Abstract
Aims
To evaluate the impact of psychiatric comorbidities on the quality of life of early stage breast cancer subjects, in the first post-surgery week, the prevalence of those psychiatric disorders in this group, the correlation between pain and adjustment disorders and between the STAI-X1 (State Trait Anxiety Inventory 1) and BDI (Beck Depression Inventory) scales and the psychopathological findings.
Patients and methods
We conducted a clinical study at the Ocological Institute “Ioan Chiricuţă” Cluj-Napoca on a group of 56 patients (mean age 53.51 years old) with nonmetastatic breast cancer, who underwent surgical intervention in the week prior to the psychiatric evaluation. Patients received a complete psychiatric evaluation during their hospitalization. The STAI-X1, BDI, Numeric Rating Scale and QLQ 30 (Quality of Life Questionnaire 30) plus BR 23 (Breast 23) questionnaires were administered.
Results
The prevalence of adjustment disorders in the group was 28.58%. The psychiatric diagnosis significantly and inversely correlated with the emotional functioning, the cognitive functioning and future perspective. The existence of psychiatric disorders significantly and directly correlated with fatigue, pain, insomnia, appetite loss, constipation, arm symptoms and global health status.
Conclusions
Depression and anxiety have an important prevalence in the breast cancer population and they significantly alter the quality of life of these patients and caregivers by reducing their functional abilities and by generating a higher level of symptomatology and subjective sufferance.
Keywords: depression, anxiety, quality of life, breast cancer, postoperative
Introduction
A number of reviews estimate the prevalence of depression in the oncologic population between 1.5 and 45% [1]. Other trials mention a prevalence of depression and anxiety of 25–40% in the first year of diagnosis [2], with significant influence on the quality of life of the subjects and on the family relations. Breast cancer is the most frequently encountered form of malignancy in women and it represents around 28% of the reported cancers in Europe [3]. In Romania over 6,000 new cases, usually at an advanced stage, are diagnosed every year, with an incidence of 56.84/100,000 and a reported mortality of 39.28/100,000. Moreover, a recent study of the International Agency for Cancer, published in 2010 in the British Medical Journal, describes a 17% increase of the mortality generated by breast cancer in Romania in the last few years [4], most probably due to the dysfunctional strategies of early detection and treatment. According to the last WHO report, in 2011 [5] 3,225 subjects died of breast cancer in Romania.
The majority of breast cancer patients experience an intense subjective emotional distress shortly after the announcement of their diagnosis, but only a subgroup develops significant psychopathological disorders [6], the reported percentage of the depressive spectrum disorders varying between 10–25% [7], according to the evaluation instruments that were used. In 2005, Burgess et al. described a decreasing annual prevalence of depression and anxiety of 50% in the first year, 25% in the second, third and fourth year and 15% in the fifth year after being diagnosed with breast cancer [8]. Having a psychiatric comorbidity predicts a worse outcome and survival rate in this population group, it generates a poorer quality of life for both patients and caregivers [9] and increases the number of somatic complaints.
Aims
The main objective of the study was to evaluate the correlation between psychiatric depressive and anxious comorbidities and a modified quality of life of early stage breast cancer subjects, in the first postoperative week. The secondary objectives were to evaluate the prevalence of those psychiatric disorders in this group, the correlation between pain and adjustment disorders and between the STAI-X1 and BDI scales and the psychopathological findings.
Patients and methods
This clinical study was carried out at the Oncological Institute ”Ioan Chiricuţă” Cluj-Napoca, from January 2011 to November 2012, in the surgery ward, on a group of 56 patients who underwent surgical intervention in the week prior to the psychiatric evaluation and who signed a written informed consent. The subjects were diagnosed in the last 12 months with nonmetastatic nonrecurring breast cancer, and they underwent sectorectomy or mastectomy in the 7 days before the evaluation. The metastatic or recurrent breast cancer patients, those with psychiatric history and those unable or unwilling to sign the informed consent were excluded from the study. Patients underwent a complete psychiatric evaluation (according to the DSM-IV [10] and ICD [11] diagnosis requirements for adjustment disorders) during their hospitalization. The study was approved by the medical ethics commission of the Iuliu Haţieganu University of Medicine and Pharmacy.
