Abstract
Alternative polyadenylation (APA), which induces shortening of the 3′UTR, is emerging as an important phenomenon in gene regulation. APA is involved in development, cancer and cell proliferation. APA may lead to disruption of microRNA-mediated gene silencing in cancer cells via detachment of microRNA binding sites. We studied the correlation between the APA profile and the tumor aggressiveness in cases of lung cancer. We selected the top 10 genes showing significant 3′UTR shortening in lung cancer, using the package of the Bioconductor for probe-level analyses of expression microarrays. We established and evaluated the APA score by quantitative RT-PCR in 147 clinical specimens of non-small cell lung cancer and compared the results with the clinical outcomes and expression levels of APA-related genes, including PABPN1, CPEB1, E2F1 and proliferation markers (MKI67, TOP2A and MCM2). High APA scores were correlated with an advanced tumor stage and a poor prognosis (P < 0.001). Multivariate analysis identified the APA score as an independent prognostic factor (hazard ratio, 3.0; P = 0.03). Both lower expression of PABPN1 and higher expression of the proliferation markers were correlated with high APA scores and a poor prognosis, with suppression of PABPN1 exerting its influence independent of gain of the proliferation markers. Moreover, the APA score was correlated with the maximum standardized uptake value of the tumors on positron emission tomography (r = 0.53; P < 0.001). Our results indicate that the loss of PABPN1, a suppressor of APA, might promote tumor aggressiveness by releasing the cancer cells from microRNA-mediated gene regulation.
Keywords: Alternative polyadenylation, lung cancer, PABPN1, prognosis, rmodel
In addition to genetic alterations in cancer cells, post-transcriptional regulation of gene expression has recently been shown to play many important roles in human cancer. MicroRNAs are broadly conserved small non-coding RNAs that bind to elements within the 3′UTR of the target mRNAs and regulate gene expression by inhibiting mRNA translation or inducing mRNA degradation.1 MicroRNA expression is altered in human cancers. While some microRNAs have oncogenic functions, in most cases, loss of microRNA expression has been reported to be correlated with cancer aggressiveness.2–6
Suppression of microRNA biosynthesis is one of the ways by which cells may escape microRNA regulation. Decreased expressions of genes involved in microRNA biosynthesis, such as Drosha, Dicer and TARBP2, are associated with cancer aggressiveness.7–11 A few reports have suggested that silencing of microRNAs by DNA methylation is associated with tumor invasiveness.12,13
Alternative polyadenylation (APA), which induces detachment of microRNA binding sites, is another way by which cells escape microRNA-mediated gene silencing (Fig.1). APA is emerging as an important player in gene regulation. More than half of mammalian genes use APA to generate multiple mRNA isoforms with different lengths of the 3′UTR.14–16 Besides escape from microRNA-mediated regulation, the shortening of the 3′UTR can influence mRNA nuclear export, localization in cytoplasm and non-microRNA-mediated changes in mRNA stability and translational efficiency.17 It has been shown that proliferating cells,18 embryonic cells,19,20 induced pluripotent stem cells21 and cancer cells22,23 express shorter transcripts. The correlation between the APA profile and tumor malignancy grade is still unclear.
Fig 1.

Schema of alternative polyadenylation and escape of microRNA regulation in cancer. (a) Loss of PABPN1 induces the use of alternative polyA site and shortening of the 3′UTR. (b) Cancer cells adopt various means to escape microRNA regulation of gene transcription. In addition to suppression of microRNA synthesis, loss of PABPN1 and 3′UTR is emerging as another mechanism of microRNA deregulation in cancer cells. CDS, coding sequence.
Several factors have been reported to be involved in the regulation of APA. While E2F24 and CPEB125 enhance APA, PABPN1 has been shown to suppress APA.26 Therefore, we investigated whether the NSCLC specific APA profile and expression levels of APA-related genes, including proliferation markers such as MKI67, TOP2A and MCM2,27–31 in clinical tumor specimens might be correlated with a poor prognosis of non-small cell lung cancer (NSCLC).
Materials and Methods
Tumor samples
Clinical lung cancer samples were collected from NSCLC patients who underwent surgical resection with curative intent at the University of Tokyo Hospital between June 2005 and May 2009. Total RNA was isolated using RNAiso Plus (TaKaRa, Shiga, Japan). The diagnoses were based on pathological evidence and the tumors were classified according to the seventh edition of the TNM classification for NSCLC.32 Patients who underwent preoperative therapy were excluded. Normal lung tissue specimens were collected from non-smoking stage I NSCLC patients without recurrence. Informed consent was obtained from all the patients, and the study was conducted with the approval of the Institutional Ethics Review Committee.
