Abstract
This study analyzed outcomes of systemic chemotherapy for advanced neuroendocrine carcinoma (NEC) of the digestive system. Clinical data from 258 patients with unresectable or recurrent NEC of the gastrointestinal tract (GI) or hepato-biliary-pancreatic system (HBP), who received chemotherapy, were collected from 23 Japanese institutions and analyzed retrospectively. Patients had primary sites in the esophagus (n = 85), stomach (n = 70), small bowel (n = 6), colorectum (n = 31), hepato-biliary system (n = 31) and pancreas (n = 31). Median overall survival (OS) was 13.4 months the esophagus, 13.3 months for the stomach, 29.7 months for the small bowel, 7.6 months for the colorectum, 7.9 months for the hepato-biliary system and 8.5 months for the pancreas. Irinotecan plus cisplatin (IP) and etoposide plus cisplatin (EP) were most commonly selected for GI-NEC and HBP-NEC. For patients treated with IP/EP (n = 160/46), the response rate was 50/28% and median OS was 13.0/7.3 months. Multivariate analysis among patients treated with IP or EP showed that the primary site (GI vs HBP; hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.35–0.97) and baseline serum lactate dehydrogenase levels (not elevated vs elevated; HR 0.65, 95% CI 0.46–0.94) were independent prognostic factors for OS, while the efficacy of IP was slightly better than for EP (HR 0.80, 95% CI 0.48–1.33; P = 0.389). IP and EP are the most common treatment regimens for NEC of the digestive system. HBP primary sites and elevated lactate dehydrogenase levels are unfavorable prognostic factors for survival. A randomized controlled trial is required to establish the appropriate chemotherapy regimen for advanced NEC of the digestive system. This study was registered at UMIN as trial number 000005176.
Keywords: Cisplatin, digestive system, etoposide, irinotecan, neuroendocrine carcinoma
Neuroendocrine neoplasms (NEN) are rare tumors that exhibit a variety of morphological, functional and behavioral characteristics.1 The World Health Organization (WHO) has proposed a grading system for NEN that divides them into three categories based on proliferation as follows: (i) neuroendocrine tumor (NET) (G1) with a mitotic count of <2/10 high power fields (HPF) and/or a Ki-67 index of ≤2%; (ii) NET (G2) with a mitotic count of 2–20/10 HPF and/or a Ki-67 index of 3–20%; and (iii) neuroendocrine carcinoma (NEC) with a mitotic count of >20/10 HPF and/or a Ki-67 index of >20%.2 Among the three categories, NEC is a poorly differentiated, high-grade malignant tumor, previously termed poorly differentiated neuroendocrine carcinoma (PDNEC), including small-cell carcinoma (SCC) and large-cell NEC. The primary sites of NEC are varied in many organs, with NEC arising in the digestive system accounting for 20–68% of cases with extra-pulmonary NEC.3–7
In treating advanced extra-pulmonary NEC, guidelines recommend chemotherapy regimens, which are suitable for small-cell lung carcinoma (SCLC).8–10 Therefore, platinum-containing regimens, such as etoposide plus cisplatin (EP), are commonly used for NEC arising from the digestive system in clinical practice worldwide and irinotecan plus cisplatin (IP) is commonly adopted in Japan. However, no randomized controlled trial has been conducted previously and retrospective reports have been limited in scope and number.11–15 Therefore, we conducted a multicenter retrospective study on the outcomes of systemic chemotherapy for advanced NEC of the digestive system to obtain useful information to prepare for a future clinical trial.
Materials and Methods
The selection criteria were as follows: (i) a histologically proven NEC such as PDNEC, SCC, mixed endocrine-exocrine carcinoma with a PDNEC component (MEEC), or a neuroendocrine tumor with a rapidly progressive clinical course (clinically-diagnosed NEC); (ii) a primary tumor arising in the digestive system (gastrointestinal tract [GI] or hepato-biliary-pancreatic system [HBP]); (iii) an unresectable or recurrent disease treated with systemic chemotherapy, which was initiated between April 2000 and March 2011; and (iv) no prior treatment, except for surgical resection. Data were collected from the medical records of patients at 23 institutions in Japan using a standardized data collection form. This study was approved by the institutional review boards of the participating institutions and registered with the UMIN Clinical Trials Registry as UMIN 000005176 (http://www.umin.ac.jp/ctr/).
Responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Progression-free survival (PFS) was defined as the time from initiation of chemotherapy to confirmation of disease progression or death due to any cause. Overall survival (OS) was defined as the time from initiation of chemotherapy to death due to any cause. Surviving patients were censored on their last follow-up date. PFS and OS were estimated using the Kaplan–Meier method and compared with the log-rank test. Among the patients treated with EP or IP, multiple variate analysis by Cox proportional hazard models was performed, and the hazard ratio (HR) and the corresponding 95% confidence interval (95% CI) for OS were calculated, using the following seven variables selected based on the results of previous investigations and our clinical experience: age (<60 years/≥60 years), sex (male/female), Eastern Cooperative Oncology Group performance status (0–1/≥2), primary site (GI/HBP), liver metastasis (yes/no), prior surgery (yes/no), baseline serum lactate dehydrogenase (LDH) levels (not elevated/elevated), and first-line chemotherapy regimens (EP/IP). Statistical analysis was performed using SPSS software, version 17.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Figure1 represents the study population flow chart. A total of 258 patients satisfied the selection criteria. Their characteristics are shown in Table1. The majority of patients were male (71%) and the most common primary site was the esophagus (33%) followed by the stomach (27%). Most patients both in the GI (84%) and HBP (88%) subgroups had Stage IV or recurrent disease.
Fig 1.
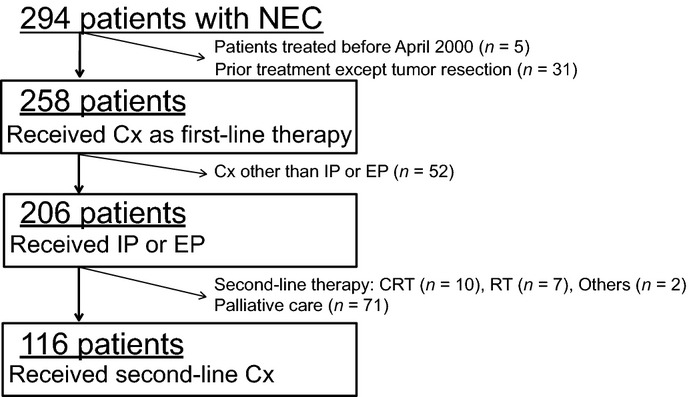
Flow chart of the study population. CRT, chemoradiotherapy; Cx, chemotherapy; EP, etoposide plus cisplatin; IP, irinotecan plus cisplatin; n, number; NEC, neuroendocrine carcinoma; RT, radiotherapy.
Table 1.
