Abstract
Although chromogranin A (CGA) is a useful marker for pancreatic neuroendocrine tumors (pNET) in the West, its usefulness in Japanese populations is unclear. To assess this, we evaluated the serum CGA levels in 189 patients with various pancreatic diseases, including proven pNET (n = 69), pancreatic cancer (PC) (n = 50), chronic pancreatitis (CP) (n = 50) and autoimmune pancreatitis (AIP) (n = 20), and 112 normal controls (controls) using an ELISA kit. The mean CGA level of patients with pNET was significantly higher than any of the other groups (407.8 ± 984.6 ng/mL [pNET] vs 91.8 ± 101.8 ng/mL [PC], 93.6 ± 57.5 ng/mL [CP], 69.9 ± 52.4 ng/mL [AIP] and 62.5 ± 48.3 ng/mL [controls]). Limiting the analysis to patients not using proton pump inhibitors (PPI), the CGA level of patients with PC or CP was not significantly different compared with the controls. Discriminant analysis revealed that the best cut-off value of CGA to distinguish patients with pNET from the controls was 78.7 ng/mL, with a sensitivity and specificity of 53.6% and 78.6%, respectively. In patients with pNET, significant factors associating with elevated CGA levels were tumor classification, tumor size, and the presence of liver metastases in univariate analysis as well as PPI use and the presence of liver metastases in multivariate analysis. We show that CGA is a useful marker for diagnosing pNET in Japanese populations and for distinguishing patients with pNET from patients with other pancreatic diseases. The increased use of CGA in Japan will likely be a helpful tool in managing these patients, as found in the West.
Keywords: Chromogranin A, Japan, pancreatic cancer, pancreatic neuroendocrine tumors, proton pump inhibitors
Pancreatic neuroendocrine tumors (pNET) are uncommon tumors that are derived from the diffuse neuroendocrine cell system.(1) It is typically an indolent slow-growing tumor.(2)
However, pNET are receiving increasing attention worldwide and are increasingly being seen in clinical practice. This is because the prevalence of pNET has been increasing over the past three decades in a number of Western countries, which could be due to the increased use of endoscopic or imaging procedures, increased clinical awareness or a real increase in the incidence.(2,3) Recent data suggest that a similar trend is also true in Japan. A nationwide epidemiological study in 2005 revealed the current status of pNET in Japan.(4) A second nationwide epidemiological study in 2010 revealed that the number of patients with pNET is increasing in Japan.(5) These reports also reveal some differences in the epidemiology between Japan and Western countries.(4,5) Unfortunately, the survival of patients with pNET, similarly to those with other gastrointestinal neuroendocrine tumors (GI-NET), has not increased,(2) which is likely because patients continue to be diagnosed late in their disease course, with an average delay in diagnosis of 5–8 years.(2,6)
Pancreatic neuroendocrine tumors are receiving increased attention not only because their frequency is increasing but also because it is increasingly recognized that a significant proportion are malignant and require treatment.(7) Although pNET have a less aggressive course than adenocarcinomas, these malignant forms are associated with considerable morbidity. Furthermore, because they have a different pathogenesis to typical adenocarcinomas, they require a different therapeutic approach as well as a different approach in their diagnosis. During the past several years, new therapeutic agents for patients with pNET have been developed that can affect malignant progression, and it is important that patients with pNET are recognized. Both everolimus and sunitinib have demonstrated the ability to prolong the progression-free survival in patients with pNET.(7–9) These agents provide a similar benefit in Japanese patients with pNET and are now approved for use in Japan.(10,11) These reports also show differences in the response to drugs between patients with pNET in Japan and those in Western countries.(10,11)
For the reasons outlined above, it is increasingly important in Japan, as in Western countries, to have reliable methods that are generally available for both diagnosing and assessing the results of treatments in patients with pNET as well as with other GI-NET, such as carcinoids. Currently, imaging modalities are generally used, but they are sometimes unfavorable for patients, frequently involve exposure to radiation and can be expensive. For some cancers, tumor markers have proven useful for this purpose (e.g. calcitonin for medullary thyroid tumor and prostate-specific antigen [PSA] for prostate cancer),(12,13) and, in the case of pNET as well as GI-NET, from studies in the West, assessment of chromogranin A (CGA) in the serum/plasma shows the most promise.(14,15)
In the USA and other Western countries, CGA is broadly used as a marker for both diagnosing and monitoring the response to therapy of patients with pNET as well as with GI-NET.(1,14–17) CGA is a hydrophilic, acidic protein that consists of 439 amino acids.(14,15,17–19) CGA is present in chromaffin granules of neuroendocrine cells in both normal tissues and in neuroendocrine tumors (NET).(17–19) Therefore, CGA is used as a general marker for all NET.
We should take into consideration that several clinical conditions, other than NET, influence the elevation of the serum CGA level when we assess the serum CGA levels in patients with pNET. For example, the elevation (especially low level increases) of serum CGA levels can occur in several non-neoplastic conditions (e.g. inflammatory bowel syndrome(20) and chronic renal failure(21,22)) or certain adenocarcinomas (e.g. breast cancer(23) and hepatocellular carcinoma(22)).(14) Particularly important for the diagnosis and assessment of pNET are reports in other countries that the serum CGA levels can be elevated in patients with other pancreatic diseases, such as pancreatic cancer (PC) or chronic pancreatitis (CP).(24) In some cases, we have difficulty distinguishing pNET from these diseases, which affects the provision of the correct therapy for patients with pNET. It is important to know the difference in the serum CGA level between patients with pNET and these diseases.
