Abstract
CD24 is a heavily glycosylated cell surface protein that is expressed in putative stem cells and is overexpressed in various human malignancies, yet the significant roles of CD24 in gastric cancer development are still elusive. We investigated the involvement of CD24 in gastric cancer aggressiveness, which is attributed to its heterogeneity. Cultured gastric cancer cells showed diverse expression patterns in CD24, whereas other defined cell surface markers, such as CD44 and CD133, were homogenous. Purely sorted CD24-negative gastric cancer cells showed strong alteration into the CD24-positive cell type in an autochthonous manner, and reached to steady expression levels. Our clinicopathological study revealed that CD24 positivity was an independent prognostic factor in both intestinal and diffuse types of gastric cancer. CD24 expression was correlated with the advanced stages, invasiveness, and lymph node metastasis of gastric cancer. Silencing of CD24 in cultured cells significantly decreased cell migration and invasion. Hypoxic treatment upregulated CD24 expression, and simultaneously induced cell motility and invasion of gastric cancer cells. Hypoxic treatment-induced CD24 expression was significantly attenuated by knockdown of hypoxia-inducible transcription factors. These data suggest that CD24-negative cells are capable of gaining cell motility and invasiveness through the induction of CD24, which is mediated by hypoxia. CD24 would be an attractive marker to define not only the heterogeneity but also the aggressiveness of gastric cancer cells. The mechanisms by which hypoxia induces CD24 expression would also be a potential therapeutic target for gastric cancer.
Keywords: CD24, gastric cancer, hypoxia, hypoxia inducible factor, invasion
Gastric cancer (GCa) is a major global malignancy that is the second leading cause of cancer mortality, and accounts for more than 700 000 deaths each year.(1) Treatment for GCa is often difficult and unsuccessful due to the strong heterogeneity.(2–4) Familial predispositions attributed to germ line mutations are rare in stomach diseases and analysis of the spectrum of somatic alterations is still a novel approach in GCa research.(5–7) Cancer stem cell theory is a new paradigm in cancer therapies and is thought to provide new avenues to eradicate the cause of the cell heterogeneity, which is associated with therapeutic resistance, relapse, and distant metastasis.(8,9) The common definition of cancer stem cell populations is by the presence or absence of various combinations of cell-surface proteins, for example, CD44 in GCa stem cells,(10) and high CD44, low CD24 as a subpopulation in breast cancer stem cells.(11) However, this concept is not universally applicable for understanding the phenotypic heterogeneity and therapeutic challenges of cancer.(12) In breast cancer, for example, there is also an ambiguity that cells expressing CD44+CD24(-/low) exhibit stem cell likeliness, while CD24+ cells are associated with poor prognosis in breast and other malignancies.(13–18) In this study, we have analyzed GCa cells with the defined cell surface markers of CD24, CD44, and CD133, which are major cancer stem cell markers.
CD24 is a cell surface protein, which consists of a core protein that comprises 27 amino acids, is extensively glycosylated, and is bound to the membrane via a phosphatidyl-inositol anchor.(19) In the immune lineage, CD24 is highly expressed on the neutrophil, and is expressed at the early stage of B-cell development, but is absent on normal T cells or monocytes.(19,20) In the normal gastrointestinal tract, CD24 is expressed in gastric parietal cells,(21) Paneth cells, and intestinal stem cells of the small intestine.(22) On human tumor cells, CD24 functions as a ligand for P-selectin, which is expressed on the cell surfaces of activated platelets and endothelial cells, in the process of tumor dissemination.(23) It has also been reported that cytoplasmic CD24 expression is associated with invasiveness and poor prognosis in diffuse-type GCa,(24) and with hypoxia in urological tumor cells as well as in endothelial cells. (25,26)
Despite many studies about the importance of CD24 in various cancers, little is known about the mechanism by which CD24 induces cancer aggressiveness, and how the CD24 expression is regulated in GCa cells. In this study, we begin our analysis by focusing on the variety of the CD24 expressions on GCa cells, and describe the importance of the influence of tumor microenvironment, such as hypoxia, on gastric cancer aggressiveness via CD24 expression.
Materials and Methods
Cell culture
Human primary tumor cells were isolated from ascites of patients with peritoneal dissemination of GCa. Written informed consent was obtained. CD45-positive hematolymphoid cells were excluded by using flowcytometric technique with CD45 antibody, shown below. GCa cell lines, TMK-1 and 44As3 were established at our Departments.(27–29) KKLS was kindlly donated by Dr. Y Kitadai (Hiroshima University, Japan). NCI-N87 was obtained from American Type Culture Collection (Manassas, VA, USA). All GCa cells were grown in RPMI supplemented with 10% FBS in a humidified atmosphere with 5% CO2 and 95% air. For hypoxic treatment, cells were incubated with 1% O2 in MCO-5MUV (SANYO Electric, Tokyo, Japan).
Flow cytometry
To identify the cancer stem cell phenotype of GCa cells, anti-CD24-FITC, anti-CD24-PE, anti-CD24-APC, anti-CD44-PE, anti-CD45-PE (BD Pharmingen, San Diego, CA, USA), anti-CD133/2-APC (MACS Miltenyi Biotec, Teterow, Germany) were used. Dead cells were detected with 7-AAD (BD Pharmingen). All flow cytometry data were acquired on BD FACS Aria for cell sorting, FACS Canto and FACS Calibur (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo software (Tree Star, San Carlos, CA, USA).
Cell proliferation assay
The viability of cells was calculated according to the manufacturer's instructions of MTS CellTiter 96 (Promega, Madison, WI, USA).
Cell adhesion assay
Cells were applied to the well coated with type I collagen and incubated at 37°C for 30 min. The adhesive cells were fixed and stained with 0.1% crystal violet in 20% methanol. After 50% ethanol was added, the optical absorbance at wavelength of 570 nm was measured.