Patient characteristics
| All patients | GI primary | HBP primary | |
|---|---|---|---|
| Number | 258 | 192 (74%) | 66 (26%) |
| Age, years | |||
| Median (range) | 62.5 (26–81) | 63 (26–81) | 58.5 (29–78) |
| Sex (%) | |||
| Male | 182 (71) | 153 (80) | 29 (44) |
| Female | 76 (29) | 39 (20) | 37 (56) |
| Performance status (%) | |||
| 0 or 1 | 240 (93) | 176 (92) | 64 (97) |
| ≧2 | 18 (7) | 16 (8) | 2 (3) |
| Baseline lactate dehydrogenase (%) | |||
| Elevated | 136 (53) | 91 (47) | 45 (68) |
| Not elevated | 95 (37 | 79 (41) | 16 (24) |
| No data | 27 (10) | 22 (11) | 5 (8) |
| Chromogranin A staining (%) | |||
| Positive | 172 (67) | 122 (64) | 50 (76) |
| Negative | 59 (23) | 51 (26) | 8 (12) |
| No data | 27 (10) | 19 (10) | 8 (12) |
| Synaptophysin staining (%) | |||
| Positive | 204 (79) | 153 (80) | 51 (77) |
| Negative | 29 (11) | 21 (11) | 8 (12) |
| No data | 25 (10) | 18 (9) | 7 (11) |
| Ki-67 index (%) | |||
| ≥55% | 43 (17) | 20 (10) | 23 (35) |
| >20%, <55% | 27 (10) | 18 (9) | 9 (14) |
| No data | 188 (73) | 154 (80) | 34 (52) |
| Histology (%) | |||
| PDNEC | 63 (24) | 37 (19) | 26 (39) |
| Small cell carcinoma | 122 (47) | 99 (52) | 23 (35) |
| MEEC | 21 (8) | 16 (8) | 5 (8) |
| Clinically diagnosed NEC | 52 (20) | 40 (21) | 12 (18) |
| Stage (%) | |||
| IV or recurrent | 219 (85) | 161 (84) | 58 (88) |
| I–III | 39 (15) | 31 (16) | 8 (12) |
| Primary site (%) | |||
| Esophagus | 85 (33) | 85 (44) | |
| Stomach | 70 (27) | 70 (36) | |
| Small bowel | 6 (2) | 6 (3) | |
| Colorectum | 31 (12) | 31 (16) | |
| Hepato-biliary system | 31 (12) | 31 (47) | |
| Pancreas | 35 (14) | 35 (53) | |
| Location of metastases (%) | |||
| Liver | 136 (53) | 95 (49) | 41 (62) |
| Lymph nodes | 131 (51) | 103 (54) | 28 (42) |
| Lung | 27 (10) | 25 (13) | 2 (3) |
| Bone | 12 (5) | 9 (5) | 3 (5) |
| Brain | 1 (0.4) | 1 (0.5) | 0 (0) |
| Others | 30 (11.6) | 26 (14) | 4 (6) |
| Prior surgery (+) (%) | 76 (29) | 66 (34) | 10 (15) |
GI, gastrointestinal tract; HBP, hepato-biliary-pancretic system; MEEC, mixed endocrine-exocrine carcinoma; NEC, neuroendocrine carcinoma; PDNEC, poorly differentiated neuroendocrine carcinoma.
Treatment
The most common regimen for first-line chemotherapy was IP (n = 160, 62%), followed by EP (n = 46, 18%) and fluoropyrimidine-based regimens (n = 37, 14%), such as 5-fluorouracil/leucovorin/oxaliplatin combination regimen (FOLFOX) and S-1 (Table2).
Table 2.
First-line chemotherapy regimens
| Eso | Stm | SB | CR | HB | P | Total (%) | |
|---|---|---|---|---|---|---|---|
| Number | 85 | 70 | 6 | 31 | 31 | 35 | 258 (100) |
| Irinotecan+Cisplatin (IP) | 71 | 54 | 2 | 15 | 7 | 11 | 160 (62) |
| Irinotecan+Carboplatin | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.4) |
| Etoposide+Cisplatin (EP) | 4 | 4 | 2 | 2 | 16 | 18 | 46 (18) |
| Etoposide+Carboplatin | 2 | 0 | 1 | 1 | 0 | 0 | 4 (2) |
| Gemcitabine-based† | 0 | 0 | 0 | 0 | 5 | 5 | 10 (4) |
| Fluoropyrimidine-based† | 6 | 11 | 1 | 13 | 3 | 3 | 37 (14) |
| Others | 2 | 1 | 0 | 0 | 0 | 0 | 3 (1) |
Overlapped. CR, colorectum; Eso, esophagus; HB, hepato-biliary system; P, pancreas; SB, small bowel; Stm, stomach.
