Abstract
Invasive lobular carcinoma (ILC) accounts for approximately 10% of all breast carcinomas and is characterized by higher levels of androgen receptor (AR) compared to invasive ductal carcinoma (IDC). Despite this potentially androgen-responsive environment, the combined importance of AR and androgen metabolism in non-neoplastic lobules and lobular carcinoma remains unknown. Therefore, in this study, we evaluated the status of pivotal androgen-producing enzymes 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5) and 5α-reductase type 1 (5αRed1) in 178 cases of ILC and surrounding histologically non-neoplastic lobular tissue using immunohistochemistry. Androgen receptor prevalence was higher but androgenic enzymes lower in ILC than non-neoplastic lobules. In ILC cases the status of 5αRed1 and 17βHSD5 was inversely correlated with tumor size (P = 0.0053) and nuclear grade (P = 0.0290), and significantly associated with better overall survival of the patients (P = 0.0059). Based on these findings, we hypothesized that androgen signaling could act as a tumor suppressor. As previous studies suggested that androgens might partially act by increasing levels of the estrogen inactivating enzyme 17β-hydroxysteroid dehydrogenase type 2 (17βHSD2) in IDC tissues, this was reasonably considered a potential mechanism of androgen actions. Significantly positive correlation was detected between the status of androgenic enzymes and 17βHSD2 (P < 0.0001) and intratumoral 17βHSD2 was inversely correlated with tumor size in ILC (P = 0.0075). These correlations suggest one protective mode of androgen action could be through modulation of estrogen metabolism. Results of our present study indicated that androgen-producing enzymes could play pivotal protective roles in AR-enriched ILC cases.
Keywords: Androgen, aromatase, breast cancer, estrogen, invasive lobular carcinoma
Invasive lobular carcinoma accounts for approximately 10% of all breast invasive carcinoma cases,(1,2) and its incidence is increasing among postmenopausal women.(3,4) Invasive lobular carcinoma is distinguished from IDC by pathological, biological, and molecular characteristics. Invasive lobular carcinoma is especially characterized by the loss of E-cadherin in carcinoma cells,(5–7) which plays a hallmark role in histopathological differentiation from IDC. In addition, ILC patients generally have larger tumor size and more abundant ER/PR content but, paradoxically, lower cell proliferation compared to IDC patients.(1,8,9) This very abundance of ER/PR has been postulated to account for a better response to adjuvant hormone therapy in ILC compared to IDC patients.(8)
In addition to estrogens and progesterones, androgens have been recently considered to play pivotal roles in both the presence and absence of ER and PR. Androgens exert their actions through AR, the presence of which is reported in the great majority of breast cancer patients, more frequently than ER and PR.(10,11) The presence of AR is particularly marked in ILC with expression in 90% of ILC patients compared to 56% in IDC patients.(8,12) In addition, the clinical or biological significance of androgen signaling is different depending on the presence or absence of ER and the equilibrium of signaling between these male and female hormones is proposed to exert an important impact upon the biological or clinical significance of the patients.(13–15) In general, androgen actions in the presence of ER antagonize ER binding, subsequently resulting in suppression of estrogen-mediated tumorigenesis. This hypothesis has been supported by results of in vitro studies indicating suppressed estrogen signaling by direct binding of AR to ER responsive element(15) and suppression of cell proliferation by androgen treatment.(16–18)
The delicate equilibrium between androgen and estrogen signaling in breast carcinoma cells is first and foremost regulated by the levels of androgen and estrogen receptors(15,19,20) but an additional level of regulation comes from intracrine metabolism of steroid hormones.(21) It is entirely true that the status of intratumoral androgen metabolism has been less frequently explored than that of estrogen, but the contribution of this intracrine system to the generation of androgen signaling within tissues is supported by the significantly increased localized levels of potent androgen DHT in postmenopausal breast cancers.(22,23) Therefore, it is also pivotal to study the status of intratumoral androgen metabolism in breast cancer. The two principle androgen metabolizing enzymes are 17βHSD5 and 5αRed1.(24) We have previously reported that the co-expression of AR and 5αRed1 in invasive ductal carcinomas was indeed associated with better clinical outcome of the patients with IDC,(25) which is consistent with the overall roles of androgens as tumor suppressors in ER-positive or luminal-type breast carcinoma.
In addition, AIs, considered the gold standard of endocrine therapy of ER-positive postmenopausal breast cancer patients, have been reported to exert antitumor effects through not only decreasing the levels of estrogens available for carcinoma cells but also increasing intratumoral androgen concentrations, most probably due to the precursor–product relationship between intratumoral androgens and estrogens.(26–28) Recently we showed that the increased androgen concentration in breast cancer tissues following AI exemestane treatment was significantly associated with an increment of 17βHSD2. The latter is well known to decrease the levels of potent estrogens, estradiol, and thus overall estrogen signaling.(26,27) In addition, we also reported an increased expression level of 17βHSD2 by DHT or exemestane treatment in a breast carcinoma cell line, suggesting 17βHSD2 expression does reflect intratumoral androgenic actions in breast cancer tissues and could possibly account for the tumor suppressive actions of androgen signaling in estrogen-dependent breast cancers.