Cell migration and invasion assay
These assays were carried out as described in a previous study.(30) The number of migrating cells was counted using Dynamic Cell Count software (BZ-H1C, KEYENCE, Osaka, Japan).
RNA interference
siRNA duplexes targeting CD24 (5′-ACAACAACTGGAACTTCAA-3′) and a nonsilencing siRNA duplexes (5′-CAGTCGCGTTTGCGACTGG-3′) were synthesized by NIPPON GENE (Tokyo, Japan). siRNA duplexes targeting HIF-1α (5′-CAAAGTTCACCTGAGCCTA-3′), HIF-2α (5′-GCAAATGTACCCAATGATA-3′) were synthesized by Sigma-Aldrich (Tokyo, Japan). Cells were transfected with 40 nM of siRNA duplexes by using Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA).
Immunoblotting
The nuclear fraction was extracted using the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA). The following antibodies were used: mouse monoclonal anti- hypoxia inducible factor (HIF)-1α antibody (Novus Biologicals, Littleton, CO, USA); goat polyclonal anti-HIF-2α antibody (R&D Systems, Minneapolis, MN, USA); mouse monoclonal anti-β-actin antibody (MBL, Nagoya, Japan).
Tissue samples
Tissue samples were obtained from 119 patients, who underwent a curative operation for GCa from 2001 to 2008 at the Hiroshima University Hospital (Hiroshima, Japan). Tumor staging was performed using the TNM classification system.(31) All of the patients gave their informed consent. The procedure to protect privacy was in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government.
Immunohistochemistry
This assay was carried out as described in a previous report.(30) CD24 (clone SN3b) antibody was purchased from Thermo Fisher Scientific (Cheshire, UK). HIF-1α (NB100-105) and HIF-2α (NB100-122) were purchased from Novus Biologicals. HISTOFINE SAB-PO (M and R) kit (Nichirei, Tokyo, Japan) and 3,3′-diaminobenzidine (DAB, Muto Pure Chemicals, Tokyo, Japan) were used for the signal detection and amplifications.
Evaluation of immunohistochemical staining
Two independent observers blinded to the clinicopathologic information of each sample evaluated CD24 expression semi-quantitatively. Staining 0–1%, 1–10%, 10–50% or more than 50% was scored as (0), (1+), (2+) or (3+), respectively. Positive CD24 expression was defined by staining of more than score (1+). HIF-1α and HIF-2α were scored according to the presence or absence of nuclear expression. Positive expression was defined by staining of more than 1% of the tumor area.
Statistical analysis
A logistic regression analysis was used to examine the association between CD24 positivity and clinicopathologic factors. χ2 test was used to examine the association of the positivity rate of CD24 and HIFs. Prognoses were estimated using the Kaplan–Meier method and compared with the log-rank test. These statistical analyses were done with the SPSS Statistics software (version 19; SPSS, Chicago, IL, USA). Other statistical analyses were carried out using Student's t-test. A P-value of <0.05 was considered as the statistically significant.
Results
Gastric cancer cells showed heterogeneous expression patterns in CD24
To discover stem-like subpopulations in GCa cells, we analyzed the cell surface marker expression patterns of CD24, CD44 and CD133, all of which are presumptive tumor stem cell phenotype identifiers for various types of cancer. Tumor cells collected from ascites of GCa patients showed high expression levels in CD44 and low or no expressions in CD133, but showed heterogeneous CD24 expression (Fig. 1a).
Fig 1.
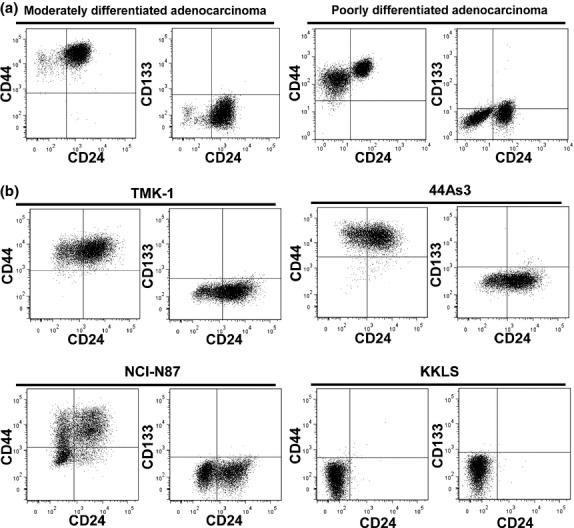
Fluorescence-activated cell sorting (FACS) analyses of representative cell surface markers in GCa cells. (a) Human primary tumor cells collected from ascites of patients. Left, moderately differentiated adenocarcinoma; right, poorly differentiated adenocarcinoma with signet ring cell. (b) GCa cell lines. Top left, TMK-1; top right, 44As3; bottom left, NCI-N87; bottom right, KKLS.
As the representatives of established GCa cell lines, TMK-1,(27,28) 44As3,(29) NCI-N87 (32) and KKLS (33) were chosen for the analyses of these three cell surface markers. Common features of these GCa cells, except for KKLS, were high CD44 expression, low or no levels of CD133, and various expression patterns of CD24 (Fig. 1b). We also investigated the expression of enzyme ALDH1 that has been proposed as a breast cancer stem cell marker.(34) Out of these four GCa cells, only KKLS was positive for ALDH1 (Table 1, Fig. S1). In addition, side population (SP) fractions were investigated in gastric cancer cell lines. TMK-1 and NCI-N87 contained a distinct fraction of SP cells (Table 1, Fig. S1), but there were no significant correlations between SP cell fraction and CD24 positivity (data not shown).
