Abstract
Objective
To asses the effects of two topical nasal corticosteroids sprays on hyposmia in patients with persistent allergic rhinitis.
Material and Methods
The study was a prospective clinical trial and it included twenty four patients with persistent allergic rhinitis (PER) and hyposmia (H). The patients were divided into two groups depending on the type of corticosteroid topical nasal spray treatment: group A, 200 micrograms dose of mometasone furoate (MF) and group B, 110 micrograms dose of fluticasone furoate (FF) both administered in the morning for 4 weeks. The olfactory function of the patients was evaluated with the extended Test battery „Sniffin’ Sticks”. The visual analogue scale (VAS) was used for the assessment of hyposmia, nasal discharge. The level of the nasal obstruction, before and after the treatment, was evaluated through the anterior rhinomanometry.
Results
The comparisons between the two types of topical corticosteroids showed a significant improvement separately between scores of the odor threshold (OT), odor discrimination (OD) and odor identification (OI) and also on the final olfactory score (SDI) before and after 4 weeks of the treatment. The comparisons of the VAS scores pre and post treatment showed a significant improvement in hyposmia and nasal obstruction. The nasal airflow and the nasal discharge scores were improved, but the differences were not statistically significant between the groups. The final statistical analysis found no significant differences between the two patients groups.
Conclusion
The study concludes that fluticasone furoate and mometasone furoate have quite the same effects on hyposmia and on the classical symptoms from PER.
Keywords: smell disorder, hyposmia, topical steroids, Sniffin’ Sticks
Introduction
Allergic rhinitis causes olfactory dysfunction in 21–23% of the patients and taste disorders in 31% of them [1,2]. In allergic rhinitis, hyposmia has a high prevalence with an underestimated role in affecting the patient’s quality of life [3,4,5]. The most common mechanisms involved in the appearance of smell disorders in allergic rhinitis are: the nasal obstruction and the histopathological changes in the nasal mucosa [3,6,7].
The new ARIA guidelines divide the allergic rhinitis in: intermittent (IAR) and persistent (PER), and in terms of severity in: mild or moderate/severe [8,9]. The impairment of olfactory function is frequently present in patients with PER [10,11,12].
It has been hypothesized that corticosteroids directly improve olfactory function in addition to the anti-inflammatory activity by modulating the function of olfactory receptor neurons through effects on the olfactory Na-K-ATPase [2]. Other studies have suggested that the most common causes of olfactory dysfunction seem to be associated with increased apoptotic death of olfactory sensory neurons, but the role of steroids in this context still remains unsolved [13].
In allergic rhinitis, topical nasal corticosteroids is considered to be the chosen medical treatment. Although the sense of smell remains ‘the Cinderella of the senses’ it is still surprising that the effect of topical steroid treatment on the nose has rarely been systematically studied in terms of olfactory function [14]. The value of steroid therapy in the differential diagnosis of olfaction appares in order to distinguish the sinonasal disease from all central causes. For the clinical assesmment of olfactory function are used different chemosensory odor tests that have been developed in different countries like the University of Pennsylvania Smell Indetification Test (UPSIT) [15], CC-SIT [16], Connecticut Chemosensory Clinical Reseach Center Indentification Test (CCCRC) [17], for USA and the „Sniffin-Sticks” test [18] and Smell Diskettes test [19] in Europe. For the „Sniffin’Sticks” test previous work has already been established through its test-retest reliability and its validity in comparison with other established measures of olfactory sensitivity attained through the UPSIT, the CCCRC and the CC-SIT tests.
The aim of the present study was to investigate the effects of mometasone furoate and fluticasone furoate taken in the head down and forward position on hyposmia regarding the odor threshold (OT), odor discrimination (OD) and odor identification (OI) and final olfactory score (SDI) in the treatment of patients with persistent allergic rhinitis.
Material and methods
Study design
Twenty six consecutive patients, between the ages of 10 and 60 (16 women and 10 men, men age: 35 years), with persistent allergic rhinitis, moderate/severe form and smell complaints, were included in this prospective, randomized, controlled study.
