Abstract
Galectin-4 is a multifunctional lectin found at both intracellular and extracellular sites. It could serve as a tumor suppressor intracellularly and promote tumor metastases extracellularly during colorectal cancer development. However, galectin-4 expression and its prognostic value for patients with hepatocellular carcinoma (HCC) have not been well investigated. Here we report that galectin-4 was significantly downregulated in early recurrent/metastatic HCC patients, when compared to non-recurrent/metastatic HCC patients. Low expression of gelectin-4 was well associated with larger tumor size, microvascular invasion, malignant differentiation, more advanced TNM stage, and poor prognosis. Cancer cell migration and invasion could be significantly reduced through overexpression of galectin-4, but upregulated by knocking down of galectin-4 in vitro. Moreover, the serum galectin-4 level could be significantly elevated solely by hepatitis B virus infection. Combined with clinicopathological features, the higher serologic level of galectin-4 was well associated with more aggressive characteristics of HCC. Taken together, galectin-4 expression closely associates with HCC progression and might have potential use as a prognostic biomarker for HCC patients.
Keywords: Early recurrence/metastasis, galectin-4, hepatitis B virus (HBV), hepatocellular carcinoma (HCC), prognostic biomarker
Hepatocellular carcinoma is one of the leading causes of cancer-related deaths worldwide, and approximately 80% of HCC patients are in Asia.(1) Although surgical resection has been proven to be an effective approach for the treatment of HCC, approximately 50–70% of HCC patients suffer from early recurrence/metastasis within 12 months in the residual liver after curative resection(2–4); the median survival time after recurrence/metastasis is only 13 months.(5) Thus, early recurrence/metastasis is the key blocking factor of the curative efficiency of hepatectomy and long-term survival. However, there is still a lack of effective prognostic biomarkers for predicting early recurrence/metastasis of HCC with high sensitivity and specificity.
Galectins, localized both intracellularly and extracellularly, constitute a family of 15 multifunctional galactoside-binding lectins.(6) It has been reported that galectins are widely expressed in various types of human cells and participate in a wide range of cellular functions including inflammatory responses, intracellular signaling, cell adhesion, tumor metastasis, and differentiation.(7) Several published reports have described galectins as being aberrantly expressed in various cancers, and well correlated with cancer invasion and metastasis.(8–11)
Galectin-4, a 36-kD divalent lectin, is predominantly expres-sed in epithelial cells from the tongue to the large intestine, but is rarely expressed in other tissues.(12) It has been reported that the aberrant expression of galectin-4 was found in several cancers. Galectin-4 was downregulated at the tissue level in colon cancer and was absent in invasive carcinomas, but its serum level was significantly higher in patients with colon and breast cancer, in particular, those with metastasis.(13,14) In contrast, it has been reported to be significantly upregulated in the pancreatic adenocarcinomas, hepatocellular carcinoma, and gastric cancer.(15) Belo et al.(16) have shown that galectin-4 was upregulated at the cell surface in early-stage pancreatic cancer acting as an “adhesin,” then downregulated in later stages of tumor progression, facilitating tumor escape by loss of cell–cell interaction, and enhancing migratory properties.
Although a few published works have reported that galectin-4 mRNA was significantly upregulated in HCC patients,(17) the clinical relevance of galectin-4 expression, especially its significance in recurrence/metastasis of HCC, remains unknown. In this study, we evaluated galectin-4 expression levels in HCC patients, and systematically analyzed the function of galectin-4 in cancer cell migration and invasion in vitro, as well as the clinicopathological correlations of galectin-4 expression in HCC patients.
Materials and Methods
Patients and follow-up
Twenty-seven fresh-frozen tumor tissues obtained from HCC patients after surgical resection were used to detect the mRNA and protein expression of galectin-4. In addition, a total of 201 formalin-fixed and paraffin-embedded HCC tissues from consecutive patients who underwent curative resection between 2005 and 2011 at the Liver Center of the First Affiliated Hospital of Fujian Medical University (Fuzhou, China) were retrieved for immunohistochemical staining. Combined with clinical diagnosis, we divided the patients into three groups according to the time of recurrence/metastasis after operation: patients who had recurrence/metastasis within 12 months after operation (R/M≤12 months group); the patients whose recurrence/metastasis occurred between 12 and 24 months after operation (R/M12–24 months group); the patients who had no recurrence/metastasis within 2 years of operation (NR/M group). The serum samples, including 20 healthy people, 29 patients with HBV infection, and 48 patients with HCC (the HCC serum samples were obtained by collecting venous blood from patients at the time of the primary diagnosis before operation), were used in this study for measuring the serological level of galectin-4 by ELISA. The project was approved for the using of human samples by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University. Written consent was received from all participants before surgery in this study. Clinical and pathologic diagnoses of HCC patients were made using the diagnostic criteria of the American Association for the Study of Liver Diseases.
Follow-up data were summarized at the end of December 2012, with a median follow-up of 25.9 months (range, 3–60 months). Time to recurrence/metastasis and OS were considered the primary endpoints. Time to recurrence/metastasis was calculated from the date of resection to the date when tumor recurrence was diagnosed; overall survival was calculated from the date of resection to the date of death or last follow-up.
