Abstract
Intimin, Tir, and EspA proteins are expressed by attaching-effacing Escherichia coli, which include enteropathogenic and enterohemorrhagic E. coli pathotypes. EspA proteins are part of the type three secretion system needle complex that delivers Tir to the host epithelial cell, while surface arrayed intimin docks the bacterium to the translocated Tir. This intimate attachment leads to attaching and effacing lesions. Recombinant forms of these effector proteins from enterohemorrhagic E. coli O157:H7 were produced by using E. coli expression vectors. Binding of intimin and Tir fragments in enzyme-linked immunosorbent assay (ELISAs) demonstrated the interaction of intimin fragments containing the C-terminal 282 or 188 amino acids to a Tir fragment containing amino acid residues 258 to 361. Recombinant intimin and EspA proteins were used to elicit immune responses in rabbits and immune phage-display antibody libraries were produced. Screening of these immune libraries by conventional phage-antibody panning and colony filter screening produced a panel of antibodies with specificity for EspA or intimin. Antibodies recognizing different C-terminal epitopes on intimin bound specifically to the gamma intimin of O157:H7 and not to other classes of intimin. Antibodies recognizing EspA from E. coli O157 also recognized the protein from the eae-deficient O157 mutant DM3 and from E. coli O111. Anti-intimin antibodies were also produced as fusion proteins coupled to the reporter molecule alkaline phosphatase, allowing the one-step detection of γ intimin. The isolated recombinant monoclonal antibodies were functional in a range of assay formats, including ELISA, Western blotting, and dot blots, thus demonstrating their diagnostic potential.
Enterohemorrhagic Escherichia coli (EHEC) presents a significant risk to human health. This enteric pathogen is associated with hemorrhagic colitis, thrombotic thrombocytopenic purpura, and hemolytic-uremic syndrome (17, 18). Serotypes causal of human disease are the prototype EHEC O157, as well as O26, O55, O91, O103, O111, and O146, with the main serotype associated with human illness in the United Kingdom and North America being E. coli O157:H7. The main facets to the virulence of this group of bacteria are intimate attachment to intestinal epithelial cells leading to attachment and effacement (A/E) lesions (7) and the production of verocytoxin (VT) (24), the toxicity of which acts at distant sites such as the kidney. Another important enteric bacterial pathogen is the closely related enteropathogenic E. coli (EPEC), the prototype A/E organism, which is an important cause of infant mortality in developing countries (24).
Both EPEC and EHEC contain a highly homologous chromosomal pathogenicity island known as the locus of enterocyte effacement, which contains genes critical for A/E lesion formation (29). The locus of enterocyte effacement can be divided into three functional regions: one encoding for a type III secretion system; a second containing the genes eae and tir; and a third containing the genes espD, espB, and espA (16). The eae gene encodes for an outer membrane protein, intimin, which is essential for intimate attachment of the bacterium to the host cell. The type III secretion system is involved in the secretion of proteins EspA, EspB, EspD, and Tir. EspA is encoded by the espA gene and forms a filamentous structure on the bacterial surface through which EspB, EspD, and Tir are secreted. The EspB and EspD proteins are thought to be incorporated into the host cell cytoplasmic membrane, where they form a pore through which other bacterial effector molecules, such as Tir, enter the host cell (5, 9). Tir is the receptor for intimin, which is translocated via the EspA filament and EspB/EspD pore into the host cell and incorporated into the membrane. As well as interacting with intimin, this protein is also involved in promoting cytoskeletal actin rearrangement in the host cell.
As two of the main components in EHEC A/E lesion formation EspA and intimin are indicators of virulence and may also provide novel targets for the disruption of bacterium-host cell interaction and therefore disease resistance strategies. Here, we use recombinant antibody technology to produce monoclonal antibody fragments against these EHEC virulence factors. The use of these antibodies in different assay systems for the detection of enteric E. coli pathogens is reported.
MATERIALS AND METHODS
Microorganisms and plasmids.
E. coli TG1 [supE thi-1 Δ(lac-proAB) Δ(mcrB-hsdSM)5 (rK− mK−) (F′ traD36 proAB lacIqZΔM15)] and BL21(DE3) [F− dcm ompT hsdS (rB− mB−) gal λ(DE3)] were from Stratagene (Cambridge, United Kingdom), and HB2151 [K-12; ara del(lac-pro), thi/F′proA+B+, lacIq, lacZΔM15] and M13KO7 helper phage were obtained from Pharmacia LKB (St. Albans, Hertfordshire, United Kingdom). The expression vectors pET22b and pET21d were from Novagen (Madison, Wis.), and pMalc2 was from NEBL (Hitchen, Herts, United Kingdom). The construction of the phagemid vector pSD3 is described by Li et al. (21). Outer membrane and whole-cell preparations from EHEC and EPEC serotypes O157:H7 (NCTC12900 [4]), O111:NM (strain B171 [28]), O127:H6 (EC2348/69 [20]), O86:H34 (1), K-12 (DH5α [Gibco-BRL]), and O157-DM3 (eae mutant in NCTC12900 (35) were produced. Briefly, 100-ml cultures were grown for 16 h in Dulbecco modified Eagle medium (D5671) plus 1% nonessential amino acids and 1% l-glutamine (Sigma) static at 37°C and 5% CO2. For whole-cell preparation, cells were harvested by centrifugation, resuspended in 10 ml of 20 mM EDTA, and incubated at 60°C for 30 min. For outer membrane preparations, cells were harvested by centrifugation at 10,000 × g for 30 min at 4°C, resuspended in 100 ml of Tris-HCl (50 mM, pH 7.2), centrifuged at 10,000 × g for 30 min, resuspended in 10 ml of 20 mM EDTA, and incubated at 60°C for 30 min. Cells were lysed by sonication (five 2-min intervals of amplitude 80 with continuous pulsing), cell debris was removed by centrifugation at 15,000 × g for 2 min, and membrane preparations were isolated by centrifugation of the resulting supernatant at 20,000 × g for 60 min at 4°C. Membrane pellet was resuspended in 2 ml of HEPES (pH 7.0) plus 2% Sarkosyl. After agitation at room temperature for 30 min at ambient temperature, the suspension was centrifuged at 20,000 × g for 60 min at 4°C, and the pellet was resuspended in 0.25 ml of water.
Molecular biology techniques.
Protocols for DNA manipulation were taken from Sambrook et al. (34) or were as recommended by the manufacturers. espA, eae, and scFv clones were sequenced by dye termination with the AmpliTaq DNA polymerase, FS, on an ABI 377 automated DNA sequencer with the following primers: T7 promoter primer (5′-TAATACGACTCACTATAGG-3′) and T7 terminator primer (5′-CTAGTTATTGCTCAGCGG-3′) for pET21d and pET22b constructs, malE (5′-GGTCGTCAGACTGTCGATGAAGCC-3′) and M13-20 (5′-GTAAAACGACGGCCAGT-3′) for pMalc2 constructs, and pSDF (5′-TATTTCAAGGAGACAGTC-3′) and pSDR (5′-ATTGGCCTTGATATTCAC-3′) for pSD3 constructs.
(i) Cloning of espA, eae, and tir genes.