As described above, there are differences between Japan and Western countries with respect to the epidemiology and therapeutic effects in patients with pNET. For this reason, there might also be differences in the serum CGA level by race. In fact, some tumor markers have been reported to have differences by race.(13,25) However, the serum CGA assessment has not previously been shown to be a useful marker for pNET in Japan. In addition, the serum CGA level in patients with other pancreatic diseases and whether CGA is useful for distinguishing pNET from these diseases in Japan have not previously been studied. Furthermore, the standard levels of CGA for Japanese people have not been well-studied. To address these issues, in the present study we studied both a group of Japanese normal controls, as well as patients with pNET and various pancreatic diseases. Our studies demonstrate that the serum CGA level is a useful marker for Japanese patients in diagnosing pNET and could be used in Japan to distinguish these patients from patients with other pancreatic diseases.
Material and Methods
We evaluated serum samples of 189 patients with pNET (n = 69), PC (n = 50), CP (n = 50) and autoimmune pancreatitis (AIP) (n = 20) who visited our institution from April 2008 to September 2012. All patients with pNET were histologically diagnosed with well-differentiated tumors corresponding to NET grade G1 or G2 according to the World Health Organization 2010 classification.(26) In 89.9% of patients, Ki67 value determination was performed and found to be G1 or G2. In the remaining 10.1% of patients, cytology was performed, but Ki67 value was not determined, and they were established to be well-differentiated tumors corresponding to G1 or G2. Patients with neuroendocrine carcinoma were excluded from this study. Each functional pNET was diagnosed by the existence of symptoms arising from oversecretion of each hormone. All patients with PC were histologically verified. All patients with CP or AIP were diagnosed using their standard diagnostic criteria in Japan, respectively.(27,28) We also evaluated serum samples of 112 controls. All controls confirmed that they were not using proton pump inhibitors (PPI), which can elevate serum CGA levels due to the gastric enterochromaffin-like cell changes these agents can cause,(15,17) and that they were not suffering from diseases of other organs, including the pancreas.
The study protocol was approved by the ethics committee at Kyushu University and written informed consent was obtained from all patients.
Blood samples were collected from each patient while fasting, centrifuged to obtain serum samples and stored at −80°C until assay. The serum CGA level was measured by using Chromoa (CIS Bioassays, GIF-SUR-YVETTE, France), which is an ELISA kit. We confirmed that the intra-assay and inter-assay coefficients of variation are 5% and 7%, respectively.
Differences in patient characteristics between each group were evaluated by 2 × 2 χ2-square test, Student t-test or Fisher's exact test. Differences in the serum CGA level between each group were evaluated using Scheffe's multiple comparisons test. Correlation coefficients were calculated to evaluate the correlations between the serum CGA and patient characteristics or other tumor markers. To determine a best cut-off value of the serum CGA to distinguish patients with pNET from the controls, discriminant function was calculated and a receiver operating characteristic curve was constructed. Univariate or multivariate analysis was conducted to determine the association between patient characteristics and elevated serum CGA level. P < 0.05 was considered statistically significant.
Results
Patient characteristics
The patient characteristics of each group are shown in Table 1. There were no significant differences between each group in terms of age and gender. In the pNET group, tumors consisted of non-functioning tumors (56.5%) with 43.5% of the functional tumor consisting primarily of gastrinomas (24.6%) and insulinomas (14.5%). All tumors were well-differentiated with histological grades of NET-G1 (58.0%) and NET-G2 (31.9%); in the remainder (10.1%), the histology was verified as well-differentiated (G1/G2) by cytology without an exact Ki67 value. In 48 patients (69.5%), a primary tumor remained in the pancreas at the time of the CGA measurement, whereas in 21 patients (30.5%), a primary tumor was resected from the pancreas and metastatic lesions were present at the time of CGA measurement. Among the patients with a primary tumor remaining in the pancreas, the maximum diameter of the primary tumor was <2 cm in 33 patients (47.8%) and >2 cm in 15 patients (21.7%).
Table 1.
Patient characteristics of this study
| Characteristics | pNET | PC | CP | AIP | Normal | P-value |
|---|---|---|---|---|---|---|
| Number | 69 | 50 | 50 | 20 | 112 | |
| Sex (%) | ||||||
| Male | 39 (56.5) | 28 (56.0) | 30 (60.0) | 17 (85.0) | 67 (59.8) | 0.709 |
| Female | 30 (43.5) | 22 (44.0) | 20 (40.0) | 3 (15.0) | 45 (40.2) | |
| Age (years) | ||||||
| Mean ± SD Range | 57.5 ± 13.9 (20–85) | 63.8 ± 9.5 (46–84) | 53.0 ± 14.0 (25–75) | 63.6 ± 11.4 (35–75) | 56.5 ± 14.3 (26–99) | 0.286 |
| PPI use (%) | ||||||
| Yes | 19 (27.5) | 19 (38.0) | 28 (56.0) | 9 (45.0) | 0 (0) | <0.0001* |
| No | 50 (72.5) | 31 (62.0) | 22 (44.0) | 11 (55.0) | 112 (100) | |
| Tumor classification (%) | ||||||
| Non-functioning | 39 (56.5) | |||||
| Functioning | 30 (43.5) | |||||
| Gastrinoma | 17 (24.6) | |||||
| Insulinoma | 10 (14.5) | |||||
| Others† | 3 (4.3) | |||||
| Histological grade | ||||||
| G1 | 40 (58.0) | |||||
| G2 | 22 (31.9) | |||||
| G1 or G2‡ (an exact Ki67 not determined) | 7 (10.1) | |||||
| Tumor size (pancreas) (%) | ||||||
| <2 cm | 33 (47.8) | |||||
| >2 cm | 15 (21.7) | |||||
| Postoperative | 21 (30.5) | |||||
| Liver metastasis (%) | ||||||
| Yes | 28 (40.6) | |||||
| No | 41 (59.4) | |||||
| Presence of MEN-1 (%) | ||||||
| Yes | 6 (8.7) | |||||
| No | 63 (91.3) | |||||
P-value was calculated using 2 × 2 χ2-test or Student t-test or Fisher's exact test.