For all patients, medical records were reviewed to extract the data on the clinicopathological characteristics. A disease status census was carried out every 6 months at our outpatient department. We chose freedom from recurrence (FFR) as the primary endpoint of the study. FFR was measured from the date of surgery until documentation of disease recurrence or metastasis, and in cases without relapse, any deaths due to causes other than lung cancer were censored.
In silico selection of alternative polyadenylation-indicator genes
In order to select genes showing significant 3′UTR shortening specifically in lung cancer, we used the Bioconductor package for probe-level analysis of expression microarrays (rmodel), as in Salisbury et al.33 Rmodel divides a probe set into segments representing the sequence boundaries of transcribed regions that change by different amounts when two sample groups are compared. Microarray data obtained on Affymetrix (Santa Clara, CA, USA) HG-U133_Plus_2 array were downloaded from the Gene Expression Omnibus resistor. We used GSE10245 containing the expression profiles of 40 lung adenocarcinomas and 18 lung squamous cell carcinomas,34 and GSM415386-8 containing the expression profiles of 3 normal lung tissues.35 These profiles of normal lung tissue were selected from the dataset GSE16538 that consists of profiles of tissue samples of pulmonary sarcoidosis and normal lung tissue.35 We reviewed the result of rmodel analysis and excluded clearly inadequate candidates (i.e. genes with no significant change of probe level and genes with probes that were mismapped).
Analysis of the alternative polyadenylation status
The APA status in the in silico-selected APA-indicator genes was analyzed by quantitative RT-PCR (qRT-PCR) using THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan), performed in triplicate. We calculated the proportion of the full-length transcripts to the total transcripts using the ddCT method. We designed primer set 1 based on the coding sequence and primer set 2 on the distal side of the 3′UTR. Primer set 2 was designed distal to the boundary between the proximal probe set and the distal probe set divided by the rmodel (Fig.2). APA score was defined as the total number of the genes in which the proportion of the full-length transcripts was less than half of that in the normal lung tissue. Expressions of the APA-related genes (PABPN1, CPEB1, E2F1, and the three proliferation markers MKI67, TOP2A and MCM2) were also determined by qRT-PCR. HPRT1 was used as the internal control, and the primer sequences are shown in Tables S1 and S2.
Fig 2.
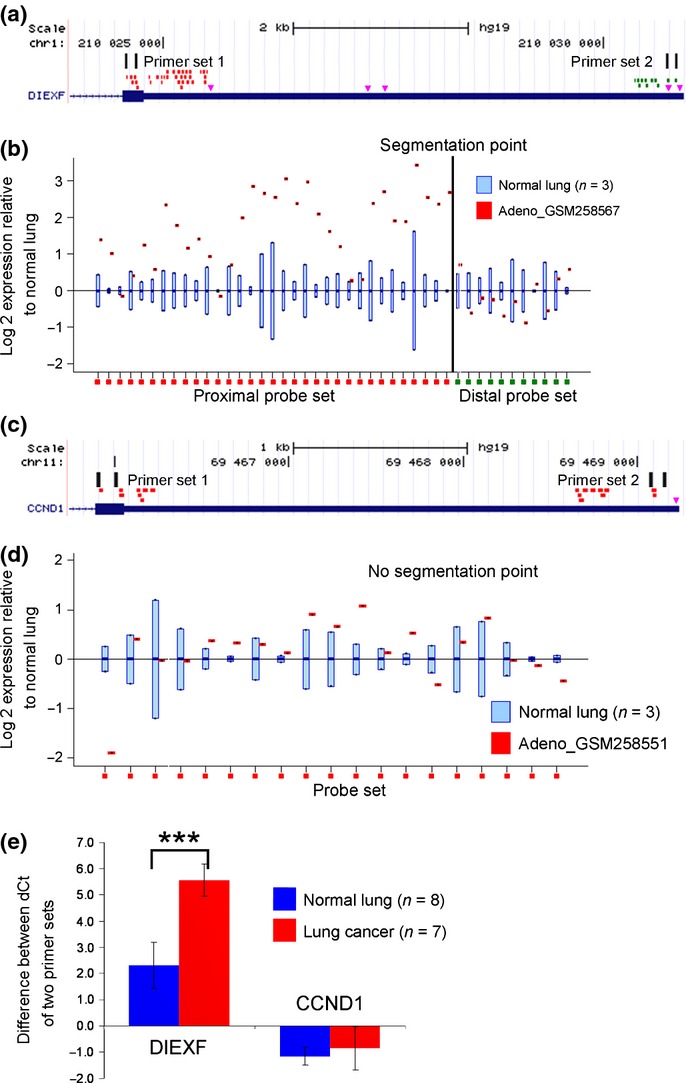
Selection of the alternative polyadenylation (APA)-indicator genes using the Rmodel. (a) The Affymetrix probes were divided into two groups by the rmodel; the proximal probe set is shown in red and the distal probe set is shown in green. The pink triangles indicate the major poly(A) signals AAUAAA and AUUAAA. Two primer sets were designed to evaluate the ratio of the transcripts with full-length 3′UTR to the total transcripts. (b) A positive example. DIEXF showed loss of signal in the 3′-terminal portion of the transcript in lung adenocarcinoma GSM258567, relative to that in the normal lung. (c and d) A negative example. CCND1 showed uniform signals along the 3′UTR. (e) Quantitative RT-PCR revealed that CCND1 was not affected by APA in the clinical samples, indicating that the observed 3′UTR shortening of other transcripts was not a result of random degradation of the transcripts in the tumor samples. ***P < 0.001.
