Abstract
Rhabdomyosarcoma is the most common soft tissue sarcoma affecting children, and the overall cure rate of children with metastatic disease remains below 30%. The CXC chemokine receptor-4 (CXCR4)/stromal cell-derived factor-1 (SDF1) axis has been implicated in the promotion of metastatic potential in several tumors. In this study, we developed a novel anti-CXCR4 mAb, CF172, and investigated its antimetastatic activity against rhabdomyosarcoma cells in vitro and in vivo, to evaluate its potential as a therapeutic antibody to treat rhabdomyosarcoma. The CF172 molecule showed a specific binding reactivity against human CXCR4, as well as a specific neutralizing activity against CXCR4/SDF1 signal transduction. Using CF172, we determined that SJCRH30 rhabdomyosarcoma cells expressed high levels of CXCR4. In addition, CF172 was found to inhibit the SDF1-induced migration activity of SJCRH30 cells in vitro. Using xenograft models of SJCRH30 cells, we carried out in vivo efficacy studies for peritoneal and lymph node metastasis, which were clinically observed in rhabdomyosarcoma. These studies indicated that CF172 significantly decreased both types of metastasis of SJCRH30. In conclusion, we found that a novel anti-CXCR4 mAb, CF172, with specific reactivity against human CXCR4, prevented peritoneal metastasis and lymph node metastasis of rhabdomyosarcoma in animal models. These results suggest that CF172 is a potential antimetastasis therapeutic antibody for rhabdomyosarcoma treatment.
Keywords: Monoclonal Antibodies, Neoplasm Metastasis, CXCR4 Receptors, Rhabdomyosarcoma, Neutralizing Antibodies
Rhabdomyosarcoma is the most common soft tissue sarcoma reported in children. During the last 30 years, the introduction of multimodal therapy has significantly improved cancer survival, with a cure rate of approximately 70% for patients with localized disease. Unfortunately, at least 15% of children with rhabdomyosarcoma have metastatic disease, and the prognoses for metastatic rhabdomyosarcoma have not improved significantly in the last 15 years, with an overall cure rate below 30%.1–6 Therefore, there is a critical need to develop therapeutics to treat metastatic rhabdomyosarcoma.
Organ-specific patterns of metastasis associated with different tumor types were first described by Paget's “seed-and-soil” hypothesis.7 This hypothesis states that tumor cells may form metastases at sites that: (i) chemoattract and arrest tumor cells by locally secreted factors; and (ii) provide a favorable microenvironment for tumor cell survival and growth.8 Chemokines are small pro-inflammatory chemoattractant proteins that bind to G-protein coupled receptors present on the cell surfaces of target cells. These chemokines are major regulators of cell trafficking, survival, and growth.9–11 The chemokine, stromal cell-derived factor 1 (SDF1), and its receptor, CXC chemokine receptor-4 (CXCR4), play a unique role in homing and migration of hematopoietic and lymphopoietic cells.8,10 In addition, research has suggested that CXCR4 may be involved in the promotion of metastatic potential in multiple tumor types, by activating migration and homing of tumors.12–16 Moreover, results of a recently published study indicated that high levels of CXCR4 expression in rhabdomyosarcoma correlated with metastatic potential and poor prognosis.17 Therefore, blocking CXCR4 would be a promising approach in the treatment of metastatic rhabdomyosarcoma.
To explore this possibility, we generated a novel anti-CXCR4 mAb, CF172, and investigated its in vitro binding and neutralizing activity against human CXCR4, and its antimetastatic efficacy against rhabdomyosarcoma cell lines.
Materials and Methods
Anti-CXCR4 antibody generation
Female BALB/c mice were immunized with CXCR4-stably expressed Ba/F3 cells. After an increase was observed in the serum titer of the anti-CXCR4 antibody, splenocytes were prepared from the killed mice and fused with myeloma cells. The resulting hybridoma cells were selected in conventional hypoxanthine–aminopterin–thymidine medium.
Anti-CXCR4 antibody was typically produced from the culture of hybridoma cells, followed by purification with protein A affinity chromatography and subsequent size-exclusion chromatography.
Flow cytometry
The binding activity of CF172 for CXCR4 expressed on cell surfaces was examined by flow cytometry. Cells were stained on ice for 30 min with either CF172 or control mouse IgG2b (R&D Systems, Minneapolis, MN, USA). After incubation with primary antibodies, cells were washed three times with cold PBS containing 2% FBS and then incubated on ice for an additional 30 min with FITC-conjugated goat anti-mouse IgG antibody (Becton Dickinson, San Jose, CA, USA). Stained cells were then analyzed using a FACSCalibur cell analyzer (Becton Dickinson).
Neutralization assay
Neutralization activity against CXCR4 was evaluated using the Tango CXCR4-bla U2OS cell-based assay system (Invitrogen, Carlsbad, CA, USA). For the assay, serially diluted antibody was pre-incubated with Tango CXCR4-bla U2OS for 30 min at 37°C. SDF1 (4 nM) was added to the cells, which were subsequently incubated for 5 h at 37°C. Cells were then loaded with LiveBLAzer FRET B/G substrate (Invitrogen) for 2 h at room temperature. Fluorescence spectra were acquired at 460 and 530 nm (excitation wavelength 409 nm), using an EnVision Multilabel Plate Reader (PerkinElmer, Waltham, MA, USA).
