Abstract
Concurrent chemoradiotherapy has become one of the standard management approaches for newly diagnosed localized nasal natural killer (NK)/T-cell lymphoma (NKTCL). Few data are available on the prognostic biomarkers of NKTCL among patients treated with concurrent chemoradiotherapy. To evaluate the prognostic significance of immunophenotypic biomarkers for patients treated with concurrent chemoradiotherapy, latent membrane protein 1 (LMP1), cutaneous lymphocyte antigen (CLA) and cell origin were examined in samples from 32 patients who were enrolled in the Japan Clinical Oncology Group 0211 trial and treated with concurrent chemoradiotherapy. LMP1 and CLA were positive in 66% (19/29) and 29% (9/31) of the cases examined, respectively. The median follow-up duration was 68 months (range, 61–94). The patients with LMP1-positive tumors showed a better overall survival (OS) than the patients with LMP1-negative tumors (hazard ratio, 0.240; 95% confidence interval [CI], 0.057–1.013; 80% CI, 0.093–0.615; P = 0.035). All five patients with LMP1-negative tumors who experienced disease progression died of lymphoma, and both patients with local failure had LMP1-negative tumors. There was no significant difference in OS according to CLA expression. A total of 27 (84%) cases were of NK-cell origin, two were of αβ T-cell origin and three were of γδ T-cell origin. In contrast to those with tumors of NK-cell origin, all five patients with NKTCL of T-cell origin were alive without relapse at the last follow up. Our results indicate that LMP1 expression is a favorable prognostic marker and suggest that a T-cell origin of the tumor may be a favorable prognostic marker for patients with localized NKTCL treated with concurrent chemoradiotherapy.
Keywords: Clinical trial, Epstein–Barr virus, extranodal NK/T-cell lymphoma, latent membrane protein 1, radiotherapy
Extranodal natural killer (NK)/T-cell lymphoma (NKTCL), nasal type, is a predominantly extranodal lymphoma associated with Epstein–Barr virus (EBV).(1,2) The tumor cells in most cases of NKTCL show an NK-cell phenotype,(1,2) while some cases show a T-cell phenotype, including γδ T-cell and αβ T-cell types.(3,4)
Tumor cells of NKTCL express P-glycoprotein, resulting in tumor multidrug resistance.(5–7) The outcomes after treatment with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or with CHOP-like chemotherapy for localized nasal NKTCL are unsatisfactory.(8–10) Based on the results of clinical trials published in 2009,(11,12) concurrent chemoradiotherapy has been recognized as one of the standard management approaches for newly diagnosed localized NKTCL.(13–15) In Japan, the Lymphoma Study Group of the Japan Clinical Oncology Group (JCOG-LSG) conducted a phase I/II study (JCOG0211) of radiotherapy (RT) and dexamethasone, etoposide, ifosfamide and carboplatin (DeVIC) (RT-DeVIC) for newly diagnosed localized nasal NKTCL.(11,16) In patients who were treated with the recommended dose (RT-2/3DeVIC), the 5-year overall survival (OS) and the 5-year progression-free survival (PFS) were 70 and 63%, respectively.(16) Subgroup analysis further revealed that both the international prognostic index(17) and the NK/T-cell lymphoma prognostic index(18) were not valid for the patient cohort of JCOG0211,(11) and similar results of a subgroup analysis were obtained in a phase II study of concurrent chemoradiotherapy in Korea.(12)
Latent membrane protein 1 (LMP1),(19–22) cutaneous lymphocyte antigen (CLA),(23,24) NK-cell origin(4,25–27) and EBV-encoded RNA (EBER) in the pretreatment bone marrow (BM), as detected by in situ hybridization,(28) have all been reported as prognostic biomarkers in patients with NKTCL who have been treated with conventional therapies. However, the prognostic significance of these biomarkers remains unclear, as most patients with NKTCL in previous studies were treated with heterogeneous treatment modalities. Because concurrent chemoradiotherapy is a new treatment modality for lymphoma, few data are available on the prognostic biomarkers of NKTCL among patients treated with concurrent chemoradiotherapy.
To evaluate the prognostic significance of immunophenotypic biomarkers among patients treated with concurrent chemoradiotherapy, we conducted an ancillary clinicopathologic study of the JCOG0211 trial.