Statistical analysis
Statistical analysis was carried out using the JMP 10 software (SAS Institute, Cary, NC, USA). The cutoff value of the APA score was determined by receiver-operating characteristic curve analysis for lung cancer recurrence. The relationships between the APA score, mRNA expression levels of the APA-related genes and the clinicopathological characteristics were analyzed using Student's t-test, Welch's method and the χ2-test. The relationship between the APA scores and the maximum standardized uptake values (SUVmax) was evaluated by calculation of the Pearson correlation coefficient. FFR was estimated using the Kaplan–Meier method and compared by the log-rank test. A multivariate analysis of various prognostic factors was carried out using the Cox proportional hazards regression model.
Results
Identification of the alternative polyadenylation-indicator genes in non-small cell lung cancer
Using the rmodel, we selected 16 genes that showed 3′UTR shortening in more than 20 of the 40 lung adenocarcinoma specimens included within the GSE10245 dataset (Table S3). All 16 genes showed 3′UTR shortening in more than 10 of the 18 lung squamous cell carcinoma specimens within the GSE10245 dataset (Table S3). The APA status of the 16 genes was confirmed in 7 clinical lung cancer specimens and 8 normal lung tissue specimens (Fig.3), and the top 10 genes which showed more marked APA in lung cancer were selected as the APA-indicator genes (C1orf52, DIEXF, ESYT2, HN1L, MUC20, NDFIP2, RBM33, SCAMP1, SMC1A and SSR1). For subsequent analysis, we defined the APA score as the total number of genes that showed 3′UTR shortening among the 10 APA-indicator genes.
Fig 3.
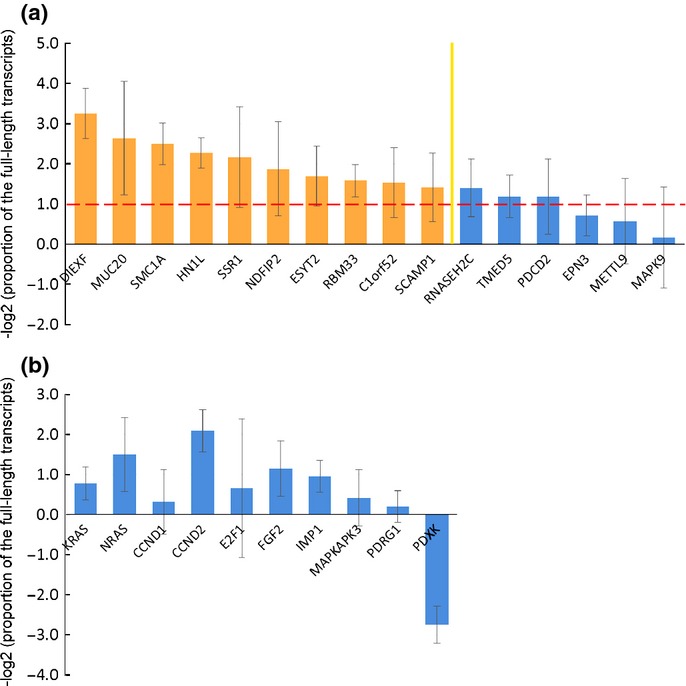
Identification of the alternative polyadenylation (APA)-indicator genes in non-small cell lung cancer. (a) The APA status of the 16 genes selected in silico was confirmed in seven clinical lung cancer specimens and eight normal lung tissue specimens, and the top 10 genes were selected as the APA-indicator genes. The APA status was evaluated on the basis of the logarithm of the proportion of the full-length transcripts of the lung cancer specimens normalized to that of the normal lung tissue specimens. Results are shown as the average ± SD. The columns over the red line indicate that the proportion of the full-length transcripts of the lung cancer specimens is less than half of that of the normal lung tissue specimens. (b) The genes selected based on the results of past studies were associated with a lower frequency of 3′UTR shortening as compared to the APA-indicator genes.
