Abstract
Although numerous studies have shown the significance of cancer-specific aerobic glycolysis, how glycolysis contributes to tumor invasion, a critical phenomenon in metastasis, remains unclear. With regard to colorectal cancer (CRC), we studied two critical gate enzymes, hexokinase 2 (HK2), which is involved in glycolysis, and phosphorylated pyruvate dehydrogenase-E1α (p-PDH), which is involved in oxidative phosphorylation (OxPhos). Immunohistochemical analyses using anti-HK2 and p-PDH antibodies were performed on surgically resected CRC samples (n = 104), and the expression in invasive front lesions of tumors was assessed. Positive HK2 expression correlated with extensive tumor diameter (P = 0.0460), advanced tumor depth (P = 0.0395), and presence of lymph node metastasis (P = 0.0409). Expression of p-PDH tended to be higher in right-sided CRCs than in left-sided CRCs (P = 0.0883). In survival analysis, the combined evaluation of positive HK2 and negative p-PDH was associated with reduced recurrence-free survival (RFS) (P = 0.0169 in all stages and P = 0.0238 in Stage II and III patients, respectively). This evaluation could predict RFS more precisely than the independent evaluation. The present study indicated that high HK2 expression combined with low p-PDH expression in the invasive front lesions of CRC tumors is predictive of tumor aggressiveness and survival of CRC cases.
Keywords: Colorectal cancer, hexokinase, invasion, metastasis, pyruvate dehydrogenase
Colorectal cancer (CRC) is the second most common cancer in the world. Each year, >1.2 million individuals develop CRC, and approximately 600 000 deaths occur.1 Although the efficacy of treatment has been gradually improving because of advances in chemotherapy or surgical technologies, the prognosis of patients with distant metastases and recurrence has not improved much. Numerous studies have shown that the activation of tumor-promoting genes and inactivation of growth-constraint tumor suppressor genes through genetic and epigenetic alterations contribute to the activation of biological phenomena, such as cell invasion, movement, and colonization, in distant organs during the metastatic process2,3; however, the precise molecular mechanisms involving biologically active metabolites in cancer are not completely understood.
Recent studies have indicated that deregulation in intratumor metabolism is involved in malignant behaviors of cancer cells.4,5 In glucose metabolism, lactate is preferentially produced in cancer cells, even in the presence of adequate oxygen in culture, a critical biological phenomenon termed aerobic glycolysis or the Warburg effect.6 Pyruvate kinase M2 (PKM2) was identified as a key molecule involved in the Warburg effect.7 Subsequently, at least three other enzymes, including phosphofructokinase 1, hexokinase 2 (HK2), and a phosphorylated form of pyruvate dehydrogenase-E1α (p-PDH), have been shown to be involved in cancer-associated metabolism, including glycolysis and oxidative phosphorylation (OxPhos) in the mitochondria.8–10 Aerobic glycolysis is thought to be beneficial for the production of biomass, such as nucleic acids and lipids, and reduced forms of glutathione, thereby conferring the advantages of low oxidative stress and selective growth.
In the initial step of glycolysis, HK converts glucose and ATP to glucose-6-phosphate and ADP. Four HK isoforms, HK1, HK2, HK3, and HK4, which are encoded by separate genes,11 are expressed in mammals. In adult tissues, HK1 is ubiquitously expressed, whereas HK2 is expressed in limited types of tissues, such as adipose tissues, skeletal muscles, and the heart.12 In cancer cells, HK2 and, to a lesser extent, HK1 are expressed,13 which suggests a preferential role of HK2 in the glucose flux of cancer cells. Recent studies have indicated that HK2 is necessary for the tumorigenicity of non-small cell lung cancer and breast cancer in humans, whereas HK2 deletion results in rapid suppression of tumor growth.9
Pyruvate dehydrogenase has a gate-keeper role in a branching point that links glycolysis to OxPhos in the citric acid cycle by converting pyruvate to acetyl-CoA in the mitochondria. The catalyzing activity of PDH is inhibited by phosphorylation at serine residue(s) by PDH kinase (PDK), whereas PDH is activated by PDH phosphatase.14,15 Reportedly, the process of aerobic glycolysis is at least partially maintained by the attenuation of mitochondrial function through PDH inhibition.16 A melanoma study showed that PDH inactivation by serine 293-phosphorylation by PDK led to high tumorigenic activity, whereas PDK depletion resulted in hypophosphorylation of PDH, regression of tumors, and further eradication of subpopulations resistant to a specific inhibitor to oncogene BRAF,V600E(17) which suggested an exclusive dependency on aerobic glycolysis of melanoma growth.
In the present study, we immunohistochemically analyzed the expression of HK2 and p-PDH in the invasive front lesions of clinical CRC samples and assessed their ability to predict tumor aggressiveness and survival. We identified an unexpected association of p-PDH with improved survival and showed that combined expression of HK2 and p-PDH was predictive of patient survival. Furthermore, we induced epithelial–mesenchymal transition (EMT) to colon cancer cell, followed by biochemical assays of HK and PDH. These results suggest a unique role of p-PDH in CRC growth at invasive fronts.
Materials and Methods
Clinical tissue samples
Colorectal tissue samples (n = 104) were collected during surgery (2007–2009) at the Department of Surgery, Osaka University. None of the patients had undergone preoperative chemotherapy or irradiation. Samples were fixed in buffered formalin at 4°C overnight, processed through graded ethanol solutions, and embedded in paraffin. The specimens were appropriately used under the approval of the ethics committee at the Graduate School of Medicine, Osaka University.