Significant difference using Fisher's exact test.
Others comprise of a glucagonoma, a somatostatinoma and a VIPoma.
Cytology was performed but not determined Ki67 value and diagnosed with well-differentiated tumor which corresponds to NET G1 or G2 according to the WHO 2010 classification. AIP, autoimmune pancreatitis; CP, chronic pancreatitis; MEN-1, multiple endocrine neoplasia type 1; PC, pancreatic cancer; pNET, pancreatic neuroendocrine tumor; PPI, proton pump inhibitor.
The proportions of the presence of liver metastases and multiple endocrine neoplasia type 1 (MEN-1) in the pNET group were 40.6% and 8.7%, respectively.
Serum chromogranin A level
The measurement result of the serum CGA level in each group is shown in Table 2. The mean serum CGA level of patients with pNET was 6.5-fold higher than in the controls and was significantly higher compared with the controls (P < 0.01). This level was also 4.5-fold higher than those of other groups and was significantly higher compared with those in the PC (P < 0.05) and CP (P < 0.05) groups but not in the AIP group (P = 0.10), which is most likely because of the small sample size. The mean serum CGA level of all patients with PC or CP was 1.5-fold higher than in the controls, but the effect was not significant (P = 0.99, respectively). Next, we conducted a subgroup analysis based on PPI use because PPI can elevate the serum CGA level, and patients with pancreatic diseases often take PPI. The serum CGA level of patients using PPI was significantly higher than that of patients not using PPI in the in the PC (P < 0.05), CP (P < 0.05) and AIP (P < 0.05) groups but not in the pNET group (P = 0.21). In patients not using PPI, the mean serum CGA level of patients with pNET was 7.1-fold higher than in the controls, which was significantly different from the controls (P < 0.01) and patients in the PC group (P < 0.05) but not patients in the CP (P = 0.11) and AIP (P = 0.28) groups. Furthermore, the serum CGA levels of patients in the PC, CP and AIP groups not taking PPI were not different from the controls (P = 0.93, P = 0.90 and P = 0.93, respectively). The distribution of the serum CGA levels in each group is shown in Figure 1.
Table 2.
Result of serum CGA level
| Characteristics | pNET | PC | CP | AIP | Normal |
|---|---|---|---|---|---|
| Total | |||||
| Number | 69 | 50 | 50 | 20 | 112 |
| CGA level (ng/mL) | |||||
| Mean ± SD | 407.8 ± 984.6* | 91.8 ± 101.8† | 93.6 ± 57.5† | 69.9 ± 52.4 | 62.5 ± 48.3 |
| PPI use (%) | |||||
| Yes | |||||
| Number | 19 (27.5) | 19 (38.0) | 28 (56.0) | 9 (45.0) | N/A |
| CGA level (ng/mL) | |||||
| Mean ± SD | 297.7 ± 389.1 | 155.9 ± 129.8‡ | 107.6 ± 66.9‡ | 98.5 ± 64.2‡ | |
| No (%) | |||||
| Number | 50 (72.5) | 31 (62.0) | 22 (44.0) | 11 (55.0) | 112 (100) |
| CGA level (ng/mL) | |||||
| Mean ± SD | 449.6 ± 1132.8* | 52.5 ± 51.2* | 75.7 ± 37.1 | 46.6 ± 24.1 | 62.5 ± 48.3 |
Significant difference between each group was evaluated by Scheffe's multiple comparison.
P < 0.01 versus normal.
P < 0.05 versus pNET. Significant difference between with or without PPI use in the same group was evaluated by Student t-test.;
P < 0.05 versus no PPI use. AIP, autoimmune pancreatitis; CGA, chromogranin A; CP, chronic pancreatitis; PC, pancreatic cancer; pNET, pancreatic neuroendocrine tumor; PPI, proton pump inhibitor.
Fig 1.
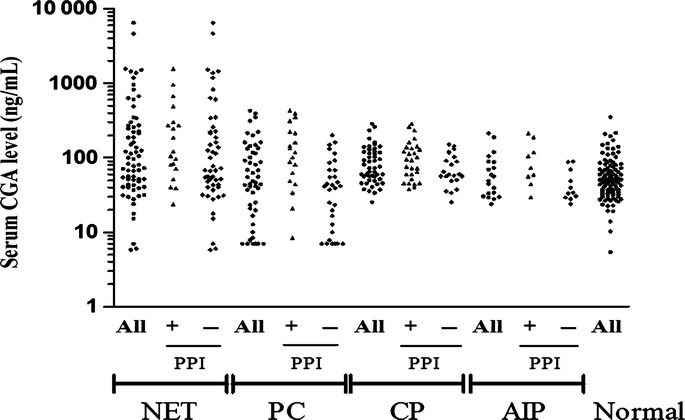
Distribution of serum chromogranin A level in this study.