Materials and Methods
Patients, treatment and tissue samples
The subjects in this study included 33 patients who were enrolled in the JCOG0211 trial. The design of the JCOG0211 trial has previously been described in detail.(11) Briefly, patients were eligible for the study if they were 20 to 69 years old and had previously untreated extranodal NKTCL, nasal type.(1) Patients were also required to have stage IE or contiguous stage IIE disease with cervical lymph node involvement and at least one measurable lymphomatous lesion in the nasal cavity and/or its adjacent sites. Patients received RT-DeVIC therapy consisting of RT of 50 Gy and three cycles of DeVIC chemotherapy. A two-thirds dose of DeVIC was selected for 27 patients who were evaluated in the phase II portion of JCOG0211. A full-dose of DeVIC was selected for six patients in the phase I portion. Among 33 cases, four cases had been included in a previous single-center study analyzing LMP1 expression in tumor cells of NKTCL.(21)
Sections of formalin-fixed, paraffin-embedded tissues of pretreatment lymphoma and BM samples were collected from the patients. The histological diagnoses of all patients were confirmed as extranodal NKTCL, nasal type by the Central Pathology Review Board.(11) All immunohistopathological examinations for the current ancillary study were performed at the Central Pathology Office of the ancillary study (Okayama University Hospital, Okayama, Japan).
The current study was approved by the JCOG Protocol Review Committee and the institutional review board at each study site. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. All data on baseline features, treatment details, response and follow up were retrieved from the original JCOG0211 dataset.
Immunohistochemical analysis
Immunohistochemical staining was performed on sections of formalin-fixed, paraffin-embedded tissues of pretreatment lymphoma samples with heat-induced or trypsin-induced epitope retrieval using an avidin–biotin complex method and an automated immunostainer (Bond-max, Leica Biosystems, Melbourne, Vic., Australia), as previously described.(29) The following primary antibodies were used to assess these samples: LMP1 (CS1-4, 1:50, Novocastra, Newcastle-upon-Tyne, UK), T-cell receptor (TCR) β (βF1; TCR1151, 8A3, 1:50, Thermo Scientific, Waltham, MA, USA), TCRγ chain constant region (CγM1; TCR1153, γ3.20, 1:80, Thermo Scientific) and CLA (HECA452, 1:10) as previously described.(23,29)
For the LMP1 antigen, samples were determined to be positive when the lymphoma cells were positive according to the methods described by Kanemitsu et al. (21). For βF1, CγM1 and CLA expression, samples in which 30% or more of the cells expressed the antigen were scored as positive, as previously described.(23,29) A preliminary evaluation for the study showed that staining for TCRγ and TCRδ was concordant in all cases.(29) Cases were considered to be of NK-cell origin if both TCRβ and TCRγ expression was not observed. Cases with positive staining for one or both of the antibodies (TCRβ and TCRγ) were determined to be of T-cell origin.(29)
In situ hybridization
Pretreatment BM specimens from patients were examined for EBER. In situ hybridization with EBER-1 probes (INFORM EBER, Leica Biosystems, Melbourne, Victoria, Australia) was performed to detect EBV.(29)
Statistical analysis
Survival estimates were calculated using the Kaplan–Meier method. Hazard ratios (HR) and 80 and 95% confidence intervals (CI) were estimated using a Cox regression. All of the analyses were performed using IBM SPSS Statistics 20.0 (IBM Japan, Tokyo, Japan).
Results
Expression of biomarkers
Pathological samples from 32 out of 33 patients were available for this study. Tissue samples from the remaining patient were exhausted during the Central Pathology Review and, therefore, were no longer available for the current study. This patient was a 33-year-old woman with stage IIE NKTCL who obtained a complete response (CR) by RT-(full-dose) DeVIC and was alive at the last follow-up examination (86 months). Pretreatment BM samples were obtained from 29 of the 32 patients.
The immunohistochemical features of these samples are shown in Figure 1. LMP1 and CLA were positive in 66% (19/29) and 29% (9/31) of the cases examined, respectively. Among the 32 cases that were examined to determine cell lineage, 27 (84%) cases were of NK-cell origin. Two (6%) cases were of αβ T-cell origin on the basis of TCRβ immunoreactivity but not TCRγ, and 3 (9%) cases were of γδ T-cell origin due to the presence of TCRγ immunoreactivity but not TCRβ immunoreactivity. Pretreatment BM samples were positive for EBER in 2 (7%) out of 29 cases examined. One of the two EBER-positive cases had two positive cells in a total field of view. Another case had three to five positive cells/high-power field. Positive cells were small to medium sized cells and were diffusely distributed in the latter case.