Cell culture
Human HCC cell line HCCLM3 was a kind gift from the Second Military Medical University of China (Shanghai, China). HCCLM3 cells were maintained as a monolayer culture in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 incubator. The confluent cells were further used for other in vitro experiments.
Quantitative real-time RT-PCR
Total RNA was isolated from 27 fresh-frozen HCC tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was carried out using a Goscript Reverse Transcription System Kit (A5001; Promega, Madison, WI, USA) according to the manufacturer's instructions. The primers for galectin-4 and the internal control gene (β-actin) were designed using the Primer-Blast Database (galectin-4 forward primer, CCCTTCTATGAGTACGGGCAC; galectin-4 reverse primer, TGGCCTCCGATGAAGTTGATT; β-actin forward primer, ATAGCACAGCCTGGATAGCAACGTAC; β-actin reverse primer, CACCTTCTACAATGAGCTGCGTGTG).
For q-PCR analysis, aliquots of double-stranded cDNA were amplified using a GoTaq q-PCR Master Mix (A6002; Promega). The cycling parameters were 45 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 30 s. The relative gene expression was normalized to the geometric mean of the housekeeping gene β-actin, and calculated according to the Livak method (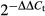 ). The experiments were independently repeated three times.
). The experiments were independently repeated three times.
Western blot analysis
After grinding, the fresh-frozen HCC tissue or HCCLM3 cells were lysed in ice-cold RIPA buffer (0.5 M Tris-HCl, pH 7.4,1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA) containing protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Tissue lysates were centrifuged at 17000 g for 30 min at 4°C. The extracts were quantified by BCA assay. Proteins of each sample (40 μg) were separated by 12% SDS-PAGE and transferred onto nitrocellulose membranes. Afterwards, the membranes were blocked for 2 h in the TBST buffer with 5% BSA and probed with the galectin-4 primary antibody (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin antibody (1:5000 dilution; Transgen, Beijing, China) overnight at 4°C. The membranes were washed three times with TBST buffer for 10 min each, then incubated with appropriate HRP-conjugated secondary antibody (1:8000 dilution; Santa Cruz Biotechnology) for 1 h at room temperature. Following washing again in the TBST buffer, the protein expression levels were detected by enhanced chemiluminescence and visualized by autoradiography.
Construction of TMAs and immunohistochemistry
Hepatocellular carcinoma samples from patients (n = 201) were used for the construction of TMAs. Using a manual tissue arrayer, tissue cores from circled areas were targeted, and then transferred to the corresponding location of the recipient block to form a TMA block. Two replicated tissue points were sampled from each HCC patient. After the successful transfer, the TMA wax was blocked with a glass slide for 1 h at 60°C, to make the TMA block surface smooth and flat, and make sure the TMA was bound tightly to the recipient wax block. Then the wax block was cooled at room temperature for 30 min and stored at 4°C for further use. The cooled wax block was fixed on the auto tissue slicer, and continuously sliced into 50 sections at 4 μm. Afterwards, the sections were extended in distilled water for 2 min, then directly mounted onto the SuperFrost Plus-treated import slide (Thermo, Pittsburgh, PA, USA), and dried on the section drier for 16 h at 60°C.
Immunohistochemical staining of galectin-4 was carried out using the two-step EnVision Plus staining technique. A representative 4-μm section TMA was deparaffinized in xylene and rehydrated in graded ethanol. The slides were incubated in 1% hydrogen peroxide for 30 min to block endogenous peroxidase activity and then rehydrated in distilled water followed by PBS. Slides were incubated in 0.1 mol/L EDTA (pH 9.0) for 3 min for high-pressure antigen retrieval. Afterwards, the liver section was incubated with the primary antibody (goat polyclonal anti-human galectin-4 antibody, 1:50 dilution; Santa Cruz Biotechnology) at 4°C overnight. After several rinses with PBS, followed by incubating with HRP-conjugated secondary antibodies (PV9003; ZSGB-BIO, Beijing, China) at room temperature for another 1 h. Finally, the liver sections were subjected to DAB coloration and hematoxylin restaining. The results were independently assessed by two pathologists double-blindly. The final score of each sample was scored as: 0, negative; 1+, weak; 2+, moderate; or 3+, strong, for the intensity and extent of staining. Each case was considered to be negative if the final score was 0 to 1, or positive if the final score was 2 to 3.
Plasmid construction and establishment of galectin-4 stable overexpressing cell lines
Complementary DNA of the galectin-4 gene (GenBank, U82953.1) was cloned into pcDNA3.1 vector for overexpression; the empty vectors were used as negative control. Afterwards, 2 μg plasmids were mixed with 6 μL Lipofectamine 2000, and then transfected into HCCLM3 cells according to the manufacturer's protocol. After 48 h of transfection, the transfected cells were cultured in the medium containing 600 μg/mL G418 for 2 weeks to select the stable overexpressing cell lines. The selected cell lines were further used for in vitro experiments as indicated.
RNA interference
To knockdown the endogenous expression of galectin-4 in HCCLM3 cells, RNAi experiments were carried out. A pool of three siRNA oligonucleotides for galectin-4 (sc-37102; Santa Cruz Biotechnology) and a negative control (sc-37007; Santa Cruz Biotechnology) were transfected into the HCCLM3 cells using Lipofectamine 2000 according to the manufacturer's instructions. After 6 h of transfection, the culture medium was replaced by fresh DMEM supplemented with 2% FBS, and the cells were further cultured in this starvation medium for 48 h. Serum-starved cells were used for the in vitro experiments as indicated.
