Abstract
Cross-resistance within a class of antimicrobial agents is a problem that is often encountered with antibacterial agents, and it is also an issue with antifungal agents. A current example is ravuconazole, a new triazole antifungal with an expanded spectrum and potency against Candida spp., Aspergillus spp., and other opportunistic fungal pathogens. The present study addresses the issue of cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict the susceptibility of Candida spp. to ravuconazole. Reference broth microdilution MIC results for 12,796 strains of Candida spp. isolated from more than 200 medical centers worldwide were used. Ravuconazole MICs and tentative interpretive categories (susceptible, ≤1 μg/ml; resistant, ≥2 μg/ml) were compared with those of fluconazole by using regression statistics and error rate bounding analyses. For all 12,796 isolates, the absolute categorical agreement rate was 92.5% (rate of false-susceptible results, or very major errors [VME], 0.1%). Ravuconazole was active (MIC, ≤1 μg/ml) against 99.9% of the fluconazole-susceptible isolates, 96% of the fluconazole-susceptible dose-dependent isolates, and 49% of the fluconazole-resistant isolates, including 99% of the Candida krusei isolates. Since ravuconazole is 16- to 32-fold more potent than fluconazole, the performance of fluconazole as a surrogate marker for ravuconazole susceptibility was improved by designating those isolates with fluconazole MICs of ≤32 μg/ml susceptible to ravuconazole, resulting in a categorical agreement rate of 98.3%, with a VME rate of 0.3% (99 and 0.4%, respectively, when C. krusei was omitted). Cross-resistance between fluconazole and ravuconazole applies most directly to fluconazole-resistant Candida glabrata and is variable among other species of Candida. Fluconazole may serve as a surrogate marker to predict the susceptibility of Candida spp. to ravuconazole.
Ravuconazole is an investigational triazole antifungal agent with broad-spectrum activity against Candida spp., Cryptococcus neoformans, Aspergillus spp., and other opportunistic fungal pathogens (1, 14, 18). The activity of ravuconazole against Candida spp. has been documented in vitro by broth dilution methods (13, 14). Although ravuconazole is active against isolates of Candida spp. with decreased susceptibility to fluconazole, evidence of cross-resistance has been demonstrated, especially with fluconazole-resistant strains of Candida glabrata (14).
The purpose of this study was to provide further documentation of cross-resistance between fluconazole and ravuconazole and to examine the usefulness of fluconazole as a surrogate marker for evaluating ravuconazole susceptibility in Candida spp. by using a large database of susceptibility test results compiled in the course of global antifungal surveillance studies (12, 15, 15a).
MATERIALS AND METHODS
Organisms.
A total of 12,796 clinical isolates of Candida spp. obtained from more than 200 medical centers worldwide were tested. The collection included 7,521 Candida albicans isolates, 1,869 Candida glabrata isolates, 1,485 Candida parapsilosis isolates, 1,185 Candida tropicalis isolates, 302 Candida krusei isolates, 128 Candida lusitaniae isolates, 103 Candida dubliniensis isolates, 84 Candida guilliermondii isolates, 34 Candida pelliculosa isolates, 28 Candida kefyr isolates, 16 Candida famata isolates, 19 Candida rugosa isolates, 6 Candida lipolytica isolates, 5 Candida zeylanoides isolates, 3 Candida inconspicua isolates, 1 Candida lambica isolate, 2 Candida sake isolates, 1 Candida norvegensis isolate, and 4 isolates of Candida spp. not otherwise identified. All of these isolates were incident isolates from individual patients, and more than 80% were obtained from blood or other normally sterile body fluids. Isolates were identified by using Vitek and API yeast identification systems (bioMerieux, Inc., Hazelwood, Mo.) and were supplemented with conventional methods as needed (4). The C. dubliniensis isolates were obtained from mucosal infections and were identified by specific probe hybridization (5). Isolates were stored as water suspensions until they were used. Prior to testing, each isolate was passaged at least twice on potato dextrose agar (Remel, Lenexa, Kans.) and CHROMagar (Hardy Laboratories, Santa Maria, Calif.) to ensure purity and viability.
Susceptibility testing.
Reference antifungal susceptibility testing of all isolates was performed by broth microdilution as described by the National Committee for Clinical Laboratory Standards (NCCLS) (8). Reference powders of fluconazole (Pfizer) and ravuconazole (Bristol-Myers Squibb) were obtained from their respective manufacturers.