Table 1.
Cell surface markers, aldehyde dehydrogenase 1 expression, and Hoechst 33342 efflux activity of human gastric cancer cell lines
| Cell line | Histological cell type | CD24 | CD44 | CD133 | ALDH1 | SP |
|---|---|---|---|---|---|---|
| TMK-1 | Poorly differentiated | ++ | +++ | – | – | + |
| 44As3 | Signet-ring cell | ++ | +++ | – | ± | ND |
| NCI-N87 | Well differentiated | + | ++ | – | – | + |
| KKLS | Undifferentiated | – | – | – | + | ND |
ALDH1, aldehyde dehydrogenase 1; SP, side population; ND, not detectable. “+++” means over 75% of the cells were positive for the indicated markers. “++” and “+” mean the positive rate of each marker were between 75% and 51%, and between 50% and 11%, respectively. “±” represents positive cell population as from 1% to 10%. “–” indicates positive cell populations were below 1%. Side population cells are defined with the high competency of Hoechst 33342 dye efflux outwards of the cells.
Single directional convergence into the CD24-positive from CD24-negative gastric cancer cells occurred spontaneously
One of the defining characteristics of GCa is its strong heterogeneity.(35) We hypothesized that CD24 could be a useful marker for the assortment of heterogenous GCa cells. After purification of GCa cells with or without CD24 expression using fluorescence-activated cell sorting (FACS), continuous culture was carried out under normal oxygen levels. Prolonged CD24-negative GCa culture cells increased into the CD24-positive from approximately 1–2% (passage 0) to 62% (passage 10) in TMK-1 cells, to 67% (passage 9) in 44As3 cells, and to 40% (passage 10) in NCI-N87 cells, respectively. The decreased levels of CD24 expression in the sorted CD24-positive GCa cells were within 10% (Fig. 2, Fig. S2).
Fig 2.
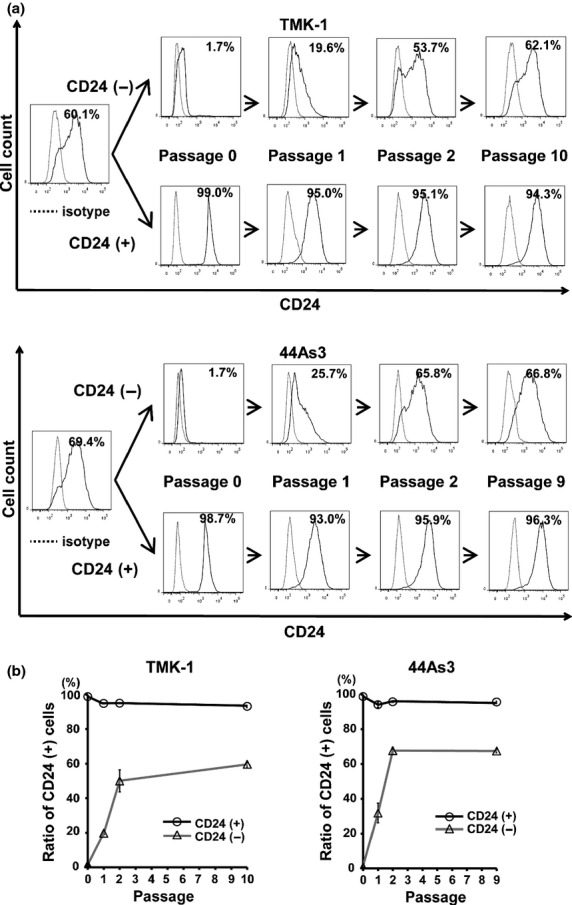
Single directional convergence into the CD24-positive from CD24-negative GCa cells under normoxia. (a) Representative histograms of chronological changes in sorted CD24-positive cells versus CD24-negative cells. Upper panels, TMK-1; lower panels, 44As3. Percentages shown in figures are the positive rate of CD24. (b) Quantitative FACS analyses of CD24-positive populations in GCa cells by the prolonged culture in normoxia.
CD24 expression facilitated cell migration and invasion of gastric cancer cells
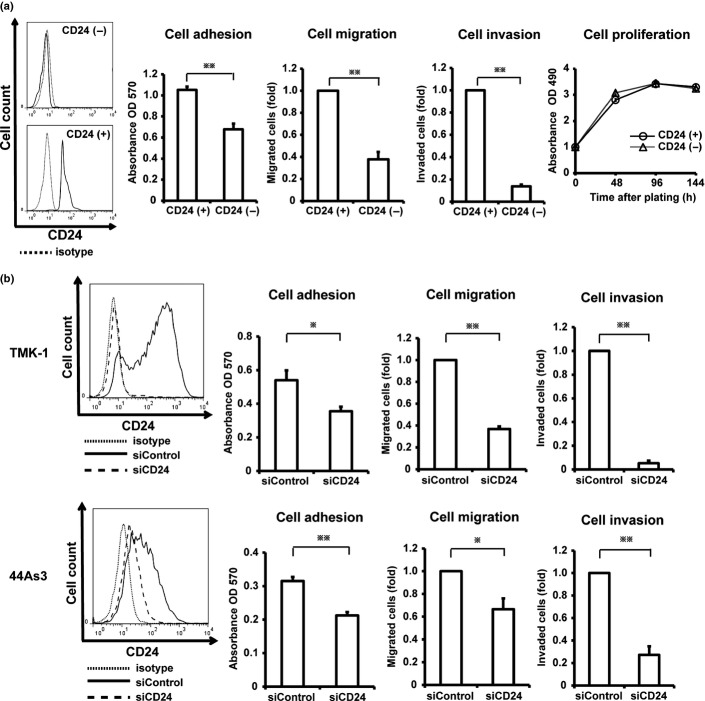
We investigated whether CD24 is involved in aggressive phenotypes of cancer cells such as cell adhesion, migration, invasion and proliferation of GCa. CD24 expression in TMK-1 cells increased cell adhesiveness toward collagen type I. The activities of cell migration and invasion of CD24-positive TMK-1 cells were higher than those of CD24-negative cells. There was no significant difference in cell growth in each population (Fig. 3a). To confirm these findings, we further performed knockdown of CD24 by RNAi in TMK-1 and 44As3 cells. Whereas control TMK-1 and 44As3 cells migrated toward collagen type I and invaded through the Matrigel, CD24 knockdown GCa cells were less capable of cell migration and invasion in the same conditions (Fig. 3b).