Immunohistochemistry
Tissue sections (3.5 μm thick) were prepared from paraffin-embedded blocks. After antigen retrieval treatment in 10 mM citrate buffer (pH 6.0) at 115°C for 15 min using Decloaking Chamber NxGen (Biocare Medical, Concord, CA, USA), immunostaining was performed using the Vectastain ABC Peroxidase Kit (Vector Laboratories, Burlingame, CA, USA). Antibodies used for immunohistochemistry were anti-HK2 rabbit antibody (2867; Cell Signaling Technology, Danvers, MA, USA), anti-pyruvate dehydrogenase E1α subunit (PDH-E1α) mouse antibody (ab110330; Abcam, Cambridge, UK), and pSer293 (p-PDH), the anti-phosphorylated form of PDH-E1α rabbit antibody (AP1062; Millipore, Darmstadt, Germany). The specificity of the antibodies was confirmed by the data showing that each antibody detected single band corresponding to the targeted protein in Western blot analysis (Suppl. Figs S1–3), and additionally, in regard to HK2 antibody, the absorption test was carried out in immunohistochemical analysis (Suppl. Fig. S4). The slides were incubated overnight at 4°C at the following dilutions: anti-HK2 antibody, 1:200; anti-p-PDH, 1:500; anti-PDH-E1α, 1:200. Sections were counterstained with hematoxylin. We rated the intensity of staining on a scale of 0 to 2: 0, negative; 1, weak; and 2, strong. We used pancreatic tissue as a positive control of HK2 according to the previous study,18 and a case of Stage I rectal cancer, the staining of which at the deepest part was strong as a positive control of p-PDH. Phosphate buffered saline instead of the antibodies was used as a negative control. We assigned colorectal tissue stained as intense as the positive control to “intensity score 2”, while unstained colorectal tissue similar to the negative control was assigned to “score 0” (Fig.1). The tissue stained weaker than the positive control but stronger than the negative control was categorized into “score 1” (Fig.1b,c). The intensity at the deepest part of the tumor was recorded in each sample. The reason why the intensity at the deepest part of the tumor was assessed was that the cancer cell was stimulated to invade into surrounding tissues at this region.19,20
Fig 1.
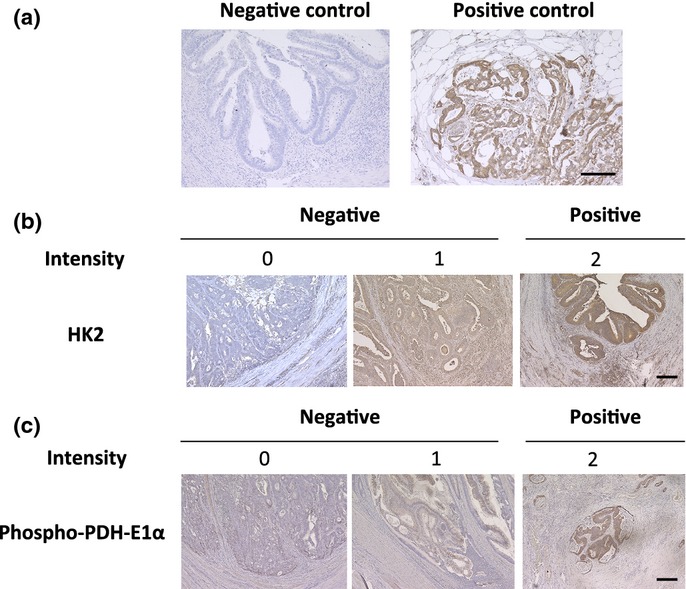
Immunohistochemical analysis of HK2 and p-PDH in clinical colorectal cancer samples. (a) Phosphate buffered saline was used as a negative control and a case of pancreatic cancer tissue was used as a positive control for HK2. (b) Staining of HK2 and (c) p-PDH at the invasive front are shown; the intensity was rated in three stages. Scale bar, 200 μm.
Assessment of tumor budding
Tumor budding was estimated according to the definition proposed by Ueno et al.21,22 An isolated cancer cell or a cluster composed of fewer than five cancer cells was defined as tumor budding. The number of buddings was counted in the field under a magnification of ×200 in the invasive front area.
Cell lines and culture
Human colon cancer cell line, SW480, was obtained from the ATCC (Manassas, VA, USA). The cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin, and grown at 37°C in a humidified incubator with 5% CO2.
Induction of EMT
Cells were seeded at the concentration of 5.0 × 104 cells/mL and incubated in a humidified atmosphere (37°C and 5% CO2) in standard medium for 48 h. After 48 h incubation, the cells were treated with transforming growth factor-β1 (TGF-β1) (2.5 ng/mL) and were incubated with MEM medium supplemented with FBS free, 10 ng/mL epidermal growth factor (Sigma-Aldrich, St Louis, MO, USA), 100 × insulin/transferring/selenium (ITS) (Life Technologies, Carlsbad, CA, USA), and 50 nmol/L hydrocortisone (Tokyo Kasei, Tokyo, Japan) for 48–72 h.
Biochemical assay
Biochemical activities of SW480 were analyzed using Hexokinase Colorimetric Assay Kit (ab136957; Abcam) for hexokinase activity and Pyruvate dehydrogenase Enzyme Activity Microplate Assay Kit (ab109902; Abcam) for pyruvate dehydrogenase activity according to the manufacturer's instructions.