For the neutralization assay against CXCR2, a PathHunter eXpress β-Arrestin GPCR assay system was used (DiscoveRx, Fremont, CA, USA). PathHunter CHO-K1 CXCR2 cells were incubated overnight at 37°C followed by 90 min incubation in the presence of CXC Chemokine Ligand 8 (CXCL8) (1.25 nM) and serially diluted antibody, followed by 1 h incubation with PathHunter detection reagent. Plates were then analyzed for a chemiluminescent signal using the EnVision Multilabel Plate Reader (PerkinElmer).
Cell cultures
SJCRH30, A-204, and A-673 rhabdomyosarcoma cell lines were obtained from ATCC (Manassas, VA, USA). The RD cell line was purchased from DS Pharma Biomedical Co., Ltd (Osaka, Japan), and RH30 cells were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). All cell lines were cultured according to the suppliers' instructions.
Cyclic AMP ELISA
SJCRH30 cells were grown as confluent monolayers in a 96-well plate. Before stimulation, cells were incubated for 30 min with mAb and 1 mM IBMX (Sigma-Aldrich Japan, Tokyo, Japan). Cells were stimulated with 100 nM SDF1 (R&D Systems) diluted in HBSS/3-isobutyl-1-methylxanthine (IBMX) for 10 min, after which they were incubated for an additional 10 min with 25 μM forskolin (Sigma-Aldrich Japan) to stimulate cyclic AMP (cAMP) production. Then, cells were washed twice in ice-cold HBSS/IBMX and solubilized, and cAMP was assayed using the cAMP Parameter Assay Kit (R&D Systems).
Migration assay
Both CIM-Plate and the xCELLigence System RTCA DP analyzer (Roche Diagnostics, Penzberg, Germany) were used to monitor real-time migrations. The xCELLigence system is an electrical impedance-based system that allows for real-time cell monitoring.18 For this assay, 4 × 104 cells with antibodies, in growth medium, were seeded into the upper chamber of a CIM-Plate. The upper chamber was then placed on the lower chamber of the CIM-Plate which contained 10 nM SDF1. Cell migration was monitored for up to 7 h. Data were analyzed using RTCA software (Roche Diagnostics).
Western blotting
Western blotting was carried out as described previously.19 The following primary antibodies were used: anti-CXCR4 (Sigma-Aldrich Japan) and anti-tubulin-α (AbD Serotec, Oxford, UK). Signals were detected using Chemi-Lumi One Super (Nacalai Tesque, Kyoto, Japan) with imaging on an LAS-4000 systems (Fujifilm, Tokyo, Japan). Images were edited with MultiGauge (Fujifilm).
Animal care
All in vivo studies described here were carried out following a protocol approved by the Chugai Institutional Animal Care and Use Committee. All animal experiments were carried out in accordance with the “Guidelines for the Accommodation and Care of Laboratory Animals” of Chugai Pharmaceutical Co. Ltd. All animals were housed in a pathogen-free environment under controlled conditions (temperature, 20–26°C; humidity, 40–70%; light:dark cycle, 12:12 h). Chlorinated water and irradiated food were provided ad libitum. The animals were allowed to acclimatize and recover from shipping-related stress for 1 week prior to the study. The health of the mice was monitored daily.
Mouse xenograft study for peritoneal metastasis
SJCRH30-luc cells (2 × 106 in HBSS) were i.p. injected into nude mice (CAnN.Cg-Foxn1<nu>/CrlCrlj nu/nu; Charles River Japan, Yokohama, Japan). On the same day of SJCRH30-luc inoculation, 25 mg/kg CF172 was i.v. injected in a single shot. On day 5 after i.p. or intraperitoneal administration of luciferase substrate (65 mg/kg VivoGlo Luciferin; Promega, Madison, WI, USA), the anesthetized mice were imaged using an in vivo imaging system (NightOWL L983; Berthold Technologies, Bad Wildbad, Germany). Luciferase signals were visualized and quantified using IndiGO version 2.0.0.22 (Berthold Technologies) imaging software for NightOWL.
Mouse xenograft study for tumor growth and lymph node metastasis
To evaluate tumor growth inhibition in vivo, SJCRH30-luc cells (3 × 106 in HBSS) were implanted s.c. into the right flank of nude mice (CAnN.Cg-Foxn1<nu>/CrlCrlj nu/nu; Charles River Japan). Tumor volume (TV) was calculated using the formula: TV = ab2/2, where a and b represent tumor length and width, respectively. Once the tumors had reached a volume of approximately 100 mm3, animals were randomly assigned to two groups (n = 5) and treatment was initiated. Twice-weekly i.v. injection of CF172 (25 mg/kg per injection) was carried out for 14 days.
For the detection of lymph node metastasis, the resected lymph nodes were sonicated and lysed with 100 μL Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA, USA) containing a complete protease inhibitor cocktail (Roche Diagnostics). The same amount of Bright-Glo luciferase assay system (Promega) was added. Assays were quantified by reading luminescence using an EnVision spectrophotometer (PerkinElmer).
Statistical analyses
Statistical tests in this study were carried out using Student's t-test or the Mann–Whitney U-test. P < 0.05 was considered significant. Statistical analyses were carried out using the SAS preclinical package (version 8.2; SAS Institute Inc., Cary, NC, USA).