Regression analyses in pancreatic neuroendocrine tumor group
A more in-depth analysis was performed on patients with pNET. First, we performed regression analysis to clarify the factors associated with an elevation of the serum CGA level. This analysis revealed that the presence of liver metastases was the only associated factor for either single or multiple regression analyses (Table 3). Gender almost reached significance in the single regression analysis (P = 0.063) and the multiple regression analysis (P = 0.061), showing a trend for females to have higher values, but it did not reach significance with this limited number of patients (Table 3).
Table 3.
Single and multiple regression analysis for CGA in patients with pNET
| Factor | Number | Single regression analysis | Multiple regression analysis (r2 = 0.44) | |||
|---|---|---|---|---|---|---|
| β | R | P-value | β | P-value | ||
| Sex | 69 | 441.11 | 0.23 | 0.063 | 551.59 | 0.061 |
| Age | 69 | −2.11 | 0.03 | 0.807 | ||
| PPI use | 69 | −151.85 | 0.07 | 0.571 | −147.60 | 0.056 |
| Tumor classification | 69 | −84.05 | 0.08 | 0.537 | −72.29 | 0.589 |
| Histological grade | 69 | 315.65 | 0.22 | 0.073 | 186.26 | 0.287 |
| Tumor size | 69 | −2.754 | 0.01 | 0.987 | ||
| Liver metastasis | 69 | 667.33 | 0.34 | 0.005* | 622.04 | 0.005* |
| Presence of MEN-1 | 69 | −83.19 | 0.02 | 0.845 | ||
Significant difference. CGA, chromogranin A; MEN-1, multiple endocrine neoplasia type 1; pNET, pancreatic neuroendocrine tumors; PPI, proton pump inhibitor.
Discriminant analysis in pancreatic neuroendocrine tumor group from normal
Next, a discriminant function was calculated to set the best cut-off value of CGA to distinguish patients with pNET from controls. The results showed that the best cut-off value of CGA for distinguishing between patients with pNET and controls was 78.7 ng/mL, with a sensitivity and specificity of 53.6% and 78.6%, respectively (Fig. 2a). A receiver operating characteristic curve was constructed to confirm the results (Fig. 2b).
Fig 2.
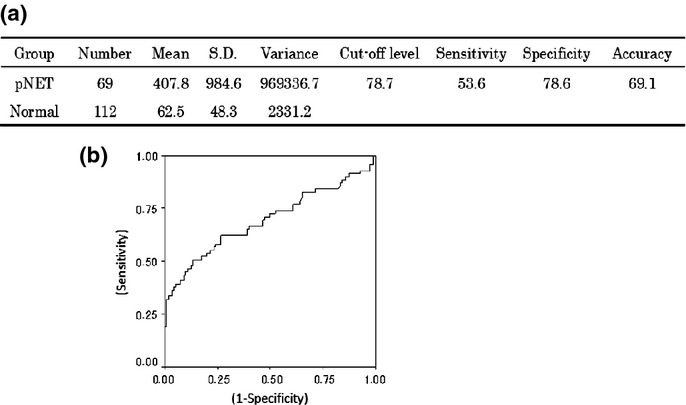
(a) Result of discriminant function calculated in this study. (b) Receiver operating characteristic curve of chromogranin A for patients with pancreatic neuroendocrine tumors versus normal.
Univariate and multivariate analyses of pancreatic neuroendocrine tumor group
Using the cut-off value calculated above, patients with pNET could be divided into two subgroups: one group with a serum CGA level above the cut-off value and the other with a serum CGA level below the cut-off value. Univariate analysis revealed that the tumor classification, the tumor size and the presence of liver metastases were significantly associated with the serum CGA levels above this cut-off value (Table 4). With respect to the tumor classification, pNET patients with non-functioning tumors or gastrinomas characteristically had high serum CGA levels exceeding the cut-off value, especially patients with gastrinomas, in which 16/17 (94%) had a high serum CGA level beyond the cut-off value. Only 1 of the 10 patients with insulinomas had a high serum CGA level above the cut-off value. With respect to the tumor size, 73% (11/15) of pNET patients with tumors larger than 2 cm had high serum CGA levels beyond the cut-off value. By contrast, only 27% (9/33) of pNET patients with tumors smaller than 2 cm had high serum CGA levels that were higher than the cut-off value. Eighty-six percent (24/28) of patients with liver metastases had serum CGA levels that exceeded the cut-off value. By contrast, only 32% (13/41) of patients without liver metastases had serum CGA levels that exceeded the cut-off value. Multivariate analysis revealed that PPI use and the presence of liver metastases were the only factors that were significantly associated with the presence of a serum CGA level beyond the cut-off value (Table 5).
Table 4.