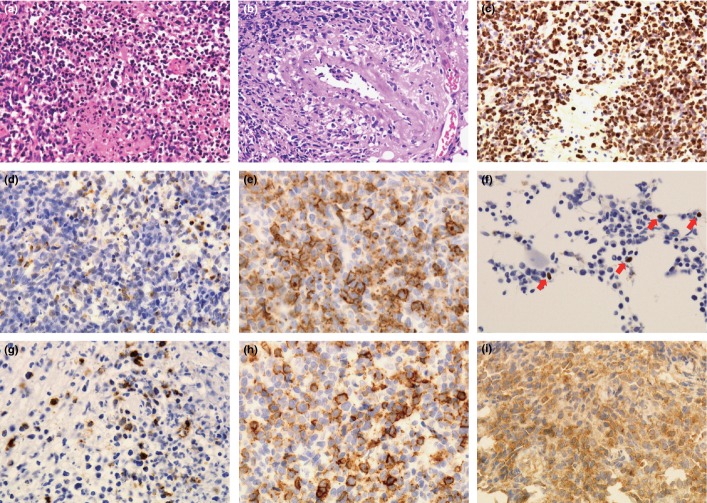
Fig 1.
(a) H&E staining in NK/T-cell lymphoma (NKTCL) of γδ T-cell origin (57-year-old male patient). Medium to large sized tumor cells with necrosis are seen. (b) H&E staining in NKTCL of γδ T-cell origin (34-year-old male patient). Tumor cells have an angiocentric angiodestructive quality. (c) In situ hybridization study for NKTCL of γδ T-cell origin (57-year-old male patient). Tumor cells are positive for Epstein–Barr virus encoded RNA (EBER). (d) Neoplastic tissue from the nasal cavity of a 39-year-old female patient with NKTCL of NK-cell origin. The neoplastic cells are positive for latent membrane protein 1 (LMP1). (e) Neoplastic tissue from the oral cavity of a 57-year-old male patient with NKTCL of NK-cell origin. The neoplastic cells are positive for cutaneous lymphocyte antigen (CLA). (f) Bone marrow tissue from a 34-year-old male patient with NKTCL of γδ T-cell origin. There are a small number of EBER-positive cells (red arrows). (g–i) Neoplastic tissue from the nasal cavity of a 55-year-old male patient with NKTCL of γδ T-cell origin. The neoplastic cells are positive for LMP1 (g), CLA (h) and T-cell receptor γ (i).
The Central Pathology Review of JCOG0211 confirmed that tumor cells in 29 out of the 32 cases were positive for EBER. Tissue samples of the remaining three cases were not evaluable for EBER. The current study revealed LMP1 expression in tumor cells of all the remaining three cases, indicating that all 32 cases were associated with EBV.
In the 29 patients whose samples were available for analysis of LMP1 and CLA expression, tumors from four patients were positive for both antigens, tumors from 15 patients were positive for LMP1 only, tumors from four patients were positive for CLA only, and tumors from six patients were negative for both antigens. In the three patients with NKTCL of γδ T-cell origin, two samples contained cells that were positive for both LMP1 and CLA, and one sample contained cells that were negative for both antigens. Of the two cases of αβ T-cell origin, one showed an LMP1-positive and CLA-negative immunophenotype, while another case was positive for both LMP1 and CLA. In two patients whose BM samples were positive for EBER, one had NKTCL of NK-cell origin that was negative for LMP1 and CLA, and the other had NKTCL of γδ T-cell origin that was positive for both LMP1 and CLA.
Clinical features according to the expression of each biomarker
The baseline clinical characteristics according to the expression of each biomarker are shown in Table 1. All CLA-positive tumors in the study were from male patients. Skin involvement prior to treatment was frequently observed in the patients with LMP1-negative tumors (odds ratio [OR], 6.533 [95% CI, 1.200–35.573]). All five patients with NKTCL of T-cell origin showed no skin involvement at baseline (OR, 0.722 [95% CI, 0.542–0.962]). No significant correlation was observed between the induction of CR and the expression of each biomarker (data not shown).
Table 1.