Results
CF172 specifically binds to and neutralizes human CXCR4
First, we generated a mAb against human CXCR4, which was designated CF172, as described in Materials and Methods.
The binding activity of CF172 to human CXCR4 was determined by FACS using human CXCR4-expressing CHO cells (Fig.1a). To evaluate the binding specificity of CF172 against human CXCR4, we carried out the FACS assay using CHO cells expressing mouse CXCR4 and human CXCR2 (the protein most closely related to CXCR4). Results of the assay indicated that CF172 did not bind to either mouse CXCR4- or human CXCR2-expressing CHO cells (Fig.1b,c, respectively).
Figure 1.
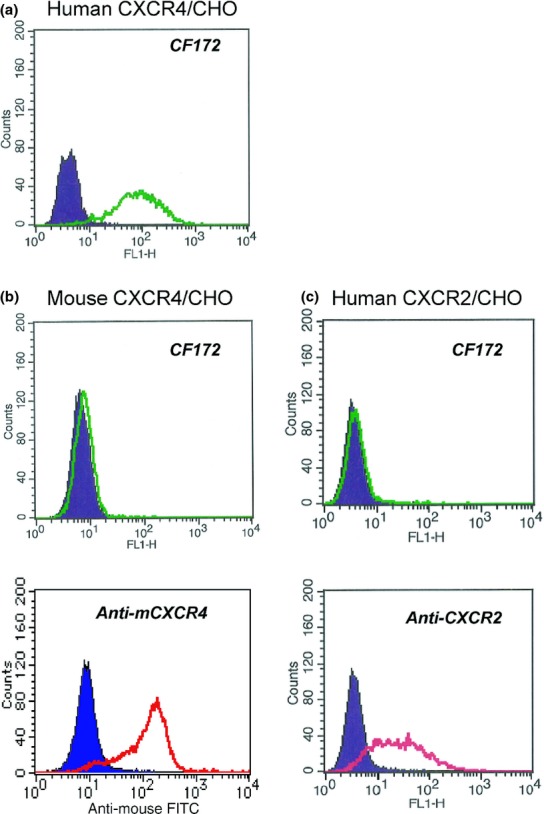
Reaction specificity of CF172 against human CXC chemokine receptor-4 (CXCR4). The binding activity of CF172 to CHO cells stably expressing (a) human CXCR4, (b) mouse CXCR4, and (c) human CXCR2, was measured by flow cytometry. Background binding, measured with isotype control (solid area), is also shown.
Following this, we investigated the neutralization activity of CF172 against human CXCR4 by using cell-based assay systems, as described in Materials and Methods. We found that CF172 dose-dependently inhibited the SDF1-mediated signaling of human CXCR4 (Fig.2a). Additionally, our results indicated that CF172 did not inhibit the CXCL8-mediated signaling of human CXCR2 (Fig.2b), which was consistent with the observed binding specificity presented in Figure1.
Figure 2.
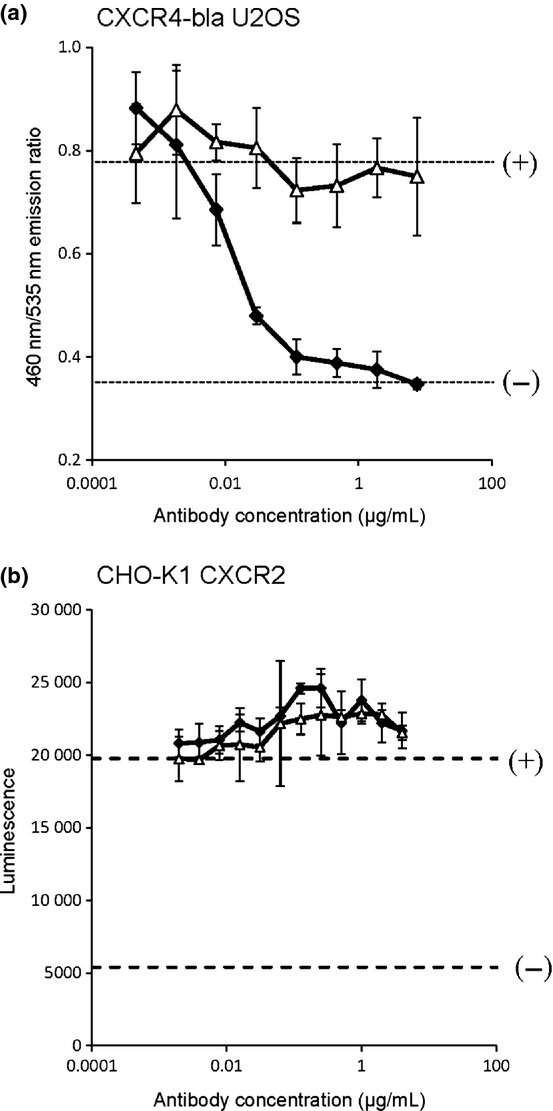
Neutralization activity of CF172 against human CXC chemokine receptor-4 (CXCR4). The neutralization activity of CF172 for CXCR4 was measured using a cell-based assay. (a) Tango CXCR4-bla U2OS cells were incubated overnight at 37°C, followed by 5 h incubation in the presence of stromal cell-derived factor-1 (SDF1) (4 nM) and serially diluted CF172 (black square) or mIgG2b control (white triangle). Samples were then incubated for 2 h with LiveBLAzer FRET B/G substrate. Signals for 4 or 0 nM SDF1 with no antibody are indicated by the (+) or (−) dotted lines, respectively. (b) PathHunter CHO-K1 CXCR2 cells were incubated overnight at 37°C followed by 90 min incubation in the presence of CXCL8 (1.25 nM) and serially diluted CF172 (black square) or mIgG2b control (white triangle). Samples were then incubated for 1 h with PathHunter detection reagent. The signals for 1.25 or 0 nM CXCL8 with no antibody are indicated by the (+) or (−) dotted lines, respectively. All points indicate the mean ± SD (n = 3).