Fig 3.
Induced cell adhesion, migration, and invasion abilities in GCa cell lines by CD24 expression. (a) The functional assays of sorted CD24-negative or CD24-positive cells from TMK-1 cells. The purity of sorted TMK-1 cells was confirmed through FACS analyses. (b) The functional assays of TMK-1 cells (upper panels) or 44As3 cells (bottom panels) transfected with siControl or siCD24. The knockdown efficiency was confirmed through FACS analyses. *P < 0.05; **P < 0.01.
CD24 expression was induced by hypoxia in gastric cancer cells
We attempted to explore the mechanisms of how CD24 expression is regulated in GCa. In the 5′-flanking region of CD24 up to −3.4 kb upstream from the transcription start site (National Center for Biotechnology Information; accession Y14692), there are some consensus sequences that might be bound by several transcriptional factors, all of which might be potential molecules to induce cancer aggressiveness (Fig. 4a, left panel). In these upstream CD24 promoter elements, we focused on the hypoxic responsive element (HRE) since low oxygen concentrations can directly influence stem cell renewal and differentiation(36) and is essential for the maintenance of those stemness.(37)
Fig 4.
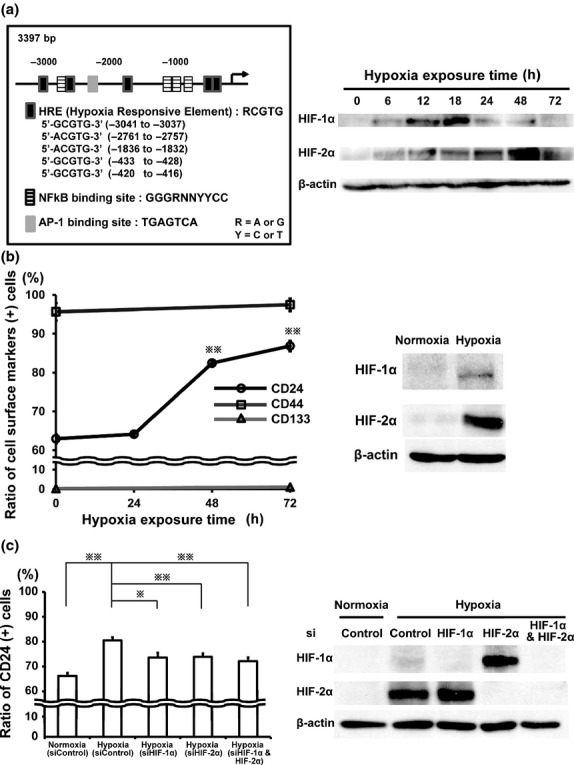
Induced CD24 expression in TMK-1 cells by hypoxia. (a) Localization of the putative binding sites of several transcriptional factors in the region of the CD24 promoter (left panel). Western blot analyses of HIF-1α and HIF-2α in TMK-1 cells under hypoxia (1% O2) for up to 72 h (right panel). (b) FACS analyses for the positive rates of CD24, CD44, and CD133 expressions in TMK-1 cells under hypoxia (1% O2, left panel). Western blot analyses of HIF-1α and HIF-2α in TMK-1 cells cultured in hypoxia (1% O2) for 48 h (right panel). (c) FACS analyses of CD24 positive population in TMK-1 cells transfected with siControl, or siHIF-1α and/or siHIF-2α and cultured in normoxia or hypoxia for 48 h (left panel). Western blot analyses of HIF-1α and HIF-2α in each corresponding cell (right panel). Paired cells were used for FACS to determine the percentage of cells in CD24-positive groups. *P < 0.05; **P < 0.01.
To examine our hypothesis that low oxygen tension would recess CD24 expression in GCa, hypoxic culture was performed on GCa. When TMK-1 was exposed to hypoxia for up to 72 h, HIF-1α was firstly stabilized within 24 h in hypoxia, and then HIF-2α was upregulated subsequently at 24 h onwards (Fig. 4a, right panel). Concomitantly with the increased HIF-2α, CD24 expression rather increased gradually from 63% to 82% (48 h; P = 0.0007) and to 87% (72 h; P = 0.0002), whereas the expression level of other cell surface markers such as CD44 and CD133 were not influenced by hypoxia (Fig. 4b, left panel). Hypoxic treatment within 72 h didn't influence the viability of TMK-1 cells (data not shown). Cellular responses to low oxygen tension were also monitored by immunoblotting to measure the stabilization of HIF-1α and HIF-2α in the nuclear fraction of TMK-1 cells at the time point of 48 h in hypoxia (Fig. 4b, right panel). The same results were observed using FACS analysis of 44As3 cells after hypoxic treatment (Fig. S3). Knockdown of HIF-1α and/or HIF-2α by using RNAi attenuated the hypoxia-induced increment of CD24 expression in TMK-1 cells (Fig. 4c, left panel). These data suggest that hypoxia-driven induction of CD24 in GCa might be regulated via both or either of HIF-1α and/or HIF-2α signaling. The stabilities of each type of HIF-α in the presence or absence of RNA interferences of HIF-1α and/or HIF-2α under hypoxia were also verified by immunoblotting analyses. Similarly with data shown in right panels of Fig. 4a and Fig. 4b, HIF-1α intensity was weaker than that of HIF-2α at the time point of 48 h of hypoxia, and it was enhanced by the knockdown of HIF-2α, and vice versa in western blot analyses (Fig. 4c, right panel).