Univariate analysis for the factors that elevate serum CGA level in patients with pNET
| Factor | Total (n = 69) | <Cut-off (n = 32) | Cut-off < (n = 37) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 57.5 ± 13.9 | 55.7 ± 15.1 | 57.2 ± 13.1 | 0.883 |
| Sex (%) | ||||
| Male | 39 | 18 (46.2) | 21 (53.8) | 1 |
| Female | 30 | 14 (46.7) | 16 (53.3) | |
| PPI use (%) | ||||
| Yes | 19 | 5 (26.3) | 14 (73.7) | 0.058 |
| No | 50 | 27 (54.0) | 23 (46.0) | |
| Tumor classification (%) | ||||
| Non-functioning | 39 | 22 (56.4) | 17 (43.6) | <0.0001* |
| Functioning (%) | ||||
| Gastrinoma | 17 | 1 (5.9) | 16 (94.1) | |
| Insulinoma | 10 | 9 (90.0) | 1 (10.0) | |
| Others | 3 | 0 (0.0) | 3 (100) | |
| Histological grade (%) | ||||
| G1 | 40 | 21 (52.5) | 19 (47.5) | 0.290 |
| G2 | 22 | 8 (36.4) | 14 (63.6) | |
| Tumor size (pancreas) (%) | ||||
| <2 cm | 33 | 24 (72.7) | 9 (27.3) | 0.004* |
| >2 cm | 15 | 4 (26.7) | 11 (73.3) | |
| Liver metastasis (%) | ||||
| Yes | 28 | 4 (14.2) | 24 (85.7) | <0.0001* |
| No | 41 | 28 (68.3) | 13 (31.7) | |
| Presence of MEN-1 (%) | ||||
| Yes | 6 | 2 (33.3) | 4 (66.6) | 0.679 |
| No | 63 | 30 (47.6) | 33 (52.3) | |
P-value was calculated using 2 × 2 χ2-test or Fisher's exact test.
Significant difference. MEN-1, multiple endocrine neoplasia type 1; PPI, proton pump inhibitor.
Table 5.
Multivariate analysis (logistic regression analysis) for the factors that elevate serum CGA level in patients with pNET
| Factor | Parameter estimate | R | P-value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| PPI use | 1.5144 | 0.16 | 0.038* | 4.55 (1.09–18.94) |
| Tumor classification | 0.5106 | 0 | 0.159 | 1.67 (0.82–3.39) |
| Liver metastasis | 2.8367 | 0.39 | 0.0001* | 17.06 (4.28–68.02) |
P-value was calculated using 2 × 2 χ2-test or Fisher's exact test.
Significant difference. MEN-1, multiple endocrine neoplasia type 1; PPI, proton pump inhibitor.
Correlation coefficient between pancreatic neuroendocrine tumor and other factors
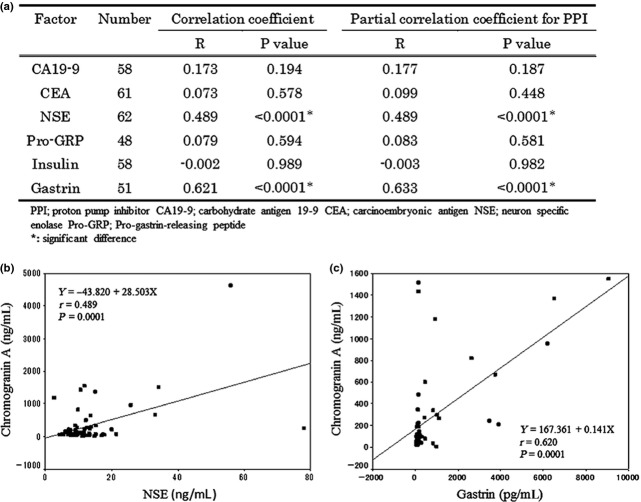
Lastly, we calculated the correlation coefficient between the serum CGA level and the level of other tumor markers or hormones (Fig. 3a). Both serum neuron specific enolase (NSE) and gastrin correlated with the serum CGA level; the correlation coefficient was 0.489 for NSE and 0.621 for gastrin. The same result was obtained by partial correlation analysis for PPI, indicating that these correlation coefficients did not interfere with PPI use. Scatter plots for the relationship between the CGA and NSE or gastrin are shown in Figure 3(b,c).
Fig 3.
(a) Correlation coefficient between serum chromogranin A levels and tumor markers in patients with pancreatic neuroendocrine tumors. Scatter plots for relationship (b) between serum chromogranin A and neuron specific enolase (c) between serum chromogranin A and gastrin.
Discussion
To the best of our knowledge, this study is the first detailed investigation of the serum CGA level in patients with pancreatic diseases, including pNET, in Japan. Our study demonstrates that the serum CGA levels in Japanese patients with pNET are higher than in patients with other pancreatic diseases and controls, demonstrating that assessing the serum CGA is a useful marker for diagnosing pNET in Japan. Previously, serum CGA was generally accepted as a useful marker for diagnosing or assessing the outcome of treatment in patients with pNET in the West and the USA.(1,14–17,29) There are two reports investigating the usefulness of the serum CGA level in an Asian population with NET,(30,31) but there are none in a Japanese population. An investigation of the potential usefulness of the serum CGA levels in Japan is important for a number of reasons. It cannot be assumed that the results of Western patients can be extrapolated to Japanese patients with possible NET because there are differences between Japan and the West with respect to the epidemiology and the therapeutic effects in patients with pNET.(4,10,11) Furthermore, studies report differences in the serum/plasma levels of other tumor markers, such as PSA levels or alpha-fetoprotein levels, between Japanese/Asian populations and Western populations.(13,25) This raises the possibility that these differences may also extend to differences between populations in the serum CGA level in patients with pNET. Furthermore, the recent development of effective therapies for treating patients with advanced pNET raises the possibility that earlier diagnosis of these tumors may increase the survival rate, which has not been reported. However, presently, in Japan, no serum marker is generally used for diagnosing pNET, which is in part because none has been verified in a Japanese population as a useful marker for pNET. For these reasons, in the present study we attempted to determine whether the serum CGA is a useful marker in Japan, as found in the West and the USA.