Western blot analysis
Total protein was extracted from the cell lines in radio immunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Rockford, IL, USA). Aliquots of protein were electrophoresed on SDS-PAGE, Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA, USA). The separated proteins were transferred to PVDF membranes using iBlot (Life Technologies). Antibodies specific to E-cadherin (3195; Cell Signaling Technology), Vimentin (5741; Cell Signaling Technology), ACTB (A2103, Sigma-Aldrich) were used in addition to the antibodies used for immunohistochemistry. The membranes were incubated with primary antibodies overnight at 4°C, at appropriate concentrations (1:1000 for E-cadherin; 1:1000 for Vimentin; 1:1000 for HK2; 1:1000 for PDH-E1α; 1:10 000 for p-PDH: 1:2000 for ACTB) followed by the incubation with horseradish-peroxidase-linked anti-rabbit or mouse IgG (GE Healthcare Biosciences, Piscataway, NJ, USA) at a dilution of 1:100 000 for 1 h at room temperature. The antigen–antibody complex was detected with the ECL Prime Western Blotting Detection Kit (GE Healthcare Biosciences).
Quantitative reverse transcription polymerase chain reaction
Total RNA was extracted from cultured cells using RNeasy Mini Kit and QIA shredder (Qiagen, Valencia, CA, USA). Complemetary DNA was synthesized with ReverTra Ace reverse transcriptase (Toyobo, Osaka, Japan). Real-time quantitative polymerase chain reactions (qRT-PCR) were conducted with the LightCycler-FastStart DNA Master SYBR Green I kit (Roche Applied Science, Basel, Switzerland). The primer sequences used in this study were: HK2 sense, 5′-CAAAGTGACAGTGGGTGTGG-3′; HK2, antisense, 5′-GCCAGGTCCTTCACTGTCTC-3′; PDK1 sense, 5′-CCAAGACCTCGTGTTGAGACC-3′; PDK1 antisense, 5′-AATACAGCTTCAGGTCTCCTTGG-3′; PDP2 sense, 5′-ACCACCTCCGTGTCTATTGG-3′; PDP2 antisense, 5′- CCAGCGAGATGTCAGAATCC-3′. Data were normalized to expression of a control gene (b-actin) for each experiment. Data represent the mean ± SD of three independent experiments.
Statistical analysis
JMP pro 10.0.2 software (SAS Institute, Cary, NC, USA) was used to perform statistical analysis. The Kaplan–Meier method was used to estimate tumor recurrence in CRC, and the log-rank test was used to determine the statistical significance. Associations between discrete variables were assessed using the χ2 test or Fisher's exact test as appropriate. Mean values were compared using the Mann–Whitney U-test. P-values <0.05 were considered to indicate statistical significance.
Results
HK2 in CRC
We performed immunohistochemical staining of HK2 in clinical samples of CRCs. The intensities of HK2 staining rated in three stages were assigned to two groups: the HK2-negative group included scores of 0 or 1, while the HK2-positive group included score of 2 (representative cases are shown in Fig.1b). As summarized in Table1, HK2 expression was significantly associated with extensive tumor diameter (P = 0.0460), advanced tumor depth (P = 0.0395), and positive lymph node metastasis (P = 0.0409), suggesting that the HK2-positive group had more patients with advanced stages than the HK2-negative group. HK2 expression was not associated with the backgrounds of the patients, including serum tumor markers, tumor locations, and histological types of tumors.
Table 1.
HK 2 expression and clinicopathological features of colorectal cancer
| Hexokinase 2 | Positive (n = 61) | Negative (n = 43) | P-value |
|---|---|---|---|
| Patient background | |||
| Gender (Male/Female) | 37/24 | 28/15 | 0.6436 |
| Age (mean ± SD) | 63.2 ± 12.4 | 66.6 ± 10.6 | 0.9265 |
| BMI (kg/m2) | 22.2 (20.5, 26.1) | 22.4 (20.3, 25.2) | 0.9579 |
| CEA (ng/mL) | 3 (2, 9) | 3 (1, 5) | 0.5232 |
| CA19-9 (U/mL) | 11 (7, 21) | 13 (5, 22) | 0.8013 |
| Tumor characteristics | |||
| Tumor diameter (mm) | 40 (25, 54) | 30 (20, 44) | 0.0460* |
| Location | |||
| C/A/T | 5/10/7 | 3/5/7 | 0.9013 |
| D/S/R | 2/13/24 | 2/7/19 | |
| Tumor type | |||
| Type 0 | 13 | 13 | 0.3008 |
| Type 1/2/3/4/5 | 3/32/12/0/1 | 3/22/4/0/1 | |
| Histological type | |||
| tub1/tub2/pap | 17/39/1 | 13/23/1 | 0.3118 |
| por/muc | 0/4 | 4/2 | |
| Depth | |||
| T0/T1/T2 | 5/5/13 | 3/10/12 | 0.0395* |
| T3/T4 | 31/7 | 15/3 | |
| Lymph node metastasis | |||
| N0 | 35 | 33 | 0.0409* |
| N1/N2/N3 | 16/7/3 | 7/0/3 | |
| Distant metastasis | |||
| M0 | 53 | 40 | 0.5190 |
| M1 | 8 | 3 | |
| Lymphatic duct invasion | |||
| ly0 | 15 | 19 | 0.2321 |
| ly1/ly2 | 34/12 | 21/3 | |
| Venous invasion | |||
| v0 | 48 | 36 | 0.6176 |
| v1/v2 | 9/4 | 7/0 | |
| Stage | |||
| 0/I/II | 5/13/15 | 3/20/9 | 0.0350* |
| IIIa/IIIb/IV | 13/7/8 | 6/2/3 | |
Statistically significant. Data are presented as median (first quartile, third quartile). A, ascending colon; BMI, body mass index; C, cecum; D, descending colon; muc, mucinous carcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; R, rectum; S, sigmoid colon; T, transverse colon; tub1, well-differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma.