Cell migration and invasion assay
The migration assays were carried out using Transwell units (No. 3422; Corning Costar, Tewksbury, MA, USA) and the invasion assays were assessed using the Transwell units coated with Matrigel (No. 354480; BD Biosciences, New York, NY, USA) according to the manufacturer's instructions. For the cell migration and invasion assay, cells (1 × 106 cells/mL, 100 μL for the migration assay; 2 × 105 cells/mL, 500 μL for the invasion assay) were cultured at the upper compartment of the chamber in serum-free DMEM supplemented with 0.5% BSA for 48 h, and the lower compartment of the chamber was filled with DMEM supplemented with 10% FBS. After 48 h of incubation, the filters were harvested, fixed with paraformaldehyde, and stained with crystal violet. Tumor cells on the upper surface of the filters were removed by wiping with cotton swabs. Cells that had passed through the filter to the lower surface were counted under a microscope in five different fields at 20× magnification.
Detection of circulating galectin-4 in HCC patients' serum
The serologic level of galectin-4 was measured by a microtiter plate reader using the commercially available Quantikine ELISA kit (SU7390; Yihanbio, Shanghai, China), according to the manufacturer's protocol. Each patient's serum sample was diluted in sample dilution buffer, or further diluted when the concentration of the target protein was too high. Fifty microliters of these diluted serum samples and standard proteins was added to the appropriate wells. Then 100 μL HRP-conjugate reagent was added to the standard wells and sample wells, and incubated for 60 min at 37°C. After washing the microtiter plate four times with PBS, chromogen solution A (50 μL/well) and chromogen solution B (50 μL/well) were added and incubated for 15 min at 37°C. Then 50 μL Stop Solution was added to each well, and the optical density at 450 nm was detected to calculate the circulating galectin-4 level.
Statistical analysis
All statistical analyses were carried out with the spss software package (version 19.0; SPSS, Chicago, IL, USA). Comparisons of quantitative data between two groups were carried out with a two-tailed unpaired Student's t-test. Categorical data were analyzed by Fisher's exact test between galectin-4 expression and clinicopathologic features. Recurrence/metastasis rates and survival rates were calculated by the Kaplan–Meier method and compared using the log–rank test. A Cox proportional hazards model was used to determine the independent factors of survival based on the variables selected in the univariate analysis. P < 0.05 was considered statistically significant.
Results
Galectin-4 is downregulated in early recurrent/metastatic HCC tumors
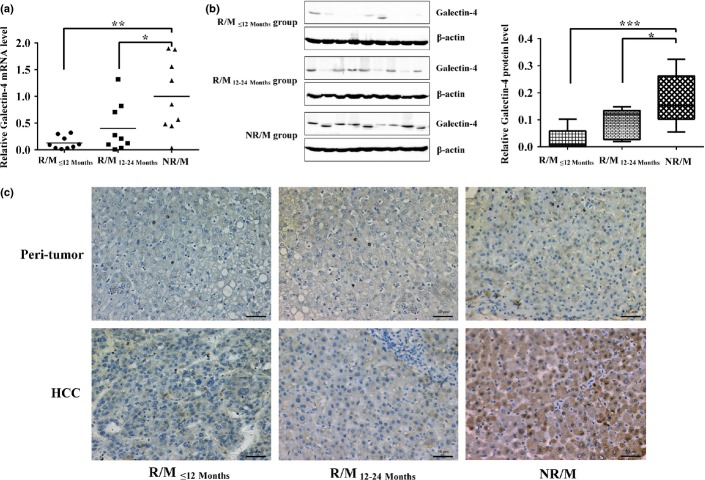
In a previous study, we applied the iTRAQ-based quantitative proteomic approach (iTRAQ-2DLC-MS/MS) to identify potential diagnostic biomarkers for the early recurrence/metastasis of HCC.(18) From that analysis, we identified an interesting protein, galectin-4, that was significantly downregulated in early recurrent/metastatic HCC tumors. To further evaluate the prognostic potential of galectin-4 in HCC patients, we assessed its expression in three HCC patient groups with different recurrence/metastasis times by q-PCR. As shown in Figure 1(a), the q-PCR analysis revealed that galectin-4 expression was downregulated 7.83-fold in the early recurrence/metastasis group (R/M≤12 months, n = 9, P = 0.002) and 2.51-fold in the late recurrence/metastasis group (R/M12–24 months, n = 9, P = 0.042) compared with the NR/M group (n = 9). To further confirm galectin-4 expression at the protein level, additional Western blot analysis was carried out. As shown in Figure 1(b), galectin-4 levels were significantly higher in the NR/M group compared to both the R/M≤12 months group and the R/M12–24 months group at the protein level. Overall, these results clearly indicated that galectin-4 expression was significantly decreased at both the mRNA and the protein level in recurrent/metastatic HCC tumors, especially in HCC tumors with early recurrence/metastasis.
Fig 1.