MIC interpretive criteria for fluconazole were those published by Rex et al. (16) and the NCCLS (8). Breakpoints were as follows: susceptible (S), ≤8 μg/ml; susceptible-dose dependent (S-DD), 16 to 32 μg/ml; resistant (R), ≥64 μg/ml. Ravuconazole has not been assigned an interpretive breakpoint. For purposes of comparison and because pharmacokinetic data indicate that achievable levels for ravuconazole in serum may range from 2 to 6 μg/ml with sustained concentrations of >1 μg/ml depending on the dosing regimen (1; D. M. Grasela, S. J. Olsen, V. Mummaenni, P. Rolan, L. Christopher, J. Norton, O. H. Hadjilabris, and M. R. Marins, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 839, p. 22, 2000), we employed breakpoints of ≤1 μg/ml (S) and ≥2 μg/ml (R).
Analysis of results.
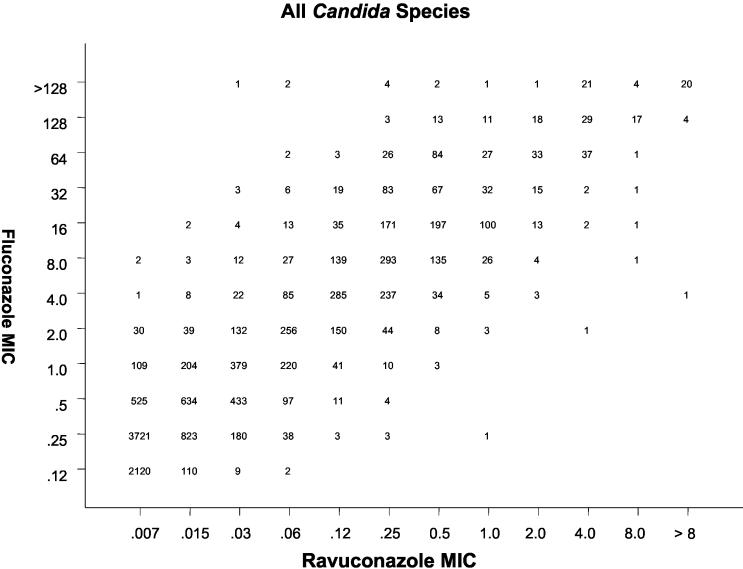
All MIC results (expressed in micrograms per milliliter) for fluconazole were directly compared with those for ravuconazole by using regression statistics and a scattergram (Fig. 1). The error rate bounding method to minimize intermethod interpretive error was also applied with the interpretive breakpoints described above. Acceptable error limits used in this comparison were those cited by the NCCLS (7) and by other authors (3, 6).
FIG. 1.
Scattergram comparing fluconazole and ravuconazole MICs (in micrograms per milliliter) for 12,796 strains of Candida spp. An excellent correlation was observed (R = 0.92; y = 4.7 + 1.0x).
The definitions of errors used in this analysis were as follows: a very major error (VME), or a false-susceptible error, was a result of S for the surrogate marker fluconazole and a result of R for ravuconazole; a major error (ME), or a false-resistant error, was a result of R for fluconazole and a result of S for ravuconazole; and a minor error was a result of S-DD for fluconazole and a result of either S or R for ravuconazole. In general, for an agent to be considered a reliable surrogate, the VME rate should be ≤1.5% of all results and the absolute categorical agreement between methods should be ≥90% (3, 6, 7).
RESULTS AND DISCUSSION
Table 1 summarizes the comparison of 12,796 strains of Candida spp. tested against ravuconazole and the surrogate marker fluconazole by using the NCCLS (8) validated broth microdilution method. Overall, for fluconazole, 11,666 (91.2%) isolates were categorized as S, 766 (6.0%) were categorized as S-DD, and 364 (2.8%) were categorized as R. Conversely, for ravuconazole, 12,567 (98.2%) were categorized as S at ≤1 μg/ml and 229 (1.8%) were categorized as R, with MICs of ≥2 μg/ml (range, 2 to >8 μg/ml) (Table 1 and Fig. 1). If the fluconazole test result category (S, S-DD, or R) was used to predict the ravuconazole category, the absolute categorical agreement between test results was 92.5%, with a VME rate of 0.1%, a ME rate of 1.4%, and a minor error rate of 6.0% (Table 2). The regression statistics (y = 4.7 + 1.0x; R = 0.92) show an excellent level of agreement between the two methods (Fig. 1).
TABLE 1.