Correlation between high alternative polyadenylation score and a poor prognosis
We determined the APA scores of the 147 primary NSCLC specimens. The specimens were divided into two groups, the training set and the test set, to determine and validate the appropriate cutoff value of the APA score. The training set comprised 47 lung cancer samples and 4 normal lung tissue samples. The test set comprised 100 lung cancer samples and 6 normal lung tissue samples. The median follow-up period was 78 months for the training set and 58 months for the test set. The clinicopathological characteristics of the corresponding patient groups are shown in Table S4.
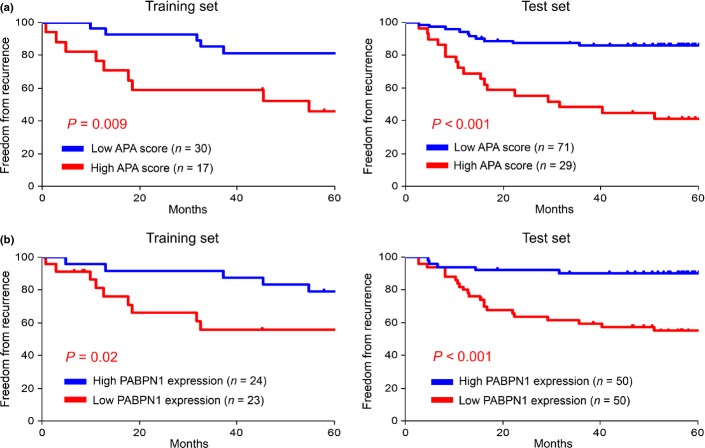
The most appropriate cutoff value of the APA score was determined to be 5 from the training set by receiver-operating characteristic curve analysis, with the same value verified as appropriate in the test set. The frequency of advanced disease, non-adenocarcinoma and adjuvant chemotherapy was higher in the high APA score group (Table1). The high APA score was significantly associated with the presence of lymph node metastasis and vascular invasion as well as with the advanced T factor. These observations indicate that abnormal APA status accumulates during the course of tumor progression and the acquisition of an invasive phenotype. The prognosis of the patients in the high APA score group was significantly poorer (Fig.4a). Univariate analysis using the log-rank test revealed the APA score, histological type, T factor, N factor, presence/absence of lymphatic invasion, presence/absence of vascular invasion and history of adjuvant chemotherapy as prognostic factors (Table2). Multivariate analysis identified the APA score, N factor and age as independent prognostic factors (Table3).
Table 1.
APA score and clinicopathological characteristics
| Training set (n = 47) | Test set (n = 100) | |||||
|---|---|---|---|---|---|---|
| High APA | Low APA | P | High APA | Low APA | P | |
| Age (years) | ||||||
| <70 | 7 | 19 | NS | 13 | 35 | NS |
| ≥70 | 10 | 11 | 16 | 36 | ||
| Sex | ||||||
| Male | 16 | 16 | 0.01 | 19 | 33 | NS |
| Female | 1 | 14 | 10 | 38 | ||
| Smoking habit | ||||||
| Negative history | 1 | 12 | 0.03 | 21 | 38 | NS |
| Positive history | 16 | 18 | 8 | 33 | ||
| Histological type | ||||||
| Adenocarcinoma | 11 | 25 | NS | 21 | 65 | 0.03 |
| Others | 6 | 5 | 8 | 6 | ||
| T factor | ||||||
| T1 | 4 | 16 | NS | 9 | 46 | 0.004 |
| T2, T3 | 13 | 14 | 20 | 25 | ||
| N factor | ||||||
| N0 | 9 | 24 | NS | 18 | 66 | <0.001 |
| N1, N2 | 8 | 6 | 11 | 5 | ||
| Lymphatic invasion | ||||||
| Negative | 10 | 24 | NS | 20 | 61 | NS |
| Positive | 7 | 6 | 9 | 10 | ||
| Vascular invasion | ||||||
| Negative | 6 | 21 | 0.04 | 12 | 57 | <0.001 |
| Positive | 11 | 9 | 17 | 14 | ||
| History of adjuvant chemotherapy | ||||||
| Negative | 10 | 23 | NS | 18 | 59 | 0.04 |
| Positive | 7 | 7 | 11 | 12 | ||
APA, alternative polyadenylation; NS, not significant.
Fig 4.