SJCRH30 and RH30 are CXCR4-expressing rhabdomyosarcoma cell lines
Next, to investigate CXCR4 expression on the cell surface of rhabdomyosarcoma cells, we carried out FACS assays of CF172 using five different rhabdomyosarcoma cell lines. We found that SJCRH30 and RH30 cells expressed human CXCR4, and the expression levels of SJCRH30 were higher than those of RH30 (Fig.3a). To confirm this expression pattern, we used Western blot analysis (Fig.3b) and real-time PCR (Fig. S1). Consistent with the cell surface expression pattern (Fig.3a), SJCRH30 showed the highest mRNA/protein expression for CXCR4 among the five rhabdomyosarcoma cell lines.
Figure 3.
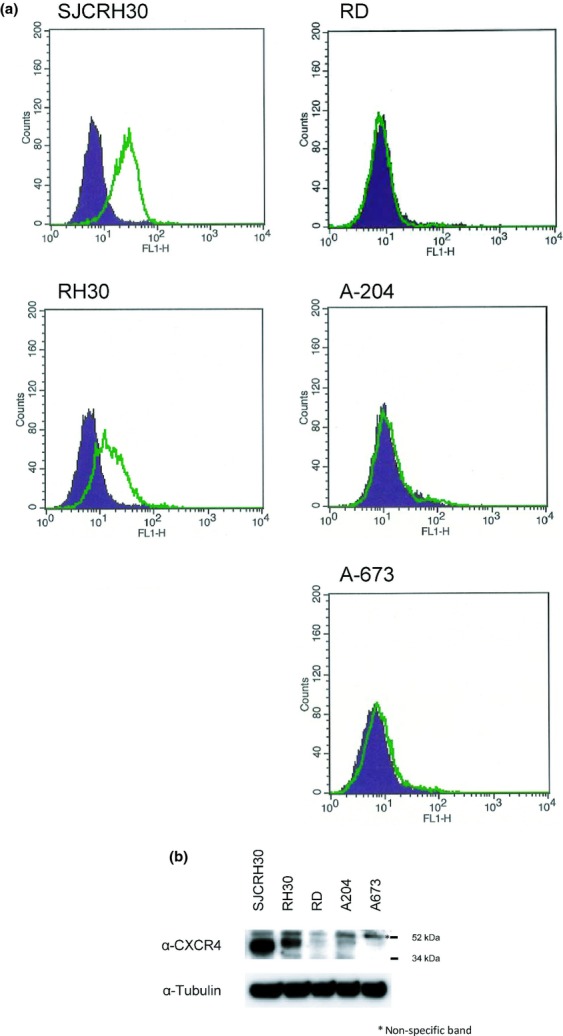
Analysis of CXC chemokine receptor-4 (CXCR4) expression in rhabdomyosarcoma cell lines. (a) CXCR4 membrane expression in rhabdomyo-sarcoma cell lines was analyzed by flow cytometry using CF172. Background binding, measured with isotype control (solid area), is also shown. (b) Expression of CXCR4 protein in five rhabdomyo-sarcoma cell lines was analyzed by Western blotting using anti-CXCR4 antibody.
CF172 inhibits the biological activity of human CXCR4 in SJCRH30 cells
To evaluate the inhibitory activity of CF172 against the biological activity of CXCR4 in rhabdomyosarcoma cells, we further analyzed SJCRH30 cells, which had shown the highest CXCR4 expression of the five rhabdomyosarcoma cells evaluated (Fig.3a,b), by determining their response against SDF1.
It is known that CXCR4 transduces signals by way of the Giα-subunit of the heterotrimeric G protein complex, after stimulation by SDF1.20 The prototypic function of Giα proteins is the binding to, and inhibition of, adenylyl cyclase-mediated cAMP production.21,22 To determine if this is also the case in human rhabdomyosarcoma cells, we assessed whether activation of CXCR4 with SDF1 could block adenylyl cyclase-mediated cAMP production in SJCRH30 cells. SJCRH30 cells were first incubated with SDF1, and then stimulated with forskolin, to upregulate cAMP production. As seen in Figure4(a) (comparison of [+] and [−] dotted lines), SDF1 inhibited the forskolin-stimulated cAMP responses in SJCRH30 cells. The subsequent addition of CF172 then abrogated the inhibition of forskolin-stimulated cAMP production by SDF1 in a dose-dependent manner (Fig.4a).
Figure 4.
