Abstract
Thymus and activation-regulated chemokine (TARC) can stimulate cancer cell proliferation and migration. The present study evaluated the clinical significance of serum TARC in gastric cancer (GC). We measured serum TARC, macrophage-derived chemokine, monocyte chemotactic protein-1 and stem cell factor (SCF) levels using a chemiluminescent immunoassay along the GC carcinogenesis (normal, high-risk, early GC [EGC] and advanced GC [AGC]) in both training (N = 25 per group) and independent validation datasets (90 normal, 30 high-risk, 50 EGC and 50 AGC). Serum levels were compared among groups using one-way analysis of variance. To evaluate the diagnostic potential of serum TARC for GC, receiver operating characteristic curve and logistic regression analyses were performed. Correlations between serum TARC and GC clinicopathological features were analyzed using Spearman's correlation. In the training dataset, serum TARC correlated with serum MDC, MCP-1 and SCF. However, only serum TARC and SCF were significantly higher in cancer groups than non-cancer groups (P < 0.001). In the validation dataset, serum TARC also increased along the GC carcinogenesis; the AGC group (167.2 ± 111.1 ng/mL) had significantly higher levels than the EGC (109.1 ± 67.7 ng/mL), the high-risk (66.2 ± 47.7 ng/mL) and the normal (67.5 ± 36.2 ng/mL) groups (Bonferroni, all P < 0.001). Receiver operating characteristic curves and logistic regression demonstrated the remarkable diagnostic potential of serum TARC as a single marker (72.0% sensitivity and 71.1% specificity; cutoff point, 0.37; logistic regression) and in a multiple-marker panel (72.6% sensitivity and 88.2% specificity; cutoff point, 0.54). Spearman's correlation showed that serum TARC was closely correlated with tumor size (γs = 0.227, P = 0.028), T-stage (γs = 0.340, P = 0.001), N-stage (γs = 0.318, P = 0.002) and M-stage (γs = 0.346, P = 0.001). Serum TARC is a promising serum biomarker for GC.
Keywords: Diagnostic, gastric cancer, prognostic, serum biomarker, thymus and activation-regulated chemokine
Previous studies indicate that chemokines and their receptors contribute to the various processes of tumor cell biology, including proliferation, angiogenesis and metastasis.1–3 Initially, chemokines were found to regulate leukocyte trafficking to sites of inflammation and recirculation in secondary lymphatics.3,4 Later, interest was gained in the roles of chemokines in tumor biology because leukocyte trafficking shares many characteristics with tumor cell infiltration and metastasis. Increasing evidence supports the roles of chemokines and their receptors in tumor growth and metastasis in both hematological and solid cancers.1–3,5–7
CC chemokine receptor 4 (CCR4), an important chemokine receptor that regulates immune homeostasis, is preferentially expressed on certain immune cells.8 It is also expressed in some hematological9,10 and solid malignancies, including gastric cancer (GC),11–15 and contributes to tumor growth and metastasis by suppression of the host immune response through stimulation of regulatory T cell accumulation in the tumor microenvironment.10,14
Thymus and activation-regulated chemokine (TARC), also known as CCL17, is a ligand of CCR4 that specifically binds to and induces chemotaxis in T cells. This chemokine is also overexpressed in the serum of patients with hematological malignancies, and potentially predicts the prognosis of these malignancies.16–19 Al-haidari et al. and Biragyn et al.20,21 report that TARC acts as a potent stimulator of cancer cell proliferation, migration and metastasis in solid tumors. In addition, Shiels22 suggests that it is overexpressed in the serum of lung cancer patients and is associated with prospective lung cancer risk.
Although the incidence of GC has decreased over the past few decades,23,24 GC remains the third most common cause of cancer-related death worldwide.25 The prognosis of advanced GC (AGC) is particularly poor, while the prognosis of early gastric cancer (EGC) is favorable.26 Thus, early detection of GC is clinically important. Endoscopic examination is an ideal, highly reliable technique for early detection of GC, but its usefulness for GC screening is somewhat limited compared with serum biomarkers because of its high cost and the risks associated with the invasive procedure. However, research is still underway to identify effective serum biomarkers for GC.
Although emerging evidence suggests that serum TARC is a potential biomarker for predicting cancer development or progression, no studies have evaluated serum TARC levels along the GC carcinogenesis sequence, correlations with GC clinicopathological parameters or their potential as a desirable biomarker for predicting cancer development or progression.
The current study is the first to evaluate serum TARC levels along the “gastritis–dysplasia–carcinoma” sequence of gastric carcinogenesis,27 and analyze the correlations between serum TARC levels and GC clinicopathological features. Furthermore, we validate serum TARC as a potential biomarker candidate for GC and compare it with serum carcinoembryonic antigen (CEA), a pre-existing biomarker for gastrointestinal tumors, using human serum samples.
Materials and Methods
Subject enrollment and disease groups
For the present study, a total of 320 subjects were enrolled from the Yonsei University Health System. Subjects were classified into four groups along the “gastritis–dysplasia–carcinoma” sequence of gastric carcinogenesis.27 The normal control group included subjects with normal gastric mucosa or simple gastritis, the high-risk group included patients with intestinal metaplasia (IM) and dysplasia, the EGC group included patients with GC confined within the submucosal layer, and the AGC group included patients with GC extending beyond the proper muscle layer.
