Abstract
Basophil activation was observed in patients with a history of carboplatin-induced severe hypersensitivity reaction (HR). However, the precise mechanism by which carboplatin induces basophil activation and the associated surrogate markers remains to be elucidated. To investigate whether IgE-dependent mechanisms, including the overexpression of FcεRI, participate in carboplatin-induced basophil activation, 13 ovarian cancer patients were enrolled: 5 with a history of carboplatin-induced severe hypersensitivity reaction within the past 2 years, and 8 with no such history. The expression levels of FcεRI, IgE, and CD203c on basophils were measured using a flow cytometer. Immunoglobulin E-dependent basophil activation was evaluated by testing for IgE passive sensitization using lactic acid, and by testing for phosphatidylinositol 3-kinase inhibition, using wortmannin. In three patients positive for carboplatin hypersensitivity, pretreatment with wortmannin almost completely inhibited carboplatin-induced basophil activation (P < 0.05). In a healthy control subject, whose own IgE showed no response to carboplatin, acquired reactivity to carboplatin when exposed to plasma from patients positive for carboplatin hypersensitivity. This did not occur when the same experiment was carried out using plasma from the patients negative for carboplatin hypersensitivity. Moreover, pretreatment with omalizumab, a monoclonal anti-IgE antibody, almost completely blocked carboplatin-induced basophil activation in the plasma of patients positive for carboplatin hypersensitivity. On further investigation, the HR-positive group had significantly higher levels of FcεRI compared with the negative group (P < 0.05). In conclusion, an IgE-dependent mechanism incorporating FcεRI overexpression participates in carboplatin-induced severe HR. These results establish the relevance of monitoring the pharmacodynamic changes of basophils to prevent carboplatin-induced severe HR.
Keywords: Basophil, biological markers, carboplatin, hypersensitivity reaction, immunoglobulin E
Carboplatin (CBDCA)-induced hypersensitivity reaction (HR) is a potentially lethal adverse event that frequently requires discontinuation of the drug. The rate of HR has been reported to be as high as 26.7% in patients receiving more than seven cycles of CBDCA.(1) As CBDCA has been widely used for many types of cancer as a first or second line treatment, clarifying the mechanism of CBDCA-induced HR is extremely important for prevention of severe HR in such cases.
The risk of CBDCA-induced severe HR is thought to be associated with a previous history of drug allergy, a prolonged platinum-free interval, or high dosages of CBDCA.(2) We previously confirmed this by showing that patients with a CBDCA-free interval >13 months had a 22-fold higher risk of HR and that patients with a maximum dose/body of CBDCA >650 mg had a 9.5-fold higher risk of HR.(3) Despite these findings, these factors have been insufficient for predicting HR in clinical practice. Alternatively, intradermal injections of CBDCA have also been used to predict CBDCA-induced severe HR.(4,5) However, there are some risks associated with the clinical application of skin testing for anticancer drugs. For example, in patients with a history of life-threatening anaphylaxis, skin testing may be contraindicated. It is also not recommended due to several other factors including potential skin damage, concerns about environmental contamination, and risks to medical and nursing professionals resulting from excessive exposure to anticancer drugs.(6)
An improved understanding of basophils is useful for understanding and diagnosing allergic diseases, due to their role in the release of various inflammatory mediators, such as histamine.(7) CD203c has been identified as a specific surface marker for basophils and mast cells of the hematopoietic lineage.(8) CD203c is expressed on resting cells at low levels and its expression is rapidly upregulated following activation.(9) In addition, it has been reported that CD203c is a useful marker for allergies induced by amoxicillin and (L)-asparaginase.(10,11) We recently confirmed that patients with a history of CBDCA-induced severe HR showed an increase in CD203c-positive basophils compared with patients without HR.(12) In addition, all five patients with grade 2 or higher HR showed increased CD203c on basophils during the months following the onset of HR, whereas no such increase was not observed in the previous courses of CBDCA.(12) Therefore, it is reasonably certain that the mechanism of CBDCA-induced severe HR somehow involves basophil activation. However, the expression of CD203c on the day prior to anaphylaxis was found to be insufficient for predicting HR in half of the patients. Recently, Caiado et al. reported that 59% of patients have CBDCA-specific IgE; this was identified using the ImmunoCAP assay (Phadia, MI, USA).(13) These findings strongly suggest that IgE-mediated basophil activation plays a key role in the development of CBDCA-induced HR.
A major goal of our study is to construct an appropriate way for predicting CBDCA-induced severe HR. To accomplish this, we attempted to confirm whether an IgE-dependent mechanism participates in CBDCA-induced basophil activation.