Survival
The median OS of all 258 patients was 11.5 months. In terms of primary site, the median overall was 13.4 months the esophagus, 13.3 months for the stomach, 29.7 months for the small bowel, 7.6 months for the colorectum, 7.9 months for the hepato-biliary system and 8.5 months for the pancreas (Fig.2). Subgroups were determined by histological analysis and the median OS in months was calculated: PDNEC (12.6, n = 63), SCC (13.0, n = 122), MEEC (12.3, n = 21) and clinically-diagnosed NEC (9.9, n = 52) (Fig.3). No statistically significant difference in OS was found between the four histology subgroups, including clinically-diagnosed NEC (P = 0.120).
Fig 2.
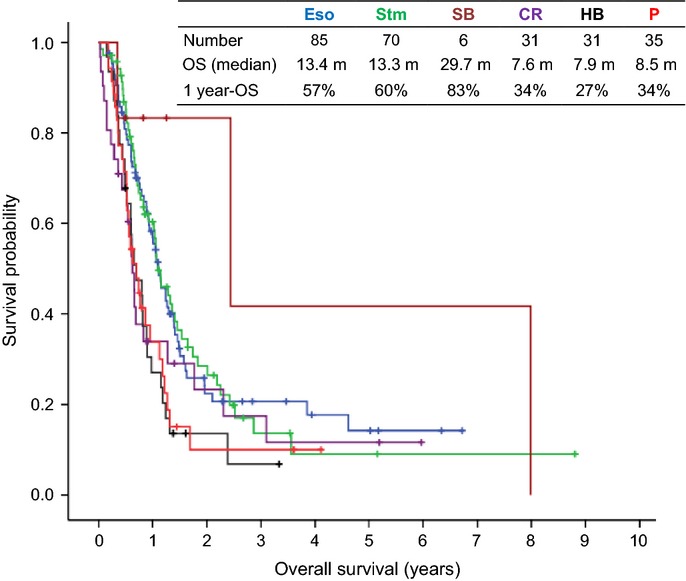
Kaplan–Meier curves for overall survival according to the primary site. CR, colorectum; Eso, esophagus; HB, hepato-biliary system; OS, overall survival; P, pancreas; SB, small bowel; Stm, stomach.
Fig 3.
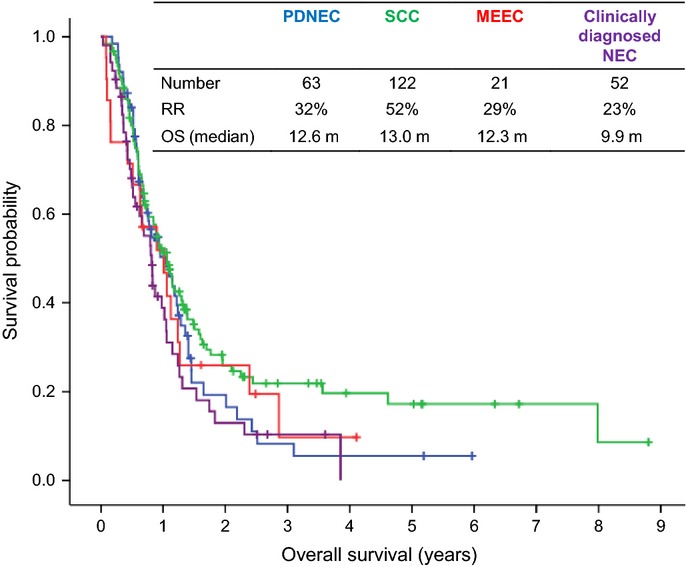
Kaplan–Meier curves for overall survival according to histology. MEEC, mixed endocrine-exocrine carcinoma; NEC, neuroendocrine carcinoma; OS, overall survival; PDNEC, poorly differentiated neuroendocrine carcinoma; RR, response rate; SCC, small cell carcinoma.