Clinical features according to each biomarker expression
| All Patients n = 32 (%) | LMP1 |
CLA |
Cell Origin |
||||
|---|---|---|---|---|---|---|---|
| Positive n = 19 | Negative n = 10 | Positive n = 9 | Negative n = 22 | NK-cell type n = 27 | T-cell type n = 5 | ||
| Age at Diagnosis, n (%) | |||||||
| ≤60 | 25 (78) | 15 (79) | 8 (80) | 8 (89) | 16 (73) | 21 (78) | 4 (80) |
| >60 | 7 (22) | 4 (21) | 2 (20) | 1 (11) | 6 (27) | 6 (22) | 1 (20) |
| Sex, n (%) | |||||||
| Male | 19 (59) | 9 (47) | 3 (30) | 9 (100) | 10 (45) | 12 (44) | 1 (20) |
| Female | 13 (41) | 10 (53) | 7 (70) | 0 (0) | 12 (55) | 15 (56) | 4 (80) |
| PS, n (%) | |||||||
| 0 or 1 | 30 (94) | 17 (89) | 10 (100) | 9 (100) | 20 (91) | 25 (93) | 5 (100) |
| >1 | 2 (6) | 2 (11) | 0 (0) | 0 (0) | 2 (9) | 2 (7) | 0 (0) |
| Serum LDH Level, n (%) | |||||||
| ≤Normal | 25 (78) | 15 (79) | 7 (70) | 7 (78) | 17 (77) | 20 (74) | 5 (100) |
| >Normal | 7 (22) | 4 (21) | 3 (30) | 2 (22) | 5 (23) | 7 (26) | 0 (0) |
| B Symptoms, n (%) | |||||||
| Absent | 20 (63) | 13 (68) | 5 (50) | 4 (44) | 15 (68) | 16 (59) | 4 (80) |
| Present | 12 (37) | 6 (32) | 5 (50) | 5 (56) | 7 (32) | 11 (41) | 1 (20) |
| Stage, n (%) | |||||||
| IE | 22 (69) | 12 (63) | 8 (80) | 5 (56) | 16 (73) | 20 (74) | 2 (40) |
| IIE | 10 (31) | 7 (37) | 2 (20) | 4 (44) | 6 (27) | 7 (26) | 3 (60) |
| Skin Involvement, n (%) | |||||||
| Absent | 18 (56) | 14 (74) | 3 (30) | 4 (44) | 14 (64) | 13 (48) | 5 (100) |
| Present | 14 (44) | 5 (26) | 7 (70) | 5 (56) | 8 (36) | 14 (52) | 0 (0) |
| IPI, n (%) | |||||||
| 0 | 19 (59) | 11 (58) | 6 (60) | 6 (67) | 12 (55) | 15 (56) | 4 (80) |
| 1 | 10 (31) | 6 (32) | 3 (30) | 3 (33) | 7 (32) | 9 (33) | 1 (20) |
| 2 | 3 (9) | 2 (11) | 1 (10) | 0 (0) | 3 (14) | 3 (11) | 0 (0) |
| NK-PI, n (%) | |||||||
| 0 | 11 (34) | 7 (37) | 3 (30) | 1 (11) | 9 (41) | 9 (33) | 2 (40) |
| 1 | 9 (28) | 4 (21) | 4 (40) | 5 (56) | 4 (18) | 8 (30) | 1 (20) |
| 2 | 9 (28) | 7 (37) | 1 (10) | 2 (22) | 7 (32) | 7 (26) | 2 (40) |
| 3 | 3 (9) | 1 (5) | 2 (20) | 1 (11) | 2 (9) | 3 (11) | 0 (0) |
CLA, cutaneous lymphocyte antigen; IPI, international prognostic index; LDH, lactate dehydrogenase; LMP1, latent membrane protein 1; NK-PI, NK/T-cell lymphoma prognostic index; PS, performance status.
Survival analysis
The median follow-up duration was 68 months (range, 61–94). The OS was better in the LMP1-positive group than in the LMP1-negative group (HR, 0.240; 95% CI, 0.057–1.013; 80% CI, 0.093–0.615; P = 0.035, Fig. 2). The OS at 5 years was 84% in the LMP1-positive group and 50% in the LMP1-negative group. The PFS at 5 years was 74% in the LMP1-positive group and 50% in the LMP1-negative group. The HR of PFS among LMP1-positive tumors was 0.421 (95% CI, 0.121–1.463; 80% CI, 0.187–0.950). There were no significant differences in the OS and the PFS between the CLA-positive and CLA-negative groups (5-year OS, 56 vs 77%; 5-year PFS, 56 vs 68%, Fig. 2). All five patients with NKTCL of T-cell origin achieved a CR by RT-DeVIC and were alive, without relapse, at the time of the last follow up (Fig. 2). The 5-year OS and PFS in the 27 patients with NKTCL of NK-cell origin were 67 and 59%, respectively. The two patients whose pretreatment BM samples were positive for EBER survived for more than 5 years without disease progression.