Full-length espA gene from E. coli O157 human isolate EC157 was amplified by PCR with primers homologous to espA regions 5′-GATACATCAAATGCAACA-3′ and 5′-TATTTACCAAGGGATATT-3′, cleaved with the restriction enzymes BamHI and HindIII, and cloned into similarly cut pET21d expression vector. A 5′-truncated espA gene (lacking the N-terminal 36 amino acids and designated EspA120) from E. coli O157:H7 (strain 140065) was amplified by PCR with primers homologous to the espA regions 5′-GCTGATATGAATGAGGC-3′ and 5′-TTTACCAAGGGATATTGC-3′, cleaved with the restriction enzymes BamHI and HindIII, and cloned into similarly cut pMalc2 and pET22b expression vectors. The pMal construct was transformed into TG1, and the pET constructs were transformed into BL21(DE3). Similarly, a 5′-truncated version of the eae gene was amplified from bovine isolate EC157 by using primers homologous to eae regions (5′-CCGTTGAAGTCGAGTACG-3′ and 5′-TTCTACACAAACCGCATA-3′) and cloned into pET21d (coding for the C-terminal 313 residues of intimin and designated intimin313). Also, two further 3′-terminal fragments of the eae gene were amplified from E. coli O157:H7 (strain 140065) and cloned into the expression vectors pMalc2 and pET, resulting in constructs that coded for the C-terminal 282 and 188 amino acid residues of intimin, designated intimin282 and intimin188, respectively. Primers used in this intimin cloning were homologous to the following eae regions: 5′-TTTGATCAAACCAAGG-3′ (for intimin282 5′ primers), 5′-GATGAACTGAAAATTG-3′ (for intimin188 5′ primers), and 5′-TTCTACACAAACCGC-3′ (3′ primers). Fragments of the tir gene coding for amino acids 258 to 361 (designated Tir103) were amplified by PCR from E. coli O157:H7 (strain 140065) with primers to the tir regions (5′-CCGGAGCCGGATAGCC-3′ and 5′-CGGTCGGGGGCTACTTTG-3′) and cloned into pMalc2 and pET22b. For espA, tir, and eae cloning, the resulting transformants were sequenced to verify the inserted gene.
(ii) Phage-library production.
The construction of an immune phage-display single-chain variable region (scFv) antibody library was carried out as described by Li et al. (21) and Gough et al. (12). Briefly, female New Zealand White rabbits were immunized subcutaneously with 50 μg of intimin or EspA (from the pET21d expression system) in phosphate-buffered saline (PBS) and emulsified with Freund complete adjuvant. The animals were boosted three times over a 55-day period with 50 μg of antigen mixed with incomplete Freund adjuvant. The spleens of the animals were removed 61 days after the initial immunizations. Total RNA was isolated from spleen tissue by using an RNA isolation kit (Qiagen, Surrey, United Kingdom) and used in the production of first-strand cDNA by using a cDNA synthesis kit (Pharmacia, Hertfordshire, United Kingdom) and a random hexanucleotide primer. The VL and VH repertoire of the rabbits were amplified by PCR with the primers listed in Table 1 based on those described by Ridder et al. (32). Purified VL PCR products were cleaved with SfiI and purified VH PCR products cleaved with PflMI. Cleaved products were purified by gel extraction, and the VL repertoire was ligated into SfiI-cut pSD3. Ligation products (5 μg) were purified and cleaved with PflMI, and the VH repertoires were ligated in. Purified pSD3:scFv ligation product was then used to transform electrocompetent TG1 cells. Library diversity was assessed by amplification of the scFv gene with primers pSD3F and pSD3R, followed by digestion of the PCR products with BstNI restriction enzyme. The resulting fragments were analyzed on a 3% (wt/vol) agarose gel.
TABLE 1.
Primers for scFv phage display library construction
| Primera | Sequence (5′→3′)b |
|---|---|
| Vλ primers | |
| Reverse | |
| RL1 | TAAGTCAATTTCAATGGCCCAACCGGCCATGGCTGAGCTCGWKMTGACCCAGACTCCA |
| RLL1 | TAAGTCAATTTCAATGGCCCAACCGGCCATGGCTCAGCCTGTGCTGACTCAGTCG |
| Forward | |
| RL2 | TATATATATAATTATGGCCTCCCTGGCCTWKGAYSWCCASCTCGGTCCC |
| RLJ | TATATATATAATTATGGCCTCCCTGGCCACCTGTGACGGTCAGCTGGGTCC |
| Vκ primers | |
| Reverse | |
| RK1 | TAAGTCAATTTCAATGGCCCAACCGGCCATGGCTGMBVNHGWKMTGACCCAGACTCCA |
| RK2 | TAAGTCAATTTCAATGGCCCAACCGGCCATGGCTGCCGAAGTAGWKMTGACCCAGACTCCA |
| RK3 | TAAGTCAATTTCAATGGCCCAACCGGCCATGGCTGCTCAAGTGCTGACCCAGAC |
| Forward | |
| RKJ1 | TATATATATAATTATGGCCTCCCTGGCCTHBRAYBWCYASHWYGGTCCC |
| RKJ2 | TATATATATAATTATGGCCTCCCTGGCCTTTGATCTCCAGCTTGGTCTC |
| VH primers | |
| Reverse primers | |
| RH3a | AATGAATAATGAAAACCAAGGAGTGGGTTCTCAGTCGBTGRAGGAGTCCRRG |
| RH3b | AATGAATAATGAAAACCAAGGAGTGGGTTCTCAGSAGCAGCTGGWGGAGTCCGG |
| Forward | |
| RH4 | AAGAAATGATAAAAACCACCAACTGGCTGACTGAYGGAGCCTTAGGTTGC |
V gene complementary sequences are underlined; restriction enzyme cleavage sites are in italics.
Redundant bases in the sequences are indicated as follows: M = A/C, R = A/G, W = A/T, S = C/G, Y = C/T, K = G/T, V = A/C/G, H = A/C/T, D = A/G/T, and B = C/G/T.
Cloning of AP-scFv fusions.
Expression vector pET22b containing the bacterial gene for alkaline phosphatase (AP; phoA, cloned between the BamHI and XhoI sites) was produced such that scFv genes in pSD3 were amplified by PCR with the primers scFvAPFor (5′-GTTATTACTCGCGGCCCAACCGGCCATGGCC-3′) and scFvAPRev (5′-TCACACTAGTTTCAGCACCCACCAACTGGCT-3′) and cloned into the pET-PhoA vector at the 5′ terminus of phoA by using restriction enzymes NcoI and SpeI (the latter introduced with the phoA gene). During construction of the vector the phoA gene was altered by site-directed mutagenesis (a T-to-C same-sense mutation at position 820) in order to remove an internal NcoI cleavage site.
Expression and purification of recombinant proteins.
For all recombinant proteins, protein concentration was estimated by using the BCA protein assay kit (Pierce, Rockford, Ill.), and protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
(i) MBP fusions.
TG1/pMalc2 clones containing the gene for maltose-binding protein (MBP)-intimin, MBP-Tir103 or MBP-EspA120 fusion were grown in 1-liter cultures to mid-log phase at 37°C. Expression was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the cells were grown for a further 4 h. Cells were harvested by centrifugation and resuspended in lysis buffer (200 mM NaCl, 10 mM Tris [pH 7.4]; 5 ml/100 ml of culture). Cells were lysed by sonication, and the supernatant collected after centrifugation. A one-step purification of the MBP fusion proteins was carried out under native conditions on an amylose resin column as recommended by the manufacturer (NEBL).
(ii) His-tagged intimin, Tir, and EspA.
BL21(DE3)/pET constructs containing the gene for poly-His-tagged intimin, Tir or EspA were grown in 1-liter cultures to mid-log phase at 37°C. Expression was induced by the addition of 1 mM IPTG, and the cells were grown for a further 4 h. Cells were harvested by centrifugation and resuspended in lysis buffer (100 mM NaH2PO4, 8 M urea, 10 mM Tris-Cl [pH 8.0]; 5 ml/100 ml of culture). Cell debris was pelleted by centrifugation, and the supernatant was collected. A one-step purification of the His-tagged proteins was carried out under denaturing conditions on a nitrilotriacetic acid (NTA)-Ni resin column as recommended by the manufacturer (Qiagen). After purification, intimin282, EspA, and Tir proteins were renatured by dialysis against PBS (pH 7.4). Urea-solubilized intimin188 was used for colony filter screening, and for all other applications PBS soluble intimin188 was produced by purification under native conditions (as described for His-tagged scFvs).
