Abstract
Breast cancer is a heterogeneous disease. Approximately 70% of breast cancers are estrogen receptor (ER) positive. Endocrine therapy has dramatically improved the prognosis of ER-positive breast cancer; however, many tumors exhibit de novo or acquired resistance to endocrine therapy. A thorough understanding of the molecular mechanisms regulating hormone sensitivity or resistance is important to improve the efficacy of and overcome the resistance to endocrine therapy. The growth factor receptor signaling pathways, particularly the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway can mediate resistance to all forms of endocrine therapy. In contrast, FOXA1 transcription factor is a key determinant of ER function and endocrine response. Intriguingly, a link between hormone resistance induced by the PI3K/Akt/mTOR pathway and the function of FOXA1 has been suggested. In this review, we focus on the PI3K/Akt/mTOR pathway and functions of FOXA1 in terms of the molecular mechanisms regulating the hormone sensitivity of breast cancer.
Keywords: Breast cancer, endocrine therapy, estrogen receptor, FOXA1, PI3K/Akt/mTOR pathway
Breast cancer is a heterogeneous disease. Approximately 70% of breast cancers are estrogen receptor (ER) positive. The ER drive tumor growth in response to their natural ligands, estrogen, and ER expression indicates the degree of estrogen dependence of breast cancer.(1) Endocrine therapy is the most efficacious treatment for ER-positive breast cancer, which is achieved by antagonizing the ligand binding to ER (tamoxifen and other selective ER modulators), downregulating ER (fulvestrant) or blocking estrogen biosynthesis (aromatase inhibitors [AI] and luteinizing hormone–releasing hormone agonists).
Many tumors exhibit de novo or acquired resistance to endocrine therapy, although it has dramatically improved the prognosis of ER-positive breast cancer. Multiple mechanisms of endocrine resistance have been proposed, including the deregulation of components of the ER pathway itself, alterations in the cell cycle and cell survival signaling molecules and the activation of escape pathways.(2–5) Activating ESR1 mutations were reported as a new factor mediating endocrine resistance.(6,7)
Understanding the molecular mechanisms regulating the hormone sensitivity or resistance is important to improve the efficacy of and overcome the resistance to endocrine therapy. Many studies have shown that the growth factor receptor (GFR) signaling pathways, particularly the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, can mediate resistance to all forms of endocrine therapy.
Recent studies using a new technology that combines chromatin immunoprecipitaion (ChIP) with high-throughput sequencing (ChIP-seq) have identified a complex network formed by the ER and its coregulators, and their genome-wide DNA binding patterns, the cistrome.(8) These studies revealed that a transcription factor, FOXA1, is a key determinant of ER function and endocrine response.(9) Intriguingly, a link between hormone resistance induced by the PI3K/Akt/mTOR pathway and the function of FOXA1 has been suggested.(10) In the present review, we focus on the PI3K/Akt/mTOR pathway and functions of FOXA1 in terms of the molecular mechanisms regulating the hormone sensitivity of breast cancer.
ER Signaling
There are two different forms of ER encoded by distinct genes, ERα and ERβ.(11) ERα is responsible for estrogen-induced mitogenic signaling in epithelial cells in the breast, uterus and ovaries and plays a crucial role in breast cancer initiation and progression. In the present review, “ER” refers to ERα unless stated otherwise.
When estradiol (E2) binds to ER, ER undergoes conformational changes and forms dimers. The ER dimers bind to the estrogen response element sequence within the promoter of target genes and attract a complex of co-factors (co-activators and co-repressors).(4,12) This classic function of ER is its nuclear function, also called its genomic activity (Fig. 1). The E2-ER complexes affect the expression of hundreds of genes involved in proliferation, differentiation, survival, invasion, metastasis and angiogenesis, which are particularly relevant for cancer. The ER can also bind to other transcription factors, such as activator protein-1 and specificity protein-1, at their specific sites on DNA and its transcriptional activity is modulated by this binding.(4) In addition, the ER signaling pathway is also regulated by membrane receptor tyrosine kinases (RTK), including epidermal GFR, HER2 and insulin-like growth factor receptor (IGF1-R).(4) These membrane RTK activate signaling pathways such as the PI3K/Akt/mTOR pathway and the mitogen-activated protein kinase pathway, which eventually result in phosphorylation of ER, thus leading to ER activation (Fig. 1).