Fig 2.
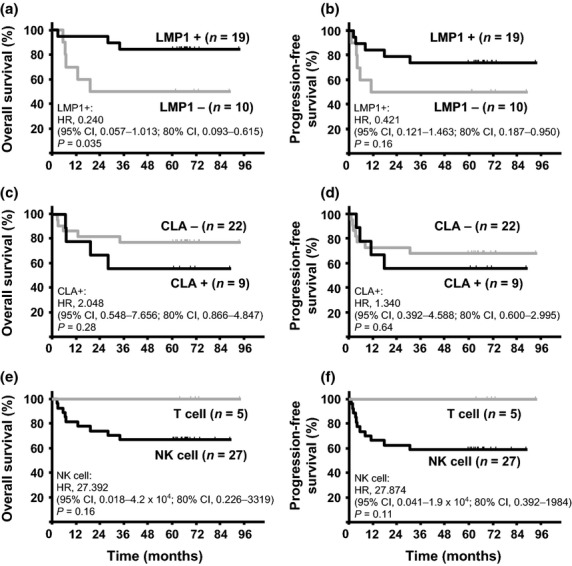
(a,c,e) Overall survival curves of patients with NK/T-cell lymphoma (NKTCL) by latent membrane protein 1 (LMP1) expression (a), cutaneous lymphocyte antigen (CLA) expression (c) and cell-of-origin (e). (b,d,f) Progression-free survival curves of patients with NKTCL by LMP1 expression (b), CLA expression (d) and cell-of-origin (f).
Characteristics and biomarker expression in patients who experienced disease progression or recurrence
During the follow-up period, 11 of the 33 patients who were enrolled in JCOG0211 experienced disease progression or recurrence. Detailed clinical information and biomarker expression for these cases are presented in Table 2. Among the 5 LMP1-positive patients, two patients achieved a second CR using L-asparaginase-containing chemotherapy followed by allogeneic hematopoietic stem cell transplantation and attained long-term survival. In contrast, all five patients whose tumors were LMP1-negative and who experienced disease progression died within 19 months of the initial registration. In JCOG0211, only two patients experienced local failure,(16) and the present study revealed that both of these cases were LMP1-negative (Table 2).
Table 2.
Characteristics and biomarker expression of the 11 patients who experienced disease progression or recurrence during follow-up
| Age/Sex | Stage | LMP1 | CLA | Overall response | Site(s) of recurrence | Salvage therapy | OS, mo | PFS, mo | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 57/F | IIEA | + | − | PD | Liver, spleen, BM | None | 3 | 2 | DOD |
| 21/M | IEA | + | − | PD | LN | Chemotherapy for ALL, CBT | 69 | 3 | AND |
| 57/F | IIEA | + | − | CR | LN, stomach | L-asp/VCR/DMS, Allo PBSCT | 68 | 8 | AND |
| 57/M | IIEB | + | − | CR | BM, PB | CMED, L-asp/VCR/DMS, EPOCH | 34 | 31 | DOD |
| 38/M | IIEB | + | + | CR | LN, liver, spleen, ascites | mPSL, ETP, FCM | 29 | 18 | DOD |
| 58/M | IEB | − | + | CR | Skin | ESHAP, RT, CHOP | 19 | 11 | DOD |
| 63/F | IEA | − | − | PD | CNS, nasal cavity†, parotid gland, PB, subcutaneous tissue | MTX/AraC it, IVAC, HD-MTX, DeVIC, CHOP | 13 | 1 | DOD |
| 57/M | IIEA | − | + | CR | LN, skin, BM | L-asp/VCR/DMS | 7 | 4 | DOD |
| 58/F | IEA | − | − | PR | Skin, kidney, LN | ESHAP | 6 | 4 | DOD |
| 60/M | IEB | − | + | SD | Eye, nasal cavity†, skin | None | 7 | 6 | DOD |
| 45/M | IIEB | ND | − | PD | Gallbladder, BM | None | 3 | 2 | DOD |
ALL, acute lymphoblastic leukemia; Allo PBSCT, allogeneic peripheral blood stem cell transplantation; AND, alive with no evidence of disease; AraC, cytarabine; BM, bone marrow; CBT, cord blood transplantation; CLA, cutaneous lymphocyte antigen; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CMED, cyclophosphamide, methotrexate, etoposide, and dexamethasone; CNS, central nervous system; CR, complete response; DeVIC, dexamethasone, etoposide, ifosfamide, and carboplatin; DOD, died of disease; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; ESHAP, etoposide, methylprednisolone, cytarabine, and cisplatin; ETP, etoposide; FCM, fludarabine, cyclophosphamide, and mitoxantrone; HD, high dose; it, intrathecal; IVAC, ifosfamide, etoposide, and cytarabine; L-asp/VCR/DMS, L-asparaginase, vincristine, and dexamethasone; LMP1, latent membrane protein 1; LN, lymph node, mPSL, methylprednisolone; MTX, methotrexate; ND, not done; OS, overall survival; PB, peripheral blood; PD, progressive disease; PFS, progression-free survival; PR, partial response; RT, radiotherapy; SD, stable disease. All 11 cases were of NK-cell origin.