Materials and Methods
Study subjects
This study included patients who were admitted to Mie University Hospital (Tsu, Japan) between December 2011 and July 2013. Thirteen female patients with ovarian cancer were enrolled in this study. Five patients had a history of CBDCA-induced grade 3 or higher anaphylaxis,(14) whereas 8 had no such history (Table 1). Upon study entry, blood samples were obtained from the patients on the day before CBDCA treatment. Five healthy control subjects with no history of treatment with CBDCA and no history of allergic diseases were also enrolled in this study. In addition, for further control, we enrolled a healthy basophil donor, whose IgE can only be recognized by polyclonal anti-IgE antibodies (Vector Laboratories, Burlingame, CA, USA), but not the positive control of the monoclonal anti-IgE antibody clone from the Allergenicity Kit is the same kit mentioned below. This study was carried out in accordance with the Declaration of Helsinki and its amendments. This study's protocol was reviewed and approved by the Ethics Committee of Mie University (certification No. 2253), and written informed consent was obtained from each subject.
Table 1.
Characteristics of ovarian cancer patients who participated in this study (n = 13)
| HR(−) group | HR(+) group | P-value | |
|---|---|---|---|
| No. of patients | 8 | 5 | – |
| Age, years† | 57.5 (50–73) | 61 (54–69) | 0.30 |
| No. of CBDCA treatments prior to study† | 7 (7–8) | 14 (11–22) | <0.01 |
| History of drug/food allergy‡ | 4 | 3 | 1.00 |
| Dose of CBDCA during study, mg† | 575 (400–690) | 500 (150–700) | 0.66 |
–, not applicable; CBDCA, carboplatin; HR, hypersensitivity reaction.
Data are expressed as median values (minimum–maximum), and statistical analyses between the two groups were carried out using the Mann–Whitney U-test.
Statistical analyses between the two groups were carried out using Fisher's exact test.
Measurement of CD203c expression on basophils
Measurement of CD203c expression on basophils was carried out within 24 h of blood sampling. The Allergenicity Kit (Beckman Coulter, Fullerton, CA, USA) was used for the quantification of basophil CD203c expression according to the instructions supplied by the manufacturer.(12) We used 50 μg/mL CBDCA (carboplatin; Sandoz, Tokyo, Japan), anti-IgE antibody (4 μg/mL) as a positive control, and 5% dextrose solution as a negative control. Leukocytes in each sample were then analyzed using a flow cytometer (FACSCalibur or FACSCanto II; Becton Dickinson Japan, Tokyo, Japan). Basophils were identified by their characteristic forward and side scatter, by the expression of CBDCA-induced basophil activation, and by the absence of CD3.
Inhibitory effect of wortmannin on CBDCA-induced basophil activation
In order to investigate whether IgE-mediated cell signaling contributes to basophil activation, we analyzed the inhibitory effect of wortmannin, which acts as a specific inhibitor of phosphatidylinositol 3-kinase (PI3-K).(15) Whole blood anticoagulated with EDTA from the three HR(+) patients was pre-incubated for 20 min at 37°C with 0.1 and 10 μM wortmannin (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the expression level of CD203c on basophils was analyzed using a flow cytometer.
Confirmation of IgE-mediated reaction on CBDCA by passive sensitization
As previously discussed, we included a healthy basophil donor, whose IgE cannot be recognized by the monoclonal anti-IgE antibody clone from the Allergenicity Kit. This basophil donor's PBMCs were isolated from whole blood anticoagulated with EDTA and incubated for 5 min on ice with an LS buffer (10 mM lactic acid, 130 mM NaCl, 5 mM KCl, pH3.9) to dissociate the IgE from FcεRI on basophils.(16,17) These acid-treated peripheral blood mononuclear cells (PBMCs) were then incubated for 2 h at 37°C in the plasma of each CBDCA patient (n = 5) for passive sensitization. To block IgE binding to basophils on passive sensitization, the plasma was pretreated for 30 min at room temperature with 1.25 mg/mL omalizumab (Novartis Pharma, Tokyo, Japan).
To confirm the dissociation of IgE from FcεRI by acid treatment and binding of IgE to FcεRI after passive sensitization, pre- and post-passive-sensitized basophils were stained with an FITC-conjugated anti-IgE (Dako, Tokyo, Japan) and R-phycoerythrin (PE)-conjugated anti-FcεRI antibody (CRA1 or CRA2; Bio Academia, Osaka, Japan) and analyzed using a flow cytometer. Subsequently, to confirm the contribution of the IgE-mediated pathway to CBDCA-induced severe HR, we evaluated the change of basophil function after passive sensitization, by analyzing the expression levels of CD203c, using the Allergenicity Kit with both 50 μg/mL CBDCA.
Measurement of FcεRIα expression on basophils
In order to detect FcεRIα, whole blood anticoagulated with EDTA was incubated at room temperature for 1 h with the following antibodies: R-phycoerythrin-cyanine 7-conjugated anti-CD3 (Medical and Biological Laboratories, Nagoya, Japan), PE-conjugated anti-CRTH2 (Beckman Coulter), and FITC-conjugated anti-FcεRIα (CRA1; eBioscience, San Diego, CA, USA). Mouse IgG2bk was used as an isotype control of anti-FcεRIα antibody. The expression levels of FcεRIα in each sample were then analyzed using a flow cytometer.