p-PDH in CRCs
Immunohistochemical staining of p-PDH was performed in a manner similar to that of immunohistochemical analysis of HK2 (Fig.1c). Correlations between p-PDH expression and clinicopathological factors are summarized in Table2. In contrast to HK2 expression, p-PDH expression did not show any correlations with tumor depth, lymph node metastasis, or other patient backgrounds. Assessment of tumor locations indicated that p-PDH expression tended to be higher in right-sided CRCs than in left-sided CRCs, but did not reach statistical significance. We also performed immunohistochemical analysis to detect the total amount of PDH-E1α regardless of phosphorylation status in 19 colorectal cancer tissues. The result showed that total PDH-E1α-positive cases showed p-PDH positive or negative expression (Fig.2; Figs S2 and S3), whereas the total PDH-E1α-negative cases (intensity score 0) was absent for p-PDH expression, although the low expression cases (score 1) could be p-PDH positive (Table3; data not shown), suggesting that the phosphorylation event is independent of the protein amount, and that phosphorylation control may be critical in clinical status of tumors.
Table 2.
p-PDH expression and clinicopathological features of colorectal cancer
| Phospho-PDH-E1α | Positive (n = 34) | Negative (n = 70) | P-value |
|---|---|---|---|
| Patient background | |||
| Gender (Male/Female) | 22/12 | 43/27 | 0.7461 |
| Age (mean ± SD) | 66.0 ± 10.4 | 63.5 ± 13.3 | 0.1669 |
| BMI (kg/m2) | 23.6 (20.6, 25.6) | 21.9 (20.3, 25.6) | 0.4316 |
| CEA (ng/mL) | 2.5 (1, 6) | 3 (2.0, 7.3) | 0.4401 |
| CA19-9 (U/mL) | 12.5 (5.0, 23.3) | 12.5 (7.0, 21.3) | 0.8538 |
| Tumor characteristics | |||
| Tumor diameter (mm) | 37 (22, 52) | 35 (22, 52) | 0.6126 |
| Location | |||
| C/A/T | 5/6/5 | 3/9/9 | 0.0883 |
| D/S/R | 3/3/12 | 1/17/31 | |
| Tumor type | |||
| Type 0 | 11 | 15 | 0.2275 |
| Type 1/2/3/4/5 | 1/16/4/0/2 | 5/38/12/0/0 | |
| Histological type | |||
| tub1/tub2/pap | 10/18/1 | 20/44/1 | 0.2893 |
| por/muc | 2/3 | 2/3 | |
| Depth | |||
| T0/T1/T2 | 3/4/7 | 5/11/18 | 0.4779 |
| T3/T4 | 16/4 | 30/6 | |
| Lymph node metastasis | |||
| N0 | 23 | 45 | 0.7354 |
| N1/N2/N3 | 5/4/2 | 18/3/4 | |
| Distant metastasis | |||
| M0 | 29 | 64 | 0.3339 |
| M1 | 5 | 6 | |
| Lymphatic duct invasion | |||
| ly0 | 12 | 22 | 0.6857 |
| ly1/ly2 | 15/7 | 40/8 | |
| Venous invasion | |||
| v0 | 26 | 58 | 0.4394 |
| v1/v2 | 7/1 | 9/3 | |
| Stage | |||
| 0/I/II | 3/9/10 | 5/24/14 | 0.7461 |
| IIIa/IIIb/IV | 4/3/5 | 15/6/6 | |
Data are presented as median (first quartile, third quartile). A, ascending colon; BMI, body mass index; C, cecum; D, descending colon; muc, mucinous carcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; R, rectum; S, sigmoid colon; T, transverse colon; tub1, well-differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma.
Fig 2.
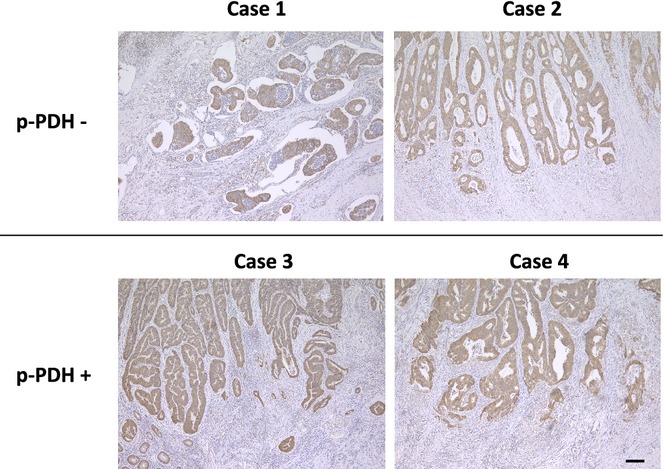
Immunohistochemical analysis of PDH-E1α. Representative images of PDH-E1α staining in p-PDH negative cases (case 1 and 2) and p-PDH positive cases (cases 3 and 4). Scale bar, 100 μm.
Table 3.