Among the 320 subjects, 100 subjects were enrolled for the initial training dataset and 220 subjects were enrolled for the following independent validation dataset. The appropriate sample size for the initial training dataset was calculated to be ≥25 subjects per group using Russ Lenth's interactive power/sample size online calculator with the assumptions that there were four comparison groups, the estimated standard deviation (SD) was 1, and the confidence level (CI) was 0.05 (one-way anova). This sample size achieved >80% statistical power. For the following independent validation dataset, 90 subjects were enrolled for the normal control group, 30 for the high-risk group, 50 for the EGC group and 50 for the AGC group, giving sample sizes that allowed achievement of >90% statistical power (one-way anova) using the Number Cruncher Statistical System Power Analysis and Sample Size (NCSS PASS) program.
All subjects underwent upper gastrointestinal endoscopy (Types XQ-260; Olympus, Tokyo, Japan) with biopsies before enrollment, and the final diagnoses were based on histological findings from biopsies or surgical specimens. All patients were diagnosed for the first time during the enrollment period, and blood samples were collected before they received any treatment.
All patients in the cancer groups underwent imagining studies, including chest X-rays, abdominal-pelvic helical computed tomography scans and whole body positron emission tomography scans for TNM staging. TNM stage was evaluated based on radiological studies or surgical findings according to the 7th International Union Against Cancer TNM stage guidelines for GC. Helicobacter pylori infection was evaluated in GC patients by staining of gastric tissue with Giemsa solution (Sigma, St. Louis, MO, USA). IM changes were diagnosed according to the updated Sydney classification system, and GC histology was classified uing the Lauren classification system (intestinal and diffuse type).
Subjects with chronic diseases such as liver cirrhosis, chronic renal disease and diabetes mellitus were excluded from this study. Subjects with other cancers and other gastric neoplasms, such as gastrointestinal stromal tumors, mucosa-associated lymphoid tissue lymphomas and neuroendocrine tumors, were also excluded. In addition, patients who had previously received any treatment for GC or its premalignant lesions were excluded.
The Institutional Review Board of the Yonsei University Health System approved the current study, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Measurement of serum carcinoembryonic antigen and cytokines/chemokines levels
Serum CEA levels were measured using a Beckman Access CEA assay (Beckman Coulter, Chaska, MN, USA). Serum TARC levels were measured with the commercially available MILLIPLEX MAP Human Cytokine/Chemokine Kit (Millipore, Billerica, MA, USA) using a chemiluminescent immunoassay method according to the manufacturer's instructions. This method allowed simultaneous quantification of several cytokines and/or chemokines.
We also measured the serum levels of macrophage-derived chemokine (MDC, CCL22), another CCR4 ligand, using the same kit simultaneously. We measured the serum levels of monocyte chemotactic protein-1 (MCP-1, CCL2) and stem cell factor (SCF) at the same time using the same kit because it has been reported that MDC, MCP-1 and TARC can be released by SCF simultaneously and play pathologic roles together.28,29
Briefly, the filter plate was pre-wetted with 200 μL assay buffer for 10 min at room temperature (RT), followed by vacuum removal of the assay buffer. Standards or controls (25 μL) were added to the appropriate wells, and 25 μL assay buffer was added to sample wells, but not the background well. Next, 25 μL of the appropriate matrix solution was added to the background, standard and control wells, followed by addition of 25 μL sample to appropriate wells. After mixing, 25 μL beads were added and the plate was incubated overnight at 4°C with shaking. After incubation, the fluid was removed and the plate was washed twice. Detection antibodies (25 μL) were added, and the plate was incubated for 1 h at RT with shaking. Streptavidin-phycoerythrin (25 μL) was added to each well containing 25 μL detection antibodies and was incubated for 1 h at RT with shaking. The fluid was then removed, the plate was washed, and 150 μL sheath fluid was added. After re-suspension for 5 min, the median fluorescent intensity was read on a Luminex 100 IS (Millipore) and analyzed using the logistic curve-fitting method to determine cytokine/chemokine concentrations.
Statistical analysis
SPSS version 20.0 (IBM, Armonk, NY, USA) was used for statistical analyses. P-values < 0.05 were considered statistically significant. All tested values were expressed as a mean with 25–75% SD. Means of each group were compared by one-way anova tests with multiple comparisons using the post-hoc Bonferroni method. Means between cancer and non-cancer conditions were compared using independent sample t-tests. Pearson's correlation (coefficient, γp) and Spearman's correlation (coefficient, γs) were performed to evaluate relationships between measured serum levels and clinicopathological parameters. To analyze the relationship between serum TARC levels and primary GC size, patients were classified into three groups based on tumor size: <3 cm, 3–5 cm and >5 cm. Receiver operating characteristic (ROC) curves were generated, and the area under the curve (AUC) was calculated to compare the diagnostic accuracy of each serum marker for GC. Logistic regression analysis was performed to ascertain the best sensitivity and specificity for prediction of GC as a single marker or as a part of a multiple-marker panel. Each marker was included as a linear term.
