Abstract
Introduction
The potential role of oxidative stress (OS) in metabolic syndrome (MetS) is rapidly evolving. Reported results support the concept that increased OS may play a key role in the development of atherosclerosis, hypertension and diabetes.
Study aim
The purpose of this study was to analyze the clinical correlates of systemic OS markers in a well characterized group of patients with MetS.
Material and method
72 hospitalized patients with a mean age 59.19+/−5.26 years were studied between October 2010 and June 2011. MetS was diagnosed based on the AHA/NHLBI/IDF 2009 definition. OS was assessed by urinary 8-isoprostaglandinF2α (8-isoPGF2α) (immunometric assays) and plasmatic uric acid (UA). Antioxidant status was evaluated by plasmatic glutathione peroxidase (GPx). These data were compared to those of 100 subjects without MetS (mean age 59.93+/−4.7 years).
Results
All biomarkers were significantly higher in MetS patients as compared with healthy individuals (p<0.05), except GPx which was significantly lower (p<0.001). GPx and UA were statistically significant correlated. In multivariate analysis 8-isoPGF2α concentrations were influenced by hypertension, fasting glucose and triglycerides, UA levels were directly influenced by hypertension, waist circumference, fasting glucose and triglycerides. GPx levels were inversely correlated with blood pressure (all p<0.05). Only GPx was influenced by the number of MetS components. Subjects with a lower level of GPx had a significantly greater risk of MetS (OR 0.85).
Conclusions
Higher 8-isoPGF2α and uric acid and lower GPx levels are associated with MetS. The OS biomarkers are differently influenced by each component of the MetS. High blood pressure seems to be the key component linking OS to MetS. Antioxidant status is influenced by the number of MetS components with GPx being a risk factor for MetS.
Keywords: oxidative stress, 8-isoprostaglandin F2α, uric acid, glutathion peroxidase, metabolic syndrome
Introduction
Metabolic syndrome (MetS) represents a cluster of risk factors associated with cardiometabolic risk. Even though a subject of debate, the importance of MetS lies in its epidemic proportions [1] and in its association with a significantly increased mortality from atherosclerotic cardiovascular diseases and diabetes [2,3,4]. How each of the risk factors included in MetS definition is involved in atherosclerosis have been areas of intense research in the last decade but are still poorly understood. A panel of experts assembled by the National Institutes of Health concluded that the study of mechanisms by which MetS components contribute to atherogenesis should be a high priority [5]. Therefore, it is of great interest to study the relationship of MetS components with oxidative stress (OS) which is considered a key mechanism in atherogenesis
Excessive production of reactive oxygen species (ROS), which surpasses antioxidant defense mechanisms, has been implicated in pathophysiological conditions that have an impact on the cardiovascular system [6]. Inflammation and OS are thought to be concurrently involved in various diseases. But few studies have focused on the role of both OS and inflammation concomitantly. Regarding this, the isoprostanes (IsoP) synthesized from arachidonic acid namely, 8-isoprostaglandin-F2alpa (8-isoPGF2α) could be a suitable parameter to investigate simultaneously OS and inflammation, in diseases where both are supposed to be involved [7]. Moreover, in a recent multi-investigator study (Bio-markers of Oxidative Stress Study, sponsored by the National Institute of Health), the most accurate method to assess in vivo OS was proved to be the quantification of plasma or urinary IsoP, which is now considered the “gold standard” for oxidative injury in vivo [8]. As the in mammalian cells, glutathione and the glutathione peroxidases (GPx) constitute the principal antioxidant defense system [9], it is reasonable to assume that the quantification of plasma GPx would accurately reflect the antioxidant status.
Uric acid (UA) is a powerful chemical antioxidant present in human plasma Despite its physiological intention, hyperuricemia is strongly associated with cardiovascular disease, kidney disease, and hypertension, increasing the risk of mortality [10]. Hyperuricemia is also common in the MetS and obesity.