Fig 1.

A schematic diagram of estrogen receptor (ER) signaling. Estrogen (E)-bound ER binds to DNA sequences in the promoter regions of target genes at estrogen response elements (ERE) and works as a transcription factor in the nucleus. The ER can also bind to other transcription factors, such as activator protein-1 (AP-1) and specificity protein-1 (SP-1) at their specific sites on DNA. The ER signaling pathway is also regulated by membrane receptor tyrosine kinases (RTK). These RTK activate signaling pathways such as the PI3K/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway that eventually result in phosphorylation of ER, leading to ER activation.
The PI3K/Akt/mTOR Pathway
Activation of the PI3K/Akt/mTOR pathway
The PI3K/Akt/mTOR pathway is frequently activated in various malignancies and plays key roles in the development, progression and therapeutic resistance of cancer. The PI3K/Akt/mTOR pathway is now considered to be an attractive and promising target for cancer therapy and many agents targeting this pathway have been developed.(13,14)
One of the major mechanisms underlying the activation of the PI3K/Akt/mTOR pathway is the activation of the membrane RTK. Among them, HER2-containing heterodimers, especially HER2–HER3 heterodimers strongly activate the PI3K/Akt pathway.(15) Akt activation is positively associated with HER2 overexpression in breast carcinomas obtained from human materials.(16–18)
Cellular activation of Akt is dependent on the generation of inositol-containing membrane lipids phosphorylated by Class I PI3K composed of a catalytic subunit (p110) and an adaptor/regulatory subunit (p85). Mutations of PIK3CA, which encodes p110, are the most common genetic alterations of this pathway in breast cancer.(19) Akt is activated by the phosphorylation at Thr308 and Ser473 and it then phosphorylates its substrates.(20) This pathway is negatively regulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and inositol polyphosphate-4-phosphate, type II.(21)
Akt is critical for cell survival, cell cycle regulation and protein synthesis via its phosphorylation of many kinds of proteins, including FOXOs, glycogen synthase kinase-3β (GSK3β) and mTOR.(22–25) mTOR forms the mTORC1 complex with raptor, which controls protein synthesis and cell growth by activating ribosomal protein S6 kinase (p70S6K1) and inhibiting the elongation-initiation factor 4E-binding protein (4E-BP). p70S6K can also phosphprylate ER (Figs 2).(26) mTOR also forms the mTORC2 complex with rictor, which phosphorylates and activates Akt at Ser473, whereas Akt is phosphorylated at Thr308 by PDK1.(27)
Fig 2.
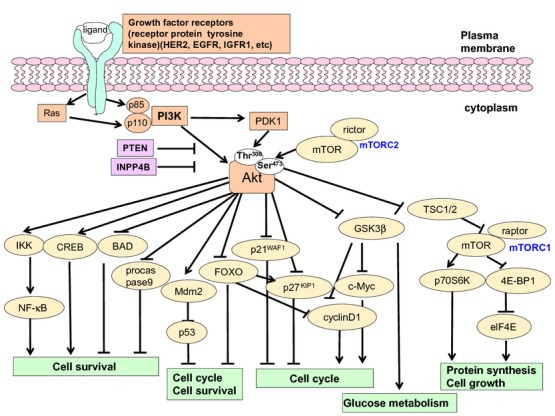
A schematic diagram of the signaling of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway involved in human cancers. Akt is activated by Class I PI3K, composed of two subunits, p110 and p85. Akt is activated by phosphorylation at Thr308 by PDK-1 and at Ser473 by mTORC2. This pathway is negatively regulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and inositol polyphosphate-4-phosphate, type II (INPP4B). Activated Akt phosphorylates its substrates, then regulates a wide range of target proteins and has multiple cellular functions, including effects on cell survival, cell cycle progression, cell growth and other processes.