Galectin-4 is significantly downregulated in early recurrent/metastatic hepatocellular carcinoma (HCC). (a) Quantitative PCR analysis of galectin-4 expression in three HCC patient groups with different recurrence/metastasis time. (b) Western blot analysis of galectin-4 expression in three HCC patient groups with different recurrence/metastasis time. (c) Different galectin-4 expression levels in HCC tumor tissues and peri-tumor tissues. Galectin-4 expression was semiquantitatively categorized into four groups: (a) negative (0), (b) weak (1+), (c) moderate (2+), and (d) strong (3+). Original magnification of 20×. *P < 0.05; **P < 0.01; ***P < 0.001. NR/M, patients with no recurrence/metastasis within 2 years of hepatectomy; R/M≤12 months, patients with recurrence/metastasis within 12 months of hepatectomy; R/M12–24 months, patients with recurrence/metastasis within 12–24 months of hepatectomy.
To further investigate the role of galectin-4 in the clinical progression of HCC, we assessed its expression in 201 patients through the immunohistochemical analysis of TMA, including 116 cases of R/M≤12 months, 48 cases of R/M12–24 months, and 37 cases of NR/M. As shown in Table 1, the positive expression of galectin-4 was detected in 26 NR/M HCC patients (of 37 patients, positive rate 70.2%); in contrast, the positive expression of galectin-4 was significantly lower in the R/M≤12 months group (41 of 116, positive rate 35.0%; P < 0.001) and the R/M12–24 months group (22 of 46, positive rate 45.8%; P = 0.029). Representative images of galectin-4 immunohistochemical staining in HCC tumors and peri-tumor tissues are shown in Figure 1(c). We further examined the association between galectin-4 expression and the clinical features of the HCC tumor tissue. As summarized in Table 2, lower expression of galectin-4 was well associated with larger tumor size (P = 0.036), poor tumor differentiation (P = 0.011), microvascular invasion (P = 0.004), and more advance TNM stage (P = 0.015), but not significantly associated with serum α-fetoprotein level, cirrhosis, or tumor encapsulation.
Table 1.
Immunohistochemical analysis of galectin-4 expression in 201 hepatocellular carcinoma tissues
| Group | n | Galectin-4 expression |
Positive expression rate (%) | P-value | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| R/M≤12 months group | 116 | 75 | 41 | 35.0 | <0.001*** |
| R/M12–24 months group | 48 | 26 | 22 | 45.8 | 0.029* |
| NR/M group | 37 | 11 | 26 | 70.2 | |
P < 0.05;
P < 0.001. NR/M, patients with no recurrence/metastasis within 2 years of hepatectomy; R/M≤12 months, patients with recurrence/metastasis within 12 months of hepatectomy; R/M12–24 months, patients with recurrence/metastasis within 12–24 months of hepatectomy.
Table 2.
Associations between galectin-4 expression and clinicopathological characteristics of 201 hepatocellular carcinoma patients
| Clinicopathological variable | Tumor galectin-4 expression |
χ2-value | P-value | |
|---|---|---|---|---|
| Negative (n = 112) | Positive (n = 89) | |||
| Sex | ||||
| Male | 99 | 75 | 0.725 | 0.412 |
| Female | 13 | 14 | ||
| Age, years | ||||
| <55 | 70 | 50 | 0.823 | 0.388 |
| ≥55 | 42 | 39 | ||
| AFP, ng/mL | ||||
| <400 | 57 | 57 | 3.494 | 0.065 |
| ≥400 | 55 | 32 | ||
| HBV DNA | ||||
| <1000 | 43 | 35 | 0.018 | 1.000 |
| ≥1000 | 69 | 54 | ||
| Cirrhosis | ||||
| Absent | 25 | 19 | 0.027 | 1.000 |
| Present | 87 | 70 | ||
| Tumor number | ||||
| Single | 101 | 77 | 0.656 | 0.505 |
| Multiple | 11 | 12 | ||
| Maximal tumor size, cm | ||||
| <5 | 13 | 21 | 5.071 | 0.036* |
| ≥5 | 99 | 68 | ||
| Tumor encapsulation | ||||
| Absent | 85 | 72 | 0.727 | 0.493 |
| Present | 27 | 17 | ||
| Tumor differentiation | ||||
| I–II | 11 | 21 | 7.029 | 0.011* |
| III–IV | 101 | 68 | ||
| Microvascular invasion | ||||
| Absent | 72 | 74 | 8.875 | 0.004** |
| Present | 40 | 15 | ||
| TNM | ||||
| I–II | 52 | 57 | 6.200 | 0.015* |
| III–IV | 60 | 32 | ||
AFP, α-fetoprotein.
P < 0.05;
P < 0.01.
Galectin-4 could inhibit HCC cancer cell migration and invasion in vitro
As cancer cell migration and invasion are tightly associated with the recurrence, metastasis, and microvascular invasion of tumors, we further examined whether abnormal expression of galectin-4 could affect HCC cancer cell migration and invasion in vitro. The HCCLM3 cell line with moderate galectin-4 expression was used for the overexpression and knockdown experiments to avoid any effects of the genetic background. As shown in Figure 2(a,d), galectin-4 was successfully overexpressed (54.3-fold) and knocked down (58%) in the HCCLM3 cell line. We carried out further in vitro migration and invasion experiments in the Transwell system, as described in “Materials and Methods”. As shown in Figure 2(b,c), HCCLM3 cells with galectin-4 overexpression migrate significantly slower, and have less ability to invade through the Matrigel; however, knockdown of galectin-4 expression in HCCLM3 cells could significantly promote cancer cell migration and invasion through the Matrigel (Fig. 2e,f). These findings are well consistent with the clinical data.