The evaluated items were the age, the living environment, the marital status, social support, financial difficulties, the histological type of cancer, the time from the announcement of the diagnosis, the type of surgical intervention, the use of tamoxifen/aromatase inhibitors, the somatic comorbidities and the chemo and radiotherapy. We administered the BDI [12], a 21-question multiple choice self report inventory, one of the most widely used instruments for measuring the severity of depression, the STAI-X1 [13], a self report scale evaluating the level of anxiety generated by a certain event, the Numeric Rating Scale for pain (NRS-11) [14], and the QLQ 30 and BR 23 questionnaires [15] for the quality of life, scored according to the authors recommendations [16]. For the statistical analysis we used the SPSS package. We used the student’s T test and correlation coefficients. The distribution of the variables was tested with Skewnes and Kurtosis, and the general data were analyzed with the Chi Square test.
Results
The mean age of the group was 53.51 years, ranging between 33 and 74, and the mean duration since diagnosis was 6 months. No statistical differences were found concerning the general demographic data; 78.6% were married, 1.8% unmarried, the other 19.6% being divorced or widows.
Most of the patients (76.8%) lived in the urban area and had in important social support (92.9%). Financial difficulties were identified in half of the subjects.
Somatic comorbidities were equally distributed in the group, 50% having no organic comorbidities, while the other 50% were suffering of different disorders (hypertension, glaucoma, diabetes, gastritis, anemia, dyslipidemia etc). The TNM staging system was used, and the breakdown of our patient group was: T1 stage: 33.92% (comprises T1N0 and T1N1 tumors), T2: 39.28% (includes T2N0, N1 and N3), and T4: 12.5% (comprises T4N0, T4N1 and T4N2 stage tumours). All of the patients were M0.
From the histological perspective, as expected, the majority (83.92%) were infiltrating ductal carcinomas, the rest of them being infiltrating lobular carcinomas (7.14%), mucinous carcinomas (1.78%) and ductal carcinomas in situ (7.14%).
A number of 13 patients (23.2%) underwent breast sectorectomy, whilst the rest of the group (76.8%) had a complete unilateral mastectomy. No patient was treated with radiotherapy, but 67.9% (38) of them had received one or more cycles of chemotherapy in the last year. In addition, 8 subjects (14.3%) were given tamoxifen in the year prior to the surgery and 4 of them (7.1%) received aromatase inhibitors.
The prevalence of adjustment disorders in the group was 28.58%, with a distribution of the corresponding subtypes as described in figure 1.
Figure 1.
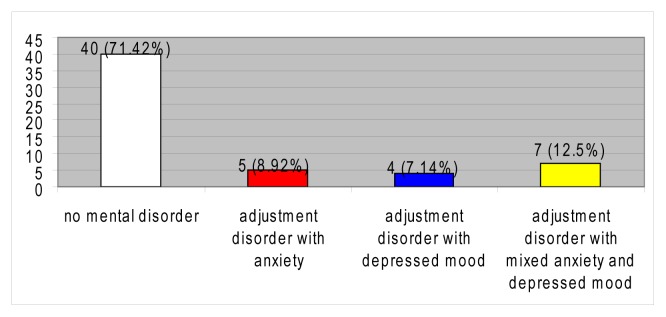
Psychiatric disorders.
No significant differences were encountered between the two subgroups regarding the age and the time since diagnosis. The STAI-X1 and BDI mean scores were significantly higher in the adjustment disorders group, as well as the mean pain score. Moreover, there was a statistically significant correlation with the psychiatric diagnosis for the above mentioned items.
The QLQ 30 and BR 23 scores were obtained for functional and symptom subscales, as recommended by the authors. The patients with no psychiatric issues had significantly higher mean scores for physical, emotional and cognitive functioning, as well as for body image and future perspective. Those means had a strong correlation with the psychiatric diagnosis for emotional and cognitive functioning and for future perspective.
The symptoms which were significantly different in the two subgroups are described in fig. 1. We obtained a positive statistical correlation between fatigue, pain, insomnia, appetite loss, constipation, arm symptoms and overall health status and the presence of the adjustment disorders in our subjects.
Discussion
Being unmarried or single [17,18], financial difficulties [19,20], comorbidities [21], pain [22] and poorer social support [23,17] are linked to a higher risk of developing an adjustment disorder in breast cancer patients. A longer time since diagnosis generates a lower rate of depression [24].
In 1983, Derogatis et al found a prevalence of adjustment disorders in cancer patients of 32% [25] and Mehnert [26] evaluated a sample of post-surgery breast cancer patients and described a prevalence of the AD of 7.1%. In our group the prevalence of AD was 28.58%, comparable to the prevalence reported by Sellik at al in 1999 [2]. The differences in the reported prevalence could be generated by different nosologic framing of the symptoms, according to the subjective experience of the examiner.