Hypoxia potentiated gastric cancer cell migration and invasion activity through the upregulation of CD24
To investigate whether hypoxia could influence migration and invasion ability through CD24 expression, TMK-1 cells were exposed to hypoxia for 48 h. The migration and invasion of GCa cells showed significant increments over those treated under normoxic controls; 1.4-fold increases in migration (P = 0.025) and 1.3-fold increases in invasion (P = 0.04), respectively (Fig. 5a,b). To define the specific requirement for CD24 function in the hypoxia-induced invasion, in vitro invasion assay was carried out for TMK-1 cells whose CD24 expressions were suppressed by the transfection of siRNA. CD24 knockdown in TMK-1 cells dramatically decreased cell invasion activities in both normal and hypoxia conditions. The effect of siRNA on CD24 expression was assessed by flow cytometry (Fig. 5c). This result indicates that CD24 is indispensable for the cell invasiveness of TMK-1 cells and that hypoxia-induced cell invasion of GCa is partially depended on the up-regulation of CD24 expression under hypoxia.
Fig 5.
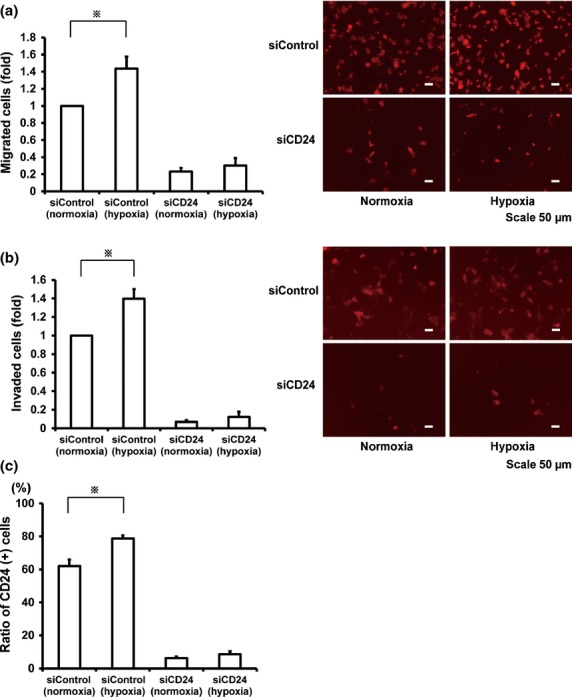
The attenuated hypoxia-induced migration and invasion by the inhibition of CD24 in TMK-1 cells. (a,b) Migration and invasion assays of cells transfected with siControl or siCD24 under normoxia or hypoxia. Relative migration and invasion activities (left panels). Representative photomicrographs of migrated cells and invaded cells (right panels). (c) FACS analyses of CD24 positive population in TMK-1 cells transfected with siControl or siCD24 and cultured in normoxia or hypoxia for 48 h. *P < 0.05.
Strong CD24 expression was a poor prognosis factor in gastric cancer after surgery
To assess the clinical relevance of CD24 expression in human GCa, the relationships between CD24 and clinicopathological characteristics were analyzed. In adjacent non-neoplastic GCa tissue, positive staining of CD24 was observed at the bottom of the mucosal epithelium (Fig. S4a, bottom panels). Almost all of the CD24-positive cells were also positive for H+K+ ATPase (a marker of parietal cells), indicating that CD24-positive cells were identical to the Parietal cells in the non-neoplastic lesion (Fig. S4b). The immunostaining of CD24 in fundic gland cells was used as the internal positive control. Figure 6, Fig. S4a and Table S1 show the representative example of expression patterns and intensity values of CD24 in human GCa samples. Two CD24 staining patterns (membranous dominant, cytoplasmic dominant) were detected in GCa cells (Table S2).
Fig 6.
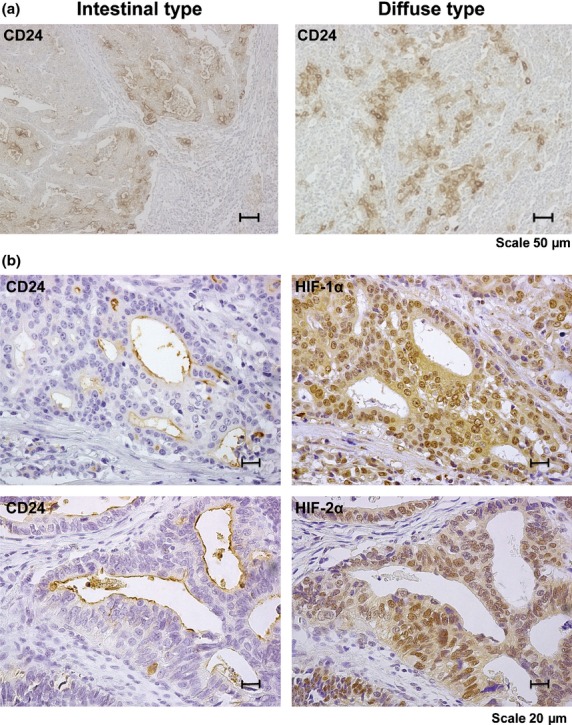
Immunohistochemical analyses of CD24 in human GCa. (a) Representative CD24 staining in both intestinal and diffuse type. (b) Representative CD24, HIF-1α and HIF-2α staining in each serial section.