Correlation between the immunohistochemical staining of phosphorylation status of PDH-E1α
| p-PDH | P | ||
|---|---|---|---|
| Positive | Negative | ||
| Total PDH-E1α | |||
| Positive | 6 | 8 | 1.0000 |
| Negative | 2 | 3 | |
Heterogeneity of HK2 or p-PDH staining in CRC and in normal mucosa
We examined whether the heterogeneity of HK2 or p-PDH staining in CRC might be observed, and moreover, the staining intensities in normal mucosa. As shown in Table4, positive correlation could be observed between the staining intensities in the deep part and in the superficial part of tumors regarding HK2 and p-PDH expression. In relation to the expression in normal mucosa, both expressions could scarcely be observed and any correlations with the staining in the deep part of tumor could not be observed.
Table 4.
Assessment of the staining heterogeneity of HK2 and p-PDH in tumor superficial and deep part and in normal mucosa
| HK2 staining in deep part | P | ||
|---|---|---|---|
| Positive | Negative | ||
| HK2 staining in superficial part | |||
| Positive | 30 | 10 | 0.0074* |
| Negative | 31 | 33 | |
| HK2 staining in normal mucosa | |||
| Positive | 1 | 1 | 1.0000 |
| Negative | 54 | 38 | |
| p-PDH staining in deep part | P | ||
|---|---|---|---|
| Positive | Negative | ||
| p-PDH staining in superficial part | |||
| Positive | 28 | 36 | 0.0024* |
| Negative | 6 | 34 | |
| p-PDH staining in normal mucosa | |||
| Positive | 0 | 0 | NA |
| Negative | 31 | 66 | |
Statistically significant. In the assessment of staining in normal mucosa, the cases that did not contain normal mucosa in paraffin section were not included.
Recurrence-free survival
We studied the correlations of HK2 or p-PDH expression with recurrence-free survival (RFS) in all the patients except for nine Stage IV patients who underwent non-curable resection. HK2 could separate the patients by prognosis, with positive HK2 expression being associated with a poor survival rate (P = 0.0290) (Fig.3a). In contrast, negative p-PDH expression tended to correlate with poor RFS, but this difference was not statistically significant (P = 0.2572) (Fig.3b). We then assessed the ability of the combination of two metabolic markers to predict aggressive phenotypes of tumors and survival of patients. We classified the patients into two groups: the combined HK2-positive and p-PDH-negative group and the group consisting of the other cases. This immunohistochemical evaluation of combined enzyme expression showed that positive HK2 expression combined with negative p-PDH expression was associated with poor RFS rates (P = 0.0169), which could be considered as more sensitive prognostic factor than HK2 alone (Fig.3a–c). In the multivariate analysis, tumor depth and lymph node metastasis were found to be independent prognostic factors, while the combined evaluation showed the statistical significance in univariate, but not in multivariate analysis (Table5).
Fig 3.
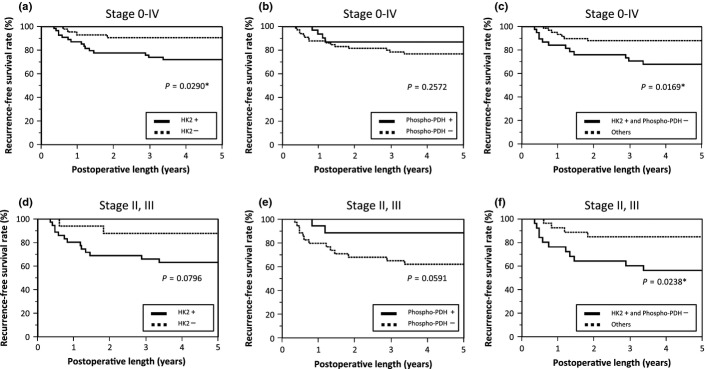
Recurrence-free survival curves of the analyzed patients. (a) Patients in all stages were analyzed based on the level of HK2 expression. (b) Patients in all stages were analyzed based on the level of p-PDH expression. (c) Patients in all stages were analyzed based on combined expression of HK2 and p-PDH. (d) Patients in stage II and III were analyzed based on the level of HK2 expression. (e) Patients in stage II and III were analyzed based on the level of p-PDH expression. (f) Patients in stage II and III were analyzed based on combined expression of HK2 and p-PDH. *Statistically significant.
Table 5.
Results of univariate and multivariate Cox regression analysis for Stage 0–IV patients
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.017 (0.977–1.063) | 0.4066 | ||
| Gender | ||||
| Female | Reference | 0.0120* | ||
| Male | 3.015 (1.004–12.963) | 0.0491* | 4.186 (1.365–18.220) | |
| Tumor diameter | 1.014 (0.994–1.032) | 0.1515 | ||
| Location | ||||
| Right-sided | Reference | 0.2371 | ||
| Left-sided | 1.806 (0.691–5.588) | |||
| Tumor type | ||||
| Type 0 | Reference | 0.0062* | ||
| Type 1/2/3/4/5 | 7.787 (1.608–140.039) | |||
| Histological type | ||||
| tub1/tub2/pap | Reference | 0.5763 | ||
| por/muc | 1.557 (0.247–5.434) | |||
| Depth | ||||
| T0/T1/T2 | Reference | 0.0028* | 0.0162* | |
| T3/T4 | 4.497 (1.631–15.781) | 3.695 (1.257–13.500) | ||
| Lymph node metastasis | ||||
| N0 | Reference | 0.0009* | 0.0190* | |
| N1/N2/N3 | 4.716 (1.892–12.704) | 3.156 (1.207–8.912) | ||
| Immunohistochemistry | ||||
| Others | Reference | 0.0198* | 0.0656 | |
| HK2+ and p-PDH- | 2.952 (1.187–7.933) | 2.383 (0.946–6.475) | ||
Statistically significant. CI, confidence interval; HR, hazard ratio.
