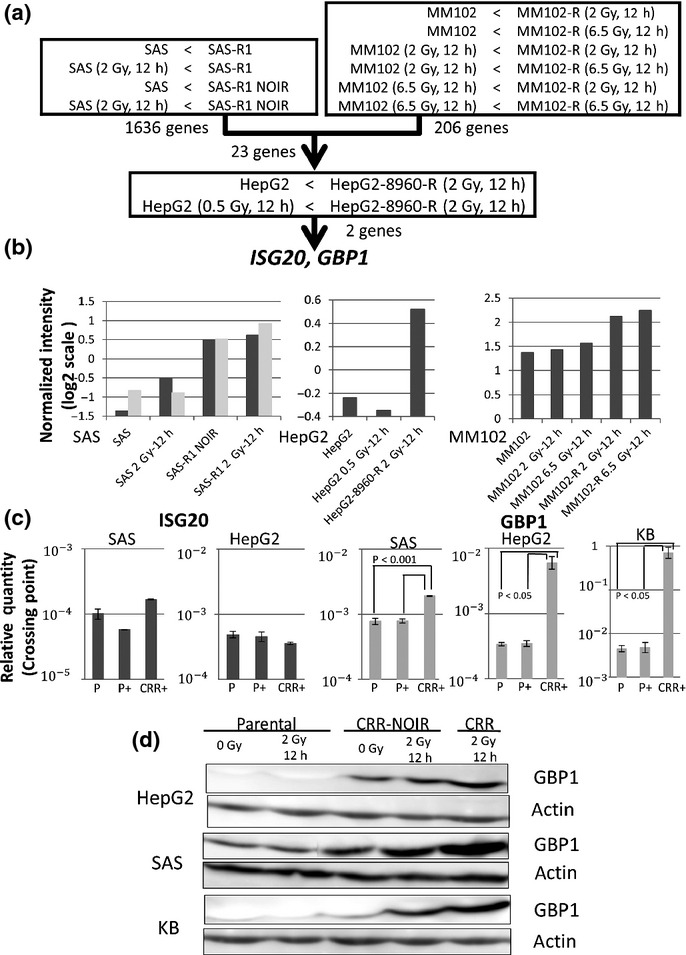
Overexpression of guanine nucleotide-binding protein 1 (GBP1) in clinically relevant radioresistant (CRR) SAS, HepG2, KB, and MM102 cells. (a,b) Compared to corresponding parental cells, overexpression of GBP1 and ISG20 was found in all CRR cells. SAS cells had two different GBP1 target probes. (c) GBP1 but not ISG20 overexpression was reconfirmed in CRR cells by RT-PCR. In all the combinations of cell lines, CRR cells 12 h after 2-Gy irradiation (CRR+) showed higher GBP1 gene expression than parental cells without irradiation (P) and parental cells 12 h after 2-Gy irradiation (P+). (d) GBP1 protein expression in CRR cells and CRR-NOIR cells 12 h after 2-Gy irradiation or without irradiation were higher than their parental cells. NOIR cells were without irradiation for over 30 days, in order to avoid the influence of maintenance radiation. RNA from three independent exposure treatments was combined for the cDNA microarray and RT-PCR analysis. cDNA microarray analysis of the SAS group was carried out in duplicate.
Verification of GBP1 overexpression by RT-PCR
Compared with their corresponding parental cells, GBP1 expression was commonly higher (Fig.1c) in HepG2-8960-R as well as SAS-R1 cells, whereas ISG20 expression was not.
Verification of GBP1 protein overexpression by Western blotting
All of the CRR cells examined showed higher GBP1 expression than their corresponding parental cells and NOIR cells (Fig.1d). HepG2 and KB showed only a trace amount of the GBP1 protein, irrespective of irradiation. GBP1 protein expression was higher in NOIR cells than in parental cells. GBP1 expression in SAS-R1 NOIR and KB NOIR tended to be higher after 2-Gy irradiation than without irradiation. SAS, the most radioresistant line, showed the highest expression of GBP1 protein among the parental cells. These results confirmed that GBP1 protein expression was correlated to radioresistance. We picked up proteins directly connected with GBP1 on the network such as GBP2, GBP3, GBP5, TAP1 and IL-15 in reference to COXPRESdb (http://coxpresdb.jp/). No associations were observed among their protein levels (Fig. S2d).