The PI3K/Akt/mTOR pathway and endocrine resistance
The PI3K/Akt/mTOR pathways activated by RTK signaling interact with ER both directly and indirectly. The phosphorylated ER by Akt or p70S6K promotes the transcription of genes encoding growth factors (GF), RTK and other target genes (Fig. 1). This crosstalk between ER and the PI3K/Akt/mTOR pathway increases estrogen-induced, tamoxifen-induced and ligand-independent ER transcriptional activity, which confers resistance to tamoxifen, fulvestrant and estrogen deprivation in ER-positive breast cancer cells.(2)
The ER-positive/progesterone receptor (PR)-negative breast cancers do not respond as well to tamoxifen compared with ER-positive/PR-positive tumors.(28) The predictive value of PR expression has long been attributed to the dependence of PR expression on ER activity, with the absence of the PR reflecting a non-functional ER. However, a recent study revealed that PR expression is inhibited in breast cancer cells via the PI3K/Akt/mTOR pathway, not via a reduction in ER levels or activity.(29) Therefore, a low PR status may serve as an indicator of activated GF signaling and resistance to endocrine therapy. We also reported that HER2 overexpression and loss of heterozygosity at the PTEN gene locus was associated with Akt activation and a lack of PR expression in breast cancer.(30) Recent research using reverse-phase protein arrays and a gene-expression array revealed that tumors with low PI3K activation have high ER levels and vice versa.(31,32) Thus, RTK and their downstream pathways can also reduce estrogen dependence by downregulating the expression of ER and PR.
So far, the only mechanism of resistance to endocrine therapy for which clinical data exist is HER2 positivity, with HER-2-positive metastatic breast cancer found to be less responsive to all types of endocrine treatment by a meta-analysis.(33–35) However, because fewer than 10% of hormone receptor-positive breast cancers are HER2 positive,(35) the mechanism(s) underlying endocrine resistance remain to be elucidated for the majority of ER-positive breast cancers. Therefore, various factors related to activation of the PI3K/Akt/mTOR pathway are considered to be potential causes of endocrine resistance. A recent study showed that treatment with fulvestrant resulted in increased HER3 expression and PI3K/mTOR signaling, while the depletion of HER3 in fulvestrant-treated tumor cells reduced PI3K/mTOR signaling, tumor cell survival and tumor growth, suggesting that upregulation of HER3 causes resistance to fulvestrant.(36) Miller et al.(32) also reported that long-term estrogen-deprived (LTED) ER-positive breast cancer cells exhibited increased PI3K/AKT/mTOR signaling, with hyperactivation of IGF-1R and/or the insulin receptor.