Furthermore, we carried out a similar analysis of RFS for the 52 patients, including 24 Stage II patients and 28 Stage III patients, who had a certain level of recurrence risk and might benefit from adequate estimation of recurrence risk in that the necessity of adjuvant therapy could be evaluated (Table6).23 HK2 and p-PDH expression tended to separate the patients by prognosis; however, significant differences were not observed (P = 0.0796 and 0.0591, respectively; Fig.3d,e). Interestingly, the immunohistochemical evaluation of combined enzyme expression showed that positive HK2 expression combined with negative p-PDH expression significantly correlated with poor RFS rates (P = 0.0238) (Fig.3f). Multivariate analysis showed the combination of positive HK2 and negative p-PDH expression was independently associated with poor prognosis (P = 0.0389) (Table7).
Table 6.
Results of immunohistochemistry for Stage II and III cases based on HK2 and p-PDH expression
| HK2 expression | ||
|---|---|---|
| Positive | Negative | |
| p-PDH expression | ||
| Positive | 10 | 6 |
| Negative | 25 | 11 |
Table 7.
Results of univariate and multivariate Cox regression analysis for Stage II and III patients
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.026 (0.976–1.077) | 0.3052 | ||
| Gender | ||||
| Female | Reference | 0.0166* | 0.0270* | |
| Male | 4.657 (1.284–29.806) | 4.740 (1.175–32.086) | ||
| Tumor diameter | 1.011 (0.989–1.030) | 0.3140 | ||
| Location | ||||
| Right-sided | Reference | 0.0974 | 0.9038 | |
| Left-sided | 2.496 (0.8526–9.011) | 0.923 (0.270–3.821) | ||
| Histological type | ||||
| tub1/tub2/pap | Reference | 0.8206 | ||
| por/muc | 1.193 (0.186–4.321) | |||
| Depth | ||||
| T0/T1/T2 | Reference | 0.3387 | ||
| T3/T4 | 2.390 (0.4803–43.287) | |||
| Lymph node metastasis | ||||
| N0 | Reference | 0.0813 | 0.1745 | |
| N1/N2/N3 | 2.616 (0.892–9.458) | 2.284 (0.705–8.957) | ||
| Immunohistochemistry | ||||
| Others | Reference | 0.0230* | 0.0389* | |
| HK2+ and p-PDH- | 3.451 (1.179–12.463) | 3.143 (1.058–11.461) | ||
Statistically significant. CI, confidence interval; HR, hazard ratio.
Budding
To confirm the reason why the combined evaluation of both HK2 and p-PDH expression strongly correlated with RFS in colorectal cancer patients especially in Stage II and III, we analyzed the association between the combined evaluation and “budding”. As a result, positive HK2 and negative p-PDH associated with the increased number of budding (P = 0.0199) (Fig.4).
Fig 4.
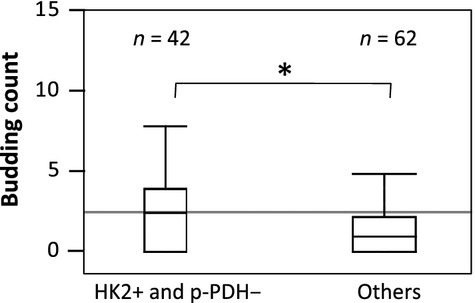
The budding count in colorectal cancer tissues. The budding count of HK2+ and p-PDH- cancers was significantly greater than that of the others (P = 0.0199). *Statistically significant.
HK2 and PDH activity analysis
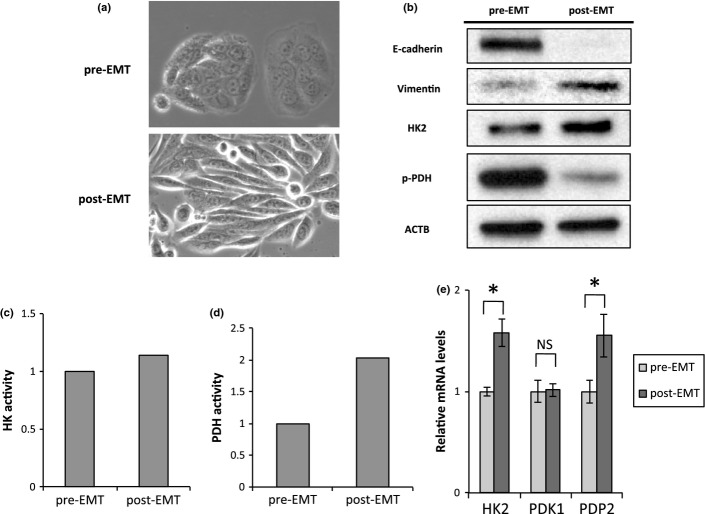
In the process of invading into stroma, cancer cells acquire the ability to detach from the epithelial lining and migrate, which is regulated by the mechanism of EMT, in a manner similar to the developmental program of an embryo. A set of pleiotropically acting genes orchestrates the EMT process by evoking loss of adherent junctions, conversion to spindly shapes, increased motility, and resistance to apoptosis in invasive front lesions of tumors.24 To study the underlined mechanism in the present observation, we induced EMT to colon cancer cell line SW480, as a model of invading colorectal cancer cells, according to the previously described procedure followed by the analyses of HK and PDH activity.25–28 In response to EMT stimulation, cell morphology changed from epithelial to fibroblastic-like spindle shape (Fig.5a) and expression of E-cadherin was decreased and Vimentin was increased (Fig.5b). By acquiring mesenchymal phenotype, HK2 expression and phosphorylation level of PDH were up-regulated (Fig.5b). Corresponding to these shifts in the expression of the two enzymes, HK activity and PDH activity were augmented in biochemical analyses (Fig.5c,d). Activity of PDH is regulated by two key PDH-modifying enzymes; PDK1 phosphorylates PDH to suppress the function, whereas PDP2 dephosphorylates PDH to stimulate it.17 We found that PDP2 expression was increased in EMT condition, but PDK1 expression was stable, which might explain why p-PDH was down-regulated (Fig.5e).