Use of fluconazole to predict ravuconazole susceptibility patterns for 12,796 clinical isolates of Candida spp. from the Global Antifungal Surveillance Program, 1992 to 2002
| Species (no. of isolates tested) | Fluconazole susceptibility category | No. (%) of isolates in ravuconazole category
|
|
|---|---|---|---|
| S (≤1 μg/ml) | R (≥2 μg/ml) | ||
| All Candida (12,796) | S | 11,656 (91.16) | 10 (0.1) |
| S-DD | 732 (5.7) | 34 (0.2) | |
| R | 179 (1.4) | 185 (1.5) | |
| C. albicans (7,521) | S | 7,441 (98.9) | 1 (<0.1) |
| S-DD | 36 (0.5) | 1 (<0.1) | |
| R | 27 (0.3) | 15 (0.2) | |
| C. glabrata (1,869) | S | 1,218 (65.2) | 6 (0.3) |
| S-DD | 452 (24.2) | 29 (1.6) | |
| R | 18 (0.9) | 146 (7.8) | |
| C. parapsilosis (1,485) | S | 1,435 (96.6) | 0 (0.0) |
| S-DD | 43 (2.9) | 0 (0.0) | |
| R | 7 (0.5) | 0 (0.0) | |
| C. tropicalis (1,185) | S | 1,159 (97.8) | 1 (0.1) |
| S-DD | 6 (0.5) | 3 (0.3) | |
| R | 1 (0.1) | 15 (1.2) | |
| C. krusei (302) | S | 8 (2.7) | 0 (0.0) |
| S-DD | 171 (56.6) | 1 (0.3) | |
| R | 120 (39.7) | 2 (0.7) | |
| C. lusitaniae (128) | S | 124 (96.9) | 0 (0.0) |
| S-DD | 3 (2.3) | 0 (0.0) | |
| R | 1 (0.8) | 0 (0.0) | |
| C. dubliniensis (103) | S | 94 (91.3) | 0 (0.0) |
| S-DD | 6 (5.8) | 0 (0.0) | |
| R | 1 (1.0) | 2 (1.9) | |
| C. guilliermondii (84) | S | 73 (86.9) | 2 (2.4) |
| S-DD | 6 (7.1) | 0 (0.0) | |
| R | 0 (0.0) | 3 (3.6) | |
| C. pellliculosa (34) | S | 34 (100) | 0 (0.0) |
| S-DD | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | |
| C. kefyr (28) | S | 28 (100) | 0 (0.0) |
| S-DD | 0 (0.0) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | |
| C. rugosa (19) | S | 15 (79) | 0 (0.0) |
| S-DD | 4 (21) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | |
| C. famata (16) | S | 12 (75) | 0 (0.0) |
| S-DD | 4 (25) | 0 (0.0) | |
| R | 0 (0.0) | 0 (0.0) | |
TABLE 2.
Absolute categorical agreement and error rates when the azole surrogate fluconazole result was used to predict ravuconazole susceptibility of Candida spp.
| Organism(s) tested | No. of isolates | Rate (%) of:
|
|||
|---|---|---|---|---|---|
| Agreement | VME | ME | Minor errors | ||
| All Candida | 12,796 | 92.5 (94.7)a | 0.1 (0.1)a | 1.4 (0.5)a | 6.0 (4.7)a |
| C. albicans | 7,521 | 99.1 | 0.1 | 0.3 | 0.5 |
| C. glabrata | 1,869 | 73.0 (97.1)b | 0.3 (1.9)b | 1.0 (1.0)b | 25.7 (0.0)b |
| C. parapsilosis | 1,485 | 96.6 | 0.0 | 0.5 | 2.9 |
| C. tropicalis | 1,185 | 99.1 | 0.1 | 0.1 | 0.8 |
| C. krusei | 302 | 3.3 | 0.0 | 39.7 | 57.0 |
| C. lusitaniae | 128 | 96.9 | 0.0 | 0.8 | 2.3 |
| C. dubliniensis | 103 | 93.2 | 0.0 | 1.0 | 5.8 |
| C. guilliermondii | 84 | 90.5 | 2.4 | 0.0 | 7.1 |
| C. pelliculosa | 34 | 100 | 0.0 | 0.0 | 0.0 |
| C. kefyr | 28 | 100 | 0.0 | 0.0 | 0.0 |
| C. rugosa | 19 | 79.0 (100)b | 0.0 (0.0)b | 0.0 (0.0)b | 21.0 (0.0)b |
| C. famata | 16 | 75.0 (100)b | 0.0 (0.0)b | 0.0 (0.0)b | 25.0 (0.0)b |
The value in parentheses is based on results for all Candida minus C. krusei (12,494 isolates).