Inclusion criteria were a history of PER based on ARIA criteria, skin test positively to clinically relevant aeroallergens and subjective smell disorders. A complete history with nasal symptoms was taken from each patient. All patients underwent examination of the nasal cavity by nasal endoscopy, anterior rhino-manometry and Sniffin’Sticks olfactometry.
Exclusion criteria were the existence of nasal diseases other than persistent allergic rhinitis, including acute or chronic rhinosinusitis, nasal polyps, severe septal deformity, upper respiratory tract infections during the previous month, specific immunotherapy and steroid or anti-leukotriene medication in the last 4 weeks and pregnancy.
Full informed consent was given by all participants and the study had the approval of the local Ethical Committee from our institution.
The study was conducted according to the Declaration of Helsinki on biomedical research involving human subjects.
Procedure
The patients were randomly divided into two groups depending on the topical nasal steroid treatment: Group A with 200 micrograms dose of mometasone furoate (MF) and group B with 110 micrograms dose of fluticasone furoate (FF), both administered in the morning during 4 weeks. All patients used sterile saline nasal water for cleaning the nose before the topical steroid treatment.
Olfactory function testing
The sense of smell was assessed by „Sniffin-Sticks” test package (Burghardt, Wedel, Germany) widely used in European Clinics. Tests were performed in a bilateral mode. The test consists in: odour threshold (OT), odour discrimination (OD) and odour identification (OI) and its overall results may be presented as a composite threshold-discrimination-identification score (SDI) [20].
The OT test was performed with n-butanol and was evaluated using a single-staircase, triple-forced choice procedure [21] and a 1:2 diluation series with 16 stages beginning with 4% was used, the dilutions were established in a geometric series, according to previous reports [22].
The OD test is composed from triplets of pens who are presented in a randomized order, with two pens containing the same odorant and the third a different one. The patient was asked to detect the different pen from all the three pens.
In the OI test the patient was asked to identify individual odorants from a list of four descriptors, this part contains 16 different odorant pens. In the first two tests the subjects were blindfolded - to avoid visual identification. The final score is the SDI, range from 0 to 48 depending on the age and on the patient. Patients can be normosmic, hyposmic and anosmic.
Skin prick tests: These were positive in all the patients included in the present study, using commercial extracts to the 20 most common airborne allergens present in our geographic area.
Nasal subjective “classical” symptoms: nasal obstruction, hyposmia and nasal discharge were recorded in each patient by using the visual analogue scale (VAS).
Nasal endoscopy: to evaluate the presence of mucopurulent discharge from the middle meatus, edema/mucosal obstruction of the ostiomeatal unit and nasal polyps, severe septal deformity and to eliminate the acute or chronic paranasal sinus disease.
Nasal airflow: was measured with anterior rhinomanometry (Rhinomanometer 200, Atmos Inc., Lenzkirch, Germany) nasal flow at 150 P) to each nasal cavity.
Statistical analysis was performed using SPSS 11.0 for Windows (SPSS 11.0, Chicago, IL), test significance at the p<0.05 level. Comparisons between groups were performed using student t test.
Results
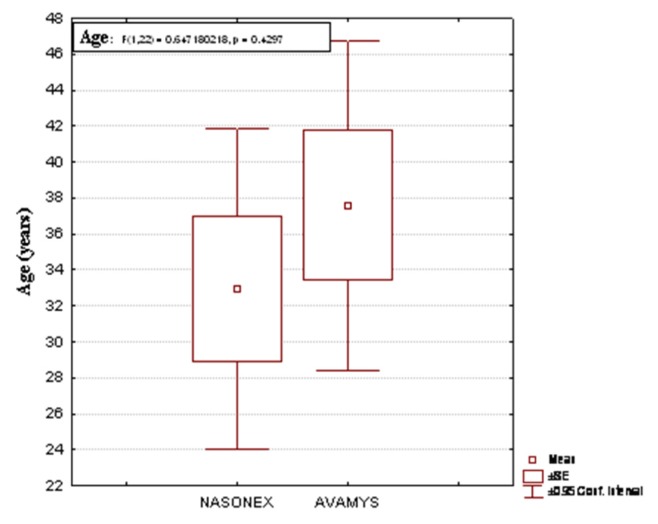
From a total of 26 patients with persistent allergic rhinitis moderate/severe form, 24 patients with hyposmia and other 2 patients with anosmia were excluded from the study. Age distribution for the both groups (Figure 1).