Local failure.
Discussion
The current study showed that LMP1 expression in tumor cells was associated with OS in patients with newly diagnosed localized NKTCL who were treated uniformly with concurrent chemoradiotherapy. Of note, all five patients with NKTCL of the T-cell type, including three patients with NKTCL of the γδ T-cell type, survived more than 5 years without recurrence.
Although NKTCL is associated with a type II EBV latency program,(30) the expression levels of LMP1 in NKTCL is variable at the single-cell level.(20,21,30) In addition, there have been conflicting results in previous studies analyzing the prognostic significance of LMP1 expression in NKTCL. A single-center study of 58 patients with NKTCL (advanced disease, n = 12) in China reported that patients with LMP1-positive tumors showed significantly worse survival.(19) Another study from China, which included 16 patients with NKTCL (advanced disease, n = 2), reported that patients whose tumors exhibited high LMP1 expression (81–100% of tumor cells) showed significantly shorter survival.(20) In contrast, one single-center study from Japan, which included 30 patients with NKTCL (advanced disease, n = 7), showed a favorable outcome for patients whose tumors were LMP1-positive compared to patients whose tumors were LMP1-negative.(21) Possible explanations for the incongruence in these reported results include the heterogeneous therapeutic approaches used, the differences in the incidence of advanced stage, and the use of different criteria for LMP1 positivity.
In the present study, the lack of LMP1 expression was associated with a short OS in patients with localized NKTCL who were treated with RT-DeVIC. Skin involvement was frequently observed in LMP1-negative cases. All five patients with LMP1-negative tumors who experienced disease progression died, and the two patients who experienced local failure in JCOG0211 had tumors that were LMP1-negative. Together, these results indicate that LMP1-negative NKTCL has an aggressive nature. The lack of detectable LMP1 in aggressive NK-cell leukemia(31) may also support this assertion. In LMP1-negative tumors, some researchers speculate that additional cellular genetic aberrations may be driving a more malignant tumor phenotype that no longer requires expression of the LMP1 oncogene.(32)
Differences in prognosis between NKTCL patients with the NK-cell type and the T-cell type have been the subject of previous research. Early studies revealed that patients with CD56-positive NKTCL show significantly worse survival than those with CD56-negative NKTCL.(25,26) A Chinese group reported that there was no difference in prognosis between these two groups.(27) However, patients in these studies were treated with various treatment modalities and different chemotherapeutic regimens. Recent immunohistochemical analyses using monoclonal antibodies against the TCRβ, γ and δ subunits have shown that some CD56-positive NKTCL cases are actually γδ T-cell lymphoma and that NKTCL of T-cell origin exhibits a trend for better OS than NKTCL of NK-cell origin.(4) In the current study, patients were uniformly treated with RT-DeVIC. Moreover, the origins of the lymphoma cells were determined immunohistochemically with monoclonal antibodies against the TCRβ and γ subunits, which constitutes a more specific method of identifying cases of the T-cell type than the previous approaches. Our current study highlighted the favorable prognosis of patients with NKTCL of T-cell origin, which suggests that a more effective therapeutic strategy is needed for NKTCL of NK-cell origin.