The value in parentheses was obtained by using the following categories for fluconazole: susceptible, MIC ≤ 32 μg/ml (S and S-DD combined); resistant, MIC ≥ 64 μg/ml.
Tables 1 and 2 also show the results for 12 individual species of Candida. With the exception of C. glabrata, C. krusei, C. rugosa, and C. famata, categorical agreement rates of 90% or better (range, 90.5 to 100%) were observed for the individual species, with few VMEs, MEs, or minor errors.
The NCCLS does not recommended that laboratories test C. krusei against fluconazole given its poor clinical response to this agent and the fact that fluconazole MICs are predictably elevated (8, 16). In contrast, ravuconazole appears to be quite active against this species (MICs for 299 of 302 isolates [99%] were ≤1 μg/ml [Table 1]). Clearly, fluconazole results are not predictive of ravuconazole susceptibility for this species (Tables 1 and 2). Thus, the C. krusei results should probably be factored out of this analysis. When the C. krusei results were excluded, the overall categorical agreement for the remaining 12,494 isolates improved to 94.7%, with VME, ME, and minor error rates of 0.1, 0.5, and 4.7%, respectively (Table 2). At this point, it appears that the susceptibility of C. krusei to ravuconazole may be predictable and the testing of this drug-organism combination will not be necessary (17). Under selected circumstances (e.g., suboptimal clinical response), specific testing of ravuconazole against C. krusei should be performed in order to determine the activity of this agent against the clinical isolate (17).
The fluconazole results also underestimated the activity of ravuconazole against C. glabrata, C. rugosa, and C. famata (Tables 1 and 2). More than 99% (range, 99.5 to 100%) of the fluconazole-susceptible isolates of these three species were also susceptible to ravuconazole at an MIC of ≤1 μg/ml (Table 1). Likewise, 89% of the fluconazole-resistant strains of C. glabrata demonstrated decreased susceptibility (MIC ≥ 2 μg/ml; range, 2 to >8 μg/ml) to ravuconazole. In contrast, 94% of the C. glabrata isolates and all isolates of C. rugosa and C. famata that were S-DD to fluconazole were susceptible (MIC, ≤1 μg/ml) to ravuconazole (Table 1). Clearly, it is most important to detect those isolates of C. glabrata that may be resistant to ravuconazole and for this purpose fluconazole performs quite well as a surrogate marker. If one uses fluconazole MICs of ≤32 μg/ml as a surrogate marker to predict susceptibility to ravuconazole (combining the S and S-DD categories) and fluconazole MICs of ≥64 μg/ml to predict the ravuconazole resistance of C. glabrata isolates, the categorical agreement rate improves to 97.1%, with VME and ME rates of 1.9 and 1.0%, respectively (Table 2). Similarly, with these criteria, the categorical agreement levels for C. rugosa and C. famata improve from 79 and 75%, respectively, to 100%. Applying these modified criteria to the entire collection of isolates (minus C. krusei) results in an overall categorical agreement rate of 99.1%, with VME and ME rates of 0.4 and 0.5%, respectively (data not shown).
The use of one drug's susceptibility test result to predict the results for another agent is a way of measuring cross-resistance and has been an important component of standardized antibacterial susceptibility testing for decades (6, 9-11). The concept of a class representative or surrogate marker is illustrated and explained in NCCLS document M100-S13 (11), in which the listing of drugs within a single box in supplemental Table 1 designates clusters of comparable agents that need not be duplicated in testing because interpretive results are usually similar and clinical efficacies are usually comparable (11). Furthermore, the joining of two or more drugs with the word “or” indicates related groups of agents with almost identical spectra of activity and interpretive results and for which cross-resistance and susceptibility are nearly complete, precluding the need to test more than one agent from the group (11). These principles can also be used to develop practical alternatives for the microbiology laboratory when diagnostic susceptibility testing reagents are not yet available (6). The example presented in the present study represents the first application of these principles to antifungal susceptibility testing.
Currently, the clinical development of ravuconazole has been suspended by the manufacturer, Bristol-Myers Squibb. Regardless, the present study serves as a proof of concept regarding the use of surrogate markers or class representatives in antifungal susceptibility testing. Previously (15a), we showed a similarly strong correlation (R = 0.9) between voriconazole and posaconazole MICs and fluconazole MICs in testing 3,932 isolates of Candida spp. With the same interpretive categories used in the present study, fluconazole proved to be an excellent surrogate marker for both voriconazole and posaconazole, with categorical agreement rates of 97 to 98% and a VME rate of 0.1% (data not shown). Thus, the class representative concept can be applied to other extended-spectrum triazoles as well as ravuconazole.