Figure 1.
Age distribution for the both groups.
The study groups, 24 hyposmic patients, were divided into two groups depending on the topical corticosteroid treatment. The results aimed at revealing what kind of smell disorders appeared in persistent moderate/severe allergic rhinitis and the effects of topical corticosteroids on the olfactory performance to the hyposmic patients after 4 weeks of treatment.
Olfactory function
For the OT, OD, OI and for the final SDI scores there was a significant correlation between the pre and post treatment scores in the both groups after 4 weeks (Table I).
Table I.
The OT, OD, OI and SDI pre and post treatment scores.
| Group | OTpre | OTpost | ODpre | ODpost |
|---|---|---|---|---|
| MF | 5.75±2.76 | 9.56±2.47 | 10.08±2.46 | 11.75±1.65 |
| FF | 5.64±2.60 | 8.54±2.51 | 10.58±1.67 | 12.08±1.64 |
| Group | OIpre | OIpost | SDIpre | SDIpost |
| MF | 9.08±1.72 | 11.00±1.59 | 25.91±3.42 | 33.02±2.63 |
| FF | 10.08±2.19 | 11.91±1.78 | 25.31±2.32 | 31.62±2.22 |
VAS for hyposmia, nasal obstruction and nasal discharge
The comparisons between VAS scores for hyposmia (Hyp), nasal obstruction (N.ob) were significant statistically (p<0.05) but for the nasal discharge (N.dis) the scores were not statistically significant (p>0.05) in both groups pre and post treatment over the period of 4 weeks (Table II).
Table II.
The VAS scores pre and post treatment for hyposmia, nasal obstruction and nasal discharge.
| Group | Hyp.pre | Hyp.post | N.ob.pre | N.ob.post | N.dis.pre | N.dis.post |
|---|---|---|---|---|---|---|
| MF | 8.35±2.40 | 5.79±3.40 | 6.10±2.06 | 2.80±2.59 | 3.40±2.50 | 3.63±2.60 |
| FF | 7.57±2.49 | 5.46±2.66 | 6.40±1.65 | 2.98±2.26 | 4.428±2.50 | 4.33±2.52 |
The final comparison between the two treatment groups (MF and FF) regarding the olfactory function and VAS scores showed a not significant p value (Table III).
Table III.
The final comparison between the all scores for the group 1 and 2.
| T-test; Grouping Drug Group 1: MF Group 2: FF |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Variable |
Mean MF |
Mean FF |
t-value | df | p |
Valid N MF |
Valid N FF |
Std.Dev. MF |
Std.Dev. FF |
F-ration Variances |
P Variances |
| OT pre | 5.75 | 5.64 | 0.094 | 22 | 0.92 | 12 | 12 | 2.76 | 2.60 | 1.12 | 0.84 |
| OD pre | 10.08 | 10.58 | −0.58 | 22 | 0.56 | 12 | 12 | 2.46 | 1.67 | 2.16 | 0.21 |
| OI pre | 10.08 | 9.08 | 1.24 | 22 | 0.22 | 12 | 12 | 2.19 | 1.72 | 1.60 | 0.44 |
| SDI pre | 25.91 | 25.31 | 0.50 | 22 | 0.61 | 12 | 12 | 3.42 | 2.32 | 2.16 | 0.21 |
| OT post | 9.56 | 8.54 | 1.00 | 22 | 0.32 | 12 | 12 | 2.47 | 2.51 | 1.03 | 0.95 |
| OD post | 11.75 | 12.08 | −0.52 | 22 | 0.60 | 12 | 12 | 1.65 | 1.44 | 1.32 | 0.65 |
| OI post | 11.91 | 11.00 | 1.32 | 22 | 0.19 | 12 | 12 | 1.78 | 1.59 | 1.24 | 0.72 |
| SDI post | 33.02 | 31.62 | 1.40 | 22 | 0.17 | 12 | 12 | 2.63 | 2.22 | 1.40 | 0.58 |
| Hyp.pre | 8.35 | 7.57 | 0.77 | 22 | 0.44 | 12 | 12 | 2.40 | 2.49 | 1.07 | 0.90 |
| Hyp.post | 5.79 | 5.46 | 0.26 | 22 | 0.79 | 12 | 12 | 3.40 | 2.66 | 1.63 | 0.42 |
| N.ob.pre | 6.10 | 6.40 | −0.39 | 22 | 0.69 | 12 | 12 | 2.06 | 1.65 | 1.55 | 0.47 |
| N.ob.post | 2.80 | 2.98 | −0.18 | 22 | 0.85 | 12 | 12 | 2.59 | 2.26 | 1.30 | 0.66 |
| N.dis.pre | 3.40 | 4.28 | −0.86 | 22 | 0.39 | 12 | 12 | 2.50 | 2.50 | 1.00 | 0.99 |
| N.dis.post | 3.63 | 4.33 | −0.66 | 22 | 0.51 | 12 | 12 | 2.60 | 2.52 | 1.06 | 0.92 |
Nasal flow increased in the MF group from 670+/−150 cm3 to 740+/−95 cm3 and in FR group from 685+/−150 cm3 to 720+/−110 cm3 at 150 Pa after 4 weeks of treatment. Differences between groups were not statistically significant (p=0.75).