Decreased radioresistance by GBP1 gene knockdown
In order to determine whether GBP1 was involved in radioresistance or not, the MHDS assay was carried out after cells were transfected with two types of GBP1 siRNA, GBP1_8 and GBP1_9 (Fig. S2a). During this period of experiments, GBP1 gene expression was suppressed (Fig. S2b). In all of the CRR cells examined, GBP1 knockdown cancelled radioresistance to the levels of their parental cells (Figs2 and S1b). Furthermore, GBP1 knockdown increased radiosensitivity in parental cells. These indicate that GBP1 is one of the key molecules commonly involved in coping with radiation exposure, both in CRR and parental cells. Because suppression with GBP1_8 was stronger than with GBP1_9, further experiments were carried out using GBP1_8.
Figure 2.
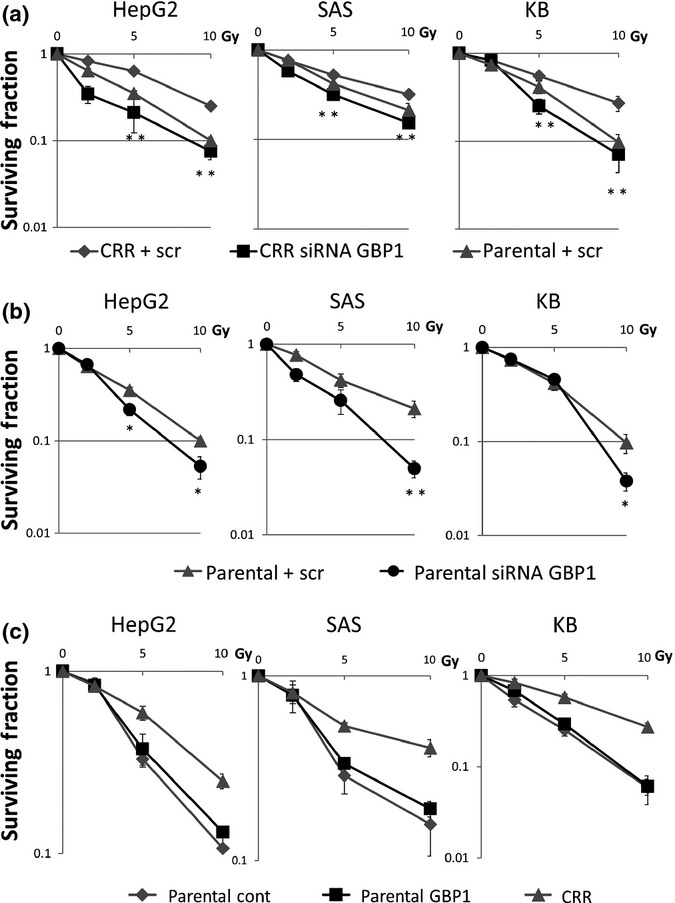
Radiosensitivity after guanine nucleotide-binding protein 1 (GBP1) knockdown and overexpression, as determined by modified high-density survival assay. (a,b) GBP1 knockdown increased radiosensitivity both in clinically relevant radioresistant (CRR) and parental cells. GBP1 knockdown was carried out with Hs_GBP1_8. (c) Parental cells transfected with GBP1 (Parental GBP1) did not show significant changes in radioresistance compared to parental cells with an empty vector (Parental cont). Exponentially growing cells were seeded in 25-cm2 flasks and incubated for 24 h; cells were then irradiated and incubated for another 72 h. Subsequently, 10% of the cells in each flask were seeded into another flask and incubated for a further 72 h and live cells were counted. scr, scramble siRNA.
Association of GBP1 overexpression and radioresistance
To determine whether GBP1 directly participates in the establishment of the radioresistant phenotype, we transfected parental cells with a GBP1 expression vector. We confirmed GBP1 protein overexpression in all transfected parental cells (Fig. S2c). The expression level was even higher in transfected cells of SAS and KB compared to their CRR cells. In all the parental cells, GBP1 transfection did not show significant change in radioresistance (Fig.2c).
Relationship between suppressing GBP1 and cell death
As GBP1 knockdown increased radiosensitivity, we examined the association of GBP1 expression and radiation-induced cell death by FACS. The frequency of apoptosis induced by 10-Gy X-rays was significantly higher in CRR cells with siGBP1 than with scrambled RNA in all cell lines examined (Fig.3a). DNA double-strand breaks (DSBs) induced by 2-Gy X-rays were determined by γH2AX staining. GBP1 knockdown increased DNA DSBs by irradiation (Figs3b,c and S3a,b).
Figure 3.