High alternative polyadenylation (APA) scores were correlated with a poor prognosis, loss of PABPN1 and CPEB1, and gain of MKI67, TOP2A and MCM2. Kaplan–Meier curves are shown for APA score (a) and PABPN1 expression (b). The cutoff value of the APA score was determined to be 5 by receiver-operating characteristic curve analysis and the median expression value of PABPN1 was used as the threshold to divide the patients into two groups. Comparison between the two groups was performed using the log-rank test.
Table 2.
Results of univariate analyses carried out to identify prognostic factors in patients with resected non-small cell lung cancer
| Training set (n = 47) | Test set (n = 100) | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| APA score ≥5 | 3.9 | 0.009 | 5.4 | <0.001 |
| Age ≥70 years | 1.1 | NS | 0.6 | NS |
| Male | 3.5 | NS | 1.7 | NS |
| Positive smoking history | 2.6 | NS | 2.3 | NS |
| Non-adenocarcinoma | 3.1 | 0.03 | 2.8 | 0.02 |
| T2, T3 | 13.7 | <0.001 | 5.2 | <0.001 |
| N1, N2 | 2.9 | 0.04 | 9.7 | <0.001 |
| Lymphatic invasion (+) | 3.3 | 0.02 | 5.8 | <0.001 |
| Vascular invasion (+) | 1.9 | NS | 8.2 | <0.001 |
| Adjuvant chemotherapy | 2.5 | NS | 4.8 | <0.001 |
| Loss of PABPN1† | 3.7 | 0.02 | 5.3 | <0.001 |
| Loss of CPEB1† | 0.7 | NS | 3.4 | 0.003 |
| Gain of MKI67† | 1.5 | NS | 4.2 | <0.001 |
| Gain of TOP2A† | 1.5 | NS | 5.3 | <0.001 |
| Gain of MCM2† | 2.9 | NS | 2.2 | 0.04 |
| Gain of E2F1† | 1.0 | NS | 2.9 | 0.008 |
The median expression value of each gene was used as the threshold to divide the patients into two groups: those with either loss or gain of expression. APA, alternative polyadenylation; NS, not significant.
Table 3.
Results of multivariate analyses carried out to determine the independent prognostic factors in patients with resected non-small cell lung cancer
| Test set (n = 100) | ||
|---|---|---|
| Hazard ratio | P | |
| APA score ≥5 | 3.0 | 0.03 |
| T2, T3 | 2.6 | NS |
| N1, N2 | 4.7 | 0.002 |
| Age ≥70 years | 0.3 | 0.02 |
| Male | 1.0 | NS |
| Positive smoking history | 2.2 | NS |
| Non-adenocarcinoma | 1.6 | NS |
| Test set (n = 100) | ||
|---|---|---|
| Hazard ratio | P | |
| Loss of PABPN1† | 3.5 | 0.01 |
| Loss of CPEB1† | 2.1 | NS |
| Gain of MKI67† | 1.7 | NS |
| Gain of TOP2A† | 2.1 | NS |
| Gain of MCM2† | 0.4 | NS |
| Gain of E2F1† | 3.9 | 0.02 |
The median expression value of each gene was used as the threshold to divide the patients into two groups; those with either loss or gain of expression. APA, alternative polyadenylation; NS, not significant.
Positron emission tomography (PET) was performed preoperatively in 130 of the 147 NSCLC patients, and the SUVmax of the primary tumor was evaluated in 115 cases. The SUVmax was found to be correlated with the APA score (r = 0.53; P < 0.001), with the SUVmax values being higher in the high APA score group than those in the low APA score group (11.8 ± 8.0 vs 5.6 ± 4.8, P < 0.001).
Correlation between high alternative polyadenylation status score and low PABPN1 expression
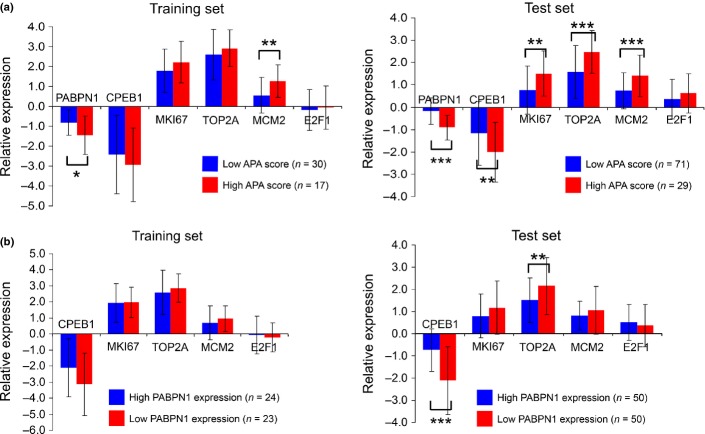
The APA scores were inversely correlated with the relative expression of PABPN1 (r = 0.53; P < 0.001). High APA scores were significantly correlated with an enhanced proliferative state of the cells (increased expression of MKI67, TOP2A and MCM2) and lower expression of PABPN1 and CPEB1 (Fig.5a), with suppression of PABPN1 exerting its influence independent of higher expression of the proliferation markers (Fig.5b). Recent studies have shown that E2F24 and CPEB125 enhance APA. We did not observe any significant relation between the APA scores and E2F1 expression. The high APA score group tended to show lower expression levels of CPEB1 than the low APA score group. Moreover, the expression of CPEB1 was markedly suppressed in the lung cancer specimens (Fig.5a).