Discussion
Our study supports the notion that the main smell dysfunction in moderate/severe form of persistent allergic rhinitis (PER) is hyposmia; the second is anosmia, and the olfactive dysfunction it is to be found in the odor threshold, discrimination and identification.
Guilemany et al. [12] have showed that in PER there is a high prevalence of smell dysfunction, loss of smell detection and identification – all particularly present in the moderate/severe form.
Topical steroid therapy is effective in the treatment of seasonal and perennial rhinitis and for the post surgical recurrence of nasal polyps. Therefore following topical treatment with nasal steroids, mometasone furoate for 2 weeks, a significant improvement has been reported on odor threshold. Moreover, it is to be mentioned that in odor identification and discrimination using the same “Sniffin’Sticks” test, no significant improvement was evidenced [23].
In our study, we confirmed a significant improvement of odor threshold after 4 weeks of topical steroid treatment in patients with PER for MF and for the first time for FF. The olfactory improvement in the hyposmic patients was also observed in these scores for odor discrimination and identification. In another study the authors have shown a significant improvement for odor identification followed by a negative result for odor threshold still using the CCCRC test, with a different assessment for odor threshold [24]. Visual analogue scale has proved a useful technique for the measurement of subjective experience [25].
All our patients with hyposmia showed improvement of the final SDI score after the topical steroid treatment with a correlation of VAS hyposmia improvement for the same patients. Another study suggests that olfactory disorders in PER patients may have a mixed etiology in which the main responsible factors are the nasal obstruction and inflammation [12]. Using the BAST-24 they showed that forced choice was more sensitive than detection and identification. In this study no topical steroid treatment was applied to the patients. The authors showed that PER has a moderate impact on the sense of smell.
Our study showed that the impairment of olfactory function in PER is mostly because of allergic inflammation and not because of a reduced nasal air flow.
Although the number of patients used in this study group is considered to be small, it still demonstrates that hyposmia is an important smell disorder and the anti-inflammatory treatment with topical nasal steroids improves the olfactory function in persistent allergic rhinitis, moderate/severe form.
To sum up, mometasone furoate and fluticasone furoate have rather the same effects on hyposmia and on the classical symptoms of PER.