Relationship between suppression of guanine nucleotide-binding protein 1 (GBP1) and cell death. (a) Apoptotic cells, as determined by annexin V staining, increased both in clinically relevant radioresistant (CRR) and parental cells after 10 Gy of irradiation. Knockdown of GBP1 increased the frequency of apoptotic cells, both in CRR and parental cells regardless of irradiation. (b,c) Knockdown of GBP1 increased the number of γH2AX foci (DNA double-strand breaks, green) induced by 2-Gy X-rays in CRR cells. In total, 50 cells were counted for the number of γH2AX foci at 0, 0.5, 6, 12, 24, and 48 h after X-ray irradiation. The nucleus was counterstained with DAPI (blue). scr, scramble siRNA.
Relationship between suppression of GBP1 and CSCs
We isolated surviving CSCs by exposure to long-term 0.5-Gy FR for 82 days from HepG2 cells.20 Microarray analysis showed that the mRNA level of GBP1 was significantly higher in HepG2 CSCs than in HepG2 cells (Fig.4a). Additionally, the protein level of GBP1 was higher in HepG2 cells after FR than in non-radiated HepG2 cells (Fig.4b). Therefore, we examined the relationship between Oct4 expression and long-term FR. The frequency of Oct4-positive cells was higher in cells exposed to 0.5-Gy FR every 12 h for 30 days than in parental cells and was the highest in CRR cells (Fig.4c). We examined whether Oct4-positive CSCs coincided with GBP1-positive cells by immunostaining. Even in CRRs, Oct4-positive cells constituted less than 8% of total cells (Fig.4d). In contrast, GBP1 was positive in all CRR cells.
Figure 4.
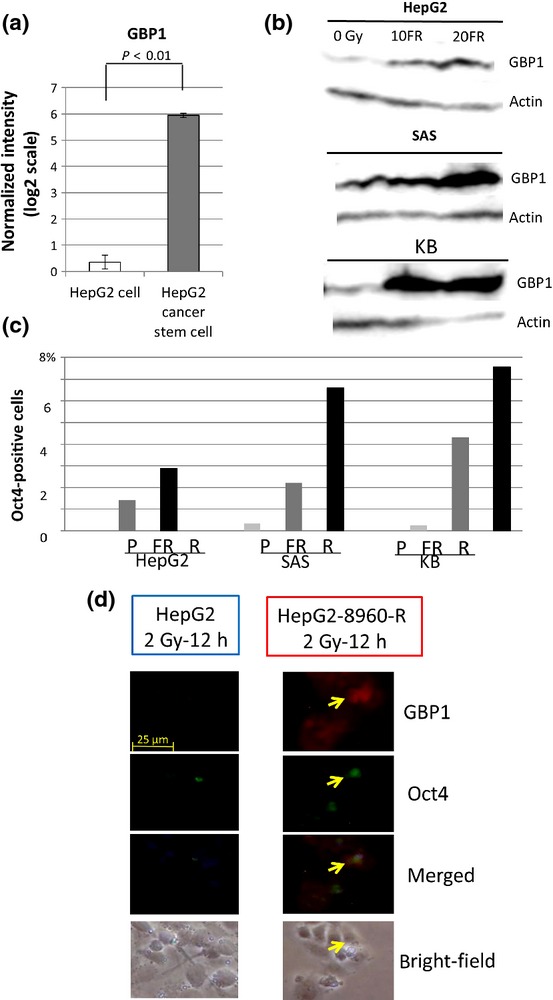
Relationship between suppression of guanine nucleotide-binding protein 1 (GBP1) and cancer stem cell markers. (a) Microarray analysis revealed significantly higher GBP1 gene expression in HepG2 cells than in HepG2 cancer stem cells (CSC). (b) GBP1 protein expression tended to correlate with the accumulation of fractionated radiation with 0.5-Gy X-rays every 12 h (FR). (c) The fraction of GBP1-positive cells was higher in cells with FR for 30 days than in Parental cells (P) and the highest in clinically relevant radioresistant (CRR) cells (R). (d) The number of Oct4 (green) and GBP1 (red) cells was higher in CRR cells than parental cells. Immunofluorescence of GBP1 staining was stronger in CRR cells than their parental cells but not all CRR cells were Oct4-positive.
GBP1 immunohistochemistry in xenotransplanted tumor tissues
SAS-R1 tumors transplanted into nude mice are also resistant to FR.16 We found overexpression of both the GBP1 protein and mRNA in SAS-R1 tumors and KB-R tumors, compared with their parental cell-derived tumors (Fig.5a). In order to determine the association of GBP1 with tumor radioresistance, we suppressed GBP1 by AteloGene-delivered siRNA into animal tissues (Fig. S4) and irradiated with FR of 2-Gy/day for 5 days (Fig.5b). GBP1 suppression alone did not affect the growth or microvessel density (MVD) of SAS and SAS-R1 tumors. However, SAS-R1 tumors shrank if combined with irradiation. In parental tumors, irradiation diminished the growth irrespective of GBP1 suppression.