Fig 5.
Epithelial–mesenchymal transition (EMT) accompanies HK2 upregulation and p-PDH downregulation. (a) Photomicrographs of the morphological change of SW480 cells. (b) Western blot assays of E-cadherin, Vimentin, HK2, p-PDH, and ACTB expression in pre-EMT and post-EMT cells. Samples of post-EMT cells were harvested at 72 h. (c) Hexokinase activity in pre-EMT and post-EMT cells. Samples of post-EMT cells were harvested at 72 h. (d) Pyruvate dehydrogenase activity in pre-EMT and post-EMT cells. Samples of post-EMT cells were harvested at 72 h. (e) Relative transcript (mRNA) levels of HK2, PDK1, and PDP2 after inducing EMT for 0 and 48 h. The values at 0 h have been normalized to 1, and the data are expressed as fold. *Statistically significant.
Discussion
An increasing amount of evidence has shown that cancer-specific alterations in metabolism, that is, enhanced glucose uptake and successive preferential conversion to lactate, are important factors in cancer metabolism (the Warburg effect)6,29 and constitute tumor growth. This system is beneficial for the biosynthetic and bioenergetic demands of proliferation by diverting glycolytic intermediates to an alternative biosynthetic, the pentose phosphate pathway. Moreover, these metabolic systems are attractive targets for possible therapeutic interventions and currently research is ongoing to demonstrate the definite mechanism of cancer metabolism.30 Although aerobic glycolysis is intimately linked to tumor growth and cancer cell proliferation, how glycolysis is involved in cellular invasion remains unclear. Invasion and metastasis are hallmarks of cancer and are closely associated with the development of pathological stages of cancer arising from precancerous lesions in epithelial tissues.24 Distant metastasis becomes clinically evident as a consequence of multistep cascades that initially occur in local invasions.31,32 Thus, the study of the effect of cancer metabolism in invasion may be beneficial for elucidating the novel mechanism of invasion and metastasis. To the best of our knowledge, this is the first study to demonstrate a significant association between the expression of the biomarkers HK2 and p-PDH and patient survival.
Although tumor heterogeneity is largely a common feature for the generation of biological plasticity, genetic diversification, and intractableness of tumors in advanced stages,33 the presence of subpopulations with a high invasive potential (termed “budding”) characterizes tumor heterogeneity in CRCs.19,20 Budding is defined as detachment from tumor tissues into single or up to five cancer cell clusters at invasive front lesions of CRCs.19,20 Previous reports, including our own, indicate that tumor budding undergoes EMT.34–36 The clinical guidelines of the European Society for Medical Oncology37 and the Japanese Society for Cancer of the Colon and Rectum38 include tumor budding. Based on these backgrounds, we aimed to examine the glycolytic characteristics of the deepest part of tumor and those of the cancer cells undergoing EMT in this study.
According to the previous studies, the association between HK2 and RFS has not been clear and consistent results could not be acquired yet.39–43 A possible explanation for this controversy is that the samples analyzed in these studies might be obtained from the superficial tissues of tumor. In considering the role of HK2 in aerobic glycolysis in the invasive front lesions of CRCs, where the cancer cells are usually located in the deep parts of tumors and are stimulated to invade and metastasize, the present study focused on samples from the invasive front lesions of CRCs. The present study showed that enhanced glucose uptake and glycolysis in the deeper parts of the tumor was associated with tumor growth and invasion, as shown by the data of lymph node metastasis samples. Thus, in invasive fronts, our results suggest that cancer metabolism may be reprogrammed and dominantly shifted toward active glycolysis.
We also studied the expression of p-PDH. Considering that p-PDH is involved in the OxPhos inhibition in the mitochondria and contributes to the establishment of aerobic glycolysis, it is possible that high p-PDH is associated with poor prognosis. Contrary to this original expectation, the present analysis of the invasive fronts of CRCs showed that low p-PDH was associated with poor prognosis, and evaluation of combined expression of p-PDH and HK2 demonstrated a clear association with patient prognosis. The results suggest that p-PDH plays a unique role in the malignant behavior of CRCs. Interestingly, recent studies have identified two subpopulations of cancer cells that are distinct in their energy-generating pathways.44–46 One subpopulation depends on anaerobic glycolysis and secretes massive lactate. The other subpopulation can use lactate from upstream glycolysis in individual cells as well as from surrounding cells, and therefore the metabolites produced by the abovementioned subpopulation. The latter subpopulation can use lactate as the energy source by employing OxPhos in the mitochondria.46 Also as suggested by our in vitro experiments, we speculate that CRC cells perform OxPhos in invasive front lesions. This hypothesis may be further supported by the observations that the citric cycle generates reactive oxygen species by OxPhos, which promote EMT and further cancer invasion.47 Assessment of combined expression of HK2 and p-PDH may be useful for detecting highly malignant CRC cells. Further investigation should be performed via more detailed mechanistic studies of cancer metabolism associated with invasion and budding to identify more accurate predictors of patient prognosis and to regulate cancer invasion and metastasis.