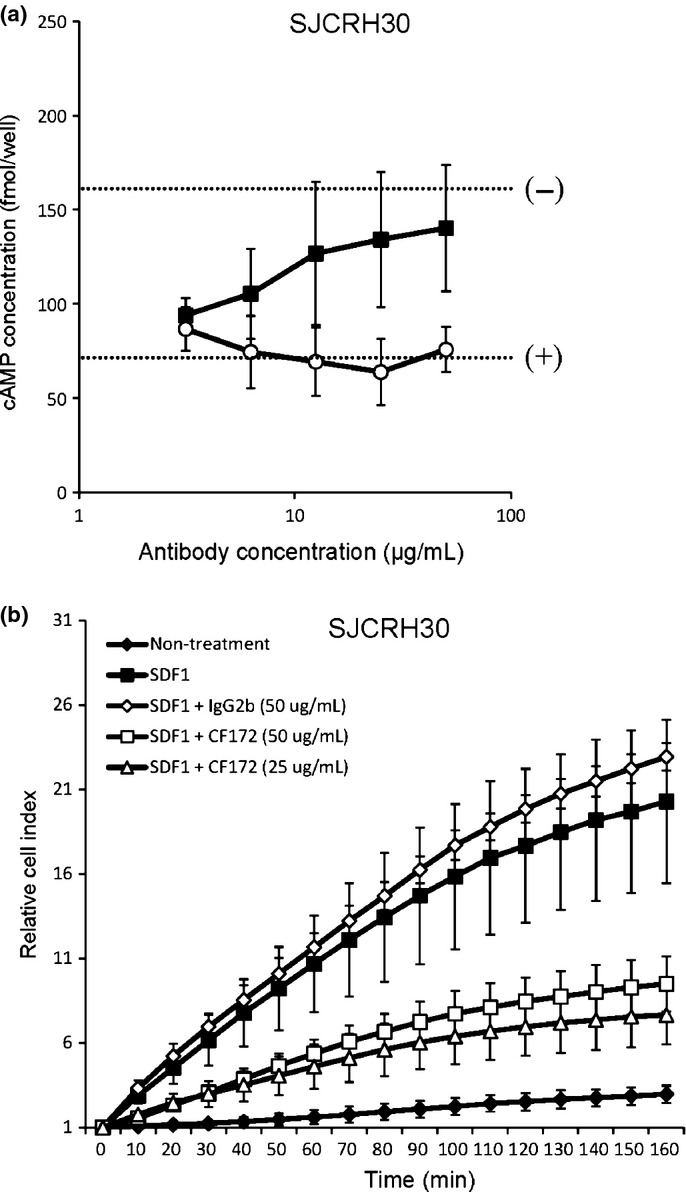
Inhibitory activity of CF172 against biological activity of CXC chemokine receptor-4 (CXCR4) in SJCRH30. The ability of CF172 to inhibit biological activity of CXCR4 was measured using (a) stromal cell-derived factor-1 (SDF1)/CXCR4-induced inhibitory activity of forskolin-stimulated cyclic AMP (cAMP) production, and (b) SDF1/CXCR4-induced migration activity. (a) SJCRH30 rhabdomyosarcoma cells were pretreated with serially diluted CF172 (black squares) or mIgG2b control (white circles). Cells were then stimulated with 100 nM SDF1 and 25 μM forskolin. Cells were harvested 10 min later, and cAMP levels were assayed by ELISA. The signals for forskolin (25 μM) with 100 or 0 nM SDF1, with no antibody, are indicated by the (+) and (−) dotted lines, respectively. (b) The xCELLigence system was used to monitor real-time cell migration. SJCRH30 cells with antibodies were seeded in the upper chamber of a CIM-Plate in the growth medium. The upper chamber was then placed on the lower chamber of the CIM-Plate containing 10 nM SDF1. Cell migration was monitored for up to 7 h. All points indicate the mean ± SD (n = 3).
The CXCR4/SDF1 signaling in tumor cells is known to enhance migration activity.12–16 To determine if this is also the case in human rhabdomyosarcoma cells, we assessed whether activation of CXCR4 with SDF1 enhanced migration activity in SJCRH30 cells. Our results indicated that SDF1 enhanced the migration activity of SJCRH30 cells (Fig.4b). When we added CF172, SDF1-induced migration activity of SJCRH30 cells was inhibited (Fig.4b). To determine whether CF172 specifically inhibited migration and did not affect the proliferation of SJCRH30 cells, we next investigated the effect of this agent on proliferation in SJCRH30 cells. As seen in Figure S2, serial concentrations of CF172 did not inhibit proliferation of SJCRH30 cells in either the presence or absence of SDF1.
CF172 inhibited in vivo peritoneal metastasis and lymph node metastasis of SJCRH30
We finally investigated the in vivo effect of CF172 against the metastasis of SJCRH30. Although there are many types of tumor metastases, we selected peritoneal metastasis and lymph node metastasis for evaluation because both are clinically observed with rhabdomyosarcoma.1 For these analyses, we generated an SJCRH30 cell line stably expressing luciferase (SJCRH30-luc) in order to carry out bioimaging in mice (Fig. S3a). Compared to the SJCRH30 parental cells, the SJCRH30-luc cells showed similar levels of CXCR4 expression in vitro (Figs3,S3b). In addition, we evaluated the inhibitory effects of SDF1-induced migration activity of SJCRH30-luc, and found that SJCRH30-luc cells were migrated by SDF1, whereas CF172 inhibited this migration activity (Fig. S3c).