Study aim
The purpose of this study was to analyze the clinical correlates of systemic OS (evaluated by 8-isoPGF2α, UA and GPx levels) in a well characterized group of patients with MetS. We also calculated the odds ratio of MetS based on the levels of the OS biomarkers.
Material and method
Patient selection and data collection
The studied group comprised 72 hospitalized patients with MetS studied between October 2010 and June 2011. All patients gave written informed consent and the study was approved by the local Ethics Committee No. 631/02.07.2012. The mean age of the study group was 59.19+/−5.26 years and the sex distribution was 46 (63.89%) females and 26 (36.11%) males. MetS was diagnosed based on the AHA/NHLBI/IDF 2009 definition, if three of the following five criteria were met: 1) abdominal obesity: waist circumference >94 cm in men and >80 cm in women; 2) hypertriglyceridemia: ≥150 mg/dl or specific treatment; 3) low levels of HDL-C: <40 mg/dl in men and <50 mg/dl in women or specific treatment; 4) high blood pressure (HBP): ≥130/85 mmHg or specific treatment; 5) high fasting glucose: ≥100 mg/dl or antidiabetes drugs [11]. The exclusion criteria were defined as following: acute infections, stroke, acute coronary syndrome, autoimmune diseases, pulmonary thrombembolism, chronic pulmonary diseases, seric creatinin ≥1,2 mg/dl, chronic liver diseases including liver cirrhosis.
Demographical details and medical history were obtained by anamnesis and by consulting medical files. Body mass index (BMI) was computed as weight divided by height squared (kg/m2). Obesity was classified based on WHO/NIH recommendations: overweight (BMI=25–30 kg/m2), class I obesity (BMI=30–34.9 kg/m2), class II obesity (BMI=35–39.9 kg/m2), and class III obesity (BMI ≥40 kg/m2) [12,13]. Waist circumference was measured with a fiberglass measuring tape at the midpoint between the lowest rib and the iliac crest [14]. Blood pressure was determined based on ESC/ESH 2007 Guidelines for the management of high blood pressure (HBP), after 10 minutes of rest, in both arms, in standing and sitting position and the highest value was taken into account [15]. Glucose and lipid parameters were determined using routine laboratory techniques.
The data obtained in the study group were compared to those of a control group comprising 100 subjects who did not meet the criteria for MetS. The mean age of the control group was 59.93+/−4.7 years with a sex distribution of 55% females and 45% males.
Blood and urine sampling and biochemical analyses
Peripheral venous blood was collected after an overnight fasting period, into 5 ml potassium EDTA tubes. Serum was separated by centrifugation (2000 rpm for 20 minutes at 40ºC), and stored at −80 °C until measurement. Morning urine (mid portion of first void) was collected and transferred into Eppendorf tubes without any addition of preservative and stored at −80 °C until measurement. UA, glucose and lipid parameters were determined using routine laboratory techniques in samples from all subjects. Urinary levels of 8-isoPGF2α were measured using a commercially available enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI) [16]. The intraassay CV was 9.7% and the detection limit 2.7 pg/ml. Urinary creatinine was quantified using a method based on the reaction of creatinine and alkaline picrate with an average intra- and interassay CV of 2% and 4%, respectively. Urinary content of 8-isoPGF2α was indexed to creatinine and expressed as ng/mmol creatinine. GPx activity was measured using a spectrophotometric method (Cayman Chemical, Ann Arbor, MI). The intra- and interassay CV were 5.7% and 7.2% and the detection limit 50 nmol/min/ml [17].
Statistical analysis
The data were analyzed using Statistica 10 (www.statsoft.com). The distribution of variables was evaluated using the Shapiro-Wilk test. The differences between groups were analyzed using Student’s t-test for normally distributed variables and the Mann Whitney rank sum test for non-normally distributed. For categorical response variables, differences between the two groups were assessed using the Chi square test or Fisher’s exact test. Pearson’s correlation or Spearman rank order correlation analyses were performed to examine the correlations between the levels of OS markers and the components of MetS. Adjusted odds ratios (ORs) with 95% confidence intervals (CI) for MetS were calculated from the logistic regression models based on the levels of OS markers. The data are expressed as mean+/− standard deviation (SD) for normally distributed variables and as median (25–75% interquartilic range) for non-normally distributed variables. A p value of <0.05 was considered significant.