Fig 2.
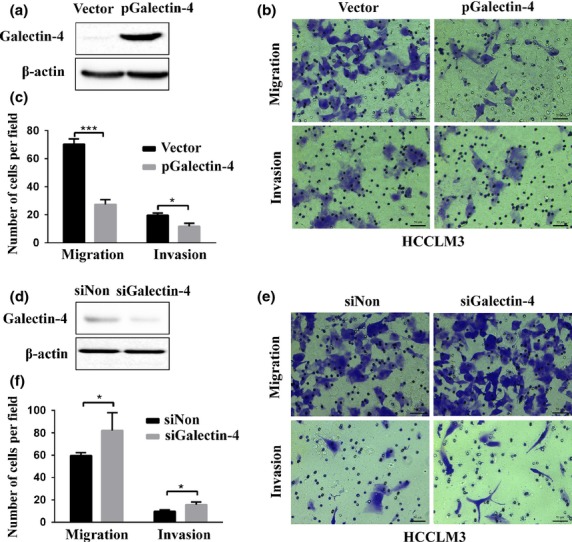
In vitro migration and invasion analysis of HCCLM3 hepatocellular carcinoma cells with galectin-4 overexpression (a) or galectin-4 knockdown (d), confirmed by Western blotting. Representative images and quantification of results of migration and invasion of galectin-4 overexpressing cells (b, c) and galectin-4 knockdown cells (e, f). *P < 0.05; **P < 0.01; ***P < 0.001. SiNon, Negative control with none target siRNA transfection.
Lower galectin-4 expression associated with poor prognosis of HCC
To further evaluate the prognostic value of galectin-4 expression in HCC, Cox's proportional hazards model and Kaplan–Meier analysis was used to calculate the effect of galectin-4 expression on TTR and OS time. As shown in Table 3, Cox's multivariate proportional hazards model showed that galectin-4 expression was an independent poor prognostic factor for TTR (P = 0.003) and OS time (P = 0.002) in HCC after curative resection. Kaplan–Meier analysis showed that patients with low galectin-4 expression had a higher recurrence/metastasis rate (log–rank, P < 0.001, Fig. 3a) and shorter OS time (log–rank, P < 0.001, Fig. 3b) than those with high galectin-4 expression. These data suggested that galectin-4 would be a very promising prognostic biomarker for HCC, but might need further larger-scale, multicenter clinical study.
Table 3.
Univariate and multivariate analysis of recurrence and survival-associated factors in 201 hepatocellular carcinoma patients
| Variable | Recurrence |
Survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | 0.834 | 0.618–1.125 | 0.235 | 0.887 | 0.617–1.276 | 0.519 | ||||||
| Sex (male vs. female) | 1.481 | 0.954–2.296 | 0.080 | 1.152 | 0.689–1.926 | 0.589 | ||||||
| AFP (<400 ng/mL vs. ≥400 ng/mL) | 1.855 | 1.377–2.500 | <0.001*** | 1.697 | 1.246–2.310 | 0.001*** | 1.744 | 1.215–2.504 | 0.003** | 1.489 | 1.031–2.150 | 0.034* |
| HBV infection (absent vs. present) | 1.318 | 0.971–1.789 | 0.076 | 1.137 | 0.787–1.643 | 0.090 | ||||||
| Cirrhosis (absent vs. present) | 1.133 | 0.792–1.622 | 0.494 | 0.859 | 0.570–1.294 | 0.468 | ||||||
| Tumor number (single vs. multiple) | 1.118 | 0.708–1.764 | 0.633 | 0.726 | 0.390–1.349 | 0.311 | ||||||
| Maximal tumor size (≤5 cm vs. >5 cm) | 2.293 | 1.486–3.539 | <0.001*** | 1.825 | 1.166–2.856 | 0.008*** | 2.208 | 1.301–3.748 | 0.003** | 1.55 | 0.895–2.684 | 0.118 |
| Tumor encapsulation(absent versus present) | 0.598 | 0.429–0.833 | 0.002** | 0.690 | 0.490–0.972 | 0.034* | 0.515 | 0.348–0.762 | 0.001** | 0.563 | 0.376–0.841 | 0.005** |
| Tumor differentiation (I–II vs. III–IV) | 0.721 | 0.575–0.903 | 0.004** | 0.744 | 0.582–0.953 | 0.019** | 0.643 | 0.491–0.843 | 0.001** | 0.632 | 0.471–0.849 | 0.002** |
| Tumor thrombus (absent vs. present) | 1.973 | 1.416–2.749 | <0.001*** | 1.173 | 0.807–1.703 | 0.403 | 1.559 | 1.084–2.243 | 0.001** | 1.069 | 0.720–1.586 | 0.741 |
| TNM (I–II vs. III–IV) | 1.417 | 1.239–1.620 | <0.001*** | 1.289 | 1.102–1.507 | 0.001** | 2.069 | 1.437–2.977 | <0.001*** | 1.652 | 1.112–2.454 | 0.013* |
| Galectin-4 expression (negative vs. positive) | 0.506 | 0.373–0.686 | <0.001*** | 0.648 | 0.450–0.845 | 0.003** | 0.510 | 0.376–0.690 | <0.001*** | 0.533 | 0.357–0.796 | 0.002** |
P < 0.05;
P < 0.01;
P < 0.001. AFP, α-fetoprotein.