Results
Correlations among serum levels of thymus and activation-regulated chemokine, macrophage-derived chemokine, monocyte chemotactic protein-1 and stem cell factor in the training dataset
The training dataset included 25 subjects in each group (normal control, high-risk, EGC and AGC groups). Table1 shows that serum TARC levels were closely correlated with serum levels of MDC (γp = 0.433, P < 0.001), MCP-1 (γp = 0.273, P = 0.006) and SCF (γp = 0.453, P < 0.001).
Table 1.
Pearson's correlations among serum TARC, MDC, MCP-1 and SCF in the initial training dataset
| TARC | MDC | MCP-1 | SCF | |
|---|---|---|---|---|
| γp (P-value) | γp (P-value) | γp (P-value) | γp (P-value) | |
| TARC | — | 0.433 (<0.001)* | 0.273 (0.006)* | 0.453 (<0.001)* |
| MDC | 0.433 (<0.001)* | — | 0.283 (0.004)* | 0.177 (0.079) |
| MCP-1 | 0.273 (0.006)* | 0.283 (0.004)* | — | 0.293 (0.003)* |
| SCF | 0.453 (<0.001)* | 0.177 (0.079) | 0.293 (0.003)* | — |
These values are statistically significant. MCP-1, monocyte chemotactic protein-1; MDC, macrophage-derived chemokine; SCF, stem cell factor; TARC, thymus activation-regulated chemokine; γp, Pearson's correlation coefficient. P < 0.05 (two-tailed) was considered statistically significant.
Serum levels of tested cytokines/chemokines and carcinoembryonic antigen along the gastric cancer carcinogenic sequence in the training dataset
Serum TARC levels increased along the GC carcinogenic sequence, with differences among groups that were statistically significant (anova, P < 0.001; Table2). Serum TARC was significantly higher in the AGC group (213.9 ± 115.0 ng/mL) compared with those of EGC (128.9 ± 71.5 ng/mL), high-risk (67.7 ± 42.5 ng/mL) and normal control (66.7 ± 35.5 ng/mL) groups (post-hoc Bonferroni, all P < 0.001). Serum TARC was also significantly higher in the EGC group compared with the high-risk (P = 0.024) and normal control (P = 0.020) groups. When serum TARC levels were compared between cancer and non-cancer groups, they were significantly higher in the cancer groups (171.4 ± 104.1 ng/mL) than in the non-cancer groups (67.2 ± 38.8 ng/mL, P < 0.001, t-test; Table2).
Table 2.
Serum levels of TARC, MDC, MCP-1, SCF and CEA according to the GC carcinogenic sequences (upper) and between cancer and non-cancer groups (lower) in the initial training dataset
| Group (N) | Normal (25) | High-risk (25) | EGC (25) | AGC (25) | P-value‡ |
|---|---|---|---|---|---|
| Serum TARC (ng/mL) | 66.7 ± 35.5† | 67.7 ± 42.5 | 128.9 ± 71.5 | 213.9 ± 115.0 | <0.001 |
| Serum MDC (ng/mL) | 658.0 ± 248.2 | 788.4 ± 326.5 | 679.0 ± 304.6 | 1002.8 ± 392.1 | 0.089 |
| Serum MCP-1 (ng/mL) | 148.9 ± 56.6 | 182.9 ± 145.0 | 163.7 ± 42.1 | 347.2 ± 839.3 | 0.298 |
| Serum SCF (ng/mL) | 4.0 ± 5.3 | 11.2 ± 7.1 | 14.2 ± 10.5 | 19.7 ± 14.2 | <0.001 |
| Serum CEA (ng/mL) | 1.8 ± 0.7 | 2.1 ± 0.9 | 1.5 ± 0.8 | 9.9 ± 11.7 | 0.044 |
| Group (N) | Non-cancer groups (50) | Cancer groups (50) | P-value§ |
|---|---|---|---|
| Serum TARC (ng/mL) | 67.2 ± 38.8† | 171.4 ± 104.1 | <0.001 |
| Serum MDC (ng/mL) | 723.2 ± 294.5 | 840.9 ± 384.0 | 0.089 |
| Serum MCP-1 (ng/mL) | 165.9 ± 110.3 | 255.5 ± 595.4 | 0.298 |
| Serum SCF (ng/mL) | 7.6 ± 7.2 | 17.0 ± 12.7 | <0.001 |
| Serum CEA (ng/mL) | 2.0 ± 0.8 | 5.7 ± 9.2 | 0.044 |
All tested values are expressed as the mean ± standard deviation.
One-way analysis of variance test with the multiple comparisons using the post-hoc Bonferroni method is applied to compare differences in means among disease groups.
Independent sample t-test is applied to compare the means between cancer and non-cancer groups. P < 0.05 (two-tailed) was considered statistically significant. AGC, advanced gastric cancer; CEA, carcinoembryonic antigen; EGC, early gastric cancer; GC, gastric cancer; MCP-1, monocyte chemotactic protein-1; MDC, macrophage-derived chemokine; SCF, stem cell factor; TARC, thymus activation-regulated chemokine.
Serum MDC, MCP-1 and SCF also increased along the GC carcinogenesis sequence, similar to serum TARC. However, serum MDC and MCP-1 levels were not significantly different between the cancer and non-cancer groups (all P > 0.05; Table2), while serum SCF levels were significantly different between cancer and non-cancer groups (P < 0.001, t-test, Table2). Serum CEA levels were only significantly elevated in the AGC group, compared with the other groups (post-hoc Bonferroni, all P < 0.05; Table2).