The results we obtained concerning the STAI-X1 and BDI mean scores confirmed the validity of the evaluation and also the efficiency of the mentioned scales despite the interfering of the physical symptoms (i.e. weight loss, fatigue etc) generated by the malignancy. Pain was more intensely perceived by the patients suffering from depression and anxiety. Considering the fact that all patients had identical types, locations and etiology for pain (the pain generated by surgery), we concluded that the psychiatric status of the patients influenced the pain perception.
Important differences resulted in the quality of life subscales scores, all indicating a lower quality of life in the adjustment disorder subgroup. As expected, the emotional functioning was inferior in the adjustment disorder group, with a statistically significant correlation. The mean reference value for local or locoregional disease was 70.4 [27], lower than the mean we obtained for the non-psychiatric group, but much higher than the mean in the adjustment disorders group. Moreover, an inferior cognitive functioning (reference value=85.6) and an altered future perspective correlated significantly with the psychiatric diagnosis.
The score means obtained for the physical functioning subscales were also lower in the depressed or anxious patients, but no correlations were confirmed. In two studies carried out in 2009 and 2010 [19,22], physical functioning and role performance also showed the expected inverse relationships with depressive symptoms, in our study the results suggesting the fact that a diminished physical performance would rather generate psychiatric symptoms than being generated by them.
Regarding the symptoms scales from QLQ 30 and BR 23, once again the classical physical complaints generated by depression and anxiety (fatigue, insomnia – reference value=26.7, appetite loss – reference value=9) generated significantly higher scores in the adjustment disorders subgroup and statistically correlated with those diagnosis. The score means for fatigue (reference value=20.60 and pain (reference value=16.7) were much higher in our study group comparing to the reported reference values. We considered those results to be influenced by the post-surgery evaluation of the patients.
A study carried out by Kim et al. in 2008 identified significant bivariate relationships between depressive symptoms and constipation (reference value=10) and arm symptoms; however, data were unclear as to direction of the relationships [20]. We found comparable results in our study, with higher scores for those symptoms in the subgroup of psychiatric disorders subjects, and significant positive correlations. Pain perception was, once again, more intense in the depressed/anxious subjects.
Finally, the global health status, which we consider to be the best reflection of the subjective perception of well being in the QLQ 30 scale, inversely and significantly correlated with the adjustment disorders in our study, confirming once again that psychiatric comorbidity in nonmetastatic post-surgery breast cancer subjects alters their quality of life. Another mention should be done comparing our mean score with the reference value (73.4), the significant difference being most probably due to the surgical intervention and the lack of appropriate psychosocial measures in managing this difficult period in one’s existence.
The limitation of our study consists in the relatively small group of evaluated participants. Further systematic evaluations need to be performed in order to accurately detect the correlation between psychiatric disorders and an altered quality of life in this group of population.
Conclusions
Depression and anxiety have an important prevalence in the breast cancer population and should be thoroughly prevented, evaluated and treated.
The adjustment disorders significantly alter the quality of life of these subjects by reducing their functional abilities and by generating a higher level of symptomatology and subjective sufferance. Pain perception seems to be higher in this subgroup of subjects.
Appropriate prevention, screening and therapeutic approach is needed in order to obtain a better collaboration with the patient and a better quality of life for both patients and caregivers.
Table 1.
Marital status.
| frequency | percent | |
|---|---|---|
| unmarried | 1 | 1.8 |
| married | 44 | 78.6 |
| divorced | 4 | 7.1 |
| widow | 7 | 12.5 |
Table 2.
Living environment, financial and social situation.
| frequency | percent | |
|---|---|---|
| Urban | 43 | 76.8 |
| Rural | 13 | 23.2 |
| Financial diff. | 28 | 50 |
| No financial diff. | 28 | 50 |
| Social support | 52 | 7.1 |
| No social support | 4 | 92.2 |
Table 3.
Histological type.
| frequency | percent | |
|---|---|---|
| Infiltrating ductal carcinoma | 47 | 83.92 |
| Infiltrating lobular carcinoma | 4 | 7.14 |
| Mucinous carcinoma | 1 | 1.78 |
| Ductal carcinoma in situ | 4 | 7.14 |
Table 4.