Reverse transcription-PCR analysis
Total RNA was prepared from whole blood samples using Nucleo Spin RNA Blood (Takara Bio, Shiga, Japan). Messenger RNA was detected by RT-PCR using ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan) with 50 ng total RNA, EagleTaq Master Mix (Roche Applied Science, Tokyo, Japan), and corresponding primer sets. Real-time PCR analysis was carried out using StepOnePlus (Applied Biosystems, Tokyo, Japan). The expression levels of the target molecules relative to GAPDH were evaluated with StepOne software version 2.2.2 (Applied Biosystems).
Statistical analysis
The non-parametric Mann–Whitney U-test and Fisher's exact test were used to assess differences between patients with and without CBDCA-induced HR. The non-parametric Dunnett's multiple comparison test was used to compare results among the three groups. We also carried out parametric one-way repeated measures anova to test for differences among several related samples. All statistical analyses were carried out using GraphPad Prism 5 (version 5.01; GraphPad Software, San Diego, CA, USA). The P-values were two-sided and P < 0.05 was considered statistically significant.
Results
Inhibitory effect of wortmannin, a PI3-K inhibitor, on basophil activation
In the three patients with a history of CBDCA-induced severe anaphylaxis, CBDCA-induced CD203c expression on basophils was almost completely inhibited by pretreatment with wortmannin in a way similar to positive control (anti-IgE antibody) exposure (Fig. 1a) (P < 0.05 and P < 0.01, for 0.1 and 10 μM wortmannin, respectively) (Fig. 1b).
Fig 1.
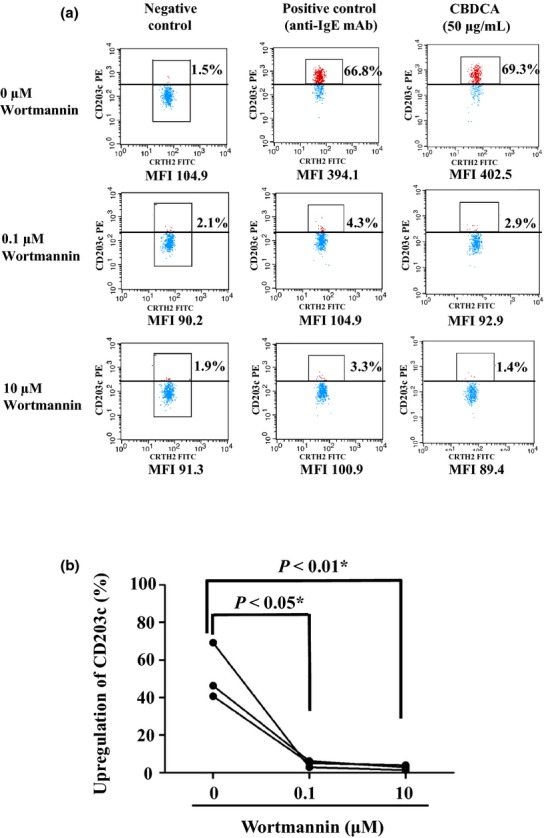
Expression levels of CD203c-positive basophils after in vitro exposure to carboplatin (CBDCA) and wortmannin, a phosphatidylinositol 3-kinase inhibitor (measured by flow cytometric analysis). Whole blood with or without wortmannin was stained for CD3, prostaglandin D2 receptor (CRTH2), and CD203c. Flow cytometer charts for CD3− and CRTH2 + cells (basophils) are shown. Upregulation of CD203c on basophils (shown as a percentage in (a)) was determined using a threshold that was defined as the expression level above which 2% of basophils in the negative control column fluoresce, on average. (a) Data are from the patient whose response to CBDCA was highest among the hypersensitivity reaction-positive patients. This patient's basophils were pretreated with the indicated concentrations of wortmannin, and subsequently exposed to the negative control, positive control, and 50 μg/mL CBDCA. Percentages shown indicate the upregulation rate of CD203c. Mean fluorescence intensities (MFIs) indicated for binding levels of CD203c on basophils. (b) Difference between the respective mean upregulation rates (n = 2) of three patients were analyzed using one-way repeated measures anova.