Invasive lobular carcinoma has been reported to have more abundant AR in carcinoma cells than IDC, as described above,(8,12) but the clinical and biological significance of androgen signaling has remained largely unexplored. In studies attempting this comparison, the interpretation of their findings has been extremely difficult due to the relative rarity of ILC and the high prevalence of AR in ILC patients. In addition, the prevalence of AR and androgen-synthesizing enzymes in non-neoplastic human lobular tissues as compared to carcinomas has been virtually unknown. Therefore, in this study, we examined the intratumoral status of AR and androgen-producing enzymes in non-neoplastic lobules and ILC tissues in order to assess the associations between increased androgen production and/or signaling and various clinicopathological factors of ILC cases, including clinical outcome of the patients. We also studied the status of the androgen-induced estrogen metabolizing enzyme 17βHSD2 and compared its status with that of the androgenic enzymes to further explore the mechanisms of androgen actions in ILC patients.
Materials and Methods
Formalin-fixed, paraffin-embedded tissues
Invasive lobular carcinoma cases examined in this study were all surgical specimens retrieved from surgical pathology files of Tohoku University Hospital (Sendai, Japan), Sagara Hospital (Kagoshima, Japan), and Tohoku Kosai hospital (Sendai, Japan). None of the patients received hormonal therapy prior to surgery; in the few patients that had received chemotherapy before surgery, results were no different from those who did not. The mean age of the patients was 57 years (range, 32–91). Clinicopathological findings including menopausal status, stage, nuclear grade, ER, PR, Her2, and Ki-67 were available in all of the cohorts examined, and data regarding the tumor size and clinical outcome available in the cohorts of Tohoku University Hospital and Kosai Hospital. Invasive ductal carcinoma cases examined in this study were also retrieved from surgical pathology files of Tohoku University Hospital. The mean age was 59 years (range, 34–82). All the specimens had been fixed with 10% formalin and embedded in paraffin. The research protocol was approved by the Ethics Committee at Tohoku University School of Medicine and review boards of all participating institutions.
Fresh frozen tissues
In addition to the 10% formalin-fixed and paraffin-embedded tissues described above, four ILC and four IDC fresh-frozen specimens were available for RT-PCR study. These specimens were obtained from Sagara Hospital and Tohoku University Hospital in 2010 and 2011. The clinicopathological findings, including age and the ER/PR and Her2 status of the patients, are summarized in Table 1. The research protocol was approved by the Ethics Committee of Tohoku University School of Medicine and the Sagara Hospital review board.
Table 1.
Characteristics of invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) cases used for RT-PCR analysis
| Age, years | ER† | PR† | Her2 | |
|---|---|---|---|---|
| IDC-1 | 47 | 5 | 5 | – |
| IDC-2 | 76 | 5 | 3 | − |
| IDC-3 | 60 | 5 | 4 | − |
| IDC-4 | 46 | 5 | 4 | − |
| ILC-1 | 60 | 5 | 5 | − |
| ILC-2 | 55 | 5 | 5 | − |
| ILC-3 | 52 | 5 | 4 | − |
| ILC-4 | 48 | 5 | 5 | − |
Positivity is presented as: 5, 90–100%; 4, 50–90%; 3, 10–50%; 2, 1–10%; 1, 0–1%; 0, 0%. –, Not detected; ER, Eestrogen receptor; PR, Pprogesterone receptor; Her2, human epidermal growth factor receptor 2.
Immunohistochemistry
Staining for 17βHSD5, 5αRed1, AR, and 17βHSD2 was carried out, as summarized in Table 2. A Histofine Kit (Nichirei Bioscience, Tokyo, Japan), based on the biotin–streptavidin method, was used for immunohistochemical staining in this study. After deparaffinization, antigen retrieval was carried out by heating the slides in an autoclave at 120°C for 5 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate [pH 6.0]) for immunostaining of 17βHSD5 and AR. The antigen–antibody complex was visualized with 3, 3′-diaminobenzidine solution (1 mM 3, 3′-diaminobenzidine, 50 mM Tris–HCl buffer [pH 7.6], and 0.006% H2O2) and counter-stained with hematoxylin.
Table 2.
Antibodies used for immunostaining of androgen-producing enzymes in 178 cases of invasive lobular carcinoma and surrounding non-neoplastic lobular tissue
| Primary antibody | Dilution | Source | Host | Antigen retrieval |
|---|---|---|---|---|
| 17βHSD5 | 1:200 | Sigma (St. Louis, MO, USA) | Mouse | Autoclave |
| 5αRed1 | 1:1000 | Abcam (Cambridge, UK) | Goat | None |
| AR | 1:50 | Dako (Kyoto, Japan) | Mouse | Autoclave |
| 17βHSD2 | 1:200 | Proteintech (Chicago, IL, USA) | Rabbit | None |
17βHSD2, 17β-hydroxysteroid dehydrogenase type 2; 17βHSD5, 17β-hydroxysteroid dehydrogenase type5; 5α-Red1, 5α-Reductase type 1; AR, Aandrogen receptor.