Pain, STAI-X1, BDI.
| Adjustment disorders | Number | Mean | Std. deviation | p | |
|---|---|---|---|---|---|
|
| |||||
| STAI-X1 | Yes | 40 | 33.7750 | 8.52895 | 0.00000 |
| No | 16 | 57.1875 | 8.42392 | ||
|
| |||||
| BDI | Yes | 40 | 8.0750 | 5.07577 | 0.00000 |
| No | 16 | 22.2500 | 4.97326 | ||
|
| |||||
| pain | Yes | 40 | 3.4000 | 2.01023 | 0.00500 |
| No | 16 | 5.1250 | 1.96214 | ||
| Spearman’s rho | Correlations | Psychiatric disorder |
|---|---|---|
|
| ||
| Psychiatric disorder | Correlation coefficient Sig. (2-tailed) | 1.000 |
|
| ||
| STAI-X1 | Correlation coefficient Sig. (2-tailed) | 0.730** |
| 0.000 | ||
|
| ||
| BDI | Correlation coefficient Sig. (2-tailed) | 0.741** |
| 0.000 | ||
|
| ||
| Pain score | Correlation coefficient Sig. (2-tailed) | 0.357** |
| 0.007 | ||
Table 5.
Functional scales.
| Psychiatric disorder | number | mean | Std. deviation | p | |
|---|---|---|---|---|---|
|
| |||||
| Physical functioning | No | 40 | 74.6638 | 15.63104 | 0.04400 |
| Yes | 16 | 61.2469 | 22.99329 | ||
|
| |||||
| Emotional functioning | No | 40 | 74.5810 | 17.18778 | 0.00100 |
| Yes | 16 | 45.8294 | 26.52864 | ||
|
| |||||
| Cognitive functioning | No | 40 | 83.3310 | 21.35149 | 0.01800 |
| Yes | 16 | 62.4969 | 29.50220 | ||
|
| |||||
| Body image | No | 40 | 71.8723 | 28.78740 | 0.04700 |
| Yes | 16 | 52.7753 | 36.68719 | ||
|
| |||||
| Future perspective | No | 40 | 51.6640 | 36.16184 | 0.04300 |
| Yes | 16 | 29.1650 | 38.27424 | ||
| Spearman’s rho | Correlations | Psychiatric disorder |
|---|---|---|
|
| ||
| Psychiatric disorder | Correlation coefficient Sig. (2-tailed) | 1.000 |
|
| ||
| Emotional functioning | Correlation coefficient Sig. (2-tailed) | −0.506** |
| 0.000 | ||
|
| ||
| Cognitive functioning | Correlation coefficient Sig. (2-tailed) | −0.364** |
| 0.006 | ||
|
| ||
| Future perspective | Correlation coefficient Sig. (2-tailed) | −0.281* |
| 0.036 | ||
Table 6.
Symptom scales.
| Adjustment disorders | Number | Mean | Std. deviation | p | |
|---|---|---|---|---|---|
|
| |||||
| Fatigue | No | 40 | 40.8193 | 23.65332 | 0.01300 |
| Yes | 16 | 59.7169 | 27.77597 | ||
|
| |||||
| Pain | No | 40 | 29.5803 | 25.17229 | 0.01400 |
| Yes | 16 | 47.9131 | 22.66697 | ||
|
| |||||
| Insomnia | No | 40 | 23.3315 | 31.30399 | 0.00500 |
| Yes | 16 | 52.0806 | 38.42931 | ||
|
| |||||
| Appetite loss | No | 40 | 17.4990 | 30.18134 | 0.00500 |
| Yes | 16 | 54.0813 | 40.31122 | ||
|
| |||||
| Constipation | No | 40 | 15.8323 | 27.20233 | 0.00500 |
| Yes | 16 | 41.6638 | 35.48469 | ||
|
| |||||
| Financial difficulties | No | 40 | 49.9965 | 33.75688 | 0.04400 |
| Yes | 16 | 70.3100 | 32.05551 | ||
|
| |||||
| Arm symptoms | No | 40 | 25.5330 | 24.41868 | 0.00800 |
| Yes | 16 | 47.2175 | 31.29262 | ||
|
| |||||
| Global health status | No | 40 | 48.9548 | 21.61448 | 0.01100 |
| Yes | 16 | 31.7681 | 23.41687 | ||
| Spearman’s rho | Correlations | Psychiatric disorder |
|---|---|---|
|
| ||
| Psychiatric disorder | Correlation coefficient Sig. (2-tailed) | 1.000 |
|
| ||
| Fatigue | Correlation coefficient Sig. (2-tailed) | 0.321* |
| 0.016 | ||
|
| ||
| Pain | Correlation coefficient Sig. (2-tailed) | 0.325* |
| 0.015 | ||
|
| ||
| Insomnia | Correlation coefficient Sig. (2-tailed) | 0.355** |
| 0.007 | ||
|
| ||
| Appetite loss | Correlation coefficient Sig. (2-tailed) | 0.429** |
| 0.001 | ||
|
| ||
| Constipation | Correlation coefficient Sig. (2-tailed) | 0.373** |
| 0.005 | ||
|
| ||
| Arm symptoms | Correlation coefficient Sig. (2-tailed) | 0.329* |
| 0.013 | ||
|
| ||
| Global health status | Correlation coefficient Sig. (2-tailed) | −0.331* |
| 0.013 | ||
References