Confirmation of passive sensitization by flow cytometry analysis
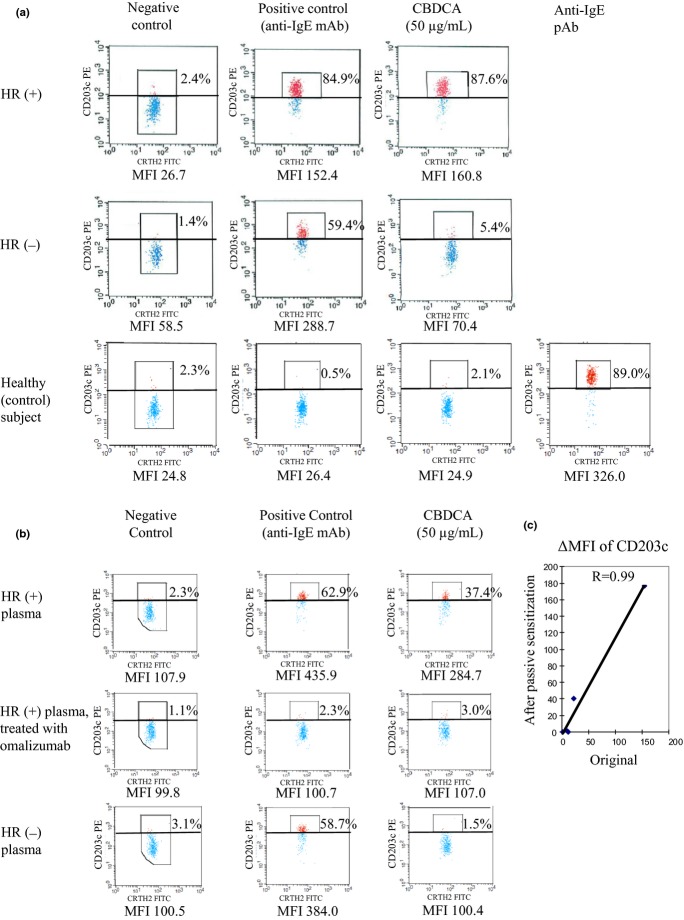
We tested the involvement of CBDCA-specific IgE on CBDCA-induced severe HR using in vitro sensitization. Dissociation of IgE from the basophils from the healthy basophil donor as well as binding of IgE to the basophils in CBDCA patients' plasma were confirmed either by staining with anti-FcεRI antibodies, CRA1 or CRA2, or with an anti-IgE antibody. As has already been shown, CRA1 reacts with a region other than the IgE binding site, whereas CRA2 reacts directly with the IgE binding site.(18) We were thus able to confirm the successful dissociation of IgE by confirming that acid treatment increased CRA2 staining levels (Fig. 2a). After passive sensitization, CRA2 staining levels decreased, thereby suggesting that IgE-dissociated FcεRI on the acid-treated basophils was occupied by IgE in patient plasma (Fig. 2a). Consistent with the results of the CRA2 staining, the level of IgE on basophils decreased after acid treatment, and increased after passive sensitization (Fig. 2b).
Fig 2.
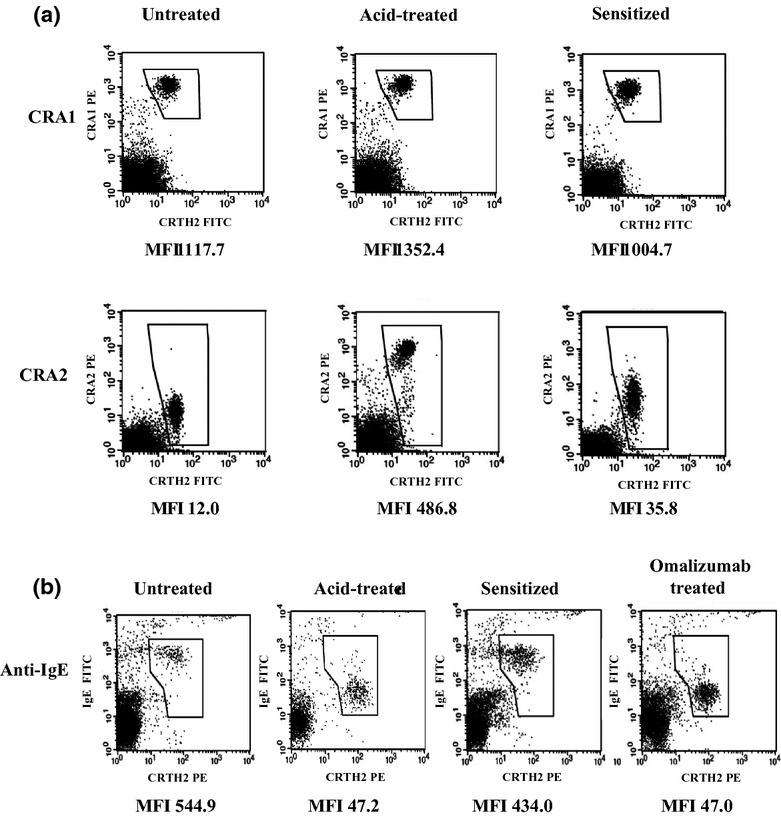
Binding levels of CRA1, CRA2, and anti-IgE antibody measured by basophil staining to confirm the passive sensitization by flow cytometric analysis. (a) Immunostained healthy subject's basophils with anti-FcεRI antibodies; PBMCs with or without acid treatment were stained for CD3, prostaglandin D2 receptor (CRTH2), and FcεRI. (b) Immunostained healthy subject's basophils with anti-IgE antibody; PBMCs with or without acid treatment were stained for CD3, CRTH2, and IgE. Flow cytometer charts for CD3− PBMCs are shown. Basophil fraction (CD3− and CRTH2+ PBMC) was gated. MFIs indicated for binding levels of anti-CRA1, anti-CRA2, and anti-IgE antibodies on basophils. MFI, mean fluorescence intensity.