References
- 1.Cowart BJ, Flynn-Rodden K, Mc Gready SL. Hyposmia in allergic rhinitis. J Allergy Clin Immunol. 1993;91:747–751. doi: 10.1016/0091-6749(93)90194-k. [DOI] [PubMed] [Google Scholar]
- 2.Rydzewski B, Pruszewicz A, Sulkowski WJ. Assessment of smell and taste in patients with allergic rhinitis. Acta Otolaryngol. 2000;120:323–326. doi: 10.1080/000164800750001189. [DOI] [PubMed] [Google Scholar]
- 3.Klimek L, Eggers G. Olfatory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. J Allergy Clin Immunol. 1997;100:158–164. doi: 10.1016/s0091-6749(97)70218-5. [DOI] [PubMed] [Google Scholar]
- 4.Fein BT, Kamin PB, Fein NN. The loss of smell in nasal allergy. Ann Allergy. 1966;24:278–283. [PubMed] [Google Scholar]
- 5.Seiden AM, Litwin A, Smith DV. Olfactory deficits in allergic rhinitis. Chem Senses. 1989;14:746–747. [Google Scholar]
- 6.Doty RL, Bartoshuk LM, Snow JB Jr, editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 529–552. [Google Scholar]
- 7.Churh JA, Bauer H, Bellanti JA, Satterly RA, Henkin RI. Hyposmia associated with atopy. Ann Allergy. 1978;40:105–109. [PubMed] [Google Scholar]
- 8.Bousquet J, Khaltaev N, Cruz A, et al. ARIA Update (In collaboration with GALEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 9.Valero A, Ferrer M, Sastre J, et al. A new criterion by which to discriminate between patients with moderate alergic rhinitis and patients with severe allergic rhinitis based on the allergic rhinitis and its impact on asthma severity items. J Allergy Clin Immunol. 2007;20:359–365. doi: 10.1016/j.jaci.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Moll B, Klimek L, Eggers G, Mann W. Comparasion of olfactory function in patients with seasonal and perennial alergic rhinitis. Allergy. 1998;53:297–301. doi: 10.1111/j.1398-9995.1998.tb03890.x. [DOI] [PubMed] [Google Scholar]
- 11.Simola M, Malmberg H. Sense of smell in allergic and nonallergic rhinitis. Allergy. 1998;53:190–194. doi: 10.1111/j.1398-9995.1998.tb03869.x. [DOI] [PubMed] [Google Scholar]
- 12.Guilemany JM, et al. Persistent Allergic Rhinitis has a moderate impact on the sense of smell, depending on both nasal congestion and inflammation. Laryngoscope. 2009;119:233–238. doi: 10.1002/lary.20075. [DOI] [PubMed] [Google Scholar]
- 13.Fong KJ, Kern RC, Foster JD, Zhao JC, Pitovski DZ. Olfactory secretion and sodium, potassium-adenosine triphosphatase: regulation by corticosteroids. Laryngoscope. 1999;109:383–388. doi: 10.1097/00005537-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kern RC, Conley DB, Haines GK, Robinson AM. Treatment of olfactory dysfunction. II. Studies with minocycline. Laryngoscope. 2004;114:2200–2204. doi: 10.1097/01.mlg.0000149458.21501.6f. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL, Shamn P, Dann MS. Development of the University of Pennsylvania Smell Indentificarion Test: a standarized microcapsulated test of olfactory function. Phys Behaw. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 16.Doty RL, Avron M, Lee WW. Development of the 12-item cross-cultural smell identification test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Cain WS, Goodspeed RB, Gent JF, Leonard G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center (CCCRC) Laryngoscope. 1988;98:83–88. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Kobal G, Hummel TH, Sekinger B, Barz S, Roscher S, Wolf S. „Sniffin’Sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 19.Briner HR, Simmen D. Smell diskettes as screening test of olfaction. Rhinology. 1999;37:145–148. [PubMed] [Google Scholar]
- 20.Wolfensberger M, Schnieper I, Welge-Lussen A. Sniffin’Sticks: a new olfactory test battery. Acta Otolaryngol. 2000;120:303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- 21.Doty RL. Olfactory system. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 175–204. [Google Scholar]
- 22.Cain WS, Rabin MD. Comparability of two tests of olfactory functioning. Chem Senses. 1989;14:479–485. [Google Scholar]
- 23.Stuck BA, et al. Mometasone furoate nasal spray improves olfactory performance in seasonal allergic rhinitis. Allergy. 2003;58:1196–1216. doi: 10.1034/j.1398-9995.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 24.Meltze EO, et al. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102:39–49. doi: 10.1016/s0091-6749(98)70053-3. [DOI] [PubMed] [Google Scholar]
- 25.Golding-Wood DG, Holmstrom M, Darby Y, Scandding GK, Lund VJ. The treatment of hyposmia with intranasal steroids. The Journal of Laryngology and Otology. 1996;110:132–135. doi: 10.1017/s0022215100132967. [DOI] [PubMed] [Google Scholar]