(iii) His-tagged scFv.
HB2151/pSD3 constructs containing the genes for poly-His-tagged scFvs were grown in 1-liter cultures to mid-log phase at 37°C. Expression was induced by the addition of 1 mM IPTG, and the cells were grown for a further 4 h. Cells were harvested by centrifugation and resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole [pH 8.0]; 5 ml/100 ml of culture). Cells were lysed with 1 mg of lysozyme/ml for 30 min on ice, followed by sonication. Cell debris was pelleted by centrifugation, and the supernatant was collected (crude cell extract). A one-step purification of the His-tagged proteins was carried out under native conditions on an NTA-Ni resin column as recommended by the manufacturer (Qiagen).
AP-scFv fusion proteins.
BL21(DE3)/pET constructs containing the gene for AP-scFv fusions were grown in 1-liter cultures to mid-log phase at 37°C. Expression was induced by the addition of 1 mM IPTG, and the cells were grown for a further 16 h. Cells were harvested by centrifugation, and the culture supernatant was used directly in immunoassays.
Library screening. (i) Phage-panning.
Phage panning was carried out as described by Marks et al. (23). Immunotubes were coated for 16 h at 22°C with 1 ml of either MBP-intimin188 plus MBP-intimin282 or MBP-EspA in PBS (pH 7.4). MBP-EspA was at concentrations of 20, 20, and 5 μg/ml for rounds 1, 2, and 3, respectively. MBP-intimin188 and MBP-intimin282 were coated at concentrations of 10, 5, and 0.5 μg/ml each for rounds 1, 2, and 3, respectively.
Monoclonal phage-displayed scFvs were obtained after three rounds of panning by plating out the TG1-infected cells to single colonies. Cultures of these single colonies were then used to produce monoclonal scFv-phage particles by rescue with M13KO7 helper phage. For the production of soluble scFvs, monoclonal scFv-phage particles were used to infect HB2151 host cells. IPTG induced expression in this alternative host cell resulted in recognition of the amber stop codon situated between the scFv and gIII phage coat-protein genes and therefore the production of soluble monoclonal antibody fragments.
(ii) Colony filter screening.
Selection of soluble scFvs by using colony filter screening was carried out essentially as described by Giovannoni et al. (13). Antigen (6 ml of a 0.15-mg/ml concentration in PBS) was coated onto polyvinylidene fluoride membrane for 6 h at 37°C. TG1/pSD3 scFv libraries were grown to mid-log phase in 2YT (16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl/liter) plus 100 μg of ampicillin/ml, 1% (wt/vol) glucose, and ∼106 CFU spread onto Durapore membrane (type GVWP; Millipore, Bedford, Mass.). This colony membrane was grown on a 14-cm plate containing 2YT agar (15 g of agar, 16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl/liter), 100 μg of ampicillin/ml, and 1% (wt/vol) glucose for 8 h at 37°C. The antigen filter was blocked with PBS plus 5% (wt/vol) Marvel for 2 h at 37°C. After a washing step, the antigen membrane was soaked in 2YT plus 100 μg of ampicillin/ml and 1 mM IPTG and placed onto a 2YT agar plate containing 100 μg of ampicillin/ml and 1 mM IPTG. This membrane was covered with the Durapore colony membrane, followed by incubation for 16 h at 37°C. After orientation of the colony filter-antigen membrane assembly, the Durapore colony filter was stored at 4°C on a fresh 2YT agar plate, and the antigen membrane was washed four times in PBS plus 0.2% (vol/vol) Tween 20 and then blocked as described previously for 6 h at 37°C. Antigen membrane-bound scFv was detected with 9E10 (anti-c-myc) antibody and anti-mouse antibody-AP conjugate (Sigma, Poole, Dorset, United Kingdom). Signals were developed by using BCIP/NBT substrate (Sigma). Positive dots on the membrane were indicative of colonies that produce c-myc-tagged scFvs which bind to the antigen. Such colonies were identified by realignment of the antigen membrane with the colony filter. Where single colonies could not be identified, cells from an area ∼0.5 cm in diameter were removed from the colony filter and replated onto a fresh Durapore membrane at ∼103 CFU/plate, and the screening was repeated.
ELISA. (i) Phage-scFv ELISA.
Maxisorb enzyme-linked immunosorbent assay (ELISA) wells (Gibco-BRL; Nunc) were coated with antigen in PBS (or 8 M urea) for 16 h at 22°C. After a rinse with PBS, the wells were blocked with PBS plus 3% (wt/vol) Marvel at 37°C for 1 h. Preblocked phage-scFv (1011 CFU) were added to the wells, and scFvs allowed to bind by incubating the wells at 37°C for 60 min. The wells were then washed three times with PBS plus 0.05% (wt/vol) Tween 20 and then three times in PBS alone. Bound phage were detected in two steps with biotin-labeled anti-fd antibody (binding to the coat proteins of fd or M13 phage) and then with extravadin-AP conjugate (both from Sigma). Incubation and washing steps were as described above. Signals were developed with p-nitrophenyl phosphate (PNPP) substrate from Sigma, and the absorbance at 405 nm was measured.
(ii) Soluble scFv ELISA.
Soluble scFv ELISAs were essentially as described for the phage-scFv ELISA. Plates were coated with antigen and scFv was added either as (i) culture supernatant produced by overnight induction of cultures with IPTG and removal of cells by centrifugation or as (ii) NTA-Ni affinity-purified scFv. The incubation and washing steps were the same as for the phage-scFv ELISAs. Bound scFvs were detected with 9E10 (anti-c-myc) antibody, anti-mouse antibody-AP conjugate, and PNPP substrate (Sigma).
(iii) Competition ELISA.
Competition of phage-displayed scFv binding to target antigen with soluble scFv was carried out by phage-scFv ELISA. Antigen concentrations were optimized for each phage-displayed scFv by assessing the binding of an excess of phage-scFv to a dilution range of antigen (0 to 1,000 ng/well). Antigen concentrations giving an optical density at 405 nm of 0.4 after 1 h with AP substrate were used in the subsequent optimization of the phage concentration. A dilution range of phage of from 4 × 108 to 2.5 × 1011 CFU/well was used in the assays. Phage concentrations just below saturation of the antigen were used for the subsequent competition ELISAs. Soluble scFv was produced as described for AP-scFv fusion production, and 100 μl was added to the phage in PBS plus 3% (wt/vol) Marvel. The mixture was preincubated for 30 min at 37°C before it was added to the antigen-coated wells. A reference uninhibited sample was produced by using a soluble scFv with irrelevant binding.
Electrophoresis and Western blots.
Discontinuous SDS-PAGE was carried out through a running gel containing 10 or 12% (wt/vol) total acrylamide by using Mini-Protean II Dual cells from Bio-Rad. Proteins were detected with 0.05% (wt/vol) Coomassie brilliant blue. For Western blots, separated proteins were transferred to nitrocellulose membranes by semidry blotting at 140 mA for 30 min. After being blocked with Tris-buffered saline plus 3% (wt/vol) Marvel, blots were probed with scFv or monoclonal antibody. Bound scFv was detected with 9E10 antibody, anti-mouse antibody-horseradish peroxidase conjugate (Dako, Ely, Cambridgeshire, United Kingdom) and BM chemiluminescence blotting substrate (Roche, Lewes, East Sussex, United Kingdom). Bound monoclonal antibody was detected by using the appropriate anti-species antibody-horseradish peroxidase conjugate and chemiluminescence substrate.
Dot blots.