Clinically, the activation of Akt has been shown to be associated with worse outcomes in endocrine-treated patients with breast cancer.(16,17,37) We also reported that Akt activation was associated with resistance to endocrine therapy in metastatic breast cancer.(38)
The prognostic and predictive value regarding endocrine resistance of PIK3CA mutations in ER-positive breast cancer remains unclear. PIK3CA mutations have been shown to result in in vitro activation of the PI3K/AKT/mTOR pathway.(39) However, in luminal tumors there are no significant relationships between PIK3CA mutations and pAkt, p70S6K and p4EBP1, which indicate activation of the PI3K/Akt pathway.(40,41) In addition, PIK3CA mutations did not have a significant effect on outcome after adjuvant tamoxifen therapy in hormone receptor-positive breast cancer patients.(41)
Targeting the PI3K/Akt pathway to overcome endocrine resistance
The combination of endocrine therapy and targeted therapy directed against the PI3K/Akt pathway has been developed to overcome endocrine resistance. The combination treatment of ER-positive/HER2-positive breast cancer cells with trastuzumab and tamoxifen significantly inhibited their growth(42) and treatment of Akt-activated breast cancer cells with mTORC1 inhibitors, rapamycin and temsirolimus led to similar growth inhibition.(43) The growth of LTED cell lines in the absence of estrogen was inhibited by treatment with the PI3K/mTOR dual inhibitor, BEZ235, or with the TORC1 inhibitor, everolimus.(32) Intriguingly, ER is required for acquired hormone-independent breast cancer cell growth in some LTED cell lines and therefore combined downregulation of ER and inhibition of PI3K induces a regression of tumors comprising these cells.(44)
Clinically, some large studies have shown the efficacy of inhibiting the PI3K/Akt/mTOR pathway to overcome endocrine resistance. For ER-positive/HER2- positive breast cancers, the utility of the combined use of trastuzumab or latatinib with AI has been shown.(45,46) In both trials, progression-free survival and the clinical benefit rate were superior in the combination arms. The efficacy of mTORC1 inhibitors was investigated in patients with ER-positive/HER2-negative tumors relapsed following previous treatment with AI. In the BOLERO-2 trial, patients were randomized to groups receiving everolimus or placebo, combined with exemestane.(47) In the TAMRAD (GINECO) study, patients were randomized to tamoxifen combined with everolimus or tamoxifen alone.(48) Statistically significant increases in progression-free survival were revealed following the addition of everolimus to the endocrine agents in both trials. In the TAMRAD study, only the secondary endocrine-resistant tumors received a benefit from everolimus.(48) In contrast, adding temsirolimus to letrozole did not improve progression-free survival as first-line therapy in patients with AI-naïve advanced breast cancer.(49) These results suggest that the strategy of co-targeting the PI3K and ER pathways may work particularly well in patients whose tumors have acquired resistance to previous endocrine therapy. The key results of these studies are shown in Table 1.
Table 1.
Major published clinical trials of the combination of endocrine agents with RTK-targeting therapies for metastatic breast cancer
| RTK-targeting therapy | Study design | Patients | Key results | Reference |
|---|---|---|---|---|
| Anti-HER2 therapy | ||||
| Trastuzumab | ANA vs ANA + TRAS randomized phase III (TAnDEM study) | n = 207 | PFS: ANA + TRAS 4.8 month; ANA 2.4 month; HR, 0.63; 95% CI, 0.47–0.84; P = 0.016 OS: ANA + TRAS 28.5 month; ANA 23.9 month; P = 0.325 70% of patients in the ANA arm crossed over to TRAS after progression OS: without crossover usage of TRAS ANA + TRAS 28.5 month; ANA 17.2 month; P = 0.048 CBR: ANA + TRAS 42.7%; 95% CI, 33.0–52.9%; ANA 27.9%; 95% CI, 19.5–37.5%; P = 0.026 | Kaufman et al.(45) |
| Lapatinib | LET vs LET + LAP randomized phase III | Overall, n = 1286 HER2 positive, n = 219 | HER2+ cases PFS: LET + LAP 8.2 month; LET + placebo 3.0 month; HR, 0.71; 95% CI, 0.53–0.96; P = 0.019 OS: LET + LAP 33.3 month; LET + placebo 32.3 month; HR, 0.74; 95% CI, 0.5–1.1; P = 0.113 CBR: LET + LAP 48%; LET + placebo 29%; OR, 0.4; 95% CI, 0.2–0.8; P = 0.003 | Johnston et al.(46) |
| mTOR inhibitors | ||||