In our immunohistological study, 61 (51%) out of 119 GCa cases exhibited positive results for CD24. CD24 expression was observed more frequently in stage III cases (23 of 35 [65.7%] cases) than in stage I/II cases (38 of 84 [45.2%] cases) (P = 0.044). CD24 expression in neoplastic GCa tissue was associated with advanced T grade (depth of invasion), lymph node metastasis, and lympho-vascular invasion (P = 0.036, P = 0.045 and P = 0.004 respectively; Table 2). The frequency of CD24 expression in HIF-1α and HIF-2α-positive GCa cases was significantly higher than that in HIF-1α and HIF-2α-negative GCa cases, respectively and irrelevant to histological classifications (Table 3, Fig. 6).
Table 2.
Relationship between CD24 expression and clinicopathologic characteristics in gastric cancer
| Characteristics | CD24 (–) n (%) | CD24 (+) n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 31 (42.5%) | 42 (57.5%) | 1 (Reference) | 0.086 |
| Female | 27 (58.7%) | 19 (41.3%) | 0.519 (0.245–1.098) | |
| Age | ||||
| ≦65 | 33 (46.5%) | 38 (53.5%) | 1 (Reference) | 0.549 |
| 65< | 25 (52.1%) | 23 (47.9%) | 0.799 (0.384–1.664) | |
| Location | ||||
| Upper | 15 (48.4%) | 16 (51.6%) | 1 (Reference) | – |
| Middle | 25 (50.0%) | 25 (50.0%) | 0.938 (0.383–2.298) | 0.888 |
| Low | 18 (47.4%) | 20 (52.6%) | 1.042 (0.403–2.692) | 0.933 |
| Lauren classification | ||||
| Diffuse type | 35 (57.4%) | 26 (42.6%) | 1 (Reference) | 0.054 |
| Intestinal type | 23 (39.7%) | 35 (60.3%) | 2.048 (0.966–4.255) | |
| Stage | ||||
| I–II | 46 (54.8%) | 38 (45.2%) | 1 (Reference) | 0.044 |
| III | 12 (34.3%) | 23 (65.7%) | 2.32 (1.022–5.266) | |
| Depth of invasion | ||||
| m-sm | 27 (61.4%) | 17 (38.6%) | 1 (Reference) | 0.036 |
| mp- | 31 (41.3%) | 44 (58.7%) | 2.254 (1.053–4.826) | |
| Lymphnode metastasis | ||||
| No | 40 (56.3%) | 31 (43.7%) | 1 (Reference) | 0.045 |
| Yes | 18 (37.5%) | 30 (62.5%) | 2.151 (1.017–4.549) | |
| Lympho-vasucular invasion | ||||
| No | 26 (68.4%) | 12 (31.6%) | 1 (Reference) | 0.004 |
| Yes | 32 (39.5%) | 49 (60.5%) | 3.318 (1.467–7.505) | |
OR, odds ratio; CI, confidence interval; m, mucosa; sm, submucosa; mp, muscularis propria. m-sm, tumor invades lamina propria or submucosa; mp-, tumor invades muscularis propria or deeper.
Table 3.
Relationship between CD24 expression and HIF-1α or HIF-2α in gastric cancer
| CD24 (–) n (%) | CD24 (+) n (%) | P-value | |
|---|---|---|---|
| All cases (n = 119) | |||
| HIF-1α | |||
| (–) | 54 (57.4%) | 40 (42.6%) | <0.001 |
| (+) | 4 (16.0%) | 21 (84.0%) | |
| HIF-2α | |||
| (–) | 45 (60.8%) | 29 (39.2%) | 0.002 |
| (+) | 13 (28.9%) | 32 (71.1%) | |
| Diffuse type (n = 61) | |||
| HIF-1α | |||
| (–) | 32 (68.1%) | 15 (31.9%) | 0.002 |
| (+) | 3 (21.4%) | 11 (78.6%) | |
| HIF-2α | |||
| (–) | 24 (68.6%) | 11 (31.4%) | 0.040 |
| (+) | 11 (42.3%) | 15 (57.7%) | |
| Intestinal type (n = 58) | |||
| HIF-1α | |||
| (–) | 22 (46.8%) | 25 (53.2%) | 0.021 |
| (+) | 1 (9.1%) | 10 (90.9%) | |
| HIF-2α | |||
| (–) | 21 (53.8%) | 18 (46.2%) | 0.001 |
| (+) | 2 (10.5%) | 17 (89.5%) | |
Finally, we examined the relationship between CD24 expression and the overall survival (OS) in GCa. The OS of CD24-positive GCa was significantly lower than that of CD24-negative GCa (P = 0.004 for all types, P = 0.046 for intestinal type, P = 0.011 for diffuse type; Fig. 7), but there were no additive effects of HIF-1α nor HIF-2α expressions to the poor prognosis of CD24-positive cases (Fig. S6). These results suggested that CD24 expression contributes directly to the malignant potential of GCa under the relatively close regulations of HIF-1α and/or HIF-2α and is also a good indicator of poor prognosis for GCa.
Fig 7.