Antigen in PBS was spotted onto polyvinylidene difluoride membrane in a volume of 1 μl. Blocking and detection steps were identical to those described for the Western blots.
RESULTS
Recombinant protein production.
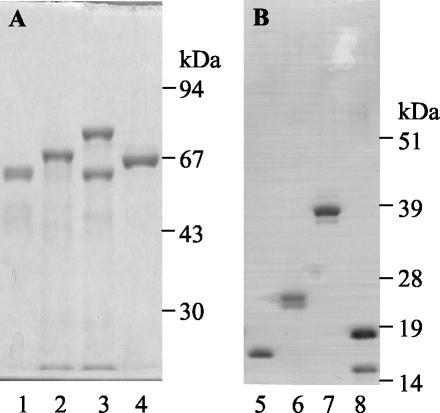
Poly-His-tagged proteins or MBP fusions—EspA, EspA120, Tir103, intimin313, intimin188, intimin282, MBP-EspA120, MBP-Tir103, MBP-intimin188, and MBP-intimin282—were expressed and purified by affinity chromatography (Fig. 1). Most of the recombinant proteins produced doublets due to the release of proteins with or without signal sequences upon cell lysis. MBP-intimin282 and His-tagged Tir also produced truncated forms of the recombinant proteins that have been reported elsewhere for EPEC proteins (8) and are most likely a consequence to premature termination of translation. For His-tagged intimin188 expression, even though the yields of purified denatured protein were high (ca. 14 mg/liter of E. coli culture), the protein could not be renatured by dialysis. Purification under native conditions produced low amounts of soluble protein (∼0.2 mg/liter of culture). For the other constructs, the yields of purified PBS soluble protein ranged from 2 mg/liter of E. coli culture for MBP-EspA120 to 23 mg/liter for MBP-Tir103.
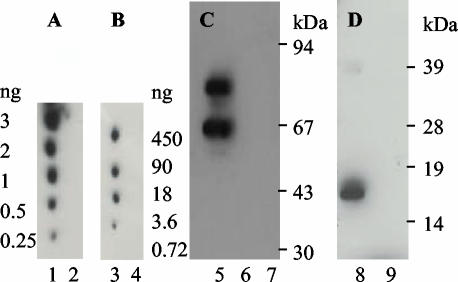
FIG. 1.
Purification of recombinant E. coli O157 proteins. Purified proteins were expressed and purified as MBP fusions from pMalc2 (A) or as His-tagged proteins from pET21b (B). EspA120 (lanes 1 and 5), intimin188 (lanes 2 and 6), intimin282 (lanes 3 and 7), and Tir103 (lanes 4 and 8) are shown. His-tagged intimin188 is in 8 M urea; all other proteins are in PBS.
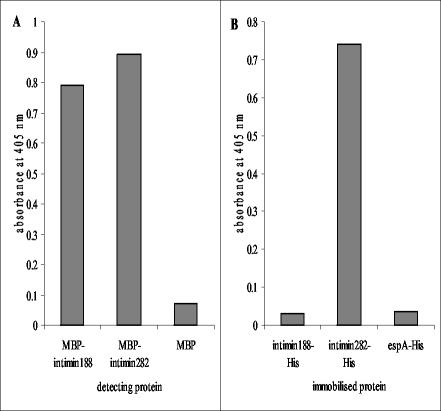
In order to determine function of the intimin188 and intimin282 constructs, their binding to Tir was assessed. The Tir protein was immobilized on Maxisorb plastic and detected with MBP-intimin fusion proteins. Interaction was detected with antibodies to MBP. Similarly, the converse interaction was studied where PBS soluble His-tagged intimin proteins were immobilized and detected with MBP-Tir protein. His-tagged immobilized Tir protein interacted with intimin188 and intimin282 proteins (Fig. 2). Also, immobilized intimin282 proteins interacted with Tir; however, immobilized intimin188 did not interact with MBP-Tir (Fig. 2).
FIG. 2.
Interaction of recombinant intimin and Tir103. (A) His-tagged Tir103 was immobilized (1 μg/well) and detected with MBP-intimin188, MBP-intimin282, or MBP (all at 10 μg/ml, 100 μl/well). (B) His-tagged intimin proteins, along with an EspA120-His control, were immobilized (1 μg/well) and detected with MBP-Tir103 (10 μg/ml, 100 μl/well). Bound MBP was detected with anti-MBP polyclonal antibody.
Isolation of phage-displayed scFvs.
Phage antibody libraries were produced from rabbits immunized with either EspA or intimin313. Library diversity was estimated by BstNI fingerprinting to be 2 × 106 for each library.
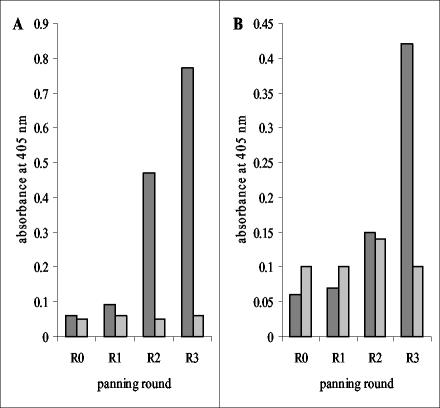
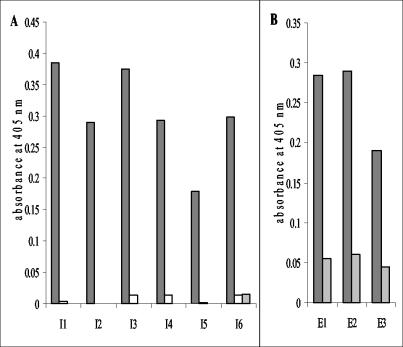
Recombinant EspA120 or a 1:1 (wt/wt) mixture of intimin282 plus intimin188 was immobilized on plastic and used as a target for antibody isolation from the relevant immune libraries. Decreasing antigen concentration coupled with increased stringency washing was used in progressive rounds in order to select for antibodies specific for the target antigens. Eluted and amplified phage after each round of selection were used in polyclonal phage ELISAs, demonstrating that enrichment had occurred for antibodies recognizing EspA and intimin (Fig. 3). Monoclonal phage-displayed scFvs were rescued from rounds 3 and selected by ELISA against target proteins used in the panning. Forty monoclonal phage isolated against either EspA120 or intimin were tested. For selection against EspA120, 6 gave positive signals; for selection against intimin282 plus intimin188, 19 were positive. Assessment of antibody diversity by BstNI fingerprinting determined that three EspA specific (designated E1 to E3) and six intimin specific (designated I1 to I6) antibodies had been isolated (Fig. 4).
FIG. 3.
Binding of polyclonal phage-displayed antibodies to intimin (A) and EspA120 (B). Three rounds of panning were carried out against MBP-intimin188 plus MBP-intimin282 or MBP-EspA120. MBP-intimin188 plus MBP-intimin282 (dark gray bars, panel A), MBP-EspA120 (dark gray bars, panel B) or MBP (light gray bars) were immobilized on ELISA plates (500 ng/well) and detected with 1012 CFU of phage/ml (100 μl/well) from panning rounds 0, 1, 2, and 3 (R0 to R3). Absorbances were measured at 405 nm.
FIG. 4.
Detection of intimin (A) and EspA (B) with monoclonal phage-displayed antibodies. MBP-intimin282 (dark gray bars, panel A), MBP-intimin188 (open bars, panel A), MBP (light gray bars), and EspA120 (dark gray bars, panel B) were immobilized (1 μg/well) and detected with 5 × 1012 CFU of monoclonal phage/ml (100 μl/well) isolated by panning against intimin (panel A, scFv I1 to I6) or EspA120 (panel B, scFv E1 to E3). Absorbances were measured at 405 nm.