Figure 5.

Distribution of guanine nucleotide-binding protein 1 (GBP1) in tumor tissues. (a) Compared with parental tumors, overexpression of the GBP1 gene and GBP1 protein was observed in clinically relevant radioresistant (CRR) tumor cells xenotransplanted into nude mice. (b) SAS-R1 tumors shrank due to fractionated radiation in combination with GBP1 suppression. SAS-R1 cells (1 × 107) were injected into the right flank and SAS cells into the left flank of a mouse. (c) Representative positive staining of GBP1 in clinical cancer tissues. IHC, immunohistochemistry; IR, fractionated radiation with 2-Gy X-rays every 24h ISH, in situ hybridization; scr, scramble siRNA; arrows, GBP1 positive cells
GBP1 immunohistochemistry of clinical samples
Immunohistochemical study of GBP1 was possible in 45 cases of HNC before treatment. Clinicopathological features of cases are shown in Table1. We defined a case GBP1-positive if the nucleus and cytoplasm of more than 5% of tumor cells were positive for GBP1 (Fig.5c). Only one GBP1-positive case and seven GBP1-negative cases out of a total of 45 cases were without recurrence or metastasis 5 years after chemoradiation treatment (Table1a). GBP1-positive cases were significantly more resistant to chemoradiotherapy than GBP1-negative cases (P < 0.05, Behrens–Fisher test).
Table 1.
Guanine nucleotide-binding protein 1 (GBP1) immunohistochemistry and clinicopathological features of head and neck cancer
| (a) Association of GBP1 expression and chemoradiotherapy resistance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recurrence or metastatis | Total | ||||||||
| + | − | ||||||||
| GBP1 | + | 20 | 1 | 21 | GBP1 positive cases were significantly more resistant to chemoradiotherapy than GBP1 negative cases (Behrens-Fisher test, P < 0.05). Relative risk, 1.345; 95% confidence limit, 1.022 −1.768. | ||||
| − | 17 | 7 | 24 | ||||||
| Total | 37 | 8 | 45 | ||||||
| (b) Clinicopathological features of 45 cases of head and neck cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample no. | Region | Age, years | Gender | Differentiation | TNM classification | Stage | Treatment | Total dose of irradiation (Gy) | Curative effect |
| GBP1(+) cases | |||||||||
| 1 | Tongue | 53 | Male | Well | T4N2bM0 | IV | Chemoradiation→operation | 40 | Recurrent |
| 2 | Tongue | 54 | Female | Well | T2N0M0 | II | Chemoradiation→operation | 40 | Recurrent and lymph node metastasis |
| 3 | Larynx | 57 | Male | Moderate | T2N0M0 | II | Radiation | 70 | Lymph node metastasis |
| 4 | Larynx | 59 | Male | Moderate | T2N0M0 | II | Chemoradiation | 70 | Recurrent |
| 5 | Buccal cavity | 59 | Male | Moderate | T1N2 cM0 | IV | Chemoradiation→operation | 70 | Lymph node metastasis |
| 6 | Buccal cavity | 60 | Male | Well | T4N2bM1 | IV | Chemoradiation→operation | 40 | Recurrent |
| 7 | Paranasal sinus | 60 | Male | Well > poor | T3N0M0 | III | Chemoradiation→operation | 70 | Recurrent and lymph node metastasis |
| 8 | Paranasal sinus | 60 | Male | Moderate | T3N0M0 | III | Chemoradiation→operation | 50 | Recurrent |
| 9 | Paranasal sinus | 60 | Female | Moderate | T3N0M0 | III | Chemoradiation→operation | 50 | Recurrent |
| 10 | Paranasal sinus | 61 | Male | Moderate | T3N0M0 | III | Chemoradiation→operation | 50 | Lymph node metastasis |
| 11 | Larynx | 67 | Male | Moderate | T2N0M0 | II | Radiation | 72 | Recurrent |
| 12 | Paranasal sinus | 69 | Male | Poor | T3N0M0 | III | Chemoradiation→operation | 50 | Pathologically no recurrent/ CR over 5 years |
| 13 | Hypopharynx | 69 | Male | Well > moderate | T4N2 cM0 | IV | Chemotherapy→operation→radiation | 63 | Recurrent and lung metastatis |