- 1.Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- 2.Sellick SM, Crooks DL. Depression and cancer: an appraisal of the literature for prevalence, detection, and practice guideline development for psychological interventions. Psychooncology. 1999;8(4):315–333. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<315::AID-PON391>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Early detection of common cancers. Breast cancer. Available from: URL: http://www.euro.who.int/en/what-we-do/health-topics/noncommunicable-diseases/cancer/news/news/2012/2/early-detection-of-common-cancers/breast-cancer.
- 4.Autier P, Boniol M, LaVecchia C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:c4480. doi: 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Statistics 2011. WHO Press; 2011. Available from : http://www.who.int/whosis/whostat/EN_WHS2011_Full.pdf. [Google Scholar]
- 6.Payne DK, Hoffman RG, Theodoulou M, Dosik M, Massie MJ. Screening for anxiety and depression in women with breast cancer. Psychiatry and medical oncology gear up for managed care. Psychosomatics. 1999;40(1):64–69. doi: 10.1016/s0033-3182(99)71273-9. [DOI] [PubMed] [Google Scholar]
- 7.Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:332. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehto US, Ojanen M, Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16(5):805–816. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington DC: 1994. [Google Scholar]
- 11.World Health Organization. International Classification of Diseases. Geneva, Switzerland: 1992. Tenth Revision. [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; 1983. [Google Scholar]
- 14.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure. Pain Pract. 2003;3(4):310–316. doi: 10.1111/j.1530-7085.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A On behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd Edition. European Organisation for Research and Treatment of Cancer Brussels; 2001. [Google Scholar]
- 17.Popoola AO, Adewuya AO. Prevalence and correlates of depressive disorders in outpatients with breast cancer in Lagos, Nigeria. Psycho-Oncology. 2012;21(6):675–679. doi: 10.1002/pon.1968. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Yang M, Chen W, et al. Prevalence and correlates of major depressive disorder in breast cancer survivors in Shanghai, China. Psychooncology. 2012;21(12):1331–1337. doi: 10.1002/pon.2075. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Zheng Y, Zheng W, et al. Prevalence of depression and its related factors among Chinese women with breast cancer. Acta Oncologica. 2009;48:1128–1136. doi: 10.3109/02841860903188650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Son BH, Hwang SY, et al. Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. Journal of Pain and Symptom Management. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Rogers LQ, Markwell SJ, Courneya KS, McAuley E, Verhulst S. Physical activity type and intensity among rural breast cancer survivors: Patterns and associations with fatigue and depressive symptoms. Journal of Cancer Survivorship. 2011;5:54–61. doi: 10.1007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karakoyun-Celik O, Gorken I, Sahin S, Orcin E, Alanyali H, Kinay M. Depression and anxiety levels in woman under follow-up for breast cancer: Relationship to coping with cancer and quality of life. Medical Oncology. 2010;27:108–113. doi: 10.1007/s12032-009-9181-4. [DOI] [PubMed] [Google Scholar]
- 23.Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: The importance of reproductive concerns. Breast Cancer Research and Treatment. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardwell WA, Fiorentino L. Risk factors for depression in breast cancer survivors. International Journal of Clinical and Health Psychology. 2012;12( 2):311–331. [Google Scholar]
- 25.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 26.Mehnert A, Koch U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology. 2007;16(3):181–188. doi: 10.1002/pon.1057. [DOI] [PubMed] [Google Scholar]
- 27.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Reference Values manual. European Organisation for Research and Treatment of Cancer. 1998 [Google Scholar]