Results
The demographic, clinical and biological characteristics of the subjects are shown in table I. The subjects in the MetS group had statistically significant higher values for SBP, DBP, BMI, waist circumference, hematological parameters (i.e., fasting glucose, TG and LDL-C), and lower HDL-C level than the control group.
Table I.
Baseline characteristics of the studied population.
| Demographic, clinical and biological characteristics | MetS group (n=72) | p* | Control group (n=100) |
|---|---|---|---|
| Age (mean +/− SD) | 59.19±5.26 | NS | 59.93±4.7 |
| Sex distribution (males/females) | 26/46 | NS | 55/45 |
| Waist circumference (cm) | 107.41±9.80 | <0.0001 | 98.82±8.73 |
| BMI (kg/m2) | 32.38±14.25 | <0.0001 | 28.96±15.51 |
| SBP (mmHg) | 156.55±24.88 | <0.0001 | 106.33±10.6 |
| DBP (mmHg) | 90.81±14.89 | <0.0001 | 71.33±6.39 |
| TGL (mg/dl) | 189.11±87.77 | <0.0001 | 103.27±44.15 |
| LDL-C (mg/dl) | 131.03 ±38.36 | 0.016 | 118.97 ±28.04 |
| HDL-C (mg/dl) | 51.92±14.16 | 0.026 | 61.00±13.96 |
| Fasting glucose (mg/dl) | 105 (99–119) | 0.002 | 92 (88–104) |
| 8-isoPGF2α (ng/mmol creat) | 118.76±53.18 | <0.0001 | 68.13±32.15 |
| UA (mg/dl) | 6.04±1.76 | 0.009 | 4.73±1.58 |
| GPx (nmol/min/ml) | 33.71±9.54 | 0.001 | 45.20±7.71 |
Data are presented as mean and SD for normally distributed variables and as median (interquartile range) for non-normally distributed variables.
p-value for continuous variables was obtained using 2-sample t-test and for categorical values using chi-square test.
The most frequent MetS feature was HBP found in 68 subjects (94.4%). Hypertensive patients were under medication as follows: 44 (64.7%) ACE inhibitors, 17 (25%) angiotensin receptors blockers, 49 (72.05% beta-blockers), 27 (39.7%) calcium channels blockers and 34 (50%) diuretics. Abdominal obesity was identified in 64 subjects (88.88%). High TGL was present in 47 subjects (65.28%), and low HDL-C 29 (40.28%). 51 (83.6%) of the dislipidemic patients were taking statins and 10 (16.39%) were taking fibrates. Impaired fasting glucose (IFG) was present in 53 of the subjects (73.61%) and 8 (15.27%) of those had overt type 2 diabetes mellitus (T2DM).
8-isoPGF2α levels were statistically significantly (p<0.001) higher in the MetS group (118.76+/−53.18 ng/mmol creatinin) as compared to the control group (68.13+/−32.15 ng/mmol creatinin). UA concentrations were also increased in MetS patients (6.03+/−1.72 mg/dl) as compared with the control group (4.74+/−1.61 mg/dl ), with statistical significance (p=0.009). GPx levels were statistically significantly (p<0.001) lower in the MetS group (33.71+/−9.54 nmol/min/ml) as compared with those without MetS (45.2 +/−7.71 nmol/min/ml). After grouping the patients according to the presence of each MetS component we found higher concentrations of 8-isoPGF2α in hypertensive patients, in those with high TGL levels, higher UA levels in obese patients, in those with HBP, high TGL and IFG. GPx levels were lower in HBP and IFG subgroups. These results are summarized in table II.