Monoclonal phage-displayed scFvs were transferred to HB2151, and soluble scFv was produced. ELISAs were carried out against both MBP fusions and His-tagged versions of EspA120, initmin188, and intimin282 (data not shown). Only one of the EspA-specific phage antibodies could be produced as a functional soluble scFv (E1). All six anti-intimin phage antibodies were produced as soluble scFvs and bound intimin282 but not intimin188.
Isolation of soluble scFvs by colony filter screening.
Colony filter screening allows the direct selection of soluble scFvs from pSD3:scFv libraries against membrane immobilized antigen. Here, we used colony filter screening first as a screening tool to isolate antibodies after phage display selection and second as an iterative selection process for antibody isolation from the pSD3:scFv libraries, providing an alternative to phage display.
Round 3-eluted phage from phage panning against intimin was transformed into TG1, and 104 colonies plated out on a filter. Selection was performed against membranes coated with His-tagged intimin282 or intimin188. For intimin188, appropriate yields of soluble antigen could only be produced in the presence of 8 M urea; therefore, antigen was immobilized on the membrane under these conditions. Positive spots were produced on the antigen membranes, and single bacterial colonies corresponding to these signals were grown, streaked out onto a fresh filter, and again selected for binding against the target antigen immobilized on a membrane (Fig. 5). Intimin binder I4 was used as a positive control. For selection against intimin282, 16 positive colonies were produced. No positive colonies were isolated against intimin188. Antibody diversity was assessed by BstNI fingerprinting and I1, I3, and I4 were identified, as well as four novel scFvs (I7 to I10).
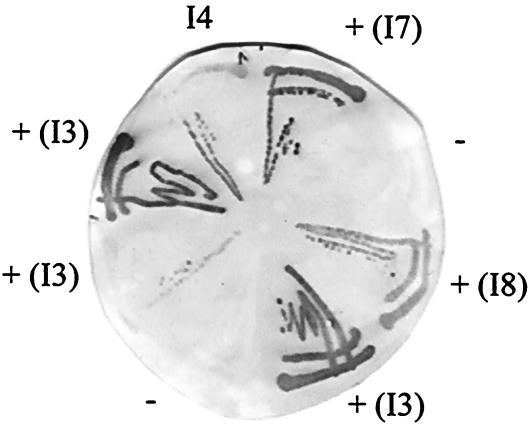
FIG. 5.
Filter screening of antibodies to intimin282. Soluble scFv were selected for binding to a filter coated with His-tagged intimin282 (0.15 mg/ml) and, after one or two rounds of colony filter screening, single colonies were streaked out and used to express monoclonal antibodies. Binding of antibodies to the filter was detected with 9E10 anti-c myc antibody. I4 monoclonal scFv, previously isolated by phage-display panning, was used as a positive control; a negative control scFv with irrelevant binding gave no signals against intimin282-coated filters. Positive clones were selected, and the designations in brackets were assigned after assessment of the genetic diversity by BstNI fingerprinting.
Iterative selection of soluble scFvs against His-tagged EspA, initmin188 (in urea), and intimin282 was carried out. Two to three rounds of selection resulted in the identification of positive single colonies. Thirty-one positive colonies against EspA were produced, as well as fourteen colonies against initmin282. An assessment of diversity revealed that 28 of 31 antibodies selected against EspA were E1; two further novel antibodies were also isolated (designated E4 and E5). Eleven novel antibodies were isolated against intimin282 (designated I11 to I21). Again, no binders were isolated against intimin188.
Characterization of anti-intimin and anti-EspA monoclonal recombinant antibodies.
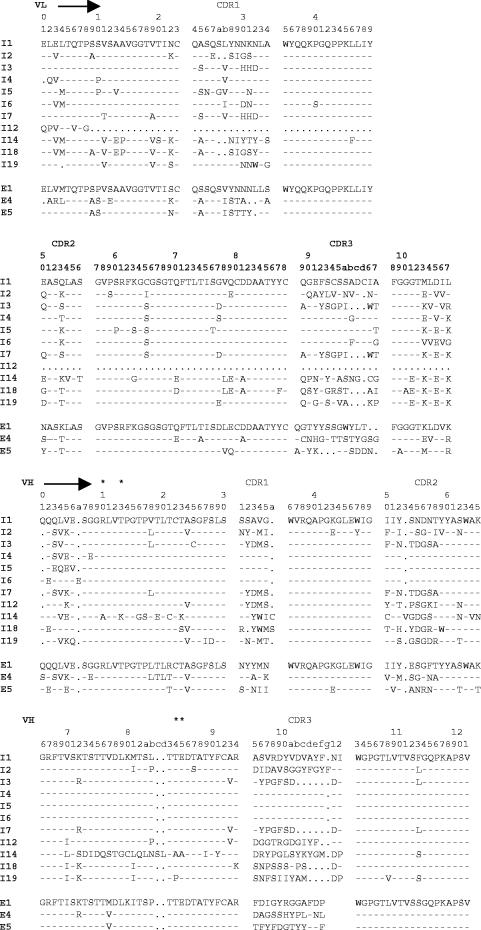
Antibodies I1 to I6, isolated by phage display selection, were analyzed by DNA sequencing. Deduced amino acid sequences (Fig. 6) show all six antibodies contain kappa light chains combined with VH1-a1 heavy chains. Heavy-chain promiscuity is also present with I1, I4, I5, and I6, all consisting of the same heavy chain coupled to distinct light chains. Five other anti-intimin scFvs were also analyzed, and one, I12, was shown to consist of a heavy chain alone, thus demonstrating the isolation of a single domain antibody by colony filter screening. All five antibodies contained distinct heavy and light chains (Fig. 6). Four of the five contained VH1-a1 heavy chains with scFv I14 containing the a3 allotype. With the exception of I12, all antibodies contained a kappa light chain.
FIG. 6.
Deduced amino acid sequences of representative scFvs against intimin (I1 to I19) and EspA (E1, E4, and E5). The alignment is according to the method of Kabat et al. (15). The VH-a1 allotype-specific residues (19) are labeled with an asterisk, dashes indicate identical residues, and dots indicate deletions.
Soluble scFvs were characterized in terms of their use in different assay systems: ELISA, Western blot, and dot blot (Table 2 and Fig. 7). Intimin-specific antibodies isolated by phage display or identified from round 3 of phage display selection by colony filter screening recognized target antigen in all of the assay systems. In contrast, although antibody I4 was also isolated by colony filter screening, all novel antibodies isolated by this method gave good signals in dot blots, but not all were functional in Western blots and none bound to their cognate epitope in ELISAs. This may reflect differences in antigen presentation in the different systems with the exposure of novel epitopes on filter-immobilized intimin that are not readily exposed upon immobilization of the antigen on plastic. For the isolation of antibodies against EspA, E1 was isolated by phage display and colony filter screening and recognized EspA in all assay systems, as did scFv E4, isolated by colony filter screening. Antibody E5 was also isolated by colony filter screening and was only functional in ELISA. Detection limits were assessed in Western blots and dot blots. For intimin and EspA detection in Western blots with I4 and E1, respectively, as little as 5 ng of target protein could be detected (data not shown); for dot blot analysis, I10 detected 0.25 ng of intimin282 and E1 detected 3.6 ng of EspA (Fig. 7).
TABLE 2.
Function of scFvs and AP-scFv fusions against E. coli O157 virulence factors in different assay systems
| Binder type | scFvs and AP-scFvs
|
||
|---|---|---|---|
| Western blot | Dot blot | ELISA | |
| Intimin binders | I1, I1-AP, I2, I2-AP, I3, I3-AP, I4, I4-AP, I5, I6, I7, I8, I9, I10, I11, I12, I13, I15, I19, I20, I21, | I1, I2, I3, I4, I5, I6, I7, I8, I9, I10, I11, I12, I13, I14, I15, I16, I17, I18, I19, I20, I21 | I1, I1-AP, I2, I2-AP, I3, I3-AP, I4, I4-AP, I5, I6, I7, I8, I9, I10 |
| EspA binders | E1, E4 | E1, E4 | E1, E4, E5 |
FIG. 7.