| Everolimus | TAM vs TAM + EVE randomized phase II (GINECO study) | After prior AI, n = 111 | CBR: TAM + EVE 61%; TAM 42%; P = 0.045 TTP: TAM + EVE 8.6 month; TAM 4.5 month; HR, 0.54; 95% CI, 0.36–0.81; P = 0.0002 Exploratory subgroup analysis in patients with secondary hormone resistance CBR: TAM + EVE 74%; TAM 48% TTP: TAM + EVE 14.8 month; TAM 5.5 month; HR, 0.46; 95% CI, 0.26–0.83; P = 0.0087 | Bachelot et al.(48) |
| EXE vs EXE + EVE randomized phase III (BOLERO-2 clinical trials) | Previously treated with NSAI in the adjuvant setting or for advanced disease (or both), n = 724 Asian patients, n = 143 | Median PFS: Local assessment: EXE + EVE 6.9 months; EXE + placebo 2.8 month; HR, 0.43; 95% CI, 0.35–0.54; P < 0.001 Central assessment: EXE + EVE 10.6 months; EXE + placebo 4.1 month; HR, 036; 95% CI, 0.27–0.47; P < 0.001 Asian patients: EXE + EVE 8.48 month; EXE + placebo 4.14 month; HR, 0.62; 95% CI, 0.41–0.94; P < 0.001 | Baselga et al.(47) | |
| Temsirolimus | LET vs LET + TEM randomized phase III | AI naïve, first-line, n = 1112 | PFS: LET + TEM 8.9 month; LET + placebo 9 month; HR, 0.90; 95% CI, 0.76–1.07; P = 0.25 OS: both NE; HR, 089; 95% CI, 0.65–1.23; P = 0.50 | Wolff et al.(49) |
Where available, P-values are indicated. AI, aromatase inhibitors; ANA, anastrozole; CBR, clinical benefit rate; CI, confidence interval; EVE, everolimus; HR, hazard ratio; LAP, lapatinib; LET, letrozole; mTOR, mammalian target of rapamycin; NE, not estimable; NSAI, non-steroidal aromatase inhibitor; OR, odds ratio; OS, overall survival; EXE: exemestane; PFS, progression-free survival; RTK, receptor tyrosine kinase; TAM, tamoxifen; TEM, temsirolimus; TRAS, trastuzumab; TTP, time-to progression.
An important finding of the previous trials of combination therapy was the observation that there was an increase in Akt activation in everolimus-treated tumors.(13) p70S6K, a molecule downstream of mTORC1, suppresses IGF-1R signaling via suppression of IRS1. The blockade of mTORC1 and the resulting inhibition of p70S6K reduce the negative feedback loop effect and the IGF-1R becomes activated, which results in increased PI3K/Akt/mTOR activation. The activation of this compensatory pathway could be, at least in part, responsible for the limited activity of this class of agents. Inhibiting or preventing activation of this compensatory pathway might improve the response to treatment.(13)
FOXA1
The functions of FOXA1
The network of the transcription factors, ER, GATA-binding protein 3 (GATA-3) and FOXA1 had attracted increasing attention, because the normal function of this network has been suggested to be required for hormone sensitivity in breast cancer.(50) FOXA1 mRNA is expressed in luminal subtype tumors, along with several other discriminatory genes, including ER and GATA-3.(51) GATA-3 regulates the lineage determination and differentiation of many cell types,(52) as well as playing a crucial role during mammary gland development.(53)
FOXA1, a member of the forkhead family of transcription factors, is expressed in many organs and plays a key role in development, chiefly in the lung and liver.(54) FOXA1 is also necessary in the postnatal development of the mammary gland and prostate.(55,56) The expression levels of FOXA1 and GATA-3 showed a significant positive correlation. A ChIP study suggested that GATA-3 may function upstream of FOXA1.(53) Although FOXA1 and GATA-3 seem to interact, they have distinct functions. A deficiency of FOXA1 causes a defect in hormone-induced mammary ductal invasion associated with a loss of terminal end bud formation and ER expression.(9,57)
FOXA1 and ER signaling
The mechanisms responsible for ER-mediated transcription are very complex.(58) Recent studies using ChIP with ChIP-seq has identified a complex network composed of ER and its coregulators and, on activation by estrogen, ER is recruited to thousands of sites across the genome of human breast cancer cells, defining its cistrome.(8) The ER frequently binds distal enhancers and FOXA1 is necessary for ER-chromatin interactions (Fig. 3).(8,9,59,60) FOXA1 works as an important pioneer factor for the interactions between ER and androgen receptor (AR) and chromatin.(9,55,56,59–61) Pioneer factors have the capacity to associate with condensed chromatin independently of other factors and can directly modulate chromatin accessibility.(62) FOXA1 interacts with the cis-regulatory regions of heterochromatin and enhances the interaction between ER and chromatin.(8,63)
Fig 3.