Evaluation of immunoreactivity
Androgen receptor immunoreactivity was assessed by LI.(29) Immunoreactivity was detected in the nuclei. We counted more than 1000 breast carcinoma cells in each case. Subsequently the percentage of immunoreactivity was determined. Labeling index was used to obtain a proportion of immunoreactive tumor cells. The cases with <10% positivity were considered negative according to our previous reports.(25) Both 17βHSD5 and 5αRed1 immunoreactivity was detected in the cytoplasm, and the cases were tentatively classified into the following two groups: negative, 0–50%; positive, 50–100%, as previously described.(28) Immunoreactivity of 17βHSD2 was also detected in the cytoplasm and classified into two groups: negative, 0–10%; and positive, 10–100%.(30)
Reverse transcription–polymerase chain reaction
Total RNA of IDC and ILC tissues were extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). Complementary DNA for RT2 qPCR was synthesized using RT2 First Strand Kit in accordance with manufacturer's protocol. All IDC and ILC cases were analyzed for 17βHSD2 expression using RT2 SYBR Green qPCR Mastermixes (Qiagen, Hilden, Germany). Polymerase chain reaction was carried out in an ABI7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Data analyses were carried out with the web-based software package for the RT2 Profiler PCR Array Data Analysis (http://www.sabiosciences.com/pcr/arrayanalysis.php).
Statistical analysis
All statistical analyses were carried out using JMP Pro 9.0.2 (SAS Institute Japan, Tokyo, Japan). The associations between androgenic enzymes and clinicopathological parameters of the cases examined were evaluated using Student's t-test or the χ2-test depending on whether the variable was continuous or categorical. Both DFS and OS were analyzed according to the Kaplan–Meier method, and the statistical significance was assessed using the log–rank test. Univariate and multivariate analyses were employed in this study using Cox's proportional hazard model. These analyses were limited to the cases with at least 5 years follow-up duration (2008 or earlier surgical date) due to the long latency period associated with this neoplasm.
Results
Immunoreactivity in ILC cases and adjacent non-neoplastic lobules
Androgen receptor immunoreactivity was detected in the nuclei of tumor cells, and 17βHSD5 and 5αRed1 immunoreactivity in the cytoplasm of tumor cells (Fig. 1). Androgen receptor was highly prevalent with positive nuclei detected in almost all the carcinoma cells in ILC. In ILC, AR-positive cases (defined as >10% LI) were 97.8% (174 cases out of 178) and the mean value of AR LI was 90.6 ± 1.4 (range, 0–100%). The proportion of 17βHSD5- and 5αRed1-positive cases (defined as >50% tumor cells of cytoplasmic immunoreactivity) were 61.8% and 53.4%, respectively, with 34.8% double-positive for both enzymes.
Fig 1.
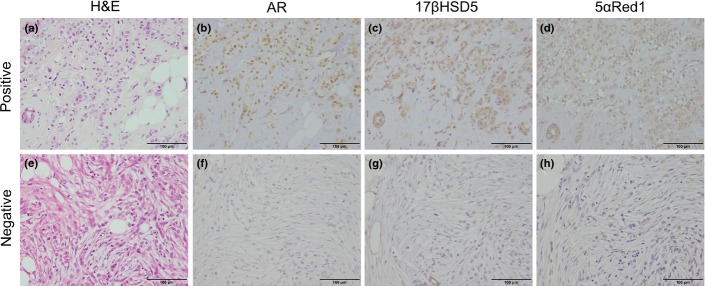
Representative illustrations of androgen receptor (AR), 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5), and 5α-Reductase type 1 (5αRed1) immunohistochemistry in AR+/17βHSD5+/5αRed1+ (b–d) and AR−/17βHSD5−/5αRed1− (f–h) invasive lobular carcinoma cases. Hematoxylin–eosin staining (a,e). Androgen receptor was immunolocalized in the nuclei of carcinoma cells (b,f), and 17βHSD5 (c,g) and 5αRed1 (d,h) immunolocalized in the cytoplasm of carcinoma cells. Scale bar = 100 μm.
The status of AR and androgenic enzymes in non-neoplastic lobular epithelial cells adjacent to ILC was also examined in this study. Androgen receptor immunoreactivity was also detected in non-neoplastic lobular epithelium but its prevalence was lower than that in carcinoma. When quantified, all non-neoplastic lobular epithelium in the cases examined (n = 28) were AR-positive (defined as >10% LI) and the mean value of AR LI was 63.2 ± 4.0 (range, 30–95%). The 17βHSD5- and 5αRed1-positive cases (defined as >50% tumor cells of cytoplasmic immunoreactivity) constituted 89.3% (25 cases out of 28) and 71.4% (20 out of 28), respectively. Immunoreactivity of both AR and androgenic enzymes were only sporadically detected in basal or myoepithelial cells of the non-neoplastic lobules adjacent to ILC.
The AR LI score was significantly higher in ILC than adjacent non-neoplastic lobular epithelium (P < 0.0001) but 17βHSD5 and 5αRed1 were significantly higher in non-neoplastic adjacent lobular epithelium than in ILC (P = 0.0028 and P = 0.0128, respectively). The status of AR in non-neoplastic lobular epithelium was similar to that in ductal epithelium, although not quantified.