It is sometimes difficult to distinguish between a pNET and other pancreatic diseases, particularly between PC and a non-functioning pNET. Therefore, in the present study, we specifically evaluated whether the assessment of the serum CGA level is a useful marker in differentiating between pNET and other pancreatic diseases. It is reported that CGA levels can be elevated in patients with PC and CP,(24) and there is a case report of a patient with AIP with an elevated serum CGA level.(32) In the present study, the average serum CGA level seems high in patients with PC, CP or AIP, but the difference is not significantly different compared with the controls. The results are conflict with those reported previously for other countries.(24)
Because PPI use is known to be a significant factor for elevating serum CGA levels,(15,33–35) we examined the effect of PPI on the serum CGA levels in our Japanese patients. We found that PPI significantly increased the serum CGA levels in our patients, which was particularly true in patients with PC, CP or AIP. In patients not using PPI, the serum CGA level of patients with these pancreatic diseases was almost equivalent to that of the controls, respectively. These results indicate that the serum CGA level is a useful marker in differentiating between pNET and other pancreatic diseases and controls; however, this is only true if patients are not taking PPI. To more accurately distinguish between pNET and other pancreatic disease, PPI should be discontinued or replaced by histamine2-receptor antagonists for 2 weeks before measurement(34,35)
In Japan, prior to this study, the normal range of CGA had not been investigated or determined. Therefore, we systematically evaluated the upper limit of the standard value of the serum CGA in Japanese people by calculating the best cut-off value of CGA, which distinguishes patients with pNET from controls. Discriminant analysis revealed that the best cut-off value of the serum CGA for distinguishing patients with pNET from controls was 78.7 ng/mL, which had a sensitivity and specificity of 53.6% and 78.6%, respectively, for identifying patients with pNET. This cut-off value can be used as the upper limit of the standard value of Japanese people if this assay is used. Previous studies in Western patients report a sensitivity of serum CGA in any neuroendocrine tumor of 53–92%,(14,16,36,37) which is 50–74% in patients with pNET;(38–40) we also observed this range, demonstrating that this tumor marker has a similar sensitivity in Japanese and Western patients for identifying pNET.
In some studies(31,37,38,41) but not in others(40,42) in the West, the extent of metastatic disease and, in particular, the presence of metastatic liver diseases is one of the factors that is most frequently associated with elevated serum CGA levels and in some studies in proportion to the magnitude of increase.(37,38,40–42) In our study, both univariate and multivariate analyses revealed that the presence of liver metastases was associated with elevation of the serum CGA level in patients with pNET. In addition, in our study, the pNET group includes patients who developed liver metastases after resection of the primary pancreatic tumor as well as those with liver metastases at presentation. These results suggest that the serum CGA may also be a useful marker for detecting relapse in postoperative Japanese patients with pNET postresection. This conclusion is supported by the results from a number of Western studies.(17,43) In the univariate analysis, tumor classification and tumor size were also significantly associated with a serum CGA level exceeding the cut-off value for distinguishing between pNET and controls. This result is in agreement with some,(1,36–38,41) but not all Western studies(39) examining the effect of the primary tumor size and/or tumor classification on the serum CGA levels. With respect to tumor classification, pNET are heterogeneous tumors with different subtypes, and they may behave differently.(44) In our study, the serum CGA level in patients with non-functioning pNET or with gastrinomas tend to be higher, and those with insulinomas have lower serum CGA levels, which indicates that assessing the serum CGA is likely to be more useful for non-functioning pNET and gastrinomas than for insulinomas. This conclusion is supported by the results of the correlation analysis between the serum CGA levels and serum gastrin levels in patients with pNET. These results are consistent with studies in Western patients that show a similar difference in the serum CGA levels for the subtype of pNET;(36–38,45) however, these findings differ from other studies in Western patients that do not report this correlation.(39) Our results demonstrate that it is important to consider tumor classification of pNET when measuring the serum CGA levels in Japanese patients.
Neuron specific enolase has been used as a marker for neuroendocrine tumors, especially neuroendocrine carcinoma.(36,39,46) In our study the correlation analysis revealed that the serum CGA levels correlated with the serum NSE levels in patients with NET G1/G2. However, when the serum cut-off value of NSE was set at the level that was defined as the upper limit of normal in our institution, the sensitivity was only 25.8%. This result indicates that the serum CGA level is a more useful marker with higher sensitivity than the serum NSE level for pNET. These results are consistent with other Western studies that generally show that assessing the serum NSE is less accurate than assessing the serum CGA level in patients with either pNET or GI-NET.(36,39,46)
In addition to the points raised above, there are a number of additional conditions that can affect the CGA level. In some cases, pNET may be part of MEN-1.(1,47) Patients with MEN-1 develop endocrine tumors in multiple organs and these can affect the serum CGA levels.(17,41,45) In our study, the serum CGA levels were not significantly different between patients with pNET and co-occurring MEN-1 and those with pNET without MEN-1. The incidence of co-occurring MEN-1 in patients with pNET is different between Japan and other countries,(4) which may be associated with the difference between the results of this study and those reported previously.(17,41,45) In addition, a number of clinical conditions can result in slight elevations of the serum CGA levels, including renal sufficiency and hepatic dysfunction;(2,14,48) such conditions were not evaluated in the current study. In addition, serum CGA levels can vary with different measurement kits or samples; that is, serum or plasma.(15,48–50) In general, blood samples for measuring the serum CGA level should be collected in the resting state because the serum CGA is released with catecholamine in neuroendocrine cells by sympathetic stimulation.(51) We should standardize the conditions when measuring the CGA levels in Japan (e.g. whether the serum or plasma should be used, whether the level should be measured by RIA or IRMA or ELISA, and whether sample collection should be limited to the resting state). In this study, we used serum samples whose collection was not limited to the resting state using an ELISA kit. The CGA levels in the serum were lower than those in plasma, but there was no conclusion regarding which was better.(50) ELISA has been reported as one of the best techniques for determining the CGA level.(49,52) Furthermore, the results of the present study were similar to those of other reports that did not demonstrate whether the sample had to be collected in the resting state.(38–40) For these reasons, we considered the conditions set in this study as applicable.