Detection of filter immobilized E. coli O157 proteins with monoclonal scFvs. Dot blots were performed against His-tagged intimin282 (lanes 1 and 4), intimin188 (lane 2), and EspA120 (lane 3). Panel A was developed with scFv I10, and panel B was developed with scFv E1. Recombinant monoclonal antibodies I4 (C) and E1 (D) were used in Western blots to detect MBP-intimin282 (lane 5), MBP-intimin188 (lane 6), MBP (lane 7), His-tagged EspA120 (lane 8), and His-tagged intimin282 (lane 9). MBP proteins were loaded at 500 ng/lane, and His-tagged proteins were loaded at 200 ng/lane. For all assays, binding of scFvs (5 ml of culture supernatant/blot) was detected with 9E10 anti-c myc antibody.
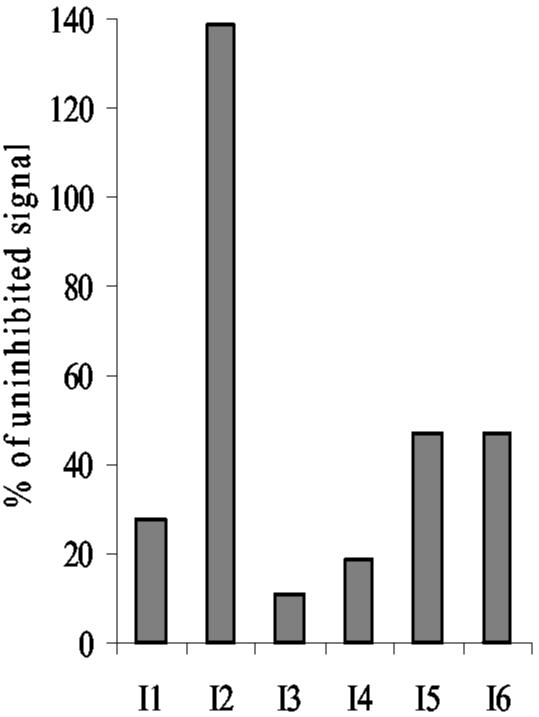
Antibodies isolated by phage display with specificity to intimin282 were assessed by competition ELISA (Fig. 8). Soluble I4 competed with phage-displayed antibodies I1, I3, I5, and I6; no competition was observed between I4 and I2, indicating that five of the six intimin-specific antibodies isolated by phage display recognized the same or overlapping epitopes.
FIG. 8.
Competition ELISA for intimin specific scFvs. MBP-intimin282 was immobilized on ELISA plates and detected with phage-displayed monoclonal scFvs I1 to I6 in the presence of competing soluble I4 scFv or a control scFv with irrelevant binding (100 μl of culture supernatant/well). Antigen and phage concentrations (both in volumes of 100 μl/well) were optimized for each scFv. Antigens were used at 200 ng/ml for I5 and I6, 625 ng/ml for I1, 1,500 ng/ml for I3, 2,000 ng/ml for I4, and 4,500 ng/ml for I2. Phage concentrations (in CFU/well) were 2 × 1010 for I1, 5 × 1010 for I2 and I4, 2 × 1011 for I3 and I5, and 1 × 1010 for I6. Phage binding was detected with anti-fd monoclonal antibody, and signals were produced in the presence of I4 expressed as a percentage of those produced in the presence of a control scFv.
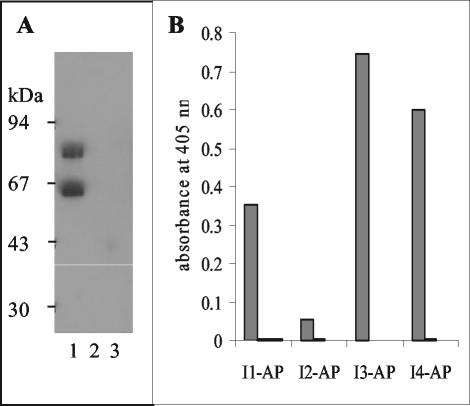
Antibodies I1, I2, I3, and I4 were also produced as fusion proteins with AP. This allowed the production of antibody fragments coupled to a functional dimeric reporter molecule. All four antibodies were functional in this format and could be used in the one-step detection of intimin by ELISA and Western blotting (Fig. 9).
FIG. 9.
One-step detection if intimin with AP-scFv fusion proteins. I3-AP fusion (panel A, 5 ml of culture supernatant/blot) was used to detect 500 ng of MBP-intimin282 (lane 1), MBP-intimin188 (lane 2), and MBP (lane 3)/lane in Western blots. Antibody-AP fusions (100 μl of culture supernatant/well) were also used to detect intimin282 (dark gray bars), intimin188 (open bars), and MBP (light gray bars) by ELISA (1 μg/well, panel B). For both assays, binding was detected by the addition of appropriate AP substrate.
Detection of E. coli O157:H7 virulence factors with monoclonal recombinant antibodies.
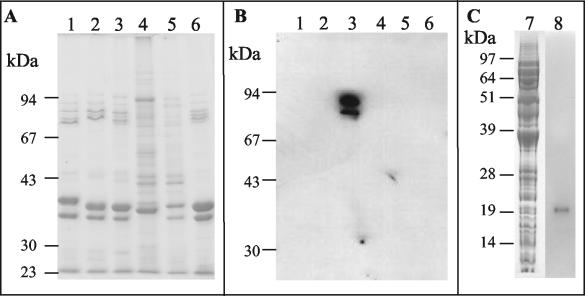
Antibodies to EspA and intimin were used in Western blots to detect these E. coli O157:H7 virulence factors in outer membrane or whole-cell preparations. Different serotypes of pathogenic EPEC and EHEC were analyzed, along with E. coli K-12 and E. coli O157:H7 DM3 (intimin mutant) controls (Fig. 10). Polyclonal immune sera after immunization with intimin313 bound to outer membrane preparations from E. coli O157:H7 only (data not shown). As expected, monoclonal scFv I4, isolated from the resulting pSD3 antibody library, was also specific for γ intimin. Polyclonal immune sera after immunization with EspA produced signals against whole-cell preparations from all of the tested pathogenic E. coli (data not shown). Monoclonal scFv E1 antibody produced relatively weak signals in Western blots against EspA in whole-cell preparations from serotypes O157 (Fig. 10) and O111 (data not shown), and no signals were produced with O127 or O86.
FIG. 10.
Detection of intimin and EspA from pathogenic E. coli with monoclonal scFvs. (A and B) Outer membrane fractions were prepared from pathogenic E. coli serotypes O127:H6 (α intimin, lane 1), O111:NM (β1 intimin, lane 2), O157:H7 (γ1 intimin, lane 3), O86:H34 (β2 intimin, lane 4), a nonpathogen K-12 strain (lane 5), and an O157:H7 intimin (−) mutant DM3 (lane 6) and then resolved on a 10% (wt/vol) SDS-PAGE gel (7.5 μl/lane, Coomassie blue-stained gel shown in panel A). A Western blot of the gel was developed with I4 (panel B, 5 ml of culture supernatant/blot). (C) Whole-cell preparations of E. coli O157:H7 were prepared and resolved on a 12% (wt/vol) SDS-PAGE gel (10 μl/lane). Lane 7 shows Coomassie blue staining, and lane 8 shows a Western blot developed with E1 (affinity-purified scFv from 25 ml of culture). ScFv binding was detected with anti-c myc antibody.