Correlations of intratumoral androgen metabolizing enzymes and clinicopathological factors in ILC cases
The AR-negative cases (n = 4) were excluded to analyze the effects of androgen metabolizing enzymes on AR-mediated actions in ILC. We tentatively classified the 174 AR-positive ILC cases into the following two groups: a 17βHSD5 and 5αRed1 double-negative cohort of patients lacking any form of androgen synthesis (45 samples); and a group encompassing all other cases (129 samples) according to the potential local production of androgens. The associations between the status of intratumoral androgenic enzymes and clinicopathological parameters of the patients are summarized in Table 3. Age, menopausal status, ER, Her2, and Ki-67 status were not correlated with the status of intratumoral androgenic enzymes. Stage and the status of PR tended to be inversely correlated with that of intratumoral androgenic enzymes (P = 0.0720 and P = 0.0663, respectively). Tumor size and nuclear grade were inversely correlated with the status of intratumoral androgen metabolizing enzymes (P = 0.0053 and P = 0.0290, respectively). The mean tumor size was also higher in the 17βHSD5–5αRed1 double-negative group of patients (Fig. 2), suggesting the correlation of tumor size with the presence of intratumoral androgenic enzymes.
Table 3.
Associations between the status of intratumoral androgenic enzymes and clinicopathological parameters in androgen receptor (AR)-positive invasive lobular carcinoma (ILC) cases (n = 174)
| 17βHSD5/5αRed1 |
|||
|---|---|---|---|
| −/− n = 45 (25.9%) | Others n = 129 (74.1%) | P-value | |
| Age,† (years) | 56.0 ± 1.9 | 57.9 ± 1.0 | 0.3632 |
| Menopausal status, n (%) | |||
| Premenopausal | 22 (12.7%) | 46 (26.4%) | 0.1173 |
| Postmenopausal | 23 (13.2%) | 83 (47.7%) | |
| Stage, n (%) | |||
| 1 | 14 (8.0%) | 60 (34.5%) | 0.0720 |
| 2 + 3 | 31 (17.8%) | 69 (39.7%) | |
| Tumor size,‡ n (%) | |||
| <20 mm | 12 (9.8%) | 53 (43.1%) | 0.0053 |
| ≥20 mm | 24 (19.5%) | 34 (27.6%) | |
| Nuclear grade, n (%) | |||
| 1 | 7 (4.0%) | 42 (24.1%) | 0.0290 |
| 2 + 3 | 38 (21.9%) | 87 (50.0%) | |
| ER status, n (%) | |||
| Negative | 2 (1.2%) | 11 (6.3%) | 0.3698 |
| Positive | 43 (24.7%) | 118 (67.8%) | |
| PR status, n (%) | |||
| Negative | 10 (5.8%) | 48 (27.6%) | 0.0663 |
| Positive | 35 (20.1%) | 81 (46.5%) | |
| Her2 status, n (%) | |||
| Negative | 45 (25.9%) | 128 (73.5%) | 0.5536 |
| Positive | 0 (0.0%) | 1 (0.6%) | |
| Ki-67 LI,† (%) | 4.3 ± 0.7 | 6.0 ± 0.8 | 0.2672 |
Data are presented as mean ± SEM. All other values represent the number of the cases and percentage. ‡Data regarding the tumor size were available in the cohorts of Tohoku University Hospital and Kosai Hospital. 17βHSD5, 17β-hydroxysteroid dehydrogenase type 5; 5αRed1, 5α-reductase type 1; ER, Eestrogen receptor; Her2, human epidermal growth factor receptor 2; LI, labeling index; PR, Progesterone receptor.
Fig 2.
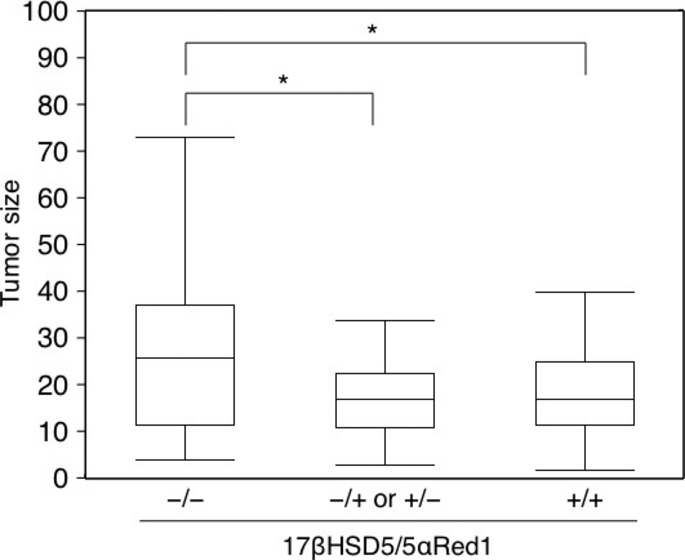
Tumor size was inversely correlated with the status of androgenic enzymes in androgen receptor-positive invasive lobular carcinoma cases. Data of tumor size were available from a subset of breast cancer patients treated at Tohoku University Hospital or Tohoku Kosai Hospital. *P < 0.05. 17βHSD5, 17β-hydroxysteroid dehydrogenase type 5; 5αRed1, 5α-reductase type 1.
Correlation between status of intratumoral androgenic enzymes and clinical outcome
Data regarding OS and DFS were available for cases from Tohoku University Hospital and Tohoku Kosai Hospital. As the cases were limited to those that had a follow-up period >5 years, the number available for this analysis was 72. Despite the relatively small number of cases examined, the absence of intratumoral androgenic enzymes was significantly associated with adverse OS (Fig. 3a) but not with DFS (Fig. 3b) and this association still remained significant in the multivariate analysis (model including ER, PR, stage, and enzyme status; data not shown).