Diagnostic accuracy of tested serum markers for prediction of gastric cancer in the training dataset
ROC curves were generated and AUC were calculated to evaluate the diagnostic accuracy of serum TARC, MDC, MCP-1 and SCF compared with serum CEA for prediction of GC in the training dataset (Fig.1). The AUC for serum TARC was 0.88 (95% CI = 0.80–0.96), whereas MDC had an AUC of 0.61 (95% CI = 0.47–0.75), MCP-1 had an AUC of 0.57 (95% CI = 0.43–0.71), SCF had an AUC of 0.70 (95% CI = 0.57–0.82) and CEA had an AUC of 0.51 (95% CI = 0.38–0.65). Collectively, serum TARC exhibited superior diagnostic potential for prediction of GC compared with serum CEA (Fig.1).
Figure 1.
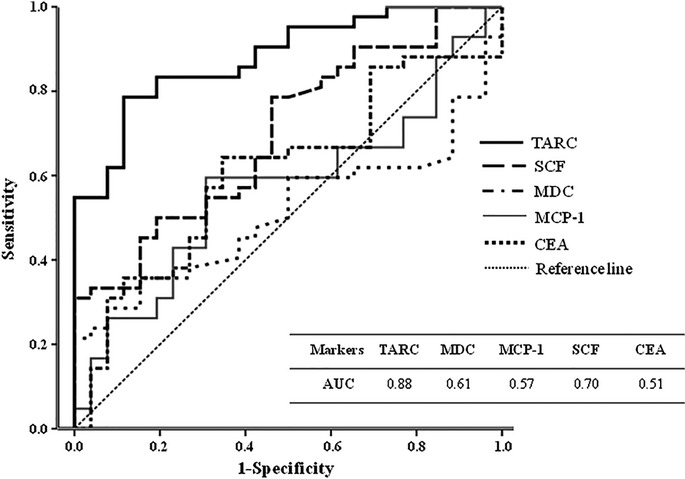
ROC curves and AUC of tested serum values for prediction of gastric cancer, compared with CEA in the training dataset. AUC, area under the ROC curve; CEA, carcinoembryonic antigen; MCP-1, monocyte chemotactic protein-1; MDC, macrophage-derived chemokine; ROC, receiver operating characteristic; SCF, stem cell factor; TARC, thymus activation-regulated chemokine.
Serum thymus and activation-regulated chemokine levels in the independent validation dataset
We used an independent validation dataset to test the reproducibility of the results from the training dataset. Serum TARC and SCF were the only values from the training dataset that were significantly different between cancer and non-cancer groups (Table2). Therefore, we only investigated serum TARC and SCF levels compared with serum CEA in the independent validation dataset.
The characteristics of patients in the validation dataset are shown in Supplementary Table S1. Consistent with the results from the training dataset, serum TARC significantly increased along the GC carcinogenic sequence (anova, P < 0.001; Table3). In the validation dataset, serum TARC was significantly higher in the AGC group (167.2 ± 111.1 ng/mL) than in EGC (109.1 ± 67.7 ng/mL), high-risk (66.2 ± 47.7 ng/mL) and normal control (67.5 ± 36.2 ng/mL) groups, respectively (post-hoc Bonferroni, all P < 0.001; Table3). The chemokine levels were also significantly higher in the EGC group than in the high-risk (P = 0.043) or normal control (P < 0.001) groups. Serum TARC levels were significantly higher in the cancer groups (145.6 ± 95.4 ng/mL) than in the non-cancer groups (62.3 ± 33.5 ng/mL; t-test, P < 0.001; Table3).
Table 3.
Serum levels of TARC, SCF and CEA according to the GC carcinogenic sequences (upper) and between cancer and non-cancer groups (lower) in the independent validation dataset
| Group (N) | Normal (90) | High-risk (30) | EGC (50) | AGC (50) | P-value‡ |
|---|---|---|---|---|---|
| Serum TARC (ng/mL) | 67.5 ± 36.2† | 66.2 ± 47.7 | 109.1 ± 67.7 | 167.2 ± 111.1 | <0.001 |
| Serum SCF (ng/mL) | 6.3 ± 6.3 | 10.4 ± 7.8 | 17.3 ± 15.1 | 22.6 ± 20.4 | <0.001 |
| Serum CEA (ng/mL) | 1.8 ± 1.4 | 2.0 ± 0.8 | 1.7 ± 1.1 | 10.7 ± 19.8 | <0.001 |
| Group (N) | Non-cancer groups (120) | Cancer groups (100) | P-value§ |
|---|---|---|---|
| Serum TARC (ng/mL) | 62.3 ± 33.5† | 145.6 ± 95.4 | <0.001 |
| Serum SCF (ng/mL) | 7.0 ± 6.6 | 20.5 ± 18.5 | <0.001 |
| Serum CEA (ng/mL) | 1.9 ± 1.3 | 6.5 ± 15.1 | 0.05 |
All tested values are expressed as the mean ± standard deviation.
One-way analysis of variance test with the multiple comparisons using the post-hoc Bonferroni method is applied to compare the means among disease groups.