DISCUSSION
The use of combinatorial scFv antibody libraries has become an important methodology for the generation of diagnostic and therapeutic molecules (31). Most applications use phage display technology in order to present and select antibodies with the desired binding properties. Recent advances have also led to an alternative selection technique called colony filter screening, which directly selects for soluble antibodies by capture on antigen-coated filters (13, 30). Here, we use both techniques to isolate antibodies to E. coli O157:H7 virulence factors: intimin and EspA.
Immune scFv libraries were produced from rabbits immunized with either a fragment of intimin composed of the 313 C-terminal amino acid residues or full-length EspA. These immunogens cover the polymorphic regions of these effector proteins of different pathotypes, therefore allowing the possibility of isolating antibodies to strain-specific epitopes. These proteins are also vital components of bacterium-host cell interaction. The C-terminal third of intimin is reported to display two independent binding activities, both of which are essential for bacterial virulence: binding to its translocated receptor Tir and binding to an as-yet-unidentified host receptor(s) (10). The formation of an extracellular polymeric EspA-associated organelle is also an essential requirement for A/E lesion formation (25).
Selection of monoclonal recombinant antibodies was carried out against three different targets: intimin282 (C-terminal 282 amino acids), intimin188 (C-terminal 188 amino acids), and EspA120 (C-terminal 120 amino acids). The C-terminal region of intimin has been reported to be involved in binding to its receptor Tir with the minimal fragment of intimin involved in this interaction being the C-terminal 181 amino acids. Furthermore, the region of Tir involved in this interaction with intimin (3, 22) has been mapped, in EPEC Tir, to amino acid residues 255 to 362 (14). In order to demonstrate function of the recombinant intimin fragments produced in the present study, we assessed intimin-Tir binding in ELISAs. Whereas both intimin282 and intimin188 bound immobilized Tir102, only intimin282 and not intimin188 demonstrated Tir binding when immobilized on the plates. The results indicate that intimin188 is conformationally distinct from the equivalent region contained within intimin282 when immobilized onto plastic, most probably due to denaturation of the shorter fragment with the additional residues in intimin282 contributing to the stability of the protein. Furthermore, intimin188 when produced fused to MBP, a highly soluble E. coli protein, was readily soluble in PBS (pH 7.4). However, when produced without such a molecular chaperone, only low amounts of intimin188 remained in solution in PBS. This may again reflect the instability of the intimin188 fragment and the contribution of the N-terminal 94 amino acids in intimin282 to the stability and function of the C-terminal domains of intimin. This finding is in agreement with studies showing that intimin fragments consisting of the C-terminal 287 amino acids had increased affinity for Tir compared to a C-terminal 181-amino-acid fragment, suggesting that regions specific to the 287 fragment contribute to the binding activity to Tir (3, 22). These interaction assays also demonstrate that the region of EHEC Tir involved in intimin binding is the equivalent region to that described in EPEC Tir-intimin interaction (14).
Antibody selection strategies yielded a number of monoclonal antibodies specific for intimin282 and EspA in ELISAs, Western blots, and dot blots with detection limits in the latter assay being 0.25 and 3.6 ng for intimin and EspA, respectively. No antibodies were isolated when selected against intimin188 by phage display panning or by colony filter screening, and no intimin282 binders cross-reacted with intimin188. Furthermore, polyclonal sera from the rabbit immunized with intimin recognized intimin282 constructs in Western blots but not intimin188 (data not shown). The isolation of a number of antibodies that bind to the 94-amino-acid fragment exclusive to intimin282 suggests the presence of immunodominant epitope(s) in this region of the protein. This view is corroborated by the demonstration that five of the six antibodies isolated by phage display panning recognized the same or overlapping epitopes in this region of the protein. The presence of immunodominant epitopes in EPEC intimin has been reported previously where polyclonal sera recognized two immunodominant regions within intimin280, one of which was situated 200 to 280 residues from the C-terminal end (2). The other immunodominant epitope was found in the region 100 to 200 residues from the C terminus, most but not all of which have equivalent EHEC residues found within the intimin188 used in these studies. It is possible that such an epitope is present within EHEC intimin but could be located toward the extreme N terminus of the EPEC fragment comprised of residues 100 to 200 from the C terminus and only part or none of the epitope is contained within the EHEC intimin188 fragment. The results here demonstrate that the residues within EHEC intimin188 did not elicit a strong immune response when the larger fragment intimin313 was used as an immunogen. Other studies have shown that polyclonal antibodies raised against EHEC intimin282 can decrease the adherence of bacteria to HEp-2 cells (6, 11). Taken together, these results suggest that antibodies capable of inhibiting bacterium-host cell interaction may well have specificities to the immunoglobulin-like domain 2 of intimin.
Anti-intimin antibodies were produced as fusions with a reporter molecule, AP allowing the one-step detection of intimin by ELISA and Western blotting. These dimeric AP-scFv fusions displayed the same binding specificities as the parental monoclonal scFvs.
Monoclonal antibodies to EspA were also described. Using both phage display selection and colony filter screening, one antibody, E1, was isolated repeatedly from the combinatorial library consisting of 106 different clones. This may be due to the inability of other in vivo anti-EspA antibodies to be cloned or to be produced as function scFvs, or it may reflect the immunodominance of this antibody and its cognate epitope in the immunization.
Both intimin and EspA polymorphisms have been reported. Using PCR-based analysis, intimin has been typed in A/E producing bacterial pathogens into α, β, γ, and δ (1, 8). More recently, δ intimin was reclassified as β2 intimin and ɛ intimin described, as well as γ intimin being further divided into γ1 and γ2 intimins (27). There are at least 11 recognized intimin types (36). For EspA, antisera to this protein from EPEC O127:H6 were reported to cross-react with EspA filaments from O55:H6 but not filaments from other EPEC or EHEC strains, whereas antisera against EHEC O157:H7 cross-reacted against only O55:H7 (26). The monoclonal antibodies isolated against intimin in the present study were tested against outer membrane preparations from A/E lesion-producing bacterial pathogens containing α (O127:H6), β (O111:NM), γ1 (O157:H7), and β2 (O86:H34) intimins, as well as an O157:H7 intimin mutant (DM3) and a nonpathogenic K-12 E. coli strain. All of the anti-intimin antibodies tested (I1 to I6) were specific for γ intimin (E. coli O157:H7) and displayed no cross-reactivity to the other intimin types. The anti-EspA antibody E1 was similarly tested against whole-cell preparations of these E. coli strains. This scFv bound to EspA from EHEC O157:H7 (wild type and DM3) and EHEC O111:NM but not to EspA from EPEC O127:H6 or EPEC O86:H34. However, the presence of E1 epitopes in EspA from O127 and O86 cannot be ruled out since the protein appeared to be present in relatively low concentrations in whole-cell preparations, which may reflect the transient nature of the proteins' expression. The polyclonal sera appeared to be much more sensitive than E1 in the Western blot analyses and could detect EspA in all of the pathogenic E. coli. The binding specificity of the polyclonal sera in the present study contrasts with the results for polyclonal sera against EHEC O157:H7 described by Neves et al. (26), which did not show cross-reactivity with EPEC O86:H34 or O127:H6 EspA filaments. Inconsistencies with the previous report may be due to the different presentation of epitopes within EspA filaments compared to denatured proteins on a Western blot or may highlight the occurrence of variations in EspA epitope recognition within different individual animals and/or immunization regimes.
The present study highlights the existence of polymorphisms within intimin, as well as the presence of potentially immunodominant epitopes within both intimin and EspA proteins. Both factors have considerable bearing when we consider targets for the development of broad range vaccines against A/E-producing pathogenic E. coli. As such, the design of such vaccines is likely to require intimin proteins derived from a range of the targeted pathogenic bacteria. The study by Neves et al. (26) indicates that the same may true for “filament-accessible” EspA epitopes; however, the present study demonstrates the production of polyclonal sera that recognizes epitope(s) present within EspA from a range of pathogenic bacteria, albeit by Western blot analysis.