CLA is a skin-homing receptor that functions in the adhesion of cells to the vascular endothelium.(33) CLA-positive lymphocytes that migrate to the skin consist mostly of T cells, but NK cells are also present in this population.(34) Patients with NKTCL often develop skin involvement during the clinical course of the disease.(1) In the present study, 5 (56%) out of nine patients with CLA-positive tumors also had skin lesions. Yoshino et al.(23) examined CLA expression in 52 patients with NKTCL, including 14 patients with advanced disease, and found that CLA expression in NKTCL was associated with skin involvement and a poor prognosis.(23) A retrospective analysis with a small number of patients showed similar results in terms of survival.(24) In the present study, CLA expression was not associated with a poor prognosis. One possible reason is that only patients with NKTCL localized within the nasal cavity or the nasopharyngeal region were included in our study. It is also possible that the prognosis of patients with CLA-positive tumors may have been improved by RT-DeVIC.
In the current study, we examined the prognostic significance of the presence of EBER in pretreatment BM samples from patients, partly because testing for BM EBER was not routinely performed in Japan during the study period (2003–2006) of the JCOG0211 trial. Two patients in our study with EBER-positive pretreatment BM survived more than 5 years without disease progression, which suggests that EBER-positive BM did not affect the prognosis of our JCOG0211 patient cohort.
The current study demonstrated, for the first time, the favorable impact of LMP1 expression on the OS of patients with newly diagnosed localized nasal NKTCL in a cohort of patients uniformly treated with concurrent chemoradiotherapy. The limitations of this study include the relatively small number of patients included, the lack of available data on the EBV DNA load in the peripheral blood, which has been reported as a useful biomarker of NKTCL,(35,36) and the possibility of intermingled TCR silent cases in our NKTCL cases of NK-cell origin. Although our evaluations were confined to a relatively small number of patients, these patients were derived from a uniform cohort and were prospectively treated with the same therapeutic strategy consisting of RT-DeVIC. The prognostic values of LMP1 expression and the T-cell origin warrant further evaluation in future studies with a larger number of patients with NKTCL who are treated with concurrent chemoradiotherapy.
Acknowledgments
We would like to thank the following institutions for providing patient data and specimens: Tohoku University, Gunma University, Saitama Cancer Center, National Cancer Center Hospital East, National Cancer Center Hospital, Kyorin University, Jikei University, Juntendo University, Tokai University, Kanazawa Medical University, Nagoya University, Mie University, Shiga Medical Center for Adults, Ehime University, University of Occupational and Environmental Health, Saga University, Kumamoto University and National Hospital Organization Kumamoto Medical Center, Japan. This work was supported in part by the National Cancer Center Research and Development Funds, Japan (21-6-3, 23-A-17, 26-A-4).
Disclosure Statement
K. Oshimi is an employee of Eisai. The remaining authors have no conflict of interest to declare.
References
- 1.Chan JKC, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 204–7. [Google Scholar]
- 2.Chan JKC, Quintanilla-Martinez L, Ferry JA, Peh S-C. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. pp. 285–8. [Google Scholar]
- 3.Gaulard P, Henni T, Marolleau JP, et al. Lethal midline granuloma (polymorphic reticulosis) and lymphomatoid granulomatosis. Evidence for a monoclonal T-cell lymphoproliferative disorder. Cancer. 1988;62:705–10. doi: 10.1002/1097-0142(19880815)62:4<705::aid-cncr2820620410>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–99. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351–6. doi: 10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93:599–606. [PubMed] [Google Scholar]
- 7.Drenou B, Lamy T, Amiot L, et al. CD3− CD56+ non-Hodgkin's lymphomas with an aggressive behavior related to multidrug resistance. Blood. 1997;89:2966–74. [PubMed] [Google Scholar]
- 8.Yamaguchi M, Ogawa S, Nomoto Y, et al. Treatment outcome of nasal NK-cell lymphoma: a report of 12 consecutively-diagnosed cases and a review of the literature. J Clin Exp Hematop. 2001;41:93–9. [Google Scholar]
- 9.Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12:349–52. doi: 10.1023/a:1011144911781. [DOI] [PubMed] [Google Scholar]