Fluconazole as a surrogate marker functioned well as a predictor of ravuconazole susceptibility among clinically significant isolates of Candida spp. The absolute categorical agreement of 94.7%, with a VME rate of 0.1%, among more than 12,000 isolates tested easily meets the recognized criteria for a reliable surrogate marker (3). Ravuconazole is 16- to 32-fold more potent than fluconazole against Candida spp. (Fig. 1), with the result that the vast majority (96%) of isolates that are S-DD to fluconazole are susceptible (MIC, ≤1 μg/ml) to ravuconazole (Table 1). The use of fluconazole as a surrogate marker for ravuconazole susceptibility could actually be improved by designating those isolates with fluconazole MICs of ≤32 μg/ml (the S and S-DD categories combined) susceptible to ravuconazole, with the resistant category staying the same at ≥64 μg/ml. The resulting categorical agreement rate of 98% and VME rate of 0.3% (99 and 0.4%, respectively, when C. krusei was omitted) is excellent for a surrogate marker.
In conclusion, cross-resistance between fluconazole and ravuconazole (and likely other extended-spectrum triazoles) is such that the fluconazole MIC result may be used as a surrogate marker for ravuconazole susceptibility. Specifically, fluconazole MICs of ≤32 μg/ml predict susceptibility and MICs of ≥64 μg/ml predict resistance to ravuconazole. This is especially true for C. glabrata. The occurrence of false-susceptible and false-resistant errors with this expanded application of the class representative concept to selected triazoles was very low and acceptable for surrogate marker testing. By using a predictor agent with a generally narrower spectrum of activity or reduced potency, such as fluconazole, a conservative and safe categorical estimation of activity can be made until specific, ravuconazole-containing, federally approved products are available (2). As commercial ravuconazole susceptibility testing products become available, their use should rapidly replace the interim use of this surrogate marker for clinical testing.
Acknowledgments
This study was supported in part by unrestricted research grants from Pfizer Pharmaceuticals and Bristol-Myers Squibb.
Linda Elliott and Sherry Roe provided excellent support in the preparation of the manuscript. We appreciate contributions of all participants in the Global Antifungal Surveillance Program. For a complete listing of participants, please go to http://www.medicine.uiowa.edu/pathology/path_folder/research/acknowledgments/artemis_participants.pdf.
REFERENCES
- 1.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, S. Killian, H. A. Norris, and M. A. Ghannoum. 1999. Multicenter comparison of the Sensitire Yeast One Colorimetric Antifungal Panel with the National Committee for Clinical Laboratory Standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeasts and yeast-like organisms. J. Clin. Microbiol. 37:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraro, M. J., and J. H. Jorgensen. 1995. Instrument-based antibacterial susceptibility testing, p. 1379-1384. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 4.Hazen, K. C., and S. A. Howell. 2003. Candida, Cryptococcus, and other yeasts of medical importance, p. 1693-1711. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 5.Joly, S., C. Pujol, M. Rysz, K. Vargas, and D. R. Soll. 1999. Development and characterization of complex DNA fingerprinting probes for the infectious yeast Candida dubliniensis. J. Clin. Microbiol. 37:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, R. N., and M. A. Pfaller, and The SENTRY Antimicrobial Surveillance Program Participants Group (USA). 2001. Can antimicrobial susceptibility testing results for ciprofloxacin or levofloxacin predict susceptibility to a newer fluoroquinolone, gatifloxacin? Report from The SENTRY Antimicrobial Surveillance Program (1997-99). Diagn. Microbiol. Infect. Dis. 39:237-243. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters, 2nd ed. Approved guideline M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests, 8th ed. Approved standard M2-A8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antmicrobial susceptibility testing. Thirteenth informational supplement, M100-S13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, H. Huynh, and R. J. Hollis. 2002. Clinical evaluation of a frozen commercially prepared microdilution panel for antifungal susceptibility testing of seven antifungal agents, including the new triazoles posaconazole, ravuconazole, and voriconazole. J. Clin. Microbiol. 40:1694-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., and D. J. Diekema. 2004. 12 years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of Candida bloodstream isolates. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 15a.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 16.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infection. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 17.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 18.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, and R. N. Jones. 2000. In vitro activities of ravuconazole (BMS-207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2883-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]