Independent sample t-test is applied to compare the means between cancer and non-cancer groups. P < 0.05 (two-tailed) was considered statistically significant. AGC, advanced gastric cancer; CEA, carcinoembryonic antigen; EGC, early gastric cancer; GC, gastric cancer; MCP-1, monocyte chemotactic protein-1; MDC, macrophage-derived chemokine; SCF, stem cell factor; TARC, thymus activation-regulated chemokine.
Diagnostic accuracy of serum thymus and activation-regulated chemokine for prediction of gastric cancer in the independent validation dataset
ROC curves with calculated AUC indicated that serum TARC was a more accurate diagnostic tool for prediction of GC compared with serum SCF and serum CEA (Fig.2). In the independent validation dataset, AUC of serum TARC was 0.83 (95% CI = 0.78–0.89), AUC of serum SCF was 0.77 (95% CI = 0.70–0.84) and AUC of CEA was 0.59 (95% CI = 0.51–0.68). The optimal cutoff value of each tested marker as a single marker was estimated from the ROC curves, and by using this optimal cutoff value, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for each tested marker were calculated through χ2 analysis (see Suppl. Table S2).
Figure 2.
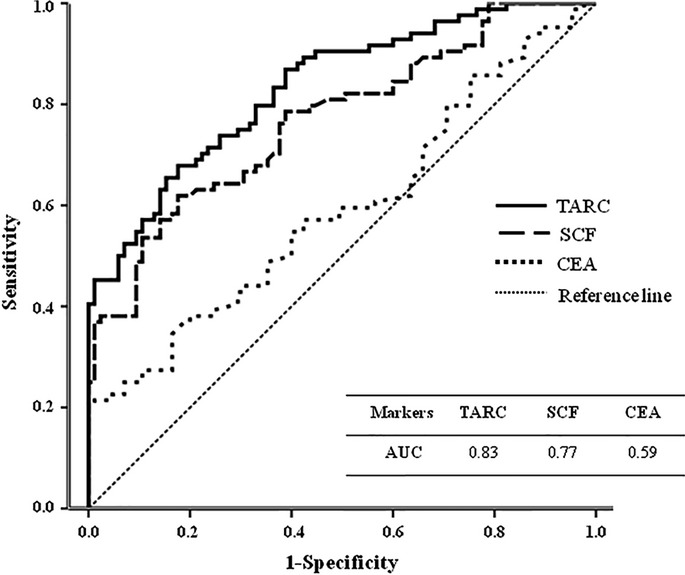
ROC curves and AUC of tested serum values for prediction of gastric cancer, compared with CEA in the independent validation dataset. AUC, area under the ROC curve; CEA, carcinoembryonic antigen; ROC, receiver operating characteristic; SCF, stem cell factor; TARC, thymus activation-regulated chemokine.
Logistic regression further confirmed the remarkable diagnostic accuracy of serum TARC for prediction of GC; the sensitivity and specificity of serum TARC for diagnosis of GC were 72.0% and 71.1%, respectively (cutoff point, 0.37; Table4), which were superior to those for CEA (57.1% sensitivity and 57.6% specificity; cutoff point, 0.44) as a single marker. Combining serum TARC with CEA or SCF increased the sensitivity and specificity, although those of SCF or CEA were not favorable as a single marker. To compare the diagnostic accuracy among panels, the cutoff point ensured a target sensitivity of approximately 72%. The best sensitivity and specificity were observed when all of three values were combined; specificity increased to almost 90% (88.2%) at a sensitivity of 72.6% (cutoff point, 0.54; Table4). Collectively, serum TARC exhibited a remarkable diagnostic accuracy for prediction of GC, both as a single marker and as a part of a multiple-marker panel (Table4).
Table 4.
Sensitivity and specificity of serum TARC, SCF and CEA as a single marker and/or as a multiple-markers panel for prediction of GC determined by logistic regression analysis in the validation dataset
| Marker panel† | Cutoffpoint (%)‡ | Sensitivity (%)§ | Specificity (%) |
|---|---|---|---|
| TARC | 0.37 | 72.0 | 71.1 |
| SCF | 0.38 | 69.9 | 66.7 |
| CEA | 0.44 | 57.1 | 57.6 |
| TARC + SCF | 0.47 | 72.0 | 85.1 |
| TARC + CEA | 0.45 | 71.4 | 80.0 |
| SCF + CEA | 0.45 | 69.0 | 72.9 |
| TARC + SCF + CEA | 0.54 | 72.6 | 88.2 |
Each marker is included as a linear term.
Cutoff point means the probability cutoff point used to classify subjects as having gastric cancer or non-cancer in a binary logistic regression.
For comparison among panels, the cut-off point ensures a target sensitivity of approximately 72%. CEA, carcinoembryonic antigen; SCF, stem cell factor; TARC, thymus activation-regulated chemokine.