Comparison of irinotecan plus cisplatin and etoposide plus cisplatin regimen efficacy
Among the 258 patients, 206 patients (80%) received either IP or EP as their first-line chemotherapy. Table3 shows the response rate, median PFS and median OS for these 206 patients. In total, 160 patients who received IP showed a better response rate (50 vs 28%, P < 0.001), longer PFS (median, 5.2 vs 4.0 months, P = 0.033) and longer OS (median, 13.0 vs 7.3 months, P < 0.001) than 46 patients who received EP. According to primary site, 142 patients (89%) in the GI subgroup received IP while 34 patients (65%) received EP in the HBP subgroup. The response rate of IP was significantly better than that for EP in the HBP subgroup (39 vs 12%, P = 0.034), but there were no statistically significant differences with respect to response rate, PFS, or OS between IP and EP in the GI subgroup.
Table 3.
Efficacy comparison IP versus EP
| IP | EP | P-value | |
|---|---|---|---|
| Total | |||
| Number | 160 | 46 | |
| RR | 50% (80/160) | 28% (13/46) | <0.001† |
| PFS (median) | 5.2 m | 4.0 m | 0.033‡ |
| OS (median) | 13.0 m | 7.3 m | <0.001‡ |
| GI | |||
| Number | 142 | 12 | |
| RR | 51% (73/142) | 75% (9/12) | 0.140† |
| PFS (median) | 5.4 m | 4.9 m | 0.585‡ |
| OS (median) | 13.4 m | 14.0 m | 0.976‡ |
| HBP | |||
| Number | 18 | 34 | |
| RR | 39% (7/18) | 12% (4/34) | 0.034† |
| PFS (median) | 4.4 m | 3.7 m | 0.056‡ |
| OS (median) | 10.1 m | 6.9 m | 0.050‡ |
χ2.
Log-rank test. EP, etoposide plus cisplatin; GI, gastrointestinal tract; HBP, hepato-biliary-pancreatic system; IP, irinotecan plus cisplatin; OS, overall survival; PFS, progression-free survival; RR, response rate.
Second-line chemotherapy
Following the failure of IP or EP, 116 patients received second-line chemotherapy. The efficacies of second-line chemotherapy according to the regimen and primary site, GI versus HBP, are shown in Table4. The efficacy of second-line chemotherapy was slightly better in GI than in HBP patients.
Table 4.
Efficacy of second-line chemotherapy
| Number | RR (%) | PFS† (median) | OS‡ (median) | |
|---|---|---|---|---|
| Regimen | ||||
| Amrubicin | 25 | 4 | 1.9 m | 8.3 m |
| EP or CE | 23 | 17 | 1.9 m | 5.0 m |
| Irinotecan | 21 | 5 | 2.2 m | 5.9 m |
| S-1 | 11 | 27 | 2.4 m | 12.2 m |
| IP | 5 | 40 | 4.8 m | 8.7 m |
| Primary site | ||||
| GI | 87 | 15 | 2.3 m | 8.1 m |
| HBP | 29 | 0 | 1.6 m | 5.1 m |
| Total | 116 | 11 | 2.1 m | 6.3 m |
PFS from second-line chemotherapy.
OS from second-line chemotherapy. CE, etoposide plus carboplatin; EP, etoposide plus cisplatin; GI, gastrointestinal tract; HBP, hepato-biliary-pancreatic system; IP, irinotecan plus cisplatin; OS, overall survival; PFS, progression-free survival; RR, response rate.
Prognostic factors
In multivariate analysis of prognostic factors for the 206 patients who received EP or IP as their first-line chemotherapy, 23 patients were excluded from the analysis because of no available data on baseline serum LDH levels. The primary site (GI vs HBP; HR 0.58, 95% CI 0.35–0.97; P = 0.039) and baseline serum LDH levels (not elevated vs elevated; HR 0.65, 95% CI 0.46–0.94) were independent prognostic factors for OS (Table5). There was a tendency towards longer survival in patients treated with the IP regimen, although the difference was not statistically significant (IP vs EP; HR 0.80, 95% CI 0.48–1.33; P = 0.389).
Table 5.