Fig 5.
The expression levels of the alternative polyadenylation (APA)-related genes is shown for the APA score (a) and for PABPN1 expression (b). Results are normalized to the normal lung tissues and shown as the average ± SD. Suppression of PABPN1 was independent of the upregulation of proliferation markers. *P < 0.05; **P < 0.01; ***P < 0.001.
We analyzed the correlations between the expression levels of the APA-related genes and the patient prognosis. The prognosis of the patients in the low PABPN1 expression group was significantly poorer (Fig.4b). Univariate analysis revealed that loss of PABPN1 and CPEB1 and gain of MKI67, TOP2A, MCM2 and E2F1 were correlated with a poor prognosis (Table2). Multivariate analysis identified loss of PABPN1 and gain of E2F1 as independent prognostic factors (Table3).
Discussion
In our study, high APA scores were correlated with advanced tumor stages and a poor prognosis of the patients. We also determined that loss of PABPN1 was correlated with high APA scores and a poor prognosis, independent of the expression of proliferation markers. Moreover, the APA scores were correlated with the SUVmax values of the tumors.
The mechanism of APA is currently under intense investigation. The 3′UTR shortening associated with APA may be the result of enhanced cell proliferation24 or deregulation of APA-regulator genes, such as PABPN1 and CPEB1,25,26 although it remains unclear whether the proposed mechanisms work in cancer cells. In our lung cancer samples, both lower expression of PABPN1 and higher expression of the proliferation markers were correlated with the APA scores and a poor patient prognosis. Importantly, suppression of PABPN1 was independent of the expression of the proliferation markers, suggesting that the pathway of APA induced by the loss of PABPN1 is independent of the pathway of APA in proliferating cells. Low expression of CPEB1 was correlated with high APA scores. This result is in conflict with a previous report which revealed that CPEB1 mediates APA.25 CPEB1 expression was markedly suppressed in the lung cancer specimens. Thus, it is likely that CPEB1 does not induce APA, at least in lung cancer.
The finding of a correlation between the SUVmax and the APA score is of interest. The SUVmax evaluated by PET represents the tumor uptake of fluorodeoxyglucose and reflects the tumor cell activity. Further interventional research is needed to clarify the causal relationship between APA and enhancement of the tumor cell proliferative activity.
Until date, there have been no consistent reports on how APA might affect the expression levels of the APA-regulated genes. Theoretically, mRNAs with shorter lengths of the 3′UTR are depleted of microRNA binding sites, resulting in increased mRNA expression. This is true for some APA-regulated genes with let-7 microRNA binding sites.22 In our study, however, expression levels of the APA-indicator genes tended to be lower in the lung cancer samples than in the normal lung tissue samples (Fig.6). There are three possibilities to explain this discrepancy. First, the expression of the APA-indicator genes was relatively suppressed as a result of upregulation of other overexpressed genes in the cancer cells. It should be noted that the APA-indicator genes are mere indicator genes, and are not oncogenes by themselves. Next, the mRNA expressions might be suppressed by negative feedback as a result of the significant increase of the protein production from mRNAs with shorter lengths of the 3′UTR. Finally, some upregulated microRNAs (oncomiRs) in the cancer cells may bind only to the target gene transcripts with full-length 3′UTR, and may result in increasing the proportion of target gene transcripts with short 3′UTR.
Fig 6.