Analysis of passive sensitization by basophil activation test
Furthermore, to verify the replacement of IgE on basophils, we examined the basophils from a healthy control subject whose own IgE was not recognized by the positive control of the Allergenicity Kit (Fig. 3a). Prior to this, all HR(+) and HR(−) patients' IgE could be recognized by the positive control (Fig. 3a). After passive sensitization, basophils from the control subject also acquired reactivity to the positive control (Fig. 3b). Subsequently, control subject basophils sensitized with plasma from HR(+) patients also acquired reactivity to CBDCA, whereas control subject basophils sensitized with plasma from HR(−) patients did not respond to CBDCA (Fig. 3b). Furthermore, we were able to confirm that omalizumab specifically blocked the binding between IgE and FcεRI (Figs 2b, 3b). Additionally, we observed a high correlation between the respective response levels to CBDCA of patients' sensitized basophils and patients' original basophils (n = 5, r = 0.99) (Fig. 3c), which suggests that a CBDCA-specific IgE is required for the activation of basophils by CBDCA in HR(+) patients.
Fig 3.
Expression levels of CD203c on basophils measured by flow cytometric analysis. (a) Original response levels in hypersensitivity reaction (HR)(+) and HR(−) patients, of the selected healthy subject for monoclonal and polyclonal anti-IgE antibodies, and for carboplatin (CBDCA). The HR(+) patient data are from those whose responses to CBDCA were highest among the CBDCA patients. (b) Response levels of healthy subject's basophils after passive sensitization with HR(+) patients' plasma with or without omalizumab treatment, or with HR(−) patients' plasma. In (a) and (b), flow cytometer charts for CD3− PBMCs are shown. Upregulation of CD203c on basophils was determined using a threshold that was defined as the expression level above which 2% of basophils in the negative control column fluoresce, on average. Mean fluorescence intensities (MFIs) indicated for binding levels of CD203c on basophils. (c) Correlation of response levels to CBDCA of sensitized basophils and of corresponding IgE donor patients' original basophils (n = 5). ΔMFI was calculated as the difference between the negative control and CBDCA-stimulated sample, with respect to the MFI of PE-labeled CD203c.
Expression levels of FcεRIα on basophils in the HR(−) and HR(+) patients
The expression levels of FcεRIα on basophils in HR(−) and HR(+) patients were measured by flow cytometry (Fig. 4). Figure 4(a,b) shows results typical of HR(−) and HR(+) patients, representing ΔMFI of 2385 and 10 193, respectively. ΔMFI is calculated as the difference in mean fluorescence intensity (MFI) between the FITC labeled anti-human FceRI a chain antibody and its isotype control. The HR(+) group had significantly higher levels of FcεRIα than both the HR(−) group (P < 0.05) and control subjects (P < 0.01) (Fig. 4c). In contrast, the expression levels of the reference protein, CRTH, on basophils were comparable among the three groups (Fig. 4d).
Fig 4.
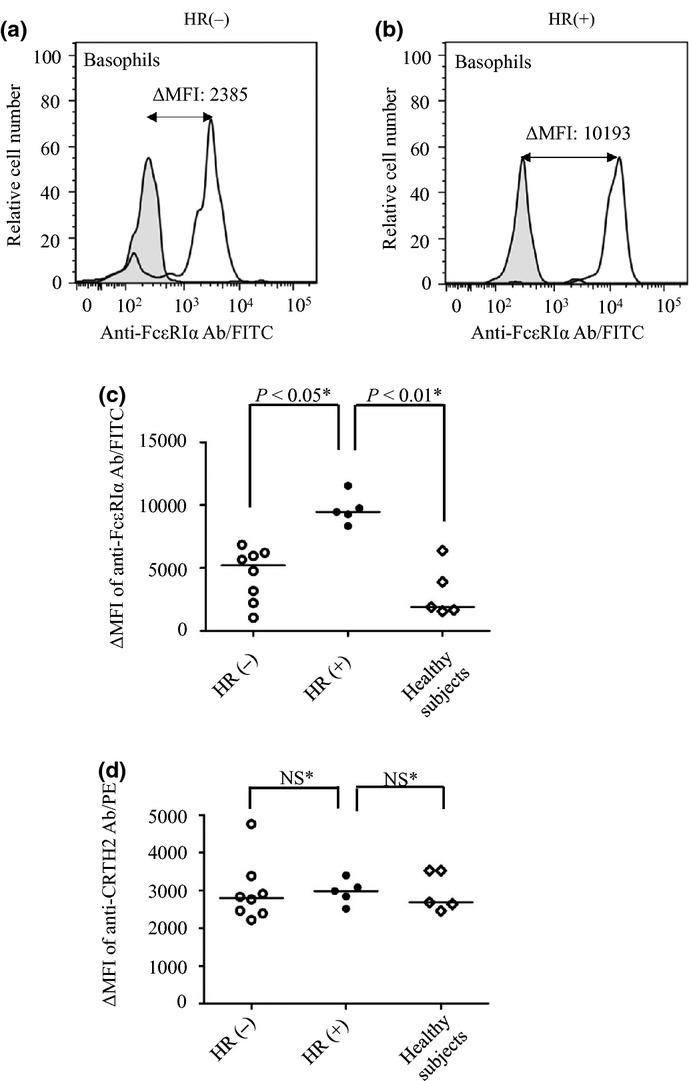
Expression levels of FcεRI on basophils in hypersensitivity reaction (HR)(+) and HR(−) patients. Flow cytometric analysis of FcεRI on basophils was performed by incubation of whole blood sample from study subjects with FITC-conjugated anti-human FcεRI α chain antibody. Histograms from a typical HR(−) (a) and HR(+) patient (b) are shown (unshaded histograms). An isotype control antibody was also used as a negative control (shaded histograms). ΔMFI is calculated as the difference in mean fluorescence intensity (MFI) between the FITC-labeled anti-human FcεRIα chain antibody and its isotype control. (c) The ΔMFI is compared between HR(+) (n = 5) and HR(−) (n = 8) patients, as well as healthy subjects (n = 5). (d) The ΔMFI of PE-conjugated anti-CRTH2 (prostaglandin D2 receptor) antibody used as a reference protein is compared between the three groups. The horizontal line inside the graph represents the median value within each group. The expressions of FcεRI and CRTH was analyzed using two or three different samples from each patient. Differences between the three groups were analyzed using Dunnett's multiple comparison tests.