Fig 3.
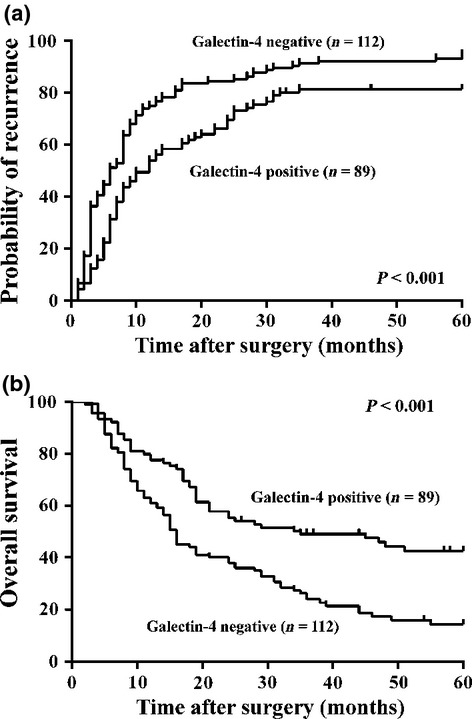
Kaplan–Meier analysis of the association between galectin-4 expression and recurrence/metastasis as well as the survival of patients with hepatocellular carcinoma. Those patients with negative galectin-4 expression (n = 112) had significantly higher recurrence rate and shorter overall survival time than those with positive galectin-4 expression (n = 89).
Serum galectin-4 level in HCC patients
Galectin-4 is not only present intracellularly, but also secreted extracellularly and could enter into the circulation system. As described above, galectin-4 was significantly downregulated in recurrent/metastatic HCC tissues and was well associated with poor prognosis. An ideal prognostic biomarker would be a protein that is secreted into the blood, which could facilitate detection with minimal invasion. Therefore, we further evaluated the galectin-4 level in the serum of HCC patients. As shown in Figure 4, the serologic level of galectin-4 was remarkably elevated in HBV-infected patients with (6134 ± 3222 pg/mL, P < 0.0001) or without (7320 ± 2172 pg/mL, P < 0.0001) HCC, but not significantly elevated in HCC patients without HBV infection (115 ± 63 pg/mL, P = 0.332), compared with healthy controls (79 ± 66 pg/mL). This result is very interesting and unexpected, but it clearly indicated that the serum galectin-4 level could be solely elevated by HBV infection due to the host immuno-defense. However, it is not clear whether the higher level of serum galectin-4 is coming from the higher secretion or the higher expression due to HBV infection. The underlying molecular mechanisms need to be further studied and clarified. As 80% of HCC patients are infected with HBV, we further evaluated the prognostic potential of serum galectin-4 as an HBV-associated HCC biomarker. Combined with a variety of clinicopathological features, we further analyzed the serologic level of galectin-4 in HBV-associated HCC patients. As shown in Table 4, the higher level of serum galectin-4 was significantly positively associated with HBV DNA, bigger tumor size, microvascular invasion, poor tumor differentiation, and more advanced TNM stage.
Fig 4.
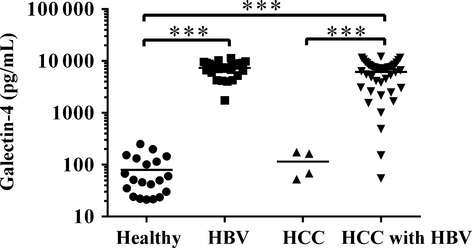
Serum galectin-4 levels in different patient groups and healthy volunteers. Serum level of galectin-4 in healthy people (n = 20), hepatitis B virus (HBV)-infected patients (n = 29), and hepatocellular carcinoma (HCC) patients without (n = 4) and with (n = 44) HBV infection were assessed by galectin-4 ELISA. ***P < 0.001.
Table 4.
Associations between serum galectin-4 level and clinicopathological characteristics of 44 hepatocellular carcinoma patients
| Clinicopathological variable | No. of patients | Galectin-4 concentration, pg/mL† | P-value |
|---|---|---|---|
| AFP, ng/mL | |||
| <400 | 27 | 5071 ± 3516 | 0.074 |
| ≥400 | 17 | 6944 ± 2582 | |
| HBV DNA | |||
| <1000 | 20 | 4928 ± 3319 | 0.028* |
| ≥1000 | 24 | 7138 ± 2827 | |
| Cirrhosis | |||
| Absent | 7 | 6972 ± 3364 | 0.459 |
| Present | 37 | 5975 ± 3218 | |
| Tumor number | |||
| Single | 33 | 6074 ± 3292 | 0.834 |
| Multiple | 11 | 6313 ± 3150 | |
| Maximal tumor size, cm | |||
| <5 | 21 | 5009 ± 2554 | 0.025* |
| ≥5 | 23 | 7160 ± 3472 | |
| Tumor encapsulation | |||
| Absent | 12 | 7032 ± 2470 | 0.262 |
| Present | 32 | 5797 ± 3436 | |
| Tumor differentiation | |||
| I–II | 12 | 3888 ± 3252 | 0.004** |
| III–IV | 32 | 6930 ± 2790 | |
| Microvascular invasion | |||
| Absent | 23 | 4796 ± 3366 | 0.003** |
| Present | 21 | 7599 ± 2354 | |
| TNM | |||
| I | 18 | 4644 ± 3437 | 0.009** |
| II–III | 26 | 7165 ± 2669 | |
Mean ± SD.