Univariate and multivariate analysis for overall survival†
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Age | ||||
| >60 years old (vs <60 years old) | 0.069 | 0.73 (0.52–1.03) | 0.541 | 0.89 (0.62–1.28) |
| Sex | ||||
| Female (vs male) | 0.143 | 0.76 (0.52–1.10) | 0.766 | 0.94 (0.61–1.43) |
| Performance status | ||||
| 0, 1 (vs 2≦) | 0.022 | 0.49 (0.26–0.90) | 0.130 | 0.55 (0.26–1.20) |
| Lactate dehydrogenase | ||||
| Notelevated (vs elevated) | 0.002 | 0.58 (0.41–0.82) | 0.021 | 0.65 (0.46–0.94) |
| Primary site | ||||
| GI (vs HBP) | <0.001 | 0.48 (0.33–0.70) | 0.039 | 0.58 (0.35–0.97) |
| Liver metastasis | ||||
| (–) (vs (+)) | 0.033 | 0.69 (0.49–0.97) | 0.119 | 0.76 (0.53–1.08) |
| First-line chemotherapy | ||||
| IP (vs EP) | 0.001 | 0.48 (0.33–0.70) | 0.389 | 0.8 (0.48–1.33) |
| Prior surgery | ||||
| (+) (vs (−)) | 0.141 | 0.71 (0.45–1.12) | 0.636 | 0.89 (0.55–1.45) |
Number = 183 (In analyzing prognostic factors, 23 patients were excluded for whom baseline serum lactate dehydrogenase level data were not available.) CI, confidence interval; EP, etoposide plus cisplatin; GI, gastrointestinal tract; HBP, hepato-biliary-pancreatic system; HR, hazard ratio; IP, irinotecan plus cisplatin.
Discussion
In 2013, the NORDIC group reported a large cohort of GI-NEC patients (NORDIC NEC study) and this study is now regarded as an important reference in the NEC field.16 The current study is also a large-scale study, conducted subsequent to the NORDIC NEC study. Therefore, it is appropriate to compare the major findings of these two recent studies. Both studies indicated that the primary site and baseline serum LDH levels were important prognostic factors. However, survival of pancreatic NEC patients was extremely poor, with a median OS of 8.6 months in our study, compared with the median OS of 15 months in the NORDIC NEC study. This discrepancy could be due to a difference in patient characteristics and/or tumor biology. In our study, 61% of the pancreatic NEC patients had a Ki-67 index ≥55% compared to only 30% for such patients in the NORDIC NEC study. It should be noted, however, that Ki-67 index data were unavailable for almost half (17/35) of the pancreatic NEC patients in our study (data not shown).
First-line chemotherapy regimens were different between the two studies. In our study, IP was the most commonly selected regimen, especially for the GI subgroup, while EP was the most commonly selected regimen in the NORDIC NEC study. This discrepancy might be caused by the different recognition of standard regimens of SCLC between Japan and other countries. In terms of treatment for extensive-stage SCLC, IP demonstrated superiority to EP in a randomized controlled trial conducted in Japan (JCOG9511).17 IP is still considered a standard therapy for extensive-stage SCLC in Japan, although two subsequent randomized controlled trials conducted outside Japan were not able to confirm these earlier results.18,19 Therefore, it is essential to determine which chemotherapy regimen, IP or EP, is more effective for NEC of the digestive system. However, the number of published reports on chemotherapy for advanced NEC is limited, and most articles investigate a small number of patients, especially for those treated with IP.11–15 The definition of NEC has also changed recently. Thus, it is difficult to arrive at a current consensus of standard treatment for advanced NEC based on previous reports.