Relationships between serum thymus and activation-regulated chemokine and clinicopathological characteristics of gastric cancer in the independent validation dataset
In the independent validation dataset, serum TARC levels were not affected by gender (Spearman's correlation; γs = −0.097, P = 0.154), age (Pearson's correlation; γp = 0.073, P = 0.282) or H. pylori infection status (γs = 0.095, P = 0.161; Table5). Histopathologically, serum TARC levels were not significantly correlated with histological type of GC (γs = −0.051, P = 0.635) or primary GC location (γs = −0.012, P = 0.908). However, serum TARC levels did closely correlate with depth of invasion (T-stage, γs = 0.340 P = 0.001), lymph node metastasis (N-stage, γs = 0.318, P = 0.002), distant metastasis (M-stage, γs = 0.346, P = 0.001) and overall stage (γs = 0.278, P = 0.008; Table5). Primary tumor size of GC was also positively correlated with serum TARC levels (γs = 0.227, P = 0.028). However, serum SCF was not correlated with TNM stage or primary tumor size of GC. Serum CEA only correlated with distant metastasis and primary tumor size (Table5). Collectively, current clinicopathological data indicates that elevated serum TARC levels in GC patients before treatment may imply a poor prognosis of GC.
Table 5.
Relationships of serum TARC or SCF with clinicopathological characteristics of GC in the validation dataset
| Clinicopathological characteristics | TARC | SCF | CEA |
|---|---|---|---|
| γs (P-value) | γs (P-value) | γs (P-value) | |
| Gender (Male:Female) | −0.097 (0.154) | −0.070 (0.305) | −0.079 (0.294) |
| Age (years)† | 0.073 (0.282) | 0.091 (0.184) | −0.031 (0.682) |
| H. pylori infection (−/+) | 0.095 (0.161) | 0.057 (0.397) | 0.009 (0.903) |
| Histology (Intestinal:Diffuse) | −0.051 (0.635) | −0.185 (0.082) | 0.090 (0.422) |
| Tumor location (Lower:Middle:Upper)‡ | −0.012 (0.908) | 0.001 (0.999) | 0.191 (0.087) |
| Tumor size (<3 cm; 3–5 cm and >5 cm)§ | 0.227 (0.028)* | 0.119 (0.255) | 0.417 (0.001)* |
| T-stage (T1a:T1b:T2:T3:T4)¶ | 0.340 (0.001)* | 0.166 (0.118) | 0.102 (0.274) |
| N-stage (N0:N1:N2:N3)¶ | 0.318 (0.002)* | 0.074 (0.485) | 0.078 (0.489) |
| Distant Metastasis (M0:M1)¶ | 0.346 (0.001)* | 0.137 (0.199) | 0.464 (0.001)* |
| Overall stage (I:II:III:IV)¶ | 0.279 (0.008)* | 0.156 (0.142) | 0.142 (0.058) |
These values are statistically significant.
This is a continuous variable. Therefore, the correlation is evaluated by Pearson's correlation (γp).
Tumor location is divided into three areas: lower third (antrum-angle), middle third (low body-middle body), and upper third (upper body-cardia).
Tumor size was classified into three groups: <3 cm, 3–5 cm, and >5 cm.
TNM stage was evaluated according to the 7th International Union Against Cancer-TNM stage. CEA, carcinoembryonic antigen; SCF, stem cell factor; TARC, thymus activation-regulated chemokine. γs, Spearman's correlation coefficient. P < 0.05 (two-tailed) was considered statistically significant.
Discussion
Chemokines and their receptors can affect tumorigenesis and metastasis by regulating angiogenesis, modulating tumor growth and inducing chemotactic attraction.2,30 Previous studies have reported that many chemokines and their receptors are expressed in GC.31 Lee et al.15 report that CCR4, a receptor for TARC, was overexpressed in human GC tissues and induced migration of GC cell lines. TARC, showing the chemotactic activity for T lymphocytes and some other leukocytes, was originally reported to be involved in skin32 and lung diseases.28,29 Increasing evidence suggests that TARC plays an important role in solid tumors,20–22 including GC.15 In the present study, we demonstrated that serum TARC levels were closely correlated with GC carcinogenesis and progression. We also validated the potential of serum TARC as a serologic biomarker for GC. To our knowledge, this is the first report showing the clinical significance of serum TARC levels in GC using human serum samples and suggesting its potential as a desirable diagnostic and prognostic biomarker for GC. This study was initially conducted in a training dataset and confirmed by a following independent validation dataset. We also followed the Standards for Reporting of Diagnostic Accuracy (STARD) statement guidelines.33
Because both TARC and MDC are ligands of CCR4, and SCF can release TARC, MDC and MCP-1 simultaneously,28,29 we expected that these chemokines would be correlated with each other and involved in GC carcinogenesis and progression through their interactions. Thus, we first evaluated the correlations among serum levels of TARC, MDC, MCP-1 and SCF and we found that serum TARC was closely correlated with MDC, MCP-1 and SCF in GC in the training dataset (Table1). However, we selectively further analyzed just serum TARC and SCF among tested chemokines in the validation dataset because only serum TARC and SCF were significantly higher in cancer groups than non-cancer conditions in the training dataset (Table2). In the validation dataset, serum TARC levels increased along the GC carcinogenic sequence and were significantly higher in cancer groups versus non-cancer groups (Table3), consistent with the results of the training dataset. Similar patterns were found in serum SCF levels (Table3).