In conclusion, this study demonstrates that the serum CGA level is a useful marker in patients with pNET in Japan as well as in Western countries, as previously reported,(1,14,16,29) especially in patients with gastrinomas or non-functioning tumors with the presence of liver metastases and larger tumors. pNET are uncommon and variable diseases, as described above; therefore, the availability of a tumor marker should be clinically helpful for diagnosis and treatment. The diagnosis of these patients is of particular importance at present because of the description of newer treatments, which are effective for patients with advanced disease. Our studies suggest that the serum CGA levels may fulfill this need; however, it is important for clinicians to remember that other clinical factors can affect serum CGA levels, such as PPI use, which should be factored into the interpretation of the results.
Acknowledgments
The authors are most grateful to N. Taki belonging to Statistical Analysis of medical science.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- 1.Öberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–81. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0934-2. [Epub ahead of print]; doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Igarashi H, Jensen RT. Zollinger-ellison syndrome: recent advances and controversies. Curr Opin Gastroenterol. 2013;29:650–61. doi: 10.1097/MOG.0b013e328365efb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941–60. doi: 10.1007/s00535-012-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Okusaka T, Ikeda M, et al. Everolimus for advanced pancreatic neuroendocrine tumours: a subgroup analysis evaluating Japanese patients in the radiant-3 trial. Jpn J Clin Oncol. 2012;42:903–11. doi: 10.1093/jjco/hys123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Okusaka T, Nishida T, et al. Phase II study of sunitinib in japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drugs. 2013;31:1265–74. doi: 10.1007/s10637-012-9910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganeshan D, Paulson E, Duran C, Cabanillas ME, Busaidy NL, Charnsangavej C. Current update on medullary thyroid carcinoma. AJR Am J Roentgenol. 2013;201:W867–76. doi: 10.2214/AJR.12.10370. [DOI] [PubMed] [Google Scholar]
- 13.Kimura T. East meets west: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–9. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Law C. Chromogranin A: a sensitive biomarker for the detection and post-treatment monitoring of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2012;6:313–34. doi: 10.1586/egh.12.15. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Igarashi H, Jensen RT. Serum pancreastatin: the long sought universal, sensitive, specific tumor marker for neuroendocrine tumors? Pancreas. 2012;41:505–7. doi: 10.1097/MPA.0b013e318249a92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967–73. doi: 10.1200/JCO.2006.10.1535. [DOI] [PubMed] [Google Scholar]
- 17.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–43. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 18.Konecki DS, Benedum UM, Gerdes H-H, Huttner WB. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987;262:17026–30. [PubMed] [Google Scholar]
- 19.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–49. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 20.Zissimopoulos A, Vradelis S, Konialis M, et al. Chromogranin A as a biomarker of disease activity and biologic therapy in inflammatory bowel disease: a prospective observational study. Scand J Gastroenterol. 2014;498:942–9. doi: 10.3109/00365521.2014.920910. [DOI] [PubMed] [Google Scholar]
- 21.Bech PR, Ramachandran R, Dhillo WS, Martin NM, Bloom SR. Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine- and amphetamine-regulated transcript. Clin Chem. 2012;585:941–3. doi: 10.1373/clinchem.2011.176099. [DOI] [PubMed] [Google Scholar]
- 22.Spadaro A, Ajello A, Morace C, et al. Serum chromogranin-A in hepatocellular carcinoma: diagnostic utility and limits. World J Gastroenterol. 2005;1113:1987–90. doi: 10.3748/wjg.v11.i13.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura N, Yoshida R, Shiraishi S, Pilichowska M, Ohuchi N. Chromogranin A and chromogranin B in noninvasive and invasive breast carcinoma. Endocr Pathol. 2002;132:117–22. doi: 10.1385/ep:13:2:117. [DOI] [PubMed] [Google Scholar]
- 24.Malaguarnera M, Cristaldi E, Cammalleri L, et al. Elevated chromogranin A (CgA) serum levels in the patients with advanced pancreatic cancer. Arch Gerontol Geriatr. 2009;48:213–7. doi: 10.1016/j.archger.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Lambe M, Trichopoulos D, Hsieh CC, Wuu J, Adami HO, Wide L. Ethnic differences in breast cancer risk: a possible role for pregnancy levels of alpha-fetoprotein? Epidemiology. 2003;14:85–9. doi: 10.1097/00001648-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th edn. Vol. 3. Lyon: IARC; 2010. [Google Scholar]
- 27.Shimosegawa T, Kataoka K, Kamisawa T, et al. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol. 2010;45:584–91. doi: 10.1007/s00535-010-0242-4. [DOI] [PubMed] [Google Scholar]