Expression levels of FcεRI mRNA in the HR(−) and HR(+) patients
The overall expression levels of FcεRI mRNA in whole blood were measured by real-time RT-PCR in HR(−) and HR(+) patients (Fig. 5). Significant differences in all chains of FcεRI mRNA were found between the two groups. The expression levels of α, β, and γ chains of FcεRI mRNA in the HR(+) group were found to be 3.2-fold (P < 0.05), 6.8-fold (P < 0.01), and 1.9-fold (P < 0.05) higher than those in the HR(−) group.
Fig 5.
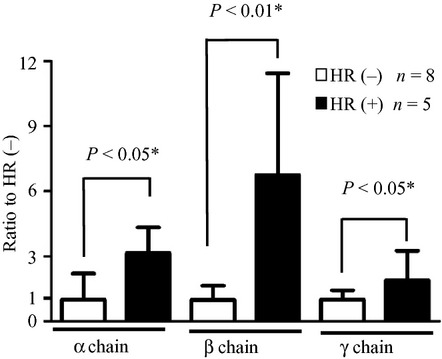
Expression levels of α, β, and γ chains of FcεRI mRNA in the peripheral blood of hypersensitivity reaction (HR)(−) and HR(+) patients. Total RNA was isolated from blood samples, and analyzed by real-time RT-PCR. Relative mRNA levels of FcεRIα, β, and γ chains were quantified relative to the expression levels of GAPDH. Data are presented as the mean ± SD. Statistical analyses between the two groups were carried out using the Mann–Whitney U-test.
Discussion
In this study, we confirmed for the first time that an IgE-dependent mechanism directly participates in CBDCA-induced basophil activation and that either CBDCA itself or a CBDCA-serum albumin conjugate can act as a complete antigen. In addition, IgE-dependent basophil activation by CBDCA was revealed to incorporate FcεRI overexpression, along all chains of FcεRI mRNA.
First, we showed that basophil activation was almost completely inhibited by pretreatment with wortmannin, a PI3-K-specific inhibitor. Previous studies also reported that wortmannin inhibited basophil activation when stimulated with an anti-IgE antibody, but not when N-formyl-L-methionyl-L-leucyl-phenylalanine was used as the basophil stimulator.(15) Furthermore, other studies have shown that, in addition to high-affinity FcεRI, human basophils also express both activating (FcγRIIA) and inhibiting (FcγRIIB) low-affinity IgG receptors, as well as minute amounts of the low-affinity IgG receptor, FcγRIIIB, while expressing neither FcγRIIIA nor high-affinity IgG receptor (FcγRI).(19) Moreover, PI3-K activation has also been reported to play distinct roles in FcγRIIA-mediated degranulation and the production of cytokines.(20) However, FcγRIIA-dependent passive systemic allergy has generally been thought to be dependent on monocytes/macrophages and neutrophils, but not on mast cells and basophils.(21) These reports are consistent with the idea that an IgE-FcεRI-mediated mechanism is primarily responsible for basophil activation by CBDCA.