Fig 3.
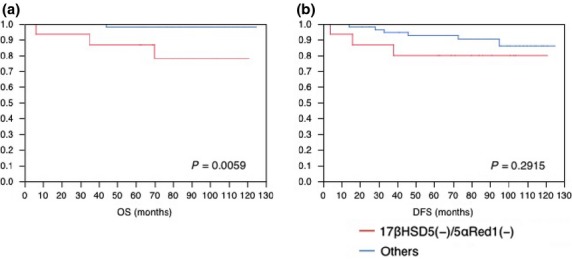
Absence of intratumoral androgenic enzymes was significantly correlated with adverse clinical outcome in breast cancer patients. Clinical information regarding overall survival (OS) and disease-free survival (DFS) was available for cases from Tohoku University Hospital and Tohoku Kosai Hospital. Only patients with survival data greater than 5 years (surgical date before 2008) were included in this study. The OS (a) and DFS (b) of these patients were analyzed according to the status of intratumoral androgenic enzymes 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5) and 5α-reductase type 1 (5αRed1) using the Kaplan–Meier method (n = 72).
Androgen and 17βHSD2-producing enzymes in ILC
It was previously reported that 17βHSD2 reflected intratumoral androgenic actions in breast cancer.(27) In this study, 17βHSD2 mRNA levels were quantified in the four ILC and four IDC cases summarized in Table 1 with RT2 qPCR, in order to compare 17βHSD2 mRNA expression between ILC and IDC cases. As illustrated in Figure 4(a), the amount of 17βHSD2 mRNA expression was 4.8-fold higher in ILC than IDC cases (P = 0.0775). The 17βHSD2 immunoreactivity was also examined in order to further explore the significance of this particular enzyme with relation to androgen actions. Fifty-four IDC cases (Tohoku University Hospital) were selected so that ER, PR, Her2, Ki-67, stage, AR, 17βHSD5, and 5αRed1 status did not differ significantly with 46 ILC cases (Tohoku University Hospital). Immunoreactivity of 17βHSD2 was detected in the cytoplasm of carcinoma cells (Fig. 4b). The number of 17βHSD2-positive cases was significantly higher in ILC than in IDC despite nearly the same status of hormone receptors between ILC and IDC cases examined (Fig. 4c).
Fig 4.
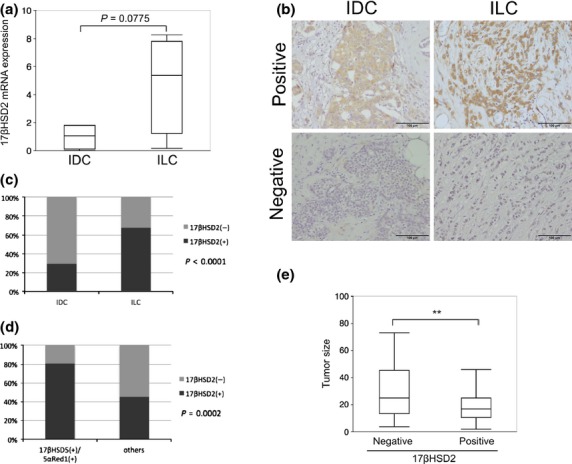
Status of 17β-hydroxysteroid dehydrogenase type 2 (17βHSD2) immunoreactivity was significantly higher in invasive lobular carcinoma (ILC) than in invasive ductal carcinoma (IDC) and inversely associated with tumor size in ILC cases. (a) 17βHSD2 mRNA expression in four IDC and four ILC cases was analyzed using RT2 quantitative PCR. (b) Representive illustrations of 17βHSD2 positive (left) and negative (right) cases in IDC (top) and ILC (bottom). 17βHSD2 was localized in the cytoplasm of carcinoma cells. Scale bar = 100 μm. (c) 17βHSD2 immunoreactivity in ILC and IDC specimens. (d) Correlation between 17βHSD2 and androgenic enzymes in androgen receptor-positive ILC cases. (e) Tumor size was inversely correlated with 17βHSD2 expression. Data of tumor size were available in a subset of breast cancer patients treated at Tohoku University Hospital or Tohoku Kosai Hospital. **P < 0.01. 17βHSD5, 17β-hydroxysteroid dehydrogenase type 5; 5αRed1, 5α-reductase type 1.
We then evaluated the status of 17βHSD2 in ILC cases using immunohistochemistry in order to analyze the correlation between androgenic enzymes and 17βHSD2 in AR-positive ILC cases. Among patients examined in this study, 101 cases (58.0%) were classified as positive for 17βHSD2. The status of 17βHSD2 was significantly correlated with that of androgenic enzymes (Fig. 4d). The associations between the status of 17βHSD2 and clinicopathological parameters of the patients are summarized in Table 4. Stage and tumor size were inversely correlated with the status of 17βHSD2 (P = 0.0049 and 0.0299, respectively), and the mean tumor size was also lower in the 17βHSD2-positive group (Fig. 4e). Nuclear grade of the cases examined was also correlated with the status of 17βHSD2 (P = 0.0110).
Table 4.