- 28.Shimosegawa T, Kanno A. Autoimmune pancreatitis in Japan: overview and perspective. J Gastroenterol. 2009;44:503–17. doi: 10.1007/s00535-009-0054-6. [DOI] [PubMed] [Google Scholar]
- 29.Nobels FR, Kwekkeboom DJ, Bouillon R, Lamberts SW. Chromogranin A: its clinical value as marker of neuroendocrine tumours. Eur J Clin Invest. 1998;28:431–40. doi: 10.1046/j.1365-2362.1998.00305.x. [DOI] [PubMed] [Google Scholar]
- 30.Chou WC, Hung YS, Hsu JT, et al. Chromogranin A is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology. 2012;95:344–50. doi: 10.1159/000333853. [DOI] [PubMed] [Google Scholar]
- 31.Paik WH, Ryu JK, Song BJ, et al. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. J Korean Med Sci. 2013;28:750–4. doi: 10.3346/jkms.2013.28.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobili E, Pezzilli R, Santini D, et al. Autoimmune pancreatitis associated with high levels of chromogranin a, serotonin and 5-hydroxyindoleacetic acid. Case Rep Gastroenterol. 2008;2:11–7. doi: 10.1159/000113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanduleanu S, De Bruïne A, Stridsberg M, et al. Serum chromogranin A as a screening test for gastric enterochromaffin-like cell hyperplasia during acid-suppressive therapy. Eur J Clin Invest. 2001;31:802–11. doi: 10.1046/j.1365-2362.2001.00890.x. [DOI] [PubMed] [Google Scholar]
- 34.Mosli HH, Dennis A, Kocha W, Asher LJ, Van Uum SH. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J Clin Endocrinol Metab. 2012;97:E1731–5. doi: 10.1210/jc.2012-1548. [DOI] [PubMed] [Google Scholar]
- 35.Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. Br J Cancer. 2011;1058:1173–5. doi: 10.1038/bjc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobels FRE, Kwekkeboom DJ, Coopmans W, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the a-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622–8. doi: 10.1210/jcem.82.8.4145. [DOI] [PubMed] [Google Scholar]
- 37.Belli SH, Oneto A, Aranda C, et al. Chromogranin A as a biochemical marker for the management of neuroendocrine tumors: a multicenter study developed in argentina. Acta Gastroenterol Latinoam. 2009;39:184–9. [PubMed] [Google Scholar]
- 38.Tomassetti P, Migliori M, Simoni P, et al. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2001;13:55–8. doi: 10.1097/00042737-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Baudin E, Gigliotti A, Ducreux M, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78:1102–7. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panzuto F, Severi C, Cannizzaro R, et al. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6–11. doi: 10.1007/BF03350903. [DOI] [PubMed] [Google Scholar]
- 41.Nehar D, Lombard-Bohas C, Olivieri S, et al. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf) 2004;60:644–52. doi: 10.1111/j.1365-2265.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 42.Cimitan M, Buonadonna A, Cannizzaro R, et al. Somatostatin receptor scintigraphy versus chromogranin A assay in the management of patients with neuroendocrine tumors of different types: clinical role. Ann Oncol. 2003;14:1135–41. doi: 10.1093/annonc/mdg279. [DOI] [PubMed] [Google Scholar]
- 43.Welin S, Stridsberg M, Cunningham J, et al. Elevated plasma chromogranin A is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology. 2009;89:302–7. doi: 10.1159/000179900. [DOI] [PubMed] [Google Scholar]
- 44.Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol. 2012;26:737–53. doi: 10.1016/j.bpg.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peracchi M, Conte D, Gebbia C, et al. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. Eur J Endocrinol. 2003;148:39–43. doi: 10.1530/eje.0.1480039. [DOI] [PubMed] [Google Scholar]
- 46.Korse CM, Taal BG, Vincent A, et al. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662–71. doi: 10.1016/j.ejca.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Niina Y, Fujimori N, Nakamura T, et al. The current strategy for managing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1. Gut Liver. 2012;6:287–94. doi: 10.5009/gnl.2012.6.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glinicki P, Jeske W. Chromogranin A (CgA) - the influence of various factors in vivo and in vitro, and existing disorders on its concentration in blood. Endokrynol Pol. 2011;62(Suppl 1):25–8. [PubMed] [Google Scholar]
- 49.Molina R, Alvarez E, Aniel-Quiroga A, et al. Evaluation of chromogranin A determined by three different procedures in patients with benign diseases, neuroendocrine tumors and other malignancies. Tumor Biol. 2011;32:13–22. doi: 10.1007/s13277-010-0085-x. [DOI] [PubMed] [Google Scholar]
- 50.Glinicki P, Kapuścińska R, Jeske W. The differences in chromogranin A (CgA) concentrations measured in serum and in plasma by IRMA and ELISA methods. Endokrynol Pol. 2010;61:346–50. [PubMed] [Google Scholar]
- 51.Louthan O. Chromogranin a in physiology and oncology. Folia Biol (Praha) 2011;57:173–81. [PubMed] [Google Scholar]
- 52.Zatelli MC, Torta M, Leon A, et al. Chromogranin A as a marker of neuroendocrine neoplasia: an italian multicenter study. Endocr Relat Cancer. 2007;142:473–82. doi: 10.1677/ERC-07-0001. [DOI] [PubMed] [Google Scholar]