| 14 | Hypopharynx | 71 | Male | Moderate | T2N0M0 | II | Chemoradiation→operation | 70 | Recurrent |
| 15 | Paranasal sinus | 74 | Male | Moderate | T4N1M0 | IV | Radiation→operation | 50 | Recurrent |
| 16 | Paranasal sinus | 75 | Female | Well | T4N2bM0 | IV | Chemoradiation | 60 | Recurrent |
| 17 | Buccal cavity | 76 | Male | Well | T2N0M0 | II | Chemoradiation→operation | 50 | Lymph node and lung metastasis |
| 18 | Buccal cavity | 79 | Female | Well | T4N2 cM0 | IV | Chemoradiation→operation | 40 | Recurrent and lymph node metastasis |
| 19 | Larynx | 79 | Male | Moderate > well | T1bN0M0 | I | Radiation | 66 | Recurrent |
| 20 | Buccal cavity | 80 | Female | Well | T4N2bM0 | IV | Chemoradiation→operation | 60 | Recurrent and lymph node metastasis |
| 21 | Larynx | 81 | Male | Well > moderate | T1aN0M0 | I | Radiation | 66 | Recurrent |
| GBP1(−) | |||||||||
| 22 | Paranasal sinus | 46 | Male | Well | T3N0M0 | III | Chemoradiationmoradiatin | 50 | Recurrent and lymph node metastasis |
| 23 | Larynx | 51 | Male | Well | T2N0M0 | I | Radiation→operation | 66 | Recurrent |
| 24 | Oropharynx | 54 | Male | Poor | T4N2 cM0 | IV | Chemoradiation→operation | 77 | Recurrent |
| 25 | Paranasal sinus | 56 | Male | Moderate > well | T3N0M0 | III | Chemoradiationoradia wel | 70 | Recurrent |
| 26 | Paranasal sinus | 57 | Male | Moderate | T3N0M0 | III | Chemoradiation→operation | 50 | Recurrent |
| 27 | Oropharynx | 57 | Male | Moderate | T2N3M0 | IV | Chemoradiation→operation | 60 | Recurrent |
| 28 | Paranasal sinus | 57 | Male | Moderate > poor | T3N2bM0 | IV | Chemoradiationmoradiatpo | 50 | Recurrent and lymph node metastasis |
| 29 | Paranasal sinus | 60 | Male | Poor | T3N0M0 | III | Chemoradiation→operation | 50 | Pathologically no recurrent/ CR over 5 years |
| 30 | Larynx | 61 | Male | Moderate > well | T2N0M0 | II | Chemoradiation | 70 | Pathologically no recurrent/ CR over 5 years |
| 31 | Rhinopharynx | 61 | Male | Moderate | T1N2bM0 | IIB | Chemoradiation→operation | 70 | Pathologically no recurrent/ CR over 5 years |
| 32 | Oropharynx | 66 | Male | Poor | T3N3M0 | IV | Chemoradiation→operation | 70 | Pathologically no recurrent/ CR over 5 years |
| 33 | Buccal cavity | 67 | Male | Well | T4N2 cM1 | IV | Chemoradiation→operation | 40 | Recurrent and lymph node metastasis |
| 34 | Tongue | 68 | Male | Moderate | T1N0M0 | I | Radiation | 70 | Pathologically no recurrent/ CR over 5 years |
| 35 | Paranasal sinus | 69 | Male | Moderate ˜ well | T4N2bM0 | IV | Chemoradiation→operation | 50 | Recurrent |
| 36 | Paranasal sinus | 71 | Male | Moderate > well | T3N0M0 | III | Chemoradiation→operation | 50 | Pathologically no recurrent/ CR over 5 years |
| 37 | Larynx | 71 | Male | Well | T1N0M0 | I | Radiation→operation | 66 | Recurrent |
| 38 | Paranasal sinus | 71 | Female | Well | T4N0M0 | IV | Chemoradiationinustionec | 50 | Recurrent |
| 39 | Paranasal sinus | 72 | Male | Moderate | T4N0M0 | IV | Chemoradiation→operation | 50 | Recurrent |
| 40 | Buccal cavity | 74 | Male | Well | T4N2bM0 | IV | Chemoradiation→operation | 40 | Recurrent |
| 41 | Buccal cavity | 75 | Female | Well | T4N1M0 | IV | Chemoradiation→operation | 48 | Recurrent |
| 42 | Paranasal sinus | 76 | Female | Moderate | T3N0M0 | III | Chemoradiation→operation | 60 | Recurrent |
| 43 | Paranasal sinus | 77 | Male | Moderate | T4N0M0 | IV | Chemoradiation→operation | 50 | Recurrent |
| 44 | Tongue | 80 | Male | Well | T2N0M0 | II | Radiation | 70 | Recurrent |
| 45 | Larynx | 82 | Male | Well | T1N0M0 | I | Radiation | 66 | Pathologically no recurrent/ CR over 5 years |
CR, complete remission; GBP1, guanine nucleotide-binding protein 1.