It is commonly believed that IgE is largely responsible for the development of type I allergy, and the activation of basophils and mast cells is prevented by the dissociation of IgE from FcεRI.(22,23) We examined the involvement of CBDCA-specific IgE at the onset of CBDCA-induced severe HR using in vitro sensitization. Flow cytometric analyses using CRA1 and CRA2 showed that pretreatment with lactic acid almost completely dissociated IgE from FcεRI on basophils, without decreasing the number of FcεRI receptors. The decrease of IgE on basophils was reversed following IgE passive sensitization from HR(+) or HR(−) patients, and the specific binding of IgE and FcεRI was confirmed by the inhibitory effect of omalizumab. Interestingly, when IgE passive sensitization was applied to plasma from HR(+) patients, it induced the acquisition of reactivity to CBDCA in the basophils of a control subject, whose own IgE showed no previous response to CBDCA. However, this acquisition of reactivity did not occur in the plasma of HR(−) patients. Furthermore, we found that the pretreatment with omalizumab almost completely blocked the basophil activation by CBDCA in the previously mentioned sample sensitized by HR(+) patient plasma. Incidentally, this was the same result produced by the monoclonal anti-IgE antibody treatment. These results are the first conclusive evidence showing that a CBDCA-specific IgE participates in basophil activation by CBDCA and that an IgE-mediated pathway plays a key role in CBDCA-induced severe HR. It is currently thought that platinum drug-specific IgE directly recognizes the primary amine groups in CBDCA and cisplatin.(13) Furthermore, in vitro basophil activation has been reported in a patient with cisplatin allergy.(24) Therefore, not only CBDCA but also cisplatin may induce severe HR, which requires an IgE-mediated activation of basophils. On the other hand, the molecular structure of oxaliplatin does not contain a primary amine. We previously confirmed that specific basophil activation did not occur by the in vitro exposure to oxaliplatin in five patients with a history of oxaliplatin-induced HR (data not shown).
In this study, we showed that patients with CBDCA-induced severe HR had significantly higher levels of FcεRI than the HR(−) group and healthy control subjects. In addition, we found that the expression levels of all chains of FcεRI mRNA were significantly elevated in the HR(+) patients compared with those in the HR(−) patients. We also noted that, among these chains, the β chain is expressed exclusively on basophils, while the α and γ chains are also expressed on monocytes and eosinophils to form trimers of FcεRI. Incidentally, it has been found that the γ chain, FcRγ, is required for the expression and function of FcεRI as well as FcγRI, FcγRIII, and FcαR.(25,26) Therefore, it is well within reason that the level of β chain specifically expressed in basophils would be significantly higher in the HR(+) patients than in the HR(−) patients. In addition, the β chain has an important role in amplifying the expression and function of FcεRI.(27,28) These results would seem to imply that a significant increase in FcεRI β-chain expression contributes substantially to the overexpression of FcεRI on basophils in HR(+) patients. It should be noted that, despite the fact that patients' respective expression levels of FcεRI were found to be associated with the number of CBDCA treatments (Table 1), the actual pharmacokinetic association is thought to be relatively insignificant. This is because the expression levels of FcεRI in the patients without CBDCA-induced HR were comparable to that of the healthy subjects, who had no history of CBDCA treatment (Fig. 4c). Indeed, these results are consistent with previous reports of an overexpression of FcεRI in type I allergy due to ragweed and peanuts.(29,30) Furthermore, in addition to circulating total IgE, FcεRI has also attracted much attention as a therapeutic target for IgE-dependent allergies.(31)
Recently, a desensitization protocol for CBDCA treatment has been developed for patients with a history of CBDCA-induced HR, who are at a high risk of developing severe HR.(5,32) As previously noted, a CBDCA-specific IgE was recently observed in patients with CBDCA-induced HR.(13) Therefore, monitoring the pharmacodynamic changes of basophils including CBDCA-specific IgE, CD203c, and FcεRI expressions in these patients must be considered to be an essential part of the process. We believe that CBDCA-induced severe HR, which has been difficult to predict up to now, will come to be a controllable side-effect in the near future. Moreover, the reactivity of basophils against CBDCA usually decline after several months from the onset of severe HR, as previously indicated.(12) Therefore, the basophil activation status following severe HR may also help identify patients for whom CBDCA is safe to administer, which is of extreme clinical significance for a large number of patients.
In conclusion, these results strongly imply that an IgE-dependent mechanism incorporating FcεRI overexpression participates in CBDCA-induced severe HR. Our results establish the relevance of monitoring the pharmacodynamic changes of basophils after repeated exposure to CBDCA and may aid in the development of a highly sensitive in vitro test for the detection of severe CBDCA-induced HR. Ultimately, we feel this work should be of considerable interest to healthcare workers concerned with the safe management of cancer chemotherapy.
Acknowledgments
The authors thank Alberto A. Gayle (Center for Medical and Nursing Education, Mie University, Tsu, Japan) for his assistance in the revision of our manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 24590188) and The Research Foundation for Pharmaceutical Sciences (Group B).
Disclosure Statement
The study was funded by Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 24590188).