We then attempted to generate an experimental peritoneal metastasis model of rhabdomyosarcoma by i.p. injecting SJCRH30-luc cells into nude mice. On day 5 after the inoculation of SJCRH30-luc cells, we detected photons in the peritonea of inoculated nude mice. The number of photons was not reduced by washing of the peritonea with PBS (data not shown). Therefore, we concluded that these results validated the experimental peritoneal metastasis model of rhabdomyosarcoma.
We then carried out an in vivo efficacy study involving i.v. injection of 25 mg/kg CF172, using the model described above. Briefly, SJCRH30-luc cells were i.p. injected into nude mice. On the same day as SJCRH30-luc inoculation, CF172 (25 mg/kg) was i.v. injected in a single shot. On day 5, peritoneal metastasis was evaluated by detection of photons, using bioimaging. Our results indicated a significant reduction in the number of photons in the CF172-injected group, when compared with the vehicle-injected group (P < 0.05, Fig.5a). Individual photographs of mice on day 5 are shown in Figure5(b) (n = 7 in the vehicle-injected group; n = 9 in the CF172-injected group). In addition, we evaluated the concentration of SDF1 in ascites fluid before and after the inoculation of SJCRH30-luc cells. As shown in Figure S4, the SDF1 concentration before the inoculation of SJCRH30-luc cells did not differ from that after the inoculation of SJCRH30-luc cells. These data indicate that inoculation of SJCRH30-luc cells had little impact on the SDF1 concentration in ascites fluid, and so the effect of CF172 against the peritoneal metastasis of SJCRH30-luc cells was not from a dramatic change in the SDF1 induced by inoculation of SJCRH30-luc cells.
Figure 5.
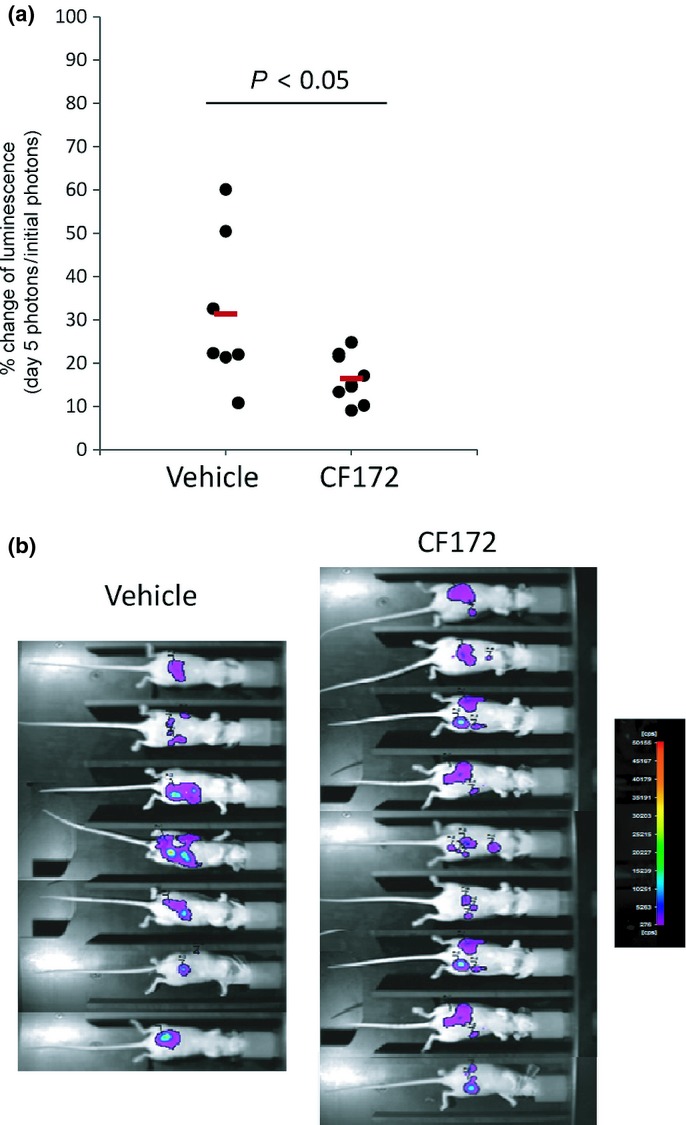
In vivo effect of CF172 against peritoneal metastasis of CXC chemokine receptor-4 (CXCR4)-expressing rhabdomyosarcoma model. (a) The anti-peritoneal metastasis activity of CF172 against SJCRH30-luc cells was evaluated based on photon detection using bioimaging. SJCRH30-luc cells were i.p. inoculated into nude mice. On the same day, CF172 (25 mg/kg) was i.v. injected in a single shot. After 5 days, metastasized tumor cells were measured by administration of luciferase substrate and detection of the photons. Data were individually plotted by the percentage change from initial photon concentrations. Statistical tests were carried out using Student's t-test. (b) Photographs of all mice on day 5.
We then examined whether CF172 inhibited the lymph node metastasis of SJCRH30-luc cells. SJCRH30-luc cells were implanted s.c. into the right flank of nude mice. Once the tumors had reached a volume of approximately 100 mm3, the mice were randomized into two groups (n = 5), and twice-weekly i.v. injection of CF172 (25 mg/kg) was carried out for 14 days. As a result, there was little difference in the size of tumors formed by SJCRH30-luc cells in the primary lesion between the CF172-injected group and the vehicle-injected group (Fig.6a). In contrast, as shown in Figure6(b), CF172 significantly inhibited the lymph node metastasis of SJCRH30-luc cells in this model. These data further indicate that CF172 decreased the metastasis of SJCRH30-luc cells by inhibiting tumor migration from the primary lesion without affecting tumor volume.