- 10.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27:6027–32. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 13.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121:4997–5005. doi: 10.1182/blood-2013-01-453233. [DOI] [PubMed] [Google Scholar]
- 14.Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin's Lymphomas, Version 2.2014. J Natl Compr Canc Netw. 2014;12:916–46. doi: 10.6004/jnccn.2014.0086. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Semin Hematol. 2014;51:42–51. doi: 10.1053/j.seminhematol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Tobinai K, Oguchi M, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan Clinical Oncology Group study JCOG0211. J Clin Oncol. 2012;30:4044–6. doi: 10.1200/JCO.2012.45.6541. [DOI] [PubMed] [Google Scholar]
- 17.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–18. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 19.Cao W, Liu Y, Zhang H, et al. Expression of LMP-1 and Cyclin D1 protein is correlated with an unfavorable prognosis in nasal type NK/T cell lymphoma. Mol Med Rep. 2008;1:363–8. [PubMed] [Google Scholar]
- 20.Mao Y, Zhang DW, Zhu H, et al. LMP1 and LMP2A are potential prognostic markers of extranodal NK/T-cell lymphoma, nasal type (ENKTL) Diagn Pathol. 2012;7:178. doi: 10.1186/1746-1596-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanemitsu N, Isobe Y, Masuda A, et al. Expression of Epstein-Barr virus-encoded proteins in extranodal NK/T-cell lymphoma, nasal type (ENKL): differences in biologic and clinical behaviors of LMP1-positive and -negative ENKL. Clin Cancer Res. 2012;18:2164–72. doi: 10.1158/1078-0432.CCR-11-2395. [DOI] [PubMed] [Google Scholar]
- 22.Komabayashi Y, Kishibe K, Nagato T, Ueda S, Takahara M, Harabuchi Y. Downregulation of miR-15a due to LMP1 promotes cell proliferation and predicts poor prognosis in nasal NK/T-cell lymphoma. Am J Hematol. 2014;89:25–33. doi: 10.1002/ajh.23570. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino T, Nakamura S, Suzumiya J, et al. Expression of cutaneous lymphocyte antigen is associated with a poor outcome of nasal-type natural killer-cell lymphoma. Br J Haematol. 2002;118:482–7. doi: 10.1046/j.1365-2141.2002.03607.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang ST, Liao YL, Lin SH, Chuang SS. NK-cell lymphoma with nodal presentation and expression of cutaneous lymphocyte-associated antigen. Pathol Res Pract. 2010;206:463–6. doi: 10.1016/j.prp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998;16:70–7. doi: 10.1200/JCO.1998.16.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Kim GE, Koom WS, Yang WI, et al. Clinical relevance of three subtypes of primary sinonasal lymphoma characterized by immunophenotypic analysis. Head Neck. 2004;26:584–93. doi: 10.1002/hed.20015. [DOI] [PubMed] [Google Scholar]
- 27.Li YX, Wang H, Feng XL, et al. Immunophenotypic characteristics and clinical relevance of CD56+ and CD56− extranodal nasal-type natural killer/T-cell lymphoma. Leuk Lymphoma. 2011;52:417–24. doi: 10.3109/10428194.2010.543718. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Suh C, Huh J, et al. Effect of positive bone marrow EBV in situ hybridization in staging and survival of localized extranodal natural killer/T-cell lymphoma, nasal-type. Clin Cancer Res. 2007;13:3250–4. doi: 10.1158/1078-0432.CCR-06-2373. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi E, Takata K, Sato Y, et al. Distinct morphologic, phenotypic, and clinical-course characteristics of indolent peripheral T-cell lymphoma. Hum Pathol. 2013;44:1927–36. doi: 10.1016/j.humpath.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int J Cancer. 1996;68:285–90. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD. Aggressive natural killer cell leukemia: report of a Chinese series and review of the literature. Int J Hematol. 2007;85:18–25. doi: 10.1532/IJH97.A10612. [DOI] [PubMed] [Google Scholar]
- 32.Fox CP, Shannon-Lowe C, Rowe M. Deciphering the role of Epstein-Barr virus in the pathogenesis of T and NK cell lymphoproliferations. Herpesviridae. 2011;2:8. doi: 10.1186/2042-4280-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pals ST, de Gorter DJ, Spaargaren M. Lymphoma dissemination: the other face of lymphocyte homing. Blood. 2007;110:3102–11. doi: 10.1182/blood-2007-05-075176. [DOI] [PubMed] [Google Scholar]
- 34.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–6. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 35.Ito Y, Kimura H, Maeda Y, et al. Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res. 2012;18:4183–90. doi: 10.1158/1078-0432.CCR-12-1064. [DOI] [PubMed] [Google Scholar]
- 36.Kwong YL, Pang AW, Leung AY, Chim CS, Tse E. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: diagnostic and prognostic significance. Leukemia. 2013;28:865–70. doi: 10.1038/leu.2013.212. [DOI] [PubMed] [Google Scholar]