Receiver operating characteristic curves and logistic regression analysis in the validation dataset indicated that serum TARC had a remarkably higher diagnostic accuracy than CEA, a pre-existing gastrointestinal tumor biomarker (Figs2, Table4, Suppl. Table S2). We found the sensitivity and specificity of serum CEA for prediction of GC to be approximately 50–60% (Table4, Suppl. Table S2), which was consistent with previous studies.34 However, both the sensitivity and the specificity of serum TARC were >70% as a single marker (Table4, Suppl. Table S2). In contrast, the sensitivity and the specificity of serum SCF were not very favorable (Table4, Suppl. Table S2). However, serum SCF and CEA could increase the diagnostic accuracy of serum TARC for prediction of GC when they were combined with serum TARC as a multiple-marker panel (Table4). Because serum TARC can be elevated in other cancers,17–22 the use of serum TARC as a single biomarker for GC may show relatively low specificity. However, combining the use of serum TARC with serum SCF or CEA as a multiple-marker panel can elevate the specificity of serum TARC close to 80–90% (Table4).
Clinicopathologically, serum TARC levels were closely correlated with depth of invasion, lymph node metastasis, distant metastasis and primary tumor size (Table5), suggesting that circulating TARC contributes to expansion, invasion and metastasis of GC, and that serum TARC is a valuable serological prognostic biomarker as well as a diagnostic biomarker for GC. However, serum TARC levels were not affected by gender, age, histological type or primary tumor site in this study (Table5). H. pylori infection can induce the chronic inflammatory milieu within the gastric mucosa, so it was expected to affect serum TARC levels. However, serum TARC levels were not affected by H. pylori infection status in the present study.
Many previous studies have suggested that cancer antigen 19-9 (CA19-9) could be another helpful serum biomarker for GC, especially in prognosis.35,36 Therefore, we also evaluated the clinical significance of serum CA19-9 in GC as a serum biomarker and compared with those of TARC using our current serum samples. Serum CA19-9 was not significantly different among normal, high-risk and EGC groups (post-hoc Bonferroni, all P > 0.05). It was just significantly higher in AGC groups, especially in the metastastic cases (data not shown). Serum CA19-9 levels were correlated with N-stage (γs = 0.216, P = 0.043) and M-stage (γs = 0.265, P = 0.012), but not T-stage (γs = 0.177, P = 0.098; data not shown). The AUC value of CA19-9 for prediction of GC was 0.62, which were inferior to that of TARC (data not shown).
Recently, endoscopic examination has been used frequently for early diagnosis of GC with high reliability. However, because the ideal screening method must be easy to use in addition to having high sensitivity, specificity and reproducibility, endoscopic examination has limitations. Accurate serum biomarkers may be more valuable than tissue markers or invasive methods. However, classic tumor markers for GC (CEA and CA19-9) and other markers (e.g. alpha fetoprotein, CA125, CA72-4 and pepsinogen) did not show a remarkably higher diagnostic accuracy until now (summarized in Suppl. Table S3).37,38 In contrast, we demonstrated that serum TARC, a proinflammatory mediator, showed remarkably higher diagnostic accuracy (AUC = 0.83) than other markers, as found in a previous study.39 Our data may suggest that serum TARC has great clinical importance as a promising serum biomarker and a novel treatment target for GC.
One of the limitations of the present study is its relatively small sample sizes, although the sample sizes of all tested datasets achieved >80% statistical power. In addition, we could not evaluate the prognosis of GC patients according to serum TARC levels directly from overall survival analysis because the observation period was too short to evaluate the survival of GC patients. Instead, we found the close relationships between serum TARC levels and N-or M-stage (Table5). Because lymph node metastasis or distant metastasis are the important prognostic indicators in GC patients,40 our results indirectly demonstrate the prognostic potential of serum TARC for GC. However, further large-scale and long-term follow-up study is needed in the future to confirm our results.
Acknowledgments
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2014 (3-2014-0115)
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Table S1. Baseline clinicopathological features of subjects in the validation dataset according to disease groups
Table S2. Sensitivity and specificity of TARC (A), SCF (B) and CEA (C) as a single marker (these are estimated through χ2 analysis by using the optimal cutoff value, which was estimated from ROC curves)
Table S3. Summary of the sensitivity and specificity of other serum biomarkers, reported in previous studies, such as alpha fetoprotein (AFP), cancer antigen (CA) 125, CA72-4, and pepsinogen (PG), compared with our current results for carcinoembryonic antigen (CEA), CA19-9 and thymus activation-regulated chemokine (TARC)
References
- Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–51. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–43. [PubMed] [Google Scholar]
- Schröttner P, Leick M, Burger M. The role of chemokines in B cell chronic lymphocytic leukaemia: pathophysiological aspects and clinical impact. Ann Hematol. 2010;89:437–46. doi: 10.1007/s00277-009-0876-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–8. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka H, Hirano T, Uda Y, Iimuro Y, Yamanaka J, Fujimoto J. Blockage of CXCR2 suppresses tumor growth of intrahepatic cholangiocellular carcinoma. Surgery. 2014;155:640–9. doi: 10.1016/j.surg.2013.12.037. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–10. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–34. [PubMed] [Google Scholar]
- Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci. 2011;102:44–50. doi: 10.1111/j.1349-7006.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Ou ZL, Yu SJ, et al. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res Treat. 2012;131:837–48. doi: 10.1007/s10549-011-1502-6. [DOI] [PubMed] [Google Scholar]
- Liu Q, Rexiati M, Yang Y, et al. Expression of chemokine receptor 4 was associated with poor survival in renal cell carcinoma. Med Oncol. 2014;31:882–7. doi: 10.1007/s12032-014-0882-y. [DOI] [PubMed] [Google Scholar]
- Yang YM, Feng AL, Zhou CJ, et al. Aberrant expression of chemokine receptor CCR4 in human gastric cancer contributes to tumor-induced immunosuppression. Cancer Sci. 2011;102:1264–71. doi: 10.1111/j.1349-7006.2011.01934.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho YS, Lee JY, et al. The chemokine receptor CCR4 is expressed and associated with a poor prognosis in patients with gastric cancer. Ann Surg. 2009;249:933–41. doi: 10.1097/SLA.0b013e3181a77ccc. [DOI] [PubMed] [Google Scholar]
- Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. Am J Pathol. 1999;154:1685–91. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niens M, Visser L, Nolte IM, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140:527–36. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- Weihrauch MR, Manzke O, Beyer M, et al. Elevated serum levels of CC thymus and activation-related chemokine (TARC) in primary Hodgkin's disease: potential for a prognostic factor. Cancer Res. 2005;65:5516–9. doi: 10.1158/0008-5472.CAN-05-0100. [DOI] [PubMed] [Google Scholar]
- Al-haidari AA, Syk I, Jirström K, Thorlacius H. CCR4 mediates CCL17 (TARC)-induced migration of human colon cancer cells via RhoA/Rho-kinase signaling. Int J Colorectal Dis. 2013;28:1479–87. doi: 10.1007/s00384-013-1712-y. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Bodogai M, Olkhanud PB, et al. Inhibition of lung metastasis by chemokine CCL17-mediated in vivo silencing of genes in CCR4+ Tregs. J Immunother. 2013;36:258–67. doi: 10.1097/CJI.0b013e318294357c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Hildesheim A, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105:1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- Stewart BW, Wild CP. World Cancer Report 2014. 3rd edn. NY: International Agency for Research on Cancer (IARC) Press; 2014. . World Health Organization, ; Chapter 1.1. [Google Scholar]
- Hundahl AS, Phillips JL, Menck HR. The National Cancer Data Base report on poor survival of US gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–32. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- Oliveira SH, Lukacs NW. Stem cell factor: a hemopoietic cytokine with important targets in asthma. Curr Drug Targets Inflamm Allergy. 2003;2:313–8. doi: 10.2174/1568010033483990. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Lukacs NW. Stem cell factor and igE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors. Inflamm Res. 2001;50:168–74. doi: 10.1007/s000110050741. [DOI] [PubMed] [Google Scholar]
- Bendall L. Chemokines and their receptors in disease. Histol Histopathol. 2005;20:907–26. doi: 10.14670/HH-20.907. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J Gastroenterol. 2014;20:1681–93. doi: 10.3748/wjg.v20.i7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarcsi A, Homey B. Chemokine networks in atopic dermatitis: traffic signals of disease. Curr Allergy Asthma Rep. 2005;5:284–90. doi: 10.1007/s11882-005-0068-y. [DOI] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003;49:1–6. doi: 10.1373/49.1.1. [DOI] [PubMed] [Google Scholar]
- Chung HW, Kim JW, Lee JH, et al. Comparison of the validity of three biomarkers for gastric cancer screening: carcinoembryonic antigen, pepsinogens, and high sensitive C-reactive protein. J Clin Gastroenterol. 2009;43:19–26. doi: 10.1097/MCG.0b013e318135427c. [DOI] [PubMed] [Google Scholar]
- Choi AR, Park JC, Kim JH, et al. High level of preoperative carbohydrate antigen 19-9 is a poor survival predictor in gastric cancer. World J Gastroenterol. 2013;19:5302–8. doi: 10.3748/wjg.v19.i32.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilege E, Mihmanli M, Demir U, et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674–7. [PubMed] [Google Scholar]
- He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87–91. doi: 10.1186/1471-230X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Pérez E, Fernández-Arjona M, Baza B, et al. Determination of the tumor marker CA 72-4 in gastric carcinoma. Rev Esp Enferm Dig. 1993;83:92–6. [PubMed] [Google Scholar]
- Lukaszewicz-Zając M, Mroczko B, Gryko M, Kędra B, Szmitkowski M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med. 2011;11:89–96. doi: 10.1007/s10238-010-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwald SN, Kim S, Klimstra DS, Brennan MF, Karpeh MS. Analysis of 154 actual five-year survivors of gastric cancer. J Gastrointest Surg. 2000;4:520–5. doi: 10.1016/s1091-255x(00)80095-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinicopathological features of subjects in the validation dataset according to disease groups
Table S2. Sensitivity and specificity of TARC (A), SCF (B) and CEA (C) as a single marker (these are estimated through χ2 analysis by using the optimal cutoff value, which was estimated from ROC curves)
Table S3. Summary of the sensitivity and specificity of other serum biomarkers, reported in previous studies, such as alpha fetoprotein (AFP), cancer antigen (CA) 125, CA72-4, and pepsinogen (PG), compared with our current results for carcinoembryonic antigen (CEA), CA19-9 and thymus activation-regulated chemokine (TARC)