We have isolated and developed recombinant antibodies that recognize EspA and intimin from EHEC O157:H7. In addition to providing diagnostic monoclonal antibodies for the detection of virulence factors from EHEC O157:H7 and, in the case of EspA, EPEC O111:NM, these antibodies also provide tools for dissecting molecular interaction and protein expression for proteins vital in bacterium-host cell interactions. These reagents may be of particular use in the analysis of the recently reported phenomenon that there exist high and low secretors of effector proteins within the E. coli O157:H7 population (33). Also, previous studies have demonstrated the potential use of polyclonal antibodies against both intimin (11) and EspA (M. A. Noguera-Obenza, H. F. Gomez, and T. G. Cleary, Abstr. Infect. Dis., p. 1529, 2002) for the disruption of bacterium-host cell interaction. As such, recombinant antibodies produced in the present study could play a role in novel disease resistance strategies. Such an assessment of these antibodies could provide the basis for future studies.
Acknowledgments
We acknowledge DEFRA for funding this research.
Y. Li (ADAS Rosemaund, Hereford, United Kingdom) contributed to the cloning of the scFv libraries, and M. P. Dibb-Fuller and A. L. Cookson (both VLA, Addlestone, Surrey, United Kingdom) helped with the cloning of the eae313 and espA genes, as well as with the immunizations.
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. Guedes Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adu-Bobie, J., L. R. Trabulsi, M. M. S. Carneiro-Sampaio, G. Dougan, and G. Frankel. 1998. Identification of immunodominant regions within the C-terminal cell binding domain of intimin α and intimin β from enteropathogenic Escherichia coli. Infect. Immun. 66:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchelor, M., S. Prasannan, S. Daniell, S. Reece, I. Connerton, G. Bloomberg, G. Dougan, G. Frankel, and S. Matthews. 2000. Structural basis for recognition of the translocated intimin receptor (Tir) by intimin from enteropathogenic Escherichia coli. EMBO J. 19:2452-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best, A., R. M. La Ragione, W. A. Cooley, C. D. O'Conner, P. Velge, and M. J. Woodward. 2003. Interaction with avian cells and colonization of SPF chicks by Shiga-toxin negative Escherichia coli O157:H7 (NCTC 12900). Vet. Microbiol. 93:207-222. [DOI] [PubMed] [Google Scholar]
- 5.Campellone, K. G., and J. M. Leong. 2003. Tails of two Tirs: actin pedestal formation by enteropathogenic Escherichia coli and enterohemorrhagic E. coli O157:H7. Curr. Opin. Microbiol. 6:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., L. J. Gansheroff, M. Mills, H. W. Moon, and A. D. O'Brien. 2002. Vaccination of pregnant dams with intiminO157 protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun. 70:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel, G., D. C. A. Candy, E. Fabiani, J. Adu-Bobie, S. Gil, M. Novakova, A. D. Phillips, and G. Dougan. 1995. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect. Immun. 63:4323-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel, G., A. D. Phillips, G. D. Rosenshine, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 10.Frankel, G., A. D. Phillips, L. R. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell: is it bound to end in Tir(s)? Trends Microbiol. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 11.Gansheroff, L. J., M. R. Wachtel, and A. D. O'Brien. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 67:6409-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gough, K. C., Y. Li, and G. C. Whitelam. 2002. Antibody phage display libraries, p. 221-236. In P. M. Golmartin and C. Bowler (ed.), Molecular plant biology, vol. 2. Oxford University Press, Oxford, United Kingdom.
- 13.Giovannoni, L., F. Viti, L. Zardi, and D. Neri. 2001. Isolation of anti-angiogenesis antibodies from a large combinatorial repertoire by colony filter screening. Nucleic Acids Res. 29:e27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartland, E. L., M. Batchelor, C. M. Delahey, Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32:151-158. [DOI] [PubMed] [Google Scholar]
- 15.Kabat, E. A., T. T. Wu, M. Reid-Miller, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest, 5th ed. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Washington, D.C.
- 16.Kaper, J. B., S. Elliot, V. Sperandio, N. T. Perna, G. F. Mayhew, and F. R. Blattner. 1998. Attaching and effacing intestinal histopathology and the locus of enterocyte effacement, p. 163-182. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 17.Karmali, M. A. 1987. Laboratory diagnosis of verotoxin-producing Escherichia coli infections. Clin. Microbiol. Newsl. 9:65-70. [Google Scholar]
- 18.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight, K. L., and R. S. Becker. 1990. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell 60:963-970. [DOI] [PubMed] [Google Scholar]
- 20.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroinvasiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., W. Cockburn, J. Kilpatrick, and G. C. Whitelam. 1999. Selection of rabbit single-chain Fv fragments against the herbicide atrazine using a new phage display system. Food Agric. Immun. 11:5-17. [Google Scholar]
- 22.Lui, H., L. Magoun, S. Luperchio, D. B. Schauer, and J. M. Leong. 1999. The Tir-binding region of enterohaemorrhagic Escherichia coli intimin is sufficient to trigger actin condensation after bacterial-induced host cell signalling. Mol. Microbiol. 34:67-81. [DOI] [PubMed] [Google Scholar]
- 23.Marks, J. D., H. R. Hoogenbomm, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization: human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 24.Nataro, J. P., and J. B. Kaper. 1998. Diarrhoeagic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves, B. C., S. Knutton, L. R. Trabulsi, V. Sperandio, J. B. Kaper, G. Dougan, and G. Frankel. 1998. Molecular and ultrastructural characterization of EspA from different enteropathogenic Escherichia coli serotypes. FEMS Microbiol. Lett. 169:73-80. [DOI] [PubMed] [Google Scholar]
- 26.Neves, B. C., R. K. Shaw, G. Frankel, and S. Knutton. 2003. Polymorphisms within EspA filaments of enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 71:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulozzi, L. J., K. E. Johnson, L. M. Kamahele, C. R. Clausen, L. W. Riley, and S. D. Helgerson. 1986. Diarrhea associated with adherent enteropathogenic Escherichia coli in an infant and toddler centre, Seattle, Washington. Pediatrics 77:296-300. [PubMed] [Google Scholar]
- 29.Perna, N. T., G. F. Mayhew, G. Posfia, S. Elliot, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pini, A., C. Ricci, and L. Bracci. 2002. Phage-display and colony filter screening for high throughput selection of antibody libraries. Combinatorial Chem. High Throughput Screening 5:503-510. [DOI] [PubMed] [Google Scholar]
- 31.Rader, C., and C. F. Barbas III. 1997. Phage display of combinatorial antibody libraries. Curr. Opin. Biotechnol. 8:503-508. [DOI] [PubMed] [Google Scholar]
- 32.Ridder, R., R. Schmitz, F. Legay, and H. Gram. 1995. Generation of rabbit monoclonal antibody fragments from a combinatorial phage display library and their production in the yeast Pichia pastoris. Bio/Technology 13:255-260. [DOI] [PubMed] [Google Scholar]
- 33.Roe, A. J., H. Yull, S. W. Naylor, A. McNally, M. J. Woodward, D. G. E. Smith, and D. L. Gally. 2003. Phase variable expression of the type III secretion translocon protein EspA of Escherichia coli O157:H7. Infect. Immun. 71:5900-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Woodward, M. J., A. Best, K. A. Sprigings, G. R. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Roe, C. Low, and R. M. La Ragione. 2003. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attached-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293:299-308. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, W. L., B. Kohler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]