References
- 1.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141–5. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 2.Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs. 2010;2010:1–11. doi: 10.1155/2010/207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugimoto H, Iwamoto T, Murashima Y, Tabata T, Sagawa N, Okuda M. Risk factors contributing to the development of carboplatin-related delayed hypersensitivity reactions in Japanese patients with gynecologic cancers. Cancer Chemother Pharmacol. 2011;67:415–9. doi: 10.1007/s00280-010-1338-5. [DOI] [PubMed] [Google Scholar]
- 4.Pagani M, Venemalm L, Bonnadona P, Vescovi PP, Botelho C, Cernadas JR. An experimental biological test to diagnose hypersensitivity reactions to carboplatin: new horizons for an old problem. Jpn J Clin Oncol. 2012;42:347–50. doi: 10.1093/jjco/hys006. [DOI] [PubMed] [Google Scholar]
- 5.Patil SU, Long AA, Ling M, et al. A protocol for risk stratification of patients with carboplatin-induced hypersensitivity reactions. J Allergy Clin Immunol. 2012;129:443–7. doi: 10.1016/j.jaci.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Vyas N, Yiannakis D, Turner A, Sewell GJ. Occupational exposure to anti-cancer drugs: a review of effects of new technology. J Oncol Pharm Pract. 2013;20:278–87. doi: 10.1177/1078155213498630. [DOI] [PubMed] [Google Scholar]
- 7.Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005;3:9. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennersdorf F, Florian S, Jakob A, et al. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 10.Hino M, Shimojo N, Ochiai H, et al. Expression of CD203c on basophils as a marker of immunoglobulin E-mediated (L)-asparaginase allergy. Leuk Lymphoma. 2014;55:92–6. doi: 10.3109/10428194.2013.794944. [DOI] [PubMed] [Google Scholar]
- 11.Abuaf N, Rostane H, Rajoely B, et al. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008;38:921–8. doi: 10.1111/j.1365-2222.2008.02960.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto T, Yuta A, Tabata T, et al. Evaluation of basophil CD203c as a predictor of carboplatin-related hypersensitivity reaction in patients with gynecologic cancer. Biol Pharm Bull. 2012;35:1487–95. doi: 10.1248/bpb.b12-00150. [DOI] [PubMed] [Google Scholar]
- 13.Caiado J, Venemalm L, Pereira-Santos MC, Costa L, Barbosa MP, Castells M. Carboplatin-, Oxaliplatin-, and Cisplatin-specific IgE: cross-reactivity and value in the diagnosis of carboplatin and oxaliplatin allergy. J Allergy Clin Immunol Pract. 2013;1:494–500. doi: 10.1016/j.jaip.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–8. [PubMed] [Google Scholar]
- 15.Aranda A, Mayorga C, Ariza A, et al. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011;66:247–54. doi: 10.1111/j.1398-9995.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka A, Tanaka T, Suzuki H, Ishii K, Kameyoshi Y, Hide M. Semi-purification of the immunoglobulin E-sweat antigen acting on mast cells and basophils in atopic dermatitis. Exp Dermatol. 2006;15:283–90. doi: 10.1111/j.0906-6705.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Yanase Y, Tsutsui T, Ishii K, Hiragun T, Hide M. Applying surface plasmon resonance to monitor the IgE-mediated activation of human basophils. Allergol Int. 2008;57:347–58. doi: 10.2332/allergolint.O-07-506. [DOI] [PubMed] [Google Scholar]
- 18.Takai T, Takahashi K, Akagawa-Chihara M, et al. Production of humanized antibody against human high-affinity IgE receptor in a serum-free culture of CHO cells, and purification of the Fab fragments. Biosci Biotechnol Biochem. 2001;65:1082–9. doi: 10.1271/bbb.65.1082. [DOI] [PubMed] [Google Scholar]
- 19.Cassard L, Jonsson F, Arnaud S, Daeron M. Fcgamma receptors inhibit mouse and human basophil activation. J Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Baskin JG, Mangan EK, et al. The unique cytoplasmic domain of human FcgammaRIIIA regulates receptor-mediated function. J Immunol. 2012;189:4284–94. doi: 10.4049/jimmunol.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson F, Mancardi DA, Zhao W, et al. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood. 2012;119:2533–44. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Verma AK, Das M, Dwivedi PD. Molecular mechanisms of IgE mediated food allergy. Int Immunopharmacol. 2012;13:432–9. doi: 10.1016/j.intimp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Viardot-Helmer A, Ott H, Sauer I, Merk HF. Basophil activation test as in vitro assay for cisplatin allergy. Hautarzt. 2008;59:883–4. doi: 10.1007/s00105-008-1653-5. [DOI] [PubMed] [Google Scholar]
- 25.Ra C, Jouvin MH, Blank U, Kinet JP. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989;341:752–4. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- 26.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 27.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–29. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 28.Hartman ML, Lin SY, Jouvin MH, Kinet JP. Role of the extracellular domain of Fc epsilon RI alpha in intracellular processing and surface expression of the high affinity receptor for IgE Fc epsilon RI. Mol Immunol. 2008;45:2307–11. doi: 10.1016/j.molimm.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Lin H, Boesel KM, Griffith DT, et al. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 30.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–5.e5. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacGlashan DW., Jr IgE-dependent signaling as a therapeutic target for allergies. Trends Pharmacol Sci. 2012;33:502–9. doi: 10.1016/j.tips.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastaminza G, de la Borbolla JM, Goikoetxea MJ, et al. A new rapid desensitization protocol for chemotherapy agents. J Investig Allergol Clin Immunol. 2011;21:108–12. [PubMed] [Google Scholar]