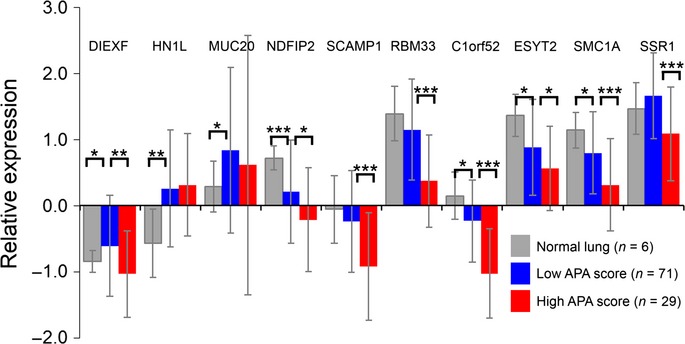
Relative expressions of alternative polyadenylation (APA)-indicator genes. APA-indicator genes tended to have lower expression in the high APA score group than in the low APA score group. Results are shown as the average ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
In most recent studies on the APA, the APA profile has been evaluated by expression microarrays or high-throughput sequencing. Correlation of shorter lengths of the 3′UTR with a poor prognosis has been shown in breast and lung cancers by analysis of Affymetrix chips.36 Certainly, analysis of a few transcripts is insufficient to estimate APA, which has a genomewide influence; however, these methods are still too expensive for clinical application. To resolve this dilemma, we selected APA-indicator genes, using the rmodel, that showed significant 3′UTR shortening specifically in lung cancer. We could evaluate the APA profile easily and efficiently by performing qRT-PCR of these selected genes. The APA profile of the 10 genes (KRAS, NRAS, CCND1, CCND2, E2F1, FGF2, IMP1, MAPKAPK3, PDRG1 and PDXK) selected based on the results of the past studies22,26,37 were evaluated in the same way (Fig.3). These genes were associated with a lower frequency of 3′UTR shortening as compared to the APA-indicator genes and were not useful as indices of APA in the clinical lung cancer samples. It has been reported that CCND1, FGF2 and IMP1 show APA in cancer cell lines, including lung cancer cell lines,22 PDXK exhibits marked APA in clinical colorectal cancer,37 and CCND2, E2F1, MAPKAPK3 and PDRG1 show APA in an osteosarcoma cell line with PABPN1 knockdown.26 Genes showing marked 3′UTR shortening may differ considerably depending on the primary site and on whether the sample is a cell line or a primary tumor. This result is consistent with a recent study carried out using the rmodel, which revealed that the characteristic signatures of 3′UTR shortening can distinguish tumor subtypes.38 Rmodel-based selection of the APA-indicator genes showing 3′UTR shortening specifically in the target disease is very useful for simple and efficient evaluation of APA.
In conclusion, we revealed significant correlations between high APA scores, loss of PABPN1 and a poor patient prognosis in cases of lung cancer. Because PABPN1 is a recently identified suppressor of APA, this observed link suggests that loss of PABPN1 might promote tumor aggressiveness by releasing the tumor cells from microRNA-mediated gene regulation. The prognostic significance of APA and PABPN1 should be validated in a large independent cohort.
Acknowledgments
This work was supported by grants from the Takeda Science Foundation and JSPS KAKENHI (23390147, 24659267, 25830098, 24791455, 23249045, 22249017 and 24390326).
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. PCR primer sequences of the selected alternative polyadenylation (APA)-indicator genes.
Table S2. PCR primer sequences of the alternative polyadenylation (APA)-related genes and HPRT1.
Table S3. Alternative polyadenylation (APA) profile of 16 genes that showed frequent 3′UTR shortening in lung cancer.
Table S4. Patient characteristics.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takai D. Disruption of the expression and function of microRNAs in lung cancer as a result of epigenetic changes. Front Genet. 2013;4:275. doi: 10.3389/fgene.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt WM, Lin YG, Han LY, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–50. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Pester RE, Chen CY, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700–4. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G, Rosato A, Ferrari F, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Melo SA, Ropero S, Moutinho C, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet. 2009;41:365–70. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karube Y, Tanaka H, Osada H, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–5. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K, Watanabe K, Emoto N, et al. CpG island methylation of microRNAs is associated with tumor size and recurrence of non-small-cell lung cancer. Cancer Sci. 2011;102:2126–31. doi: 10.1111/j.1349-7006.2011.02101.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Emoto N, Hamano E, et al. Genome structure-based screening identified epigenetically silenced microRNA associated with invasiveness in non-small-cell lung cancer. Int J Cancer. 2012;130:2580–90. doi: 10.1002/ijc.26254. [DOI] [PubMed] [Google Scholar]
- Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–12. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F, Kapranov P, Foissac S, et al. Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell. 2010;143:1018–29. doi: 10.1016/j.cell.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PJ, Choi EA, Lu J, Flanagan LA, Hertel KJ, Shi Y. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-Seq. RNA. 2011;17:761–72. doi: 10.1261/rna.2581711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–8. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–7. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Lee JY, Pan Z, Jiang B, Tian B. Progressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc Natl Acad Sci USA. 2009;106:7028–33. doi: 10.1073/pnas.0900028106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen S, Azzam G, Kaschula R, Williams LS, Alonso CR. Developmental RNA processing of 3'UTRs in Hox mRNAs as a context-dependent mechanism modulating visibility to microRNAs. Development. 2010;137:2951–60. doi: 10.1242/dev.047324. [DOI] [PubMed] [Google Scholar]
- Ji Z, Tian B. Reprogramming of 3′ untranslated regions of mRNAs by alternative polyadenylation in generation of pluripotent stem cells from different cell types. PLoS ONE. 2009;4:e8419. doi: 10.1371/journal.pone.0008419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–84. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sun Y, Li Y, et al. Differential genome-wide profiling of tandem 3′ UTRs among human breast cancer and normal cells by high-throughput sequencing. Genome Res. 2011;21:741–7. doi: 10.1101/gr.115295.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon R, Drost J, van Haaften G, et al. E2F mediates enhanced alternative polyadenylation in proliferation. Genome Biol. 2012;13:R59. doi: 10.1186/gb-2012-13-7-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava FA, Eliscovich C, Ferreira PG, et al. CPEB1 coordinates alternative 3′-UTR formation with translational regulation. Nature. 2013;495:121–5. doi: 10.1038/nature11901. [DOI] [PubMed] [Google Scholar]
- Jenal M, Elkon R, Loayza-Puch F, et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell. 2012;149:538–53. doi: 10.1016/j.cell.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Elias JM. Cell proliferation indexes: a biomarker in solid tumors. Biotech Histochem. 1997;72:78–85. doi: 10.3109/10520299709082216. [DOI] [PubMed] [Google Scholar]
- Oda M, Arakawa Y, Kano H, et al. Quantitative analysis of topoisomerase IIalpha to rapidly evaluate cell proliferation in brain tumors. Biochem Biophys Res Commun. 2005;331:971–6. doi: 10.1016/j.bbrc.2005.03.224. [DOI] [PubMed] [Google Scholar]
- Torres-Rendon A, Roy S, Craig GT, Speight PM. Expression of Mcm2, geminin and Ki67 in normal oral mucosa, oral epithelial dysplasias and their corresponding squamous-cell carcinomas. Br J Cancer. 2009;100:1128–34. doi: 10.1038/sj.bjc.6604967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaginis C, Vgenopoulou S, Vielh P, Theocharis S. MCM proteins as diagnostic and prognostic tumor markers in the clinical setting. Histol Histopathol. 2010;25:351–70. doi: 10.14670/HH-25.351. [DOI] [PubMed] [Google Scholar]
- Jakobsen JN, Sorensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer. 2013;79:1–7. doi: 10.1016/j.lungcan.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumours. 7th edn. New York: Wiley-Liss; 2010. [Google Scholar]
- Salisbury J, Hutchison KW, Wigglesworth K, Eppig JJ, Graber JH. Probe-level analysis of expression microarrays characterizes isoform-specific degradation during mouse oocyte maturation. PLoS ONE. 2009;4:e7479. doi: 10.1371/journal.pone.0007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R, Muley T, Meister M, et al. Global gene expression analysis reveals specific patterns of cell junctions in non-small cell lung cancer subtypes. Lung Cancer. 2009;63:32–8. doi: 10.1016/j.lungcan.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Crouser ED, Julian MW, Crawford M, et al. Differential expression of microRNA and predicted targets in pulmonary sarcoidosis. Biochem Biophys Res Commun. 2012;417:886–91. doi: 10.1016/j.bbrc.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Di Cunto F, Provero P. Shortening of 3′UTRs correlates with poor prognosis in breast and lung cancer. PLoS ONE. 2012;7:e31129. doi: 10.1371/journal.pone.0031129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR, Bos A, Diosdado B, et al. Alternative cleavage and polyadenylation during colorectal cancer development. Clin Cancer Res. 2012;18:5256–66. doi: 10.1158/1078-0432.CCR-12-0543. [DOI] [PubMed] [Google Scholar]
- Singh P, Alley TL, Wright SM, et al. Global changes in processing of mRNA 3′ untranslated regions characterize clinically distinct cancer subtypes. Cancer Res. 2009;69:9422–30. doi: 10.1158/0008-5472.CAN-09-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PCR primer sequences of the selected alternative polyadenylation (APA)-indicator genes.
Table S2. PCR primer sequences of the alternative polyadenylation (APA)-related genes and HPRT1.
Table S3. Alternative polyadenylation (APA) profile of 16 genes that showed frequent 3′UTR shortening in lung cancer.
Table S4. Patient characteristics.