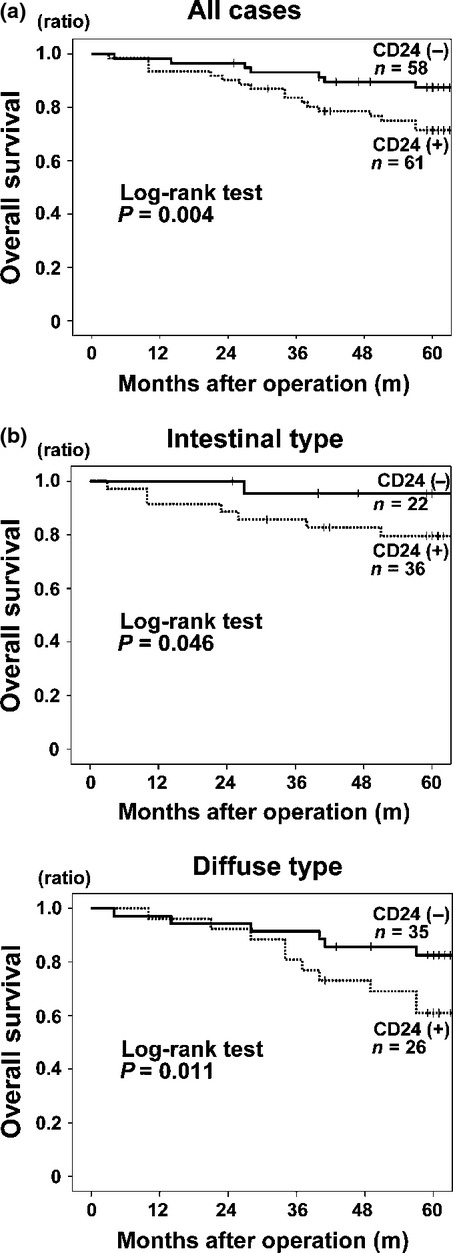
Association of CD24 expression with poor prognosis. (a) all cases; (b, upper panel) intestinal type; (b, lower panel) diffuse type.
Discussion
The chaotic complex of tumor cells is thought to be a consequence of clonal cell division as well as a continuous diversification that eventually leads to high invasiveness and metastatic potentials. This concept such as a hierarchical lineage and distinct subset within tumor cells is the basis for cancer stem cell research.(8,9) Partially based on this concept, although as an independent strategy, we began our experiments by culturing FACS-sorted CD24-negative GCa cells. CD24-negative GCa cells exhibited unique chronological changes, converting into the CD24-positive cell type by long-term culture. Highly malignant phenotypes of cancer cells including cell adhesion, migration, and invasion, were stimulated by CD24 expression in GCa cells. Samely as the previous reports,(25,26) hypoxic treatment upregulated CD24 expression in GCa as well. GCa expression of CD24 was shown to be at least, in part, an effect of hypoxia as sensed by the HIF-1α and/or HIF-2α systems under the long cascade of hypoxia-oriented intra-cellular signalings in cultured cells of GCa. Furthermore, there was a tendency for CD24 to be expressed in GCa cells expressing HIF-α isoform in clinical samples. Therefore, one proposal from this study is that hypoxia-regulated CD24 expression is an important factor to define the heterogeneity and aggressiveness of GCa, and may also be relevant in the resistance to conventional therapies of GCa. As shown in Figure S5, for example, CD24 expression did not affect the sensitivity of GCa cells to a common chemotherapeutic agent of GCa, 5-FU, whereas CD24 was upregulated by 5-FU treatment on the transcriptional level. This result indicates the probability that conventional treatments using 5-FU might bring some unfavorable outcomes to advanced or recurrent GCa patients.
HIF-1α and HIF-2α are known to play critical roles for the gaining more malignant phenotypes by highly tumorigenic cancer stem cells endowed with stem-like properties.(36,38) Since CD24-negative GCa cells showed the autochthonous shift into the CD24 positive, the negativity for the CD24 was postulated to be an indicator of differentiation potential of GCa cells, which is attributable to stemness properties. However, sorted CD24-negative cells derived from TMK-1 cells did not show clear stem-like activities, by measuring in vitro as the spherogenicity of single cells in non-serum containing media and in vivo as tumorigenicity in NOD/SCID mice (data not shown). Another finding of this analysis is that some small population out of the sorted CD24-positive GCa cell showed alteration into the CD24-negative, although the convergence rates were within 10%. This kind of fluctuation of CD24 might also be the hallmark of heterogeneity of GCa.
Our clinicopathologic study confirmed that CD24 expression in GCa was correlated with aggressiveness in advanced stages, invasiveness and lymph node metastasis, but was not related to the histological cell types of GCa. CD24 was a poor prognostic factor of GCa (Fig. 7). Recent research has reported potential efficacies of targeting CD24 in several cancers using anti-CD24 antibody or siRNA.(17,18) The targeting therapy against CD24 might also be applicable to GCa. Another crucial point we might have to pay attention to especially in GCa, is that CD24-positive cells can be generated from a CD24-negative population. Even if the cells were CD24 negative, or even if CD24 positive cells are eliminated by treatments, GCa cells can change their phenotype from the CD24-negative into the CD24-positive one. Hence, a combinatorial strategy, such as molecular targeting for CD24 plus other treatment based on the mechanism how CD24 is evoked on the CD24-negative cells, is essential for GCa treatment. To overcome these complicated characteristics of GCa, detailed molecular mechanisms as to how CD24 expression is regulated should be intensively investigated in GCa.
Acknowledgments
The authors thank Dr M Hattori (Hiroshima University, Japan) for statistical analysis. This work was carried out with the kind cooperation of the Analysis Center of Life Science, Hiroshima University, Hiroshima, Japan. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 22591453, 22800047, 25462023.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Doc. S1. Supporting Material and Methods.
Fig. S1. FACS analysis of candidate cell surface marker expressions in GCa cells.
Fig. S2. Representative histograms of chronological changes in purely sorted CD24-positive cell versus CD24-negative cells under normal oxygen levels in NCI-N87 cells.
Fig. S3. Induced CD24 expression in 44As3 cells by hypoxia.
Fig. S4. Representative CD24 staining in both intestinal type and diffuse type of GCa.
Fig. S5. Induced CD24 expression in TMK-1 cells by 5-FU treatment.