Discussion
Radiotherapy with FR is one of the major modalities for cancer treatment. Although tumors receive a large total dose, they sometimes recur and demonstrate radioresistance. In this study, we looked for genes whose overexpression was responsible for the radioresistant phenotype of CRR cells. In microarray analyses, we found two such candidate genes in common with human and murine CRR cells. We finally picked up only one gene, GBP1, whose overexpression in CRR cells was confirmed by RT-PCR. The translational level of GBP1 was closely correlated to the transcriptional step (Fig.1d). The expression levels of GBP1 protein were not different between parental cells with and without 2-Gy irradiation. All the CRR and NOIR cells examined showed GBP1 overexpression, compared with their corresponding parental cells. This indicates that GBP1 protein overexpression is associated with radioresistance and is mainly regulated at the transcriptional step.
Knockdown of GBP1 cancelled radioresistance to the level of parental cells in all the CRR cells including HepG2 and KB with naturally low GBP1 expression (Figs2a,b and S1b,c). This radiosensitization by GBP1 gene knockdown was also observed in transplanted SAS-R1 tumors (Fig.5b). However, parental cells with GBP1 transfection did not demonstrate significant changes of radioresistance even in SAS and KB cells with much more GBP1 protein than their corresponding CRR cells (Figs2c and S2c). These results indicate that GBP1 is essential to cope with radiation, but is not enough to shift the cell into CRR. Only GBP1 knockdown in CRR cells did not enhance apoptosis but induced apoptosis through increased radiation-induced DNA DSBs in an additive manner (Figs3 and S3a,b). Sublethal damage that is substantiated by FR is suggested to be restored by homologous recombination repair (HRR) of DSBs.21 Therefore, prolongation of γH2AX foci in CRR cells by GBP1 knockdown is suggested to represent the deficiency in HRR of radiation-induced DSBs.22 A member of GTPases, RECQ5/QE DNA helicase is activated by GTP binding.23 These suggest that GBP1 is involved in radioresistance through HRR of DSBs. However, the failure of enhancing radioresistance by GBP1 overexpression in parental cells indicated that HRR requires multiple molecules other than GBP1.
Overexpression of GBP1 activates class III β-tubulin and ultimately leads to paclitaxel resistance in ovarian cancer cells.12,13 Nevertheless, GBP1 protein expression was not significantly changed by treatment with cisplatin or 5-fluorouracil (Fig. S5). Extrapolating from these findings, GBP1 plays a role in radioresistance but not in chemoresistance.
The GBP1 gene is located at tight junctions of intestinal epithelial cells of inflammatory bowel disease and its knockdown enhances apoptosis.24 We previously reported that inhibition of the AKT/cyclin D1 pathway suppresses acquired radioresistance through increased apoptosis. We need to study the relationship between GBP1 and the AKT/cyclin D1 pathway.25 In this study, GBP1 knockdown enhanced radiation-induced early apoptosis in CRR cells through the delay of DSB repair. We also reported that hyperinduction of autophagy is involved in radiation-induced cell death later than 5 days after radiation.3 Combined induction of apoptosis and autophagy hyperinduction would be effective to conquer CRR cancers.
Recently, we reported that exposure to FR with 0.5-Gy X-rays every 12 h for 82 days enriched CSCs, owing to their radioresistance.20 Microarray analysis showed that GBP1 gene expression increased along with the accumulation of total dose (Fig.4b). Immunofluorescence of GBP1 staining was stronger in all CRR cells than their corresponding parental cells but not all CRR cells were Oct4-positive (Fig.4c). These results indicate that radiotherapy, in combination with targeting GBP1-positive cancer cells, is more efficient in impeding cancer than targeting CSCs.