Our study is the largest study to compare the efficacy of EP and IP. The efficacy of IP was slightly better than EP for the treatment of NEC, even after adjusting patient background by multivariate analysis. Although it can be expected that IP might bring more favorable outcomes than EP, especially in the HBP subgroup, there was a considerable confounding bias between chemotherapy regimens and primary sites. Indeed, most patients in the GI subgroup received IP whereas most patients in the HBP subgroup received EP primarily because of different treatment policies among the institutions. Consequently, it remains difficult to determine which regimen was more effective and whether the optimal chemotherapy regimen depends on the primary site for treating advanced NEC based on the results of our retrospective analysis. According to the consensus report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials planning meeting, GI-NET and pancreatic NET should be examined separately in clinical trials.20 Although, NET and NEC are different disease entities and there is still no consensus with regard to NEC, prognosis was poorer in pancreatic NEC compared with GI-NEC in the current study. Further study is required to determine the appropriateness of treating all digestive NEC with the same chemotherapy regimen, and whether pancreatic NEC should be investigated separately.
Our analysis indicated only a limited efficacy of second-line chemotherapy. Oral topotecan monotherapy has been recommended for patients with platinum refractory or relapsed SCLC.8,21–23 Recently, amrubicin was considered a promising regimen in this setting for SCLC, because it significantly improved the response rate compared with topotecan (31 vs 17%).24 Based on these more recent results, amrubicin was the most commonly-used regimen for second-line chemotherapy in our study. However, its response rate and median PFS were only 4% and 1.9 months, respectively. Amrubicin does not appear to be a promising treatment for platinum-refractory NEC. It is also necessary to establish effective treatment in the second-line setting for NEC of the digestive system.
The present study had several limitations. First, there was wide variation in the quality of pathological diagnosis. In the 2010 WHO classification, the importance of the Ki-67 index is emphasized in the grading of NEN. However, Ki-67 index information was not obtained for 73% of the patients in the present study because many of the subjects in this study had been treated before the recent WHO criteria were published in 2010. Recently, histological differentiation has been recognized as important for diagnosis of NEC and it is well known that poor differentiation is related to poor prognosis. Moreover, the present study included clinically-diagnosed NEC patients. In practice, there are some unavoidable cases where tumor grades are estimated according to histological differentiation and tumor growth velocity because adequate specimens are unavailable for histological grading, particularly specimens obtained by endoscopic ultrasound-guided fine-needle aspiration. In the present study, the prognoses of clinically-diagnosed NEC patients were as poor as for patients in the other histology subgroups. This finding may be one rationale for treating clinically-diagnosed NEC patients in accordance with the treatment of histologically-diagnosed NEC patients. Second, we did not collect toxicity data. These limitations can only be resolved by a well-designed prospective clinical trial. We are currently planning a randomized phase III trial comparing IP with EP for the treatment of advanced NEC of the digestive system.
In conclusion, IP and EP are the most commonly selected treatment regimens in Japan for NEC of the digestive system. The primary site and baseline serum LDH levels are independent prognostic factors for NEC, and IP showed a slightly better tendency for efficacy compared to EP. A prospective randomized controlled trial is required to establish the most appropriate chemotherapy regimen for advanced NEC of the digestive system.
Acknowledgments
The authors extend their sincere appreciation to Dr H. Ishii (Cancer Institute Hospital of Japanese Foundation for Cancer Research), Dr T. Yoshino, Dr M. Ikeda, Dr K. Nakachi (National Cancer Center Hospital East), Dr T. Funakoshi (Shizuoka Cancer Center), Dr T Denda, Dr K. Nakamura (Chiba Cancer Center), Dr H. Nishisaki (Hyogo Cancer Center), Dr C. Katada, Dr M. Kida (Kitasato University Hospital), Dr T. Okuno, Dr A. Ikeda, Dr S. Asari (Kobe University Graduate School of Medicine), Dr I. Hyodo, Dr T. Moriwaki (Institute of Clinical Medicine, Tsukuba University Hospital), Dr H. Kawai, Dr S. Hijioka, Dr T. Hasegawa (Aichi Cancer Center), Dr S. Ohkawa, Dr S. Kobayashi (Kanagawa Cancer Center), Dr A. Hosokawa (Toyama University Hospital), Dr S. Tokunaga (Osaka City General Hospital), Dr Y. Kojima, Dr E. Yokota (National Center for Global Health and Medicine), Dr T. Tsuda (St. Marianna University School of Medicine), Dr M. Matsuda, Dr Y Horita (Toyama Prefectural General Hospital), Dr S. Nakazuru (Osaka National Hospital), Dr K. Nakajima (Miyazaki University Hospital), Dr Y. Komatsu, Dr H. Hayashi (Hokkaido University Hospital), Dr K Matsumoto (Faculty of Medicine, Tottori University), Dr T. Ito, Dr Y. Shiina (Kyushu University Hospital), Ms. K. Kondo, Ms. R. Mukouyama and Ms. H. Hosoi (National Cancer Center Hospital).