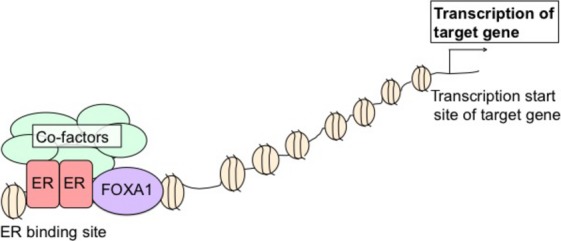
Estrogen receptor (ER)-mediated transcription involving FOXA1. FOXA1 interacts with cis-regulatory regions in heterochromatin and in combination with adjacent DNA binding elements, such as estrogen response elements, to facilitate the interaction of ER with chromatin. Subsequent to the association with ER, the recruitment of cofactors occurs at these distal enhancer sites and the transcription of the target gene is initiated.
These studies also revealed that GFR signaling results in the redirection of ER binding. The GF-stimulated ER cistrome is different from that induced by estrogen. Interestingly, the GF-dependent, ligand-independent ER cistrome regulates a set of genes found to be overexpressed in HER2-positive tumors.(64) This GF-stimulated ER cistrome might be related to endocrine resistace.
Association of FOXA1 and endocrine response
FOXA1 can influence various interactions between ER and chromatin and is required for almost all ER binding events and ER transcription activity in breast cancer cells. As such, FOXA1 is a major determinant of the endocrine response in breast cancer cells.(9) Tamoxifen functions by inhibiting estrogen-ER activity in breast cancer cells, where tamoxifen-ER is recruited to chromatin.(65) Intriguingly, FOXA1 is required for the action of tamoxifen; in tamoxifen-resistant cells, ER binding was independent of the ligand but depended on FOXA1.(9) Ross-Innes and colleagues reported important findings using clinical breast cancer samples.(10) They analyzed the ER ChIP-seq data from primary ER-positive breast tumors with a good prognosis (ER positive/PR positive/HER2 negative) and a poor prognosis (ER positive/PR positive/HER2 positive or ER positive/PR negative/HER2 negative) and samples from distant metastases. Interestingly, the signal of ER binding was lowest in the patients with a good prognosis and highest in the metastatic samples, suggesting that ER-binding intensity might correspond to disease progression of ER-positive breast cancer. The tamoxifen-resistant cancers still recruited ER to chromatin, with the acquisition of unique ER-binding regions. The increased ER binding in tamoxifen-resistant cell lines, which have the same motifs observed in the poor outcome ER-binding events in primary tumors, are probably due to the FOXA1-mediated reprogramming of ER binding. The distinctive ER cistrome reveals gene signatures that can predict the clinical outcome in ER-positive breast cancers. The different types of ER binding at distinct cis-regulatory elements is functionally and biologically relevant, resulting in altered gene expression profiles that contribute to differences in the endocrine response and outcome (Fig. 4).(10)
Fig 4.