The growth rate of transplanted murine mammary tumors transfected with GBP1 is slower accompanied with lower MVD compared with those without transfection.26 Overexpression of GBP1 in human endothelial cells decreases apoptosis but inhibits cell proliferation.11 Patients with colorectal cancer with GBP1-positive stromal cells showed better prognosis than those with GBP1-negative cases.27 In this study, CRR tumors revealed higher MVD compared to their parental cell tumors, suggesting that GBP1 of cancer cells is not involved in tumor vasculature. It is interesting to study the expression and role of GBP1 in normal cells, especially in endothelial cells after FR.
Reports of the association of GBP1 with chemotherapeutic taxanes are conflicting; GBP1 is a marker of paclitaxel-resistant cancer cells12 and a marker of docetaxel-sensitive cancer cells.28 In this study, GBP1-positive HNC was clinically resistant to radiotherapy but was not associated with resistance to cisplatin and 5-fluorouracil. These results suggest that GBP1 plays a role in radioresistance, but not in chemoresistance, and may be a useful marker for screening of specifically radioresistant tumors before treatment.
The precise mechanism of GTP hydrolyzing activity of GBP1 and its role in signal transduction remains unknown.29,30 Our study is the first to reveal another aspect of GBP1, namely radioresistance. Our results suggest that the suppression of GBP1 transcription may overcome tumor radioresistance and that GBP1 could be used for screening radioresistant malignant tumors.
Acknowledgments
This study was supported in part by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology, a Grant-in-Aid for Cancer Research from the Japanese Ministry of Health, Labor and Welfare, and a grant from the Research Seeds Quest Program, Japan Science and Technology Agency.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Fig. S1. Radioresistance of clinically relevant radioresistant (CRR)-NOIR cells, and the effect of GBP1 knockdown.
Fig. S2. Western blot analyses of guanine nucleotide-binding protein 1 (GBP1) and GBP1-associated proteins
Fig. S3. DNA double-strand breaks after GBP1 knockdown in combination with irradiation.
Fig. S4. Association of guanine nucleotide-binding protein 1 (GBP1) with tumor radioresistance in SAS and SAS-R1.
Fig. S5. Guanine nucleotide-binding protein 1 (GBP1) protein expression after treatment with cisplatin or 5-fluorouracil.
References
- Tannock I. The Basic Science of Oncology. 4th edn. New York, NY: McGraw-Hill, Medical Publications; 2005. [Google Scholar]
- Kuwahara Y, Li L, Baba T, et al. Clinically relevant radioresistant cells efficiently repair DNA double-strand breaks induced by X-rays. Cancer Sci. 2009;100:747–52. doi: 10.1111/j.1349-7006.2009.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Kakuda S, Ochiai Y, et al. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene. 2010;29:4826–37. doi: 10.1038/onc.2010.238. [DOI] [PubMed] [Google Scholar]
- Naschberger E, Bauer M, Sturzl M. Human guanylate binding protein-1 (hGBP-1) characterizes and establishes a non-angiogenic endothelial cell activation phenotype in inflammatory diseases. Adv Enzyme Regul. 2005;45:215–27. doi: 10.1016/j.advenzreg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Olszewski MA, Gray J, Vestal DJ. In silico genomic analysis of the human and murine guanylate-binding protein (GBP) gene clusters. J Interferon Cytokine Res. 2006;26:328–52. doi: 10.1089/jir.2006.26.328. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem. 1983;258:7746–50. [PubMed] [Google Scholar]
- Guenzi E, Topolt K, Cornali E, et al. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 2001;20:5568–77. doi: 10.1093/emboj/20.20.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi E, Topolt K, Lubeseder-Martellato C, et al. The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression. EMBO J. 2003;22:3772–82. doi: 10.1093/emboj/cdg382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeseder-Martellato C, Guenzi E, Jorg A, et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am J Pathol. 2002;161:1749–59. doi: 10.1016/S0002-9440(10)64452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsui Y, Sakamoto N, Kurosaki M, et al. Expressional screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J Viral Hepat. 2006;13:690–700. doi: 10.1111/j.1365-2893.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Pammer J, Reinisch C, Birner P, Pogoda K, Sturzl M, Tschachler E. Interferon-alpha prevents apoptosis of endothelial cells after short-term exposure but induces replicative senescence after continuous stimulation. Lab Invest. 2006;86:997–1007. doi: 10.1038/labinvest.3700461. [DOI] [PubMed] [Google Scholar]