Associations between the 17β-hydroxysteroid dehydrogenase type 2 (17βHSD2) expression and clinicopathological parameters in androgen receptor (AR)-positive invasive lobular carcinoma (ILC) cases (n = 174)
| 17βHSD2 |
|||
|---|---|---|---|
| Negative n = 73 (42.0%) | Positive n = 101 (58.0%) | P-value | |
| Age,† (years) | 56.6 ± 1.5 | 57.9 ± 1.1 | 0.4722 |
| Menopausal status, n (%) | |||
| Premenopausal | 33 (19.0%) | 35 (20.1%) | 0.1592 |
| Postmenopausal | 40 (23.0%) | 66 (37.9%) | |
| Stage, n (%) | |||
| 1 | 22 (12.6%) | 52 (29.9%) | 0.0049 |
| 2 + 3 | 51 (29.3%) | 49 (28.2%) | |
| Tumor size,‡ n (%) | |||
| <20 mm | 16 (13.0%) | 49 (39.9%) | |
| ≥20 mm | 25 (20.3%) | 33 (26.8%) | 0.0299 |
| Nuclear grade, n (%) | |||
| 1 | 28 (16.1%) | 21 (12.1%) | 0.0110 |
| 2 + 3 | 45 (25.8%) | 80 (46.0%) | |
| ER status, n (%) | |||
| Negative | 4 (2.3%) | 9 (5.2%) | 0.3956 |
| Positive | 69 (39.6%) | 92 (52.9%) | |
| PR status, n (%) | |||
| Negative | 24 (13.8%) | 34 (19.5%) | 0.9135 |
| Positive | 49 (28.2%) | 67 (38.5%) | |
| Her2 status, n (%) | |||
| Negative | 73 (41.9%) | 100 (57.5%) | 0.3939 |
| Positive | 0 (0.0%) | 1 (0.6%) | |
| Ki-67 LI,† (%) | 5.5 ± 0.9 | 5.6 ± 0.9 | 0.9847 |
Data are presented as mean ± SEM. All other values represent the number of the cases and percentage.
Data regarding the tumor size were available in the cohorts of Tohoku University Hospital and Kosai Hospital. ER, Eestrogen receptor; Her2, human epidermal growth factor receptor 2; LI, labeling index; PR, progesterone receptor.
Discussion
This study showed the presence of AR in almost all cases of ILC (97.8%) and the presence of androgen synthesizing enzymes (17βHSD5, 61.8%; 5αRed1, 53.4%) in the great majority of carcinomas. The prevalence of AR-positive cases in ILC is consistent with that of previously reported studies,(8,12) but the presence of androgenic enzymes in a significant proportion of ILCs represents an entirely novel finding. The high prevalence of AR in ILC excluded a direct assessment of the correlation between AR and clinicopathological characteristics of the patients but the prevalence of androgen synthesizing enzymes, which were detected in approximately 50% of the patients examined, allowed us to examine their effects on clinical outcome of the ILC patients in this study. Results of this particular analysis indicated that the tumors enriched in androgenic pathways (AR-positive/enzyme-positive) were strongly associated with smaller tumor sizes and better clinical outcomes. However, the association between the intratumoral status of AR/enzymes and tumor cell proliferation in ILC could not be assessed due to the inherently low levels of cell proliferation in ILC tissues.(1,31) This aside, the overall findings of our study suggest the importance of androgen signaling and synthesizing enzymes in determining the clinical outcome of ILC patients.
The importance of AR in influencing tumor biology has not been previously evaluated in a large and exclusively ILC cohort. In previous studies examining IDC or mixed IDC/ILC cohorts the expression of AR in cancer tissues has been mostly reported to be associated with a relatively favorable clinical outcome in both ER-positive(15,32) and ER-negative cancers.(33) Results of in vitro studies reported in ER-positive breast carcinoma cell lines have also indicated the potent androgen DHT as an inhibitor of breast carcinoma cell proliferation,(15–18) although the underlying molecular biology of the AR in ER-negative cancers is only starting to be elucidated.(13,14) The majority of ILC patients are ER-positive (e.g. 92.7% in this study) and it is reasonable to hypothesize that, as in IDC, AR has protective effects, possibly more so in ILC cases given their more abundant ER content and more favorable clinical outcome compared to IDC.
The similarities between IDC and ILC are also apparent in a comparison of the normal lobules as compared to ILC areas. The present study showed increased numbers of AR-positive carcinoma cells alongside a decrease in androgenic enzymes detected in ILC compared to non-neoplastic adjacent lobules of the same breast tissue. Higher expression of AR in ILC than in non-neoplastic lobules is also consistent with the reported findings in IDC, that is, higher than in adjacent non-neoplastic ducts,(13) as is the association of AR and enzymes with clinical outcome of the patients(14,25) and the decrease of androgenic enzymes with cancer progression,(29) although changes between non-neoplastic condition and carcinoma initiation are less clear.(34) In addition, the inverse association of PR, commonly considered an estrogen-dependent gene, and androgenic enzymes in ILC detected in our present study suggests that, as in IDC, AR actions may disrupt estrogen signaling in ILC. In non-human primates, AR expression in both lobular and ductal tissues showed similar patterns of prevalence over the reproductive cycle(35) suggesting AR expression in lobules and ducts may be regulated in the same fashion, but this awaits further investigation for clarification.