P < 0.05;
P < 0.01. AFP, α-fetoprotein.
All of these results indicate that the downregulation of galectin-4 at the tissue level and upregulation at the serum level may contribute to HCC progression, including recurrence and metastasis.
Discussion
The poor prognosis of patients with HCC after curative resection is mainly due to intrahepatic recurrence and/or extrahepatic metastasis.(19) Thus, it is crucial to screen the biomarkers and investigate the molecular mechanisms underlying the recurrence/metastasis of HCC. Accumulating evidence has indicated that galectins are frequently dysregulated in the pathogenesis of HCC and associated with HCC recurrence and metastasis(20); overexpression of galectin-1, -3, and -4, and downregulation of galectin-8 and -9 have already been reported in HCC cells.(17,21–23) However, their exact functions and how galectins associate with HCC progression remain unclear. In this study, we provide evidence that the expression of galectin-4 was downregulated in recurrent/metastatic HCC patients, compared to non-recurrent/metastatic patients. Clinicopathological association analysis indicated that low expression of gelectin-4 in tissue sections was strongly associated with increased tumor size, microvascular invasion, malignant differentiation, and more advanced TNM stage. Additionally, HCC patients with lower galectin-4 expression levels were significantly associated with a poorer 5-year survival. Furthermore, Cox's multivariate proportional hazards model revealed that the galectin-4 expression level could serve as an independent prognostic marker to identify patients with poor clinical outcomes. Taken together, these data indicated that galectin-4 could be used as a potential prognosis marker for predicting the recurrence/metastasis of HCC patients.
It has been reported that galectin-4 functions as a tumor suppressor that was significantly downregulated in colon cancer, through targeting the Wnt signaling pathways.(24) Overexpression of galectin-4 could induce cell cycle arrest and retarded cell migration and motility; in contrast, silencing galectin-4 resulted in increased cell proliferation, migration, and motility.(13) In pancreatic cancer, galectin-4 acts at an adhesion molecule to stabilize cell–cell contact and inhibits metastasis formation through preventing the release of tumor cells.(16) In our study, overexpression of galectin-4 in a highly metastatic HCC cell line (HCCLM3) could significantly reduce cell migration and invasion; these metastatic properties could be significantly upregulated by knocking down galectin-4. Therefore, galectin-4 might function by enhancing cell–cell contact then inhibiting the detachment of tumor cells from the primary site, to further retard the metastasis of HCC. These results could explain our finding that galectin-4 is significantly downregulated in recurrent/metastatic HCC tumor tissues.
Furthermore, galectin-4 has been shown to be function through both intracellular and extracellular actions. We observed significantly elevated levels of serum galectin-4 in patients with HBV infection. Paclik et al.(12,25) have shown that galectin-4 could bind to activated T cells to induce T cell apoptosis and decrease the secretion of pro-inflammatory cytokines under inflammatory conditions. Likewise, the elevated serum galectin-4 levels in HBV-infected patients, perhaps as a feedback response, contribute to the control of liver inflammation. We also found that the higher serologic level of galectin-4 was well associated with more aggressive characteristics of HCC. Several studies have reported that the increased circulation of galectins in the serum of cancer patients can promote the adhesion of invaded tumor cells to the blood vascular endothelium and spread to remote organs through binding cancer-associated Thomsen-Friedenreich disaccharides on trans-membrane mucin protein MUC1.(14,26,27) Furthermore, overexpression of MUC1 is a common feature in HCC.(28) So the increased interactions between circulating galectin-4 and MUC1 may enhance the ability of cell heterotypic adhesion and homotypic aggregation,(26) like galectin-3, and contribute to the recurrence/metastasis of HCC. These results suggested that higher serum galectin-4 might be associated with increased recurrence/metastasis risk in HCC patients. Consistent with these findings, our results suggested that intra- and extracellular galectin-4 may play opposite roles in the progression of HCC. Intracellular galectin-4 prevented the recurrence/metastasis of HCC, whereas extracellular galectin-4 promoted the aggressive characteristics of HCC.
In conclusion, this study clearly shows that galectin-4 is significantly downregulated in recurrent/metastatic HCC tumor tissues, and the higher serum galectin-4 level of HCC patients is associated with more aggressive characteristics of HCC. Thus, galectin-4 might represent a novel potential prognosis biomarker for HCC.
Acknowledgments
This work was supported by the key clinical specialty discipline construction program of Fujian Province, China, the key project of National Science and Technology of China (Grant Nos. 2012ZX10002010-001-006 and 2012ZX10002016-013), the National Natural Science Foundation of China (Grant No. 31201008), the Scientific Foundation of Fuzhou Health Department (Grant Nos. 2013-S-wq20, 2013-S-wp1, and 2014-S-w23).