In conclusion, combined expression of HK2 and p-PDH, as a novel cancer metabolomics-associated biomarker measure, may be clinically useful for predicting tumor aggressiveness and survival in CRC.
Acknowledgments
We thank the members of our laboratories for their helpful discussions. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology; a Grant-in-Aid from the Third Comprehensive 10-year Strategy for Cancer Control, Ministry of Health, Labor, and Welfare; a grant from the Kobayashi Cancer Research Foundation; a grant from the Princess Takamatsu Cancer Research Fund, Japan; a grant from the National Institute of Biomedical Innovation; and a grant from the Osaka University Drug Discovery Funds.
Disclosure Statement
A.H. is a research fellow of the Japan Society for the Promotion of Science. M.K., N.N., J.K., M.M., and H.I. received partial support from Chugai Co., Ltd., Yakult Honsha Co., Ltd., Merck Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Science Foundation and Takeda Medical Research Foundation through institutional endowments.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Western blot analysis of extracts from HEK293, HeLa cells and three clinical samples of colorectal cancer using Hexokinase 2 antibody.
Fig. S2. Western blot analysis of extracts from HEK293 cell and three clinical samples of colorectal cancer using p-PDH antibody.
Fig. S3. Western blot analysis of extracts from HEK293 cell and three clinical samples of colorectal cancer using PDH-E1α antibody.
Fig. S4. Absorption test of HK2 antibody on pancreatic cancer (A) and colorectal cancer tissues (B–D). Scale bar, 100 μm.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Vaiopoulos AG, Athanasoula KC, Papavassiliou AG. Epigenetic modifications in colorectal cancer: molecular insights and therapeutic challenges. Biochim Biophys Acta. 2014;1842:971–80. doi: 10.1016/j.bbadis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Ribelles N, Santonja A, Pajares B, Llacer C, Alba E. The seed and soil hypothesis revisited: current state of knowledge of inherited genes on prognosis in breast cancer. Cancer Treat Rev. 2014;40:293–9. doi: 10.1016/j.ctrv.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–4. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–28. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Shan C, Kang HB, et al. Tyr Phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;20:534–48. doi: 10.1016/j.molcel.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–96. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–57. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–12. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem J. 2001;358:69–77. doi: 10.1042/0264-6021:3580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche TE, Baker JC, Yan X, et al. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog Nucleic Acid Res Mol Biol. 2001;70:33–75. doi: 10.1016/s0079-6603(01)70013-x. [DOI] [PubMed] [Google Scholar]
- Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–81. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–12. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- Lyshchik A, Higashi T, Hara T, et al. Expression of glucose transporter-1, hexokinase-II, proliferating cell nuclear antigen and survival of patients with pancreatic cancer. Cancer Invest. 2007;25:154–62. doi: 10.1080/07357900701208931. [DOI] [PubMed] [Google Scholar]
- Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer. 2012;106:1713–7. doi: 10.1038/bjc.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Hase K, Hashiguchi Y, et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol. 2013 doi: 10.1007/s00535-013-0881-3. ; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–32. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66:9583–90. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- Yokobori T, Iinuma H, Shimamura T, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 2013;73:2059–69. doi: 10.1158/0008-5472.CAN-12-0326. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–74. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Ziyadeh FN, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–28. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleterry J, Sreedhar A, Zhao Y. Components of cancer metabolism and therapeutic interventions. Mitochondrion. 2014;6:50–55. doi: 10.1016/j.mito.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro R, Yamamoto H, Takahashi H, et al. C4.4A is associated with tumor budding and epithelial-mesenchymal transition of colorectal cancer. Cancer Sci. 2012;103:1155–64. doi: 10.1111/j.1349-7006.2012.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelzer VH, Karamitopoulou E, Dawson H, Kondi-Pafiti A, Zlobec I, Lugli A. Geographic analysis of RKIP expression and its clinical relevance in colorectal cancer. Br J Cancer. 2013;108:2088–96. doi: 10.1038/bjc.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA Zlobec I. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010;1:651–61. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- Galamb O, Spisak S, Sipos F, et al. Reversal of gene expression changes in the colorectal normal-adenoma pathway by NS398 selective COX2 inhibitor. Br J Cancer. 2010;102:765–73. doi: 10.1038/sj.bjc.6605515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, He X, Ji H, Shi L, Davis RW, Zhong S. Reproducibility probability score–incorporating measurement variability across laboratories for gene selection. Nat Biotechnol. 2006;24:1476–7. doi: 10.1038/nbt1206-1476. [DOI] [PubMed] [Google Scholar]
- Skrzypczak M, Goryca K, Rubel T, et al. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS ONE. 2010;5:e13091. doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Deane NG, Wu F, et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 2010;138:958–68. doi: 10.1053/j.gastro.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub E, Groene J, Heinze M, et al. An expression module of WIPF1-coexpressed genes identifies patients with favorable prognosis in three tumor types. J Mol Med. 2009;87:633–44. doi: 10.1007/s00109-009-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–33. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118:3835–7. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–48. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012;22:107–15. doi: 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Western blot analysis of extracts from HEK293, HeLa cells and three clinical samples of colorectal cancer using Hexokinase 2 antibody.
Fig. S2. Western blot analysis of extracts from HEK293 cell and three clinical samples of colorectal cancer using p-PDH antibody.
Fig. S3. Western blot analysis of extracts from HEK293 cell and three clinical samples of colorectal cancer using PDH-E1α antibody.
Fig. S4. Absorption test of HK2 antibody on pancreatic cancer (A) and colorectal cancer tissues (B–D). Scale bar, 100 μm.