It has been reported that 17βHSD2 is one of the most important androgen-induced genes in IDC,(27) playing pivotal roles in the putative protective effects of androgen in breast cancer patients. As an enzyme that converts estradiol to estrone or testosterone to androstenedione in breast cancer tissues, 17βHSD2 was reported to be significantly associated with better recurrence-free survival in breast cancer patients.(36) In addition, a significantly inverse correlation was reported between 17βHSD2 expression and intratumoral estradiol concentration, suggesting that this enzyme plays important roles as an estrogen-inactivating enzyme. In this study, we showed a higher expression of 17βHSD2 in ILC than in IDC tissues and an association between androgen pathways and 17βHSD2 in ILC tissues, indicating an androgenic induction of 17βHSD2 in ILC compared to IDC. The potential protective effect of 17βHSD2 in ILC cancers was evaluated by examining the correlations between the status of 17βHSD2 and clinicopathological factors of the cases examined. Results indicated that 17βHSD2 status was inversely correlated with the majority of adverse clinical factors, indicating that 17βHSD2 status of carcinoma cells was associated with less aggressive phenotypes of ILC. This is consistent with the suggestion that androgens may act, at least partially, through the upregulation of 17βHSD2 and thus modify the balance of estrogen production in ILC tissues, although further investigations are required for clarification.
Invasive lobular carcinoma cases are reported to express aromatase at similar or greater levels than IDC.(37) While controversies still exist as to the potential efficacy of AIs in ILC patients,(38,39) Metzger et al.(40) suggested that AI treatment was especially effective in ILC patients. This is interesting with regard to the findings of our study as one of the well documented consequences of aromatase inhibition is a shift in the equilibrium of androgens and estrogens towards a greater abundance of androgens and androgen signaling.(26–28) Results of our present study indicated a protective effect of androgens potentiated by alterations of local intracrine signaling and subsequent favoring androgen over estrogen signaling in ILC at a level at least comparable with that of IDC. Therefore, AIs should be at least as effective in ILC patients, especially given the high levels of ER- and AR-positive cases observed in ILC samples.
In this study, we showed that androgen-producing enzymes were involved in tumor suppressive roles of androgens in AR-enriched ILC tissues. In addition, we established the significant correlation of androgen-producing enzymes with 17βHSD2, one of the markers of androgenic actions in breast carcinoma cells, implying that 17βHSD2 reflects intratumoral androgenic actions and acts as suppressor of estrogenic actions. These results could be related to the relatively low proliferative status of carcinoma cells in ILC despite more abundant ER and the presence of intratumoral aromatase, and suggests further benefit of AI treatment against ILC by accumulation of intratumoral androgens.
Acknowledgments
Keely McNamara was supported in part by a Japan Society for the Promotion of Science–Australian Academy of Science postdoctoral fellowship. We would also like to acknowledge the support and assistance of the members of the Department of Pathology, Tohoku University School of Medicine.
Glossary
Abbreviations
- 17βHSD2
17β-hydroxysteroid dehydrogenase type 2
- 17βHSD5
17β-hydroxysteroid dehydrogenase type 5
- 5αRed1
5α-reductase type 1
- AI
aromatase inhibitor
- AR
androgen receptor
- DFS
disease-free survival
- DHT
dihydrotestosterone
- ER
estrogen receptor
- Her2
human epidermal growth factor receptor 2
- IDC
invasive ductal carcinoma
- ILC
invasive lobular carcinoma
- OS
overall survival
- PR
progesterone receptor
- qPCR
quantitative PCR
Disclosure Statement
The authors have no conflicts of interest.
References
- 1.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharat A, Gao F, Margenthaler JA. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg. 2009;198:516–9. doi: 10.1016/j.amjsurg.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000;88:2561–9. [PubMed] [Google Scholar]
- 4.Biglia N, Mariani L, Sgro L, Mininanni P, Moggio G, Sismondi P. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr Relat Cancer. 2007;14:549–67. doi: 10.1677/ERC-06-0060. [DOI] [PubMed] [Google Scholar]
- 5.Korkola JE, DeVries S, Fridlyand J, et al. Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res. 2003;63:7167–75. [PubMed] [Google Scholar]
- 6.Zhao H, Langerød A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–36. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008;44:73–83. doi: 10.1016/j.ejca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17:1862–9. doi: 10.1245/s10434-010-0953-z. [DOI] [PubMed] [Google Scholar]
- 10.Moinfar F, Okcu M, Tsybrovskyy O, et al. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–11. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa Y, Hai E, Matsumoto K, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–5. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 12.Riva C, Dainese E, Caprara G, et al. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005;447:695–700. doi: 10.1007/s00428-005-0003-6. [DOI] [PubMed] [Google Scholar]