Glossary
Abbreviations
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- NR/M
patients with no recurrence/metastasis within 2 years of hepatectomy
- OS
overall survival
- q-PCR
quantitative PCR
- R/M≤12 months
patients with recurrence/metastasis within 12 months of hepatectomy
- R/M12–24 months
patients with recurrence/metastasis within 12–24 months of hepatectomy
- TMA
tissue microarray
- TTR
time to recurrence/metastasis
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Lai E, Lau W. The continuing challenge of hepatic cancer in Asia. Surgeon. 2005;3:210–5. doi: 10.1016/s1479-666x(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 2.Choi GH, Han DH, Kim DH, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009;198:693–701. doi: 10.1016/j.amjsurg.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Ng KM, Yan TD, Black D, Chu FC, Morris DL. Prognostic determinants for survival after resection/ablation of a large hepatocellular carcinoma. HPB (Oxford) 2009;11:311–20. doi: 10.1111/j.1477-2574.2009.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SA, Wei AC, Cleary SP, et al. Prognosis and results after resection of very large (>or=10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589–95. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–6. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 6.Balan V, Nangia-Makker P, Raz A. Galectins as cancer biomarkers. Cancers. 2010;2:592–610. doi: 10.3390/cancers2020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumilla KM, Erickson LA, Erickson AK, Lloyd RV. Galectin-4 expression in carcinoid tumors. Endocr Pathol. 2006;17:243–9. doi: 10.1385/ep:17:3:243. [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi K, Shimura T, Masuda N, et al. Galectin-3 expression in colorectal cancer: relation to invasion and metastasis. Anticancer Res. 2007;27:2289–96. [PubMed] [Google Scholar]
- 9.Wu M-H, Hong T-M, Cheng H-W, et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311–8. doi: 10.1158/1541-7786.MCR-08-0297. [DOI] [PubMed] [Google Scholar]
- 10.Kim H-J, Do I-G, Jeon H-K, et al. Galectin 1 expression is associated with tumor invasion and metastasis in stage IB to IIA cervical cancer. Hum Pathol. 2013;44:62–8. doi: 10.1016/j.humpath.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Chen Z-G, Liu S-H, et al. Galectin-3 gene silencing inhibits migration and invasion of human tongue cancer cells in vitro via downregulating β-catenin. Acta Pharmacol Sin. 2013;34:176–84. doi: 10.1038/aps.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paclik D, Danese S, Berndt U, Wiedenmann B, Dignass A, Sturm A. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE. 2008;3:e2629. doi: 10.1371/journal.pone.0002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SW, Park KC, Jeon SM, et al. Abrogation of galectin-4 expression promotes tumorigenesis in colorectal cancer. Cell Oncol (Dordr) 2013;36:169–78. doi: 10.1007/s13402-013-0124-x. [DOI] [PubMed] [Google Scholar]
- 14.Barrow H, Guo X, Wandall HH, et al. Serum galectin-2,-4, and-8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17:7035–46. doi: 10.1158/1078-0432.CCR-11-1462. [DOI] [PubMed] [Google Scholar]
- 15.Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J. 2003;20:247–55. doi: 10.1023/B:GLYC.0000025819.54723.a0. [DOI] [PubMed] [Google Scholar]
- 16.Belo AI, van der Sar AM, Tefsen B, van Die I. Galectin-4 reduces migration and metastasis formation of pancreatic cancer cells. PLoS ONE. 2013;8:e65957. doi: 10.1371/journal.pone.0065957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondoh N, Wakatsuki T, Ryo A, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–6. [PubMed] [Google Scholar]
- 18.Huang X, Zeng Y, Xing X, et al. Quantitative proteomics analysis of early recurrence/metastasis of huge hepatocellular carcinoma following radical resection. Proteome Sci. 2014;12:22. doi: 10.1186/1477-5956-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L, Huang W, Tian D, et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–24. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–49. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spano D, Russo R, Di Maso V, et al. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16:102–15. doi: 10.2119/molmed.2009.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y, Shimosegawa T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098–111. doi: 10.1111/j.1872-034X.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z-Y, Dong J-H, Chen Y-W, et al. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503–9. doi: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 24.Satelli A, Rao PS, Thirumala S, Rao US. Galectin-4 functions as a tumor suppressor of human colorectal cancer. Int J Cancer. 2011;129:799–809. doi: 10.1002/ijc.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hokama A, Mizoguchi E, Sugimoto K, et al. Induced Reactivity of Intestinal CD4+ T Cells with an Epithelial Cell Lectin, Galectin-4, Contributes to Exacerbation of Intestinal Inflammation. Immunity. 2004;20:681–93. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Barclay M, Hilkens J, et al. Research Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrow H, Rhodes JM, Yu LG. The role of galectins in colorectal cancer progression. Int J Cancer. 2011;129:1–8. doi: 10.1002/ijc.25945. [DOI] [PubMed] [Google Scholar]
- 28.Yuan S-F, Li K-Z, Wang L, et al. Expression of MUC1 and its significance in hepatocellular and cholangiocarcinoma tissue. World J Gastroenterol. 2005;11:4661–6. doi: 10.3748/wjg.v11.i30.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]