Fig. S6. Association of CD24, HIF-1α and HIF-2α expressions with prognosis in human GCa.
Table S1. CD24 expression scores in human gastric cancer tissues.
Table S2 CD24 staining patterns in human gastric cancer tissues.
References
- 1.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–6. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 2.Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33:606–12. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim B, Bang S, Lee S, et al. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248–55. [PubMed] [Google Scholar]
- 4.Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–85. doi: 10.1053/j.gastro.2011.04.042. 85 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun N, Ford JM. Genetic testing by cancer site: stomach. Cancer J. 2012;18:355–63. doi: 10.1097/PPO.0b013e31826246dc. [DOI] [PubMed] [Google Scholar]
- 6.Zang ZJ, Cutcutache I, Poon SL, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–4. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo J, Soderberg O, Simoes-Correia J, Grannas K, Suriano G, Seruca R. The importance of E-cadherin binding partners to evaluate the pathogenicity of E-cadherin missense mutations associated to HDGC. Eur J Hum Genet. 2013;21:301–9. doi: 10.1038/ejhg.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 10.Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 13.Kristiansen G, Schluns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Br J Cancer. 2003;88:231–6. doi: 10.1038/sj.bjc.6600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen G, Winzer KJ, Mayordomo E, et al. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906–13. [PubMed] [Google Scholar]
- 15.Darwish NS, Kim MA, Chang MS, et al. Prognostic significance of CD24 expression in gastric carcinoma. Cancer Res Treat. 2004;36:298–302. doi: 10.4143/crt.2004.36.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weichert W, Denkert C, Burkhardt M, et al. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005;11:6574–81. doi: 10.1158/1078-0432.CCR-05-0606. [DOI] [PubMed] [Google Scholar]
- 17.Sagiv E, Kazanov D, Arber N. CD24 plays an important role in the carcinogenesis process of the pancreas. Biomed Pharmacother. 2006;60:280–4. doi: 10.1016/j.biopha.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71:3802–11. doi: 10.1158/0008-5472.CAN-11-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer GF, Majdic O, Gadd S, Knapp W. Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol. 1990;144:638–41. [PubMed] [Google Scholar]
- 20.Pirruccello SJ, LeBien TW. The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol. 1986;136:3779–84. [PubMed] [Google Scholar]
- 21.Duckworth CA, Clyde D, Pritchard DM. CD24 is expressed in gastric parietal cells and regulates apoptosis and the response to Helicobacter felis infection in the murine stomach. Am J Physiol Gastrointest Liver Physiol. 2012;303:G915–26. doi: 10.1152/ajpgi.00068.2012. [DOI] [PubMed] [Google Scholar]
- 22.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol. 2010;298:G590–600. doi: 10.1152/ajpgi.00470.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aigner S, Sthoeger ZM, Fogel M, et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385–95. [PubMed] [Google Scholar]
- 24.Chou YY, Jeng YM, Lee TT, et al. Cytoplasmic CD24 expression is a novel prognostic factor in diffuse-type gastric adenocarcinoma. Ann Surg Oncol. 2007;14:2748–58. doi: 10.1245/s10434-007-9501-x. [DOI] [PubMed] [Google Scholar]
- 25.Scheurer SB, Rybak JN, Rosli C, Neri D, Elia G. Modulation of gene expression by hypoxia in human umbilical cord vein endothelial cells: a transcriptomic and proteomic study. Proteomics. 2004;4:1737–60. doi: 10.1002/pmic.200300689. [DOI] [PubMed] [Google Scholar]
- 26.Thomas S, Harding MA, Smith SC, et al. CD24 is an effector of HIF-1-driven primary tumor growth and metastasis. Cancer Res. 2012;72:5600–12. doi: 10.1158/0008-5472.CAN-11-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochiai A, Yasui W, Tahara E. Growth-promoting effect of gastrin in human gastric carcinoma cell line TMK-1. Jpn J Cancer Res. 1985;76:1064–71. [PubMed] [Google Scholar]
- 28.Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000;50:767–77. doi: 10.1046/j.1440-1827.2000.01117.x. [DOI] [PubMed] [Google Scholar]
- 29.Yanagihara K, Takigahira M, Tanaka H, et al. Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci. 2005;96:323–32. doi: 10.1111/j.1349-7006.2005.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Oue N, Sato A, et al. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–46. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 31.Sobin L, Gospodaroxicz M, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumors. 7th edn. Oxford: Wiley Blackwell; 2009. [Google Scholar]
- 32.Park JG, Frucht H, LaRocca RV, et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990;50:2773–80. [PubMed] [Google Scholar]
- 33.Tsuchiya Y, Sato H, Endo Y, et al. Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res. 1993;53:1397–402. [PubMed] [Google Scholar]
- 34.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Aretxabala X, Yonemura Y, Sugiyama K, et al. Gastric cancer heterogeneity. Cancer. 1989;63:791–8. doi: 10.1002/1097-0142(19890215)63:4<791::aid-cncr2820630431>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc. S1. Supporting Material and Methods.
Fig. S1. FACS analysis of candidate cell surface marker expressions in GCa cells.
Fig. S2. Representative histograms of chronological changes in purely sorted CD24-positive cell versus CD24-negative cells under normal oxygen levels in NCI-N87 cells.
Fig. S3. Induced CD24 expression in 44As3 cells by hypoxia.
Fig. S4. Representative CD24 staining in both intestinal type and diffuse type of GCa.
Fig. S5. Induced CD24 expression in TMK-1 cells by 5-FU treatment.
Fig. S6. Association of CD24, HIF-1α and HIF-2α expressions with prognosis in human GCa.
Table S1. CD24 expression scores in human gastric cancer tissues.
Table S2 CD24 staining patterns in human gastric cancer tissues.