- 13.Hickey TE, Robinson JLL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26:1252–67. doi: 10.1210/me.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol. 2013;133:66–76. doi: 10.1016/j.jsbmb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Peters AA, Buchanan G, Ricciardelli C, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–40. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 16.Andò S, De Amicis F, Rago V, et al. Breast cancer: from estrogen to androgen receptor. Mol Cell Endocrinol. 2002;193:121–8. doi: 10.1016/s0303-7207(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 17.Lapointe J. Role of the cyclin-dependent kinase inhibitor p27Kip1 in Androgen-Induced Inhibition of CAMA-1 breast cancer cell proliferation. Endocrinology. 2001;142:4331–8. doi: 10.1210/endo.142.10.8417. [DOI] [PubMed] [Google Scholar]
- 18.Poulin R, Baker D, Labrie F. Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75-1 human breast cancer cell line. Breast Cancer Res Treat. 1988;12:213–25. doi: 10.1007/BF01805942. [DOI] [PubMed] [Google Scholar]
- 19.Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Andò S. Endogenous coactivator ARA70 interacts with estrogen receptor alpha (ERalpha) and modulates the functional ERalpha/androgen receptor interplay in MCF-7 cells. J Biol Chem. 2005;280:20421–30. doi: 10.1074/jbc.M413576200. [DOI] [PubMed] [Google Scholar]
- 20.Hu R, Dawood S, Holmes MD, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–74. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki T, Miki Y, Nakamura Y, et al. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12:701–20. doi: 10.1677/erc.1.00834. [DOI] [PubMed] [Google Scholar]
- 22.Labrie F, Luu-The V, Labrie C, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–82. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 23.Recchione C, Venturelli E, Manzari A, Cavalleri A, Martinetti A, Secreto G. Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J Steroid Biochem Mol Biol. 1995;52:541–6. doi: 10.1016/0960-0760(95)00017-t. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Miki Y, Takagi K, et al. Androgens in human breast carcinoma. Med Mol Morphol. 2010;43:75–81. doi: 10.1007/s00795-010-0494-3. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Miki Y, Moriya T, et al. 5Alpha-reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer. 2007;120:285–91. doi: 10.1002/ijc.22317. [DOI] [PubMed] [Google Scholar]
- 26.Takagi K, Ishida T, Miki Y, et al. Intratumoral concentration of estrogens and clinicopathological changes in ductal carcinoma in situ following aromatase inhibitor letrozole treatment. Br J Cancer. 2013;109:100–8. doi: 10.1038/bjc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi K, Miki Y, Nagasaki S, et al. Increased intratumoral androgens in human breast carcinoma following aromatase inhibitor exemestane treatment. Endocr Relat Cancer. 2010;17:415–30. doi: 10.1677/ERC-09-0257. [DOI] [PubMed] [Google Scholar]
- 28.Chanplakorn N, Chanplakorn P, Suzuki T, et al. Increased 5α-reductase type 2 expression in human breast carcinoma following aromatase inhibitor therapy: the correlation with decreased tumor cell proliferation. Horm Cancer. 2011;2:73–81. doi: 10.1007/s12672-010-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibahara Y, Miki Y, Sakurada C, et al. Androgen and androgen-metabolizing enzymes in metastasized lymph nodes of breast cancer. Hum Pathol. 2013;44:2338–45. doi: 10.1016/j.humpath.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Verma MK, Miki Y, Abe K, et al. Intratumoral localization and activity of 17β-hydroxysteroid dehydrogenase type 1 in non-small cell lung cancer: a potent prognostic factor. J Transl Med. 2013;11:167. doi: 10.1186/1479-5876-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverstein MJ, Lewinsky BS, Waisman JR, et al. Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673–7. doi: 10.1002/1097-0142(19940315)73:6<1673::aid-cncr2820730620>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Lie TH, Morgan FJ. Androgen receptors in breast cancer. Cancer. 1984;54:2436–40. doi: 10.1002/1097-0142(19841201)54:11<2436::aid-cncr2820541121>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.McNamara KM, Yoda T, Miki Y, et al. Androgenic pathway in triple negative invasive ductal tumors: its correlation with tumor cell proliferation. Cancer Sci. 2013;104:639–46. doi: 10.1111/cas.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibuya R, Suzuki T, Miki Y, et al. Intratumoral concentration of sex steroids and expression of sex steroid-producing enzymes in ductal carcinoma in situ of human breast. Endocr Relat Cancer. 2008;15:113–24. doi: 10.1677/ERC-07-0092. [DOI] [PubMed] [Google Scholar]
- 35.Cheng G, Li Y, Omoto Y, et al. Differential regulation of estrogen receptor (ER)alpha and ERbeta in primate mammary gland. J Clin Endocrinol Metab. 2005;90:435–44. doi: 10.1210/jc.2004-0861. [DOI] [PubMed] [Google Scholar]
- 36.Gunnarsson C, Olsson BM, Stål O. Abnormal expression of 17beta-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res. 2001;61:8448–51. [PubMed] [Google Scholar]
- 37.Sasano H. Aromatase and 17 beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab. 1996;81:4042–6. doi: 10.1210/jcem.81.11.8923858. [DOI] [PubMed] [Google Scholar]
- 38.van de Water W, Fontein DBY, van Nes JGH, et al. Influence of semi-quantitative oestrogen receptor expression on adjuvant endocrine therapy efficacy in ductal and lobular breast cancer - a TEAM study analysis. Eur J Cancer. 2013;49:297–304. doi: 10.1016/j.ejca.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Montemurro F. Upfront adjuvant aromatase inhibitors in women with lobular breast cancer. Eur J Cancer. 2013;49:3376–7. doi: 10.1016/j.ejca.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Metzger O, Giobbie-Hurder A, Mallon E, et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. Cancer Res. 2012;72(1024):S1–1. doi: 10.1200/JCO.2015.60.8133. Available from: http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/72/24_MeetingAbstracts/S1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