- De Donato M, Mariani M, Petrella L, et al. Class III beta-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J Cell Physiol. 2012;227:1034–41. doi: 10.1002/jcp.22813. [DOI] [PubMed] [Google Scholar]
- Duan Z, Foster R, Brakora KA, Yusuf RZ, Seiden MV. GBP1 overexpression is associated with a paclitaxel resistance phenotype. Cancer Chemother Pharmacol. 2006;57:25–33. doi: 10.1007/s00280-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Chang KP, Chang YJ, et al. Identification of guanylate-binding protein 1 as a potential oral cancer marker involved in cell invasion using omics-based analysis. J Proteome Res. 2011;10:3778–88. doi: 10.1021/pr2004133. [DOI] [PubMed] [Google Scholar]
- Kuwahara Y, Mori M, Oikawa T, et al. The modified high-density survival assay is the useful tool to predict the effectiveness of fractionated radiation exposure. J Radiat Res. 2010;51:297–302. doi: 10.1269/jrr.09094. [DOI] [PubMed] [Google Scholar]
- Kuwahara Y, Oikawa T, Ochiai Y, et al. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011;2:e177. doi: 10.1038/cddis.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Yang D. Cloning, eukaryotic expression of human ISG20 and preliminary study on the effect of its anti-HBV. J Huazhong Univ Sci Technolog Med Sci. 2008;28:11–3. doi: 10.1007/s11596-008-0103-z. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki K, Kodama S, Watanabe M. Phosphorylated histone H2AX foci persist on rejoined mitotic chromosomes in normal human diploid cells exposed to ionizing radiation. Radiat Res. 2006;165:269–76. doi: 10.1667/rr3508.1. [DOI] [PubMed] [Google Scholar]
- Toda Y, Kono K, Abiru H, et al. Application of tyramide signal amplification system to immunohistochemistry: a potent method to localize antigens that are not detectable by ordinary method. Pathol Int. 1999;49:479–83. doi: 10.1046/j.1440-1827.1999.00875.x. [DOI] [PubMed] [Google Scholar]
- Shimura T, Noma N, Oikawa T, et al. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis. 2012;1:e12. doi: 10.1038/oncsis.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi H, Elkind MM. Requirement for repair of DNA double-strand breaks by homologous recombination in split-dose recovery. Radiat Res. 2001;155:680–6. doi: 10.1667/0033-7587(2001)155[0680:rfrodd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shibata A, Moiani D, Arvai AS, et al. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol Cell. 2014;53:7–18. doi: 10.1016/j.molcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Maruyama S, Nakayama M, Matsumoto K, Shibata T. Drosophila melanogaster RECQ5/QE DNA helicase: stimulation by GTP binding. Nucleic Acids Res. 2002;30:3682–91. doi: 10.1093/nar/gkf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M, Betanzos A, Weber DA, Parkos CA. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M. Targeting the AKT/GSK3beta/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:540–8. doi: 10.1016/j.ijrobp.2010.12.065. [DOI] [PubMed] [Google Scholar]
- Lipnik K, Naschberger E, Gonin-Laurent N, et al. Interferon gamma-induced human guanylate binding protein 1 inhibits mammary tumor growth in mice. Mol Med. 2010;16:177–87. doi: 10.2119/molmed.2009.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naschberger E, Croner RS, Merkel S, et al. Angiostatic immune reaction in colorectal carcinoma: impact on survival and perspectives for antiangiogenic therapy. Int J Cancer. 2008;123:2120–9. doi: 10.1002/ijc.23764. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Geck P, Parkin C, Carpinito G, Makarovskiy AN. Gene expression profiling of the androgen independent prostate cancer cells demonstrates complex mechanisms mediating resistance to docetaxel. Cancer Biol Ther. 2011;11:204–12. doi: 10.4161/cbt.11.2.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle M, Staeheli P. The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J Biol Chem. 1994;269:11299–305. [PubMed] [Google Scholar]
- Praefcke GJ, Geyer M, Schwemmle M. Robert Kalbitzer H, Herrmann C. Nucleotide-binding characteristics of human guanylate-binding protein 1 (hGBP1) and identification of the third GTP-binding motif. J Mol Biol. 1999;292:321–32. doi: 10.1006/jmbi.1999.3062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Radioresistance of clinically relevant radioresistant (CRR)-NOIR cells, and the effect of GBP1 knockdown.
Fig. S2. Western blot analyses of guanine nucleotide-binding protein 1 (GBP1) and GBP1-associated proteins
Fig. S3. DNA double-strand breaks after GBP1 knockdown in combination with irradiation.
Fig. S4. Association of guanine nucleotide-binding protein 1 (GBP1) with tumor radioresistance in SAS and SAS-R1.
Fig. S5. Guanine nucleotide-binding protein 1 (GBP1) protein expression after treatment with cisplatin or 5-fluorouracil.