Disclosure Statement
Junji Furuse serves as a consultant to Bayer, Chugai Pharma, Eisai, Taiho Pharmaceutical, Ono, Zeria, Boehringer Ingelheim and Kyowa Hakko Kirin and received honoraria from Bayer, Lilly, Taiho Pharmaceutical, Chugai Pharma, Novartis and Yakult; Narikazu Boku received a research grant from Taiho Pharmaceutical, Chugai Pharma, Yakult, Ono, Takeda, Bristol-Myers Squibb and Merck Serono and received honoraria from Yakult, Shionogi, Merck Serono, Ono, Taiho Pharmaceutical, Takeda, Chugai Pharma and Diichi-Sankyo; Takuji Okusaka received honoraria from Lilly, Taiho Pharmaceutical, Novartis, Chugai Pharma, Bayer, Pfizer and Sumitomo and received a research grant from Taiho Pharmaceutical, Lilly, Chugai Pharma, Boehringer Ingelheim, Takeda Bio, Yakult, Kyowa Hakko Kirin, Ono, Otsuka, Eisai, Shizuoka Sangyo, Merck Serono, Astra Zeneca and OncoTherapy Science. The study was funded by the National Cancer Center Research and Development Fund (23-A-22); The corresponding author had full access to all of the data in the study and all authors had final responsibility for the decision to submit for publication.
References
- Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1–18. doi: 10.1677/erc.0.0110001. [DOI] [PubMed] [Google Scholar]
- Bosman TF, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press; 2010. [Google Scholar]
- Terashima T, Morizane C, Hiraoka N, et al. Comparison of chemotherapeutic treatment outcomes between advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology. 2012;96:324–32. doi: 10.1159/000338794. [DOI] [PubMed] [Google Scholar]
- Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116:888–95. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]
- Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970–2004. BMC Cancer. 2009;9:209. doi: 10.1186/1471-2407-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider K, Shahid RK, Finch D, et al. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer. 2006;107:2262–9. doi: 10.1002/cncr.22235. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee JL, Ryu MH, et al. Extrapulmonary small cell carcinoma: single center experience with 61 patients. Acta Oncol. 2007;46:846–51. doi: 10.1080/02841860601071893. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network National. Comprehensive Cancer Network (NCCN) guidelines. Available from URL: www.nccn.org (Ver. 1 2013) [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799–800. doi: 10.1097/MPA.0b013e3181ebb56f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–32. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–5. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40:313–8. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- Chin K, Baba S, Hosaka H, et al. Irinotecan plus cisplatin for therapy of small-cell carcinoma of the esophagus: report of 12 cases from single institution experience. Jpn J Clin Oncol. 2008;38:426–31. doi: 10.1093/jjco/hyn041. [DOI] [PubMed] [Google Scholar]
- Okita NT, Kato K, Takahari D, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161–5. doi: 10.1007/s10120-011-0025-5. [DOI] [PubMed] [Google Scholar]
- Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–5. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–43. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- Sørensen M, Pijls-Johannesma M, Felip E, Group EGW. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v120–5. doi: 10.1093/annonc/mdq172. [DOI] [PubMed] [Google Scholar]
- Jotte R, Von Pawel J, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan (Topo) as second-line treatment for small cell lung cancer SCLC (abstract #7000) J Clin Oncol. 2011;29:453s. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]