Schematic representation of different estrogen receptor (ER) binding events involving FOXA1 facilitating the transcription of different genes. The different ER binding events at distinct cis-regulatory elements ocurr with FOXA1 in different situations. For example, situations 1 (a) and 2 (b). The different ER binding at distinct cis-regulatory elements is functionally and biologically relevant, resulting in altered gene expression profiles that contribute to differences in the endocrine response and outcome. (a) Situation 1. Endocrine-responsive breast cancer with a good outcome, for example, ER positive/progesterone receptor (PR) positive/HER2 negative tumors. (b) Situation 2. Endocrine-resistant breast tumor with a poor prognosis, for example, ER positive/PR positive/HER2 positive or ER positive/PR negative/HER2 negative, or with active growth factor receptor (GFR) signaling.
Importantly, the majority of metastases that arise from an ER-positive breast cancer retain ER and FOXA1 expression, regardless of the sites of metastasis, which suggests the parallel redistribution of ER and FOXA1 binding events in drug-resistant cells.(10) These data indicate that FOXA1 plays a key role in hormone-resistant cancers; therefore, a specific FOXA1 inhibitor might provide a useful clinical tool for the treatment of ER-positive, hormone-resistant breast cancer.(62)
Interstingly, MCF-7 cells overexpressiong Akt exhibit a unique ER cistrome related to the Akt-dependent expression profile.(66) FOXA1 could contribute alteration of the ER cistrome induced by GFR signaling, which occurs in ER-positive breast cancers with acquired endocrine resistance.
Clinical impacts of FOXA1 expression in breast cancer
In breast cancer, FOXA1 expression positively correlates with that of ER and another transcription factor, GATA-3.(67,68) The expression of both GATA-3 and FOXA1 is associated with luminal subtypes and a good prognosis in patients with ER-positive breast cancers. Of note, FOXA1 is an independent prognostic factor for ER-positive breast cancer, probably because the presence of FOXA1 indicates the presence of a functional ER complex, which will respond well to endocrine therapy.(67,69–71)
A high expression of FOXA1 in the primary site could predict a good prognosis of ER-positive breast cancer after adjuvant endocrine therapy. However, the expression of ER and FOXA1 is retained in the metastatic sites.(10) This is an interesting and important finding, although the mechanism underlying this finding is still unclear. It would be meaningful to evaluate the relationships between FOXA1 expression in the primary and metastatic sites and the levels of downstream proteins, such as the PR and cyclin D1, in future studies.
Conclusions
Endocrine therapy is essential for ER-positive breast cancer. In the adjuvant setting, it is difficult to determine the necessity of chemotherapy or the duration of adjuvant endocrine therapy. In the metastatic setting, the indications for chemotherapy are dependent on how the endocrine resistance is judged. In order to resolve these problems, a better understanding of the mechanisms defining the sensitivity or resistance to endocrine therapy is important. Recent research has been unveiling these factors step by step. The combination of endocrine therapy with agents that overcome the resistance or improve the sensitivity to endocrine therapy could be expected to maximize the effects of treatment.
Acknowledgments
This study was supported by grants from the Ministry of Education, Culture, Sports Science, and Technology of Japan.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Robinson JL, Holmes KA, Carroll JS. FOXA1 mutations in hormone-dependent cancers. Front Oncol. 2013;3:20. doi: 10.3389/fonc.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–61. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–70s. [PubMed] [Google Scholar]
- 6.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–45. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 9.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross-Innes CS, Stark R, Teschendorff AE, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–93. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–81. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 12.Renoir JM, Marsaud V, Lazennec G. Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem Pharmacol. 2013;85:449–65. doi: 10.1016/j.bcp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):12–9. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 14.Tokunaga E, Oki E, Egashira A, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 15.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–87. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stal O, Perez-Tenorio G, Akerberg L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003;5:R37–44. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokunaga E, Kimura Y, Oki E, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–9. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Tan M, Stone Hawthorne V, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–88. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 19.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 20.Ananthanarayanan B, Ni Q, Zhang J. Signal propagation from membrane messengers to nuclear effectors revealed by reporters of phosphoinositide dynamics and Akt activity. Proc Natl Acad Sci U S A. 2005;102:15081–6. doi: 10.1073/pnas.0502889102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 24.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–45. [PubMed] [Google Scholar]