Figure 6.
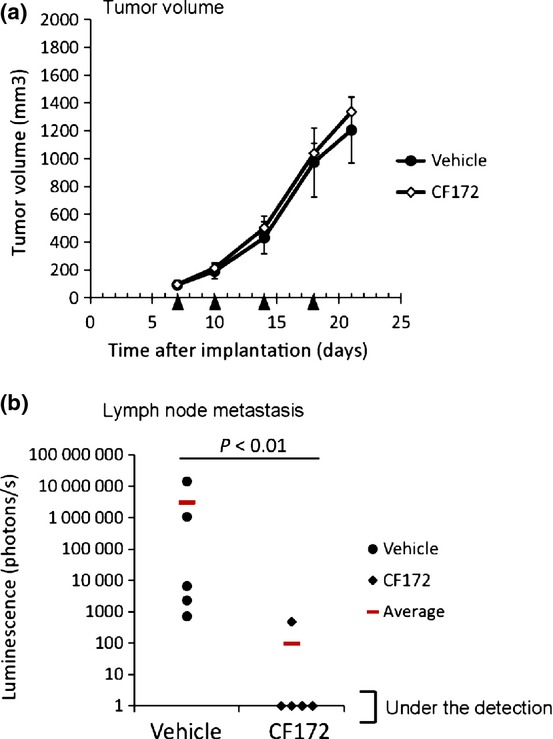
In vivo effect of CF172 against tumor growth and lymph node metastasis of CXC chemokine receptor-4 (CXCR4)-expressing rhabdomyosarcoma model. (a) Antitumor activity activity of CF172 against SJCRH30-luc cells was evaluated using s.c. implanted SJCRH30-luc cells. Once the tumors had reached a volume of approximately 100 mm3, mice were randomly assigned to two groups (n = 5) and twice-weekly i.v. injection of CF172 (25 mg/kg) was carried out for 14 days. The mean tumor volume is shown. Black triangle indicates treatment day. (b) The anti-metastasis activity of CF172 against lymph node metastasis of SJCRH30-luc was evaluated by detection of photons from lysed lymph nodes. Statistical tests were carried out using the Wilcoxon test.
Discussion
Tumor metastasis is an important clinical determinant in the prognosis of many tumors; the overall cure rate of metastatic rhabdomyosarcoma remains very low, compared to localized rhabdomyosarcoma.1–6 Therefore, therapies capable of inhibiting tumor metastasis would be highly beneficial in the treatment of rhabdomyosarcoma. High CXCR4 expression correlates with metastatic incidence and poor prognosis of rhabdomyosarcoma,17 suggesting that the SDF1/CXCR4 axis is very important to the metastatic process. These findings also suggest that anti-CXCR4 would be a promising agent in metastatic rhabdomyosarcoma treatment.
In this study, we generated a novel anti-CXCR4 mAb, CF172, which showed a specific binding reactivity, and a neutralizing activity, against human CXCR4. Several studies have shown that SDF1 activates downstream signaling pathways by interacting with the second extracellular loop of CXCR4.23–27 Compared to the amino acid sequence between human and mouse, the second extracellular loop of CXCR4 shows lower sequence identity (67%) than other extracellular regions.28 Additionally, CF172 did not bind to mouse CXCR4 (Fig.1b). Collectively, these results suggested that CF172 binds to the second extracellular loop of CXCR4, and so this agent showed specific binding reactivity and inhibited SDF1-mediated signal transduction.
By using CF172, we showed that SJCRH30 and RH30 were the CXCR4-expressing rhabdomyosarcoma cells among the five different cell lines evaluated (Fig.3). Both of these cells are histologically characterized in alveolar-type rhabdomyosarcoma, whereas the remaining three cell lines are characterized in embryonal-type.29 Alveolar-type is known as to be a more aggressive rhabdomyosarcoma than the embryonal-type,2,30,31 and our finding that CXCR4 is preferentially expressed in alveolar-type is consistent with previously published data indicating that high CXCR4 expression correlates with poor prognosis in rhabdomyosarcoma.17 These findings also suggested the importance of CXCR4 in rhabdomyosarcoma.
One of the most important findings was that CF172 inhibited metastasis in two experimental rhabdomyosarcoma models (Figs6). Previous research has shown that the SDF1 concentration in the peritoneum and lymph node is high,13,32 suggesting that this high concentration of SDF1 enhances peritoneal and lymph node metastasis in rhabdomyosarcoma, and our finding that blocking the CXCR4/SDF1 axis by CF172 inhibited these metastases is consistent with this hypothesis and strongly supports previously published conclusions that the CXCR4/SDF1 axis is important for tumor metastasis.12–16 These findings further suggest that the importance of the CXCR4/SDF1 axis in tumor metastasis is also applicable to rhabdomyosarcoma.
Compared to localized rhabdomyosarcoma, high mortality rates are still observed in patients with metastatic rhabdomyosarcoma, despite aggressive multimodality therapy.1–6 Therefore, new therapeutic agents for metastatic rhabdomyosarcoma are still necessary, and our finding that CF172 has potential as a therapeutic antibody for metastatic rhabdomyosarcoma is of significance.