- 25.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 26.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 28.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–61. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 29.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23:7721–35. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Tokunaga E, Oki E, Kimura Y, et al. Coexistence of the loss of heterozygosity at the PTEN locus and HER2 overexpression enhances the Akt activity thus leading to a negative progesterone receptor expression in breast carcinoma. Breast Cancer Res Treat. 2007;101:249–57. doi: 10.1007/s10549-006-9295-8. [DOI] [PubMed] [Google Scholar]
- 31.Creighton CJ, Fu X, Hennessy BT, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10:5670–6. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 34.De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–8. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 35.Ellis MJ, Tao Y, Young O, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–25. doi: 10.1200/JCO.2005.04.3034. [DOI] [PubMed] [Google Scholar]
- 36.Morrison MM, Hutchinson K, Williams MM, et al. ErbB3 downregulation enhances luminal breast tumor response to antiestrogens. J Clin Invest. 2013;123:4329–43. doi: 10.1172/JCI66764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Tenorio G, Stal O Southeast Sweden Breast Cancer G. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–5. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokunaga E, Kataoka A, Kimura Y, et al. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–35. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–1000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argiris A, Wang CX, Whalen SG, DiGiovanna MP. Synergistic interactions between tamoxifen and trastuzumab (Herceptin) Clin Cancer Res. 2004;10:1409–20. doi: 10.1158/1078-0432.ccr-1060-02. [DOI] [PubMed] [Google Scholar]
- 43.de Graffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10:8059–67. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 44.Miller TW, Balko JM, Fox EM, et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–51. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 46.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 49.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badve S, Nakshatri H. Oestrogen-receptor-positive breast cancer: towards bridging histopathological and molecular classifications. J Clin Pathol. 2009;62:6–12. doi: 10.1136/jcp.2008.059899. [DOI] [PubMed] [Google Scholar]
- 51.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–7. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 55.Bernardo GM, Keri RA. FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep. 2012;32:113–30. doi: 10.1042/BSR20110046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson JL, Carroll JS. FoxA1 is a key mediator of hormonal response in breast and prostate cancer. Front Endocrinol (Lausanne) 2012;3:68. doi: 10.3389/fendo.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernardo GM, Lozada KL, Miedler JD, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–54. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7:713–22. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- 59.Carroll JS, Liu XS, Brodsky AS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–70. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12:381–5. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- 63.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–14. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 64.Lupien M, Meyer CA, Bailey ST, et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010;24:2219–27. doi: 10.1101/gad.1944810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–52. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 66.Bhat-Nakshatri P, Wang G, Appaiah H, et al. AKT alters genome-wide estrogen receptor alpha binding and impacts estrogen signaling in breast cancer. Mol Cell Biol. 2008;28:7487–503. doi: 10.1128/MCB.00799-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badve S, Turbin D, Thorat MA, et al. FOXA1 expression in breast cancer–correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13:4415–21. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- 68.Habashy HO, Powe DG, Rakha EA, et al. Forkhead-box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer. 2008;44:1541–51. doi: 10.1016/j.ejca.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 69.Hisamatsu Y, Tokunaga E, Yamashita N, et al. Impact of FOXA1 expression on the prognosis of patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012;19:1145–52. doi: 10.1245/s10434-011-2094-4. [DOI] [PubMed] [Google Scholar]
- 70.Mehta RJ, Jain RK, Leung S, et al. FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res Treat. 2012;131:881–90. doi: 10.1007/s10549-011-1482-6. [DOI] [PubMed] [Google Scholar]
- 71.Hisamatsu Y, Tokunaga E, Yamashita N, et al. Impact of GATA-3 and FOXA1 expression in patients with hormone receptor-positive/HER2-negative breast cancer. Breast Cancer, in press. 2014 doi: 10.1007/s12282-013-0515-x. in press. [DOI] [PubMed] [Google Scholar]