In conclusion, the results of the present study implicated CXCR4 signaling in the promotion of metastasis in rhabdomyosarcoma, and CF172 was identified as a potential therapeutic antibody against metastatic rhabdomyosarcoma.
Acknowledgments
The authors thank Taeko Masuda and Sachiya Yamamoto for their technical support, Tsukasa Suzuki and Takeshi Baba for antibody generation, and Keiko Esaki, Akihisa Sakamoto, Manabu Wada, Masakazu Hasegawa, Junichi Nezu, Kunihiro Hattori, Masahiro Aoki, and Osamu Kondoh for helpful discussions.
Disclosure Statement
All of the authors are employees of Chugai Pharmaceutical Co., Ltd.
Supporting Information
Fig. S1. mRNA expression of CXCR4 in rhabdomyosarcoma cell lines.
Fig. S2. Growth inhibition activity of CF172 against SJCRH30 rhabdomyosarcoma cells.
Fig. S3. Comparison of the profiles of SJCHR30 and SJCRH30-luc rhabdomyosarcoma cells.
Fig. S4. Comparison of SDF1 concentration in ascites fluid of normal mice and SJCRH30-luc-xenografted mice.
References
- Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma–a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–9. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology–SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–28. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- Koscielniak E, Rodary C, Flamant F, et al. Metastatic rhabdomyosarcoma and histologically similar tumors in childhood: a retrospective European multi-center analysis. Med Pediatr Oncol. 1992;20:209–14. doi: 10.1002/mpo.2950200305. [DOI] [PubMed] [Google Scholar]
- Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59:5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–45. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Strahm B, Durbin AD, Sexsmith E, Malkin D. The CXCR4-SDF1alpha axis is a critical mediator of rhabdomyosarcoma metastatic signaling induced by bone marrow stroma. Clin Exp Metastasis. 2008;25:1–10. doi: 10.1007/s10585-007-9094-6. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Ohishi Y, Basaki Y, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: their correlation with activated Akt. LRP/MVP and P-glycoprotein expression. Cancer Sci. 2007;98:1020–6. doi: 10.1111/j.1349-7006.2007.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomedi-Camassei F, McDowell HP, De Ioris MA, et al. Clinical significance of CXC chemokine receptor-4 and c-Met in childhood rhabdomyosarcoma. Clin Cancer Res. 2008;14:4119–27. doi: 10.1158/1078-0432.CCR-07-4446. [DOI] [PubMed] [Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- Kashima K, Watanabe M, Satoh Y, Hata J, Ishii N, Aoki Y. Inhibition of lymphatic metastasis in neuroblastoma by a novel neutralizing antibody to vascular endothelial growth factor-D. Cancer Sci. 2012;103:2144–52. doi: 10.1111/cas.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Ogawa H, Barrett KE, Kagnoff MF. SDF-1/CXCL12 regulates cAMP production and ion transport in intestinal epithelial cells via CXCR4. Am J Physiol Gastrointest Liver Physiol. 2004;286:G844–50. doi: 10.1152/ajpgi.00112.2003. [DOI] [PubMed] [Google Scholar]
- Zhou N, Luo Z, Luo J, et al. Structural and functional characterization of human CXCR4 as a chemokine receptor and HIV-1 co-receptor by mutagenesis and molecular modeling studies. J Biol Chem. 2001;276:42826–33. doi: 10.1074/jbc.M106582200. [DOI] [PubMed] [Google Scholar]
- Kofuku Y, Yoshiura C, Ueda T, et al. Structural basis of the interaction between chemokine stromal cell-derived factor-1/CXCL12 and its G-protein-coupled receptor CXCR4. J Biol Chem. 2009;284:35240–50. doi: 10.1074/jbc.M109.024851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Shen J, Cui M, et al. Molecular dynamics simulations on SDF-1alpha: binding with CXCR4 receptor. Biophys J. 2003;84:171–84. doi: 10.1016/S0006-3495(03)74840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doranz BJ, Orsini MJ, Turner JD, et al. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–61. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275:23736–44. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Nakajima T, Tachibana K, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci USA. 1996;93:14726–9. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Gilbert E, Delattre O, Gauthier-Rouviere C. Variation in cadherins and catenins expression is linked to both proliferation and transformation of Rhabdomyosarcoma. Oncogene. 2004;23:2420–30. doi: 10.1038/sj.onc.1207382. [DOI] [PubMed] [Google Scholar]
- Newton WA, Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification–an Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76:1073–85. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Leuschner I, Harms D. [Pathology of childhood and adolescent rhabdomyosarcoma] Pathologe. 1999;20:87–97. doi: 10.1007/s002920050326. [DOI] [PubMed] [Google Scholar]
- Yasumoto K, Koizumi K, Kawashima A, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181–7. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. mRNA expression of CXCR4 in rhabdomyosarcoma cell lines.
Fig. S2. Growth inhibition activity of CF172 against SJCRH30 rhabdomyosarcoma cells.
Fig. S3. Comparison of the profiles of SJCHR30 and SJCRH30-luc rhabdomyosarcoma cells.
Fig. S4. Comparison of SDF1 concentration in ascites fluid of normal mice and SJCRH30-luc-xenografted mice.