Table II.
OS biomarkers levels in MetS subgroups (divided by the presence/absence of each MetS component).
| Variables/Subgroups | 8-isoPGF2α (ng/mmol creat) | UA (mg/dl) | GPx (nmol/min/ml) |
|---|---|---|---|
| Abdominal obesity (n=154) | 112.119±53.854 | 5.933±1.751 | 35.567±10.359 |
| No abdominal obesity (n=18) | 73.040±35.698 | 3.818±1.707 | 39.130±8.649 |
| p | 0.112 (NS) | 0.009 | 0.454 (NS) |
|
| |||
| HTA (n=103) | 120.564±54.959 | 6.083±1.728 | 33.472±9,532 |
| No HTA (n=69) | 79.054±35.214 | 4.957±1.662 | 42.377±9.540 |
| p | <0.0001 | 0.01 | <0.0001 |
|
| |||
| TGL>150 mg/dl (n=77) | 119.838±53.165 | 6.434±1.672 | 34.434±9.485 |
| TGL<150 mg/dl (n=95) | 96.825±52.040 | 4.969±1.555 | 37.519±11.066 |
| p | 0.049 | <0.0001 | 0.171 (NS) |
|
| |||
| Low HDL-C (n=69) | 115.429±55.486 | 6.006±1.555 | 35.213±9.881 |
| Normal HDL-C (n=103) | 98.991±48.886 | 5.401±2.128 | 36.866±11.04 |
| p | 0.182 (NS) | 0.138 (NS) | 0.485 (NS) |
|
| |||
| Fasting glucose>100 mg/dl (n=83) | 117.215±56.065 | 6.101±1.684 | 33.0223±8.427 |
| Fasting glucose <100 mg/dl (n=89) | 94.767±45.508 | 5.199±1.821 | 41.3854±11.461 |
| p | 0.069 (NS) | 0.026 | <0.0001 |
Data showed normal distribution and are presented as mean±SD; p<0.05 is statistically significant.
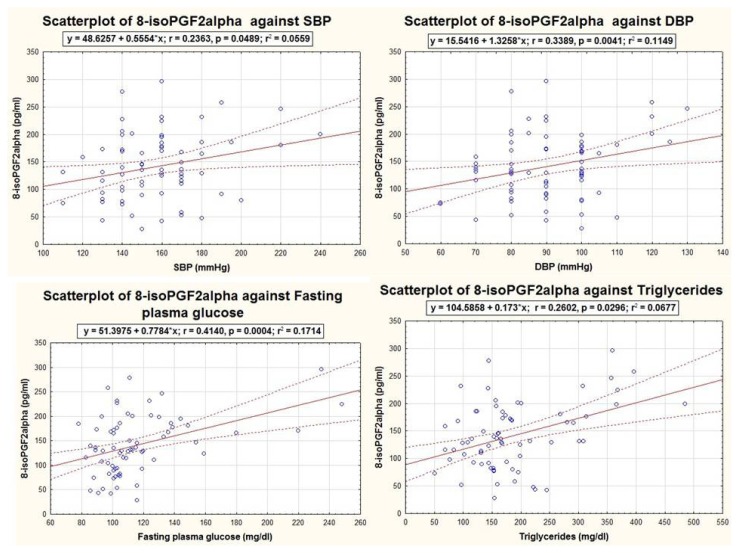
All studied biomarkers were well correlated to SBP and/or DBP, being influenced by the presence of HBP. 8-isoPGF2α concentrations were influenced by SBP (r=0.236, p=0.048), DBP (r=0.339, p=0.004), fasting glucose (r=0.441, p<0.001) and triglycerides (r=0.260, p=0.029), as shown in Figure 1 and did not correlate with HDL-C (r=−0.180, p=0.880) and waist circumference (r=0.139, p=0.250).
Figure 1.
Graphic representations of the significant correlations of 8-isoPGF2α with MetS components, within the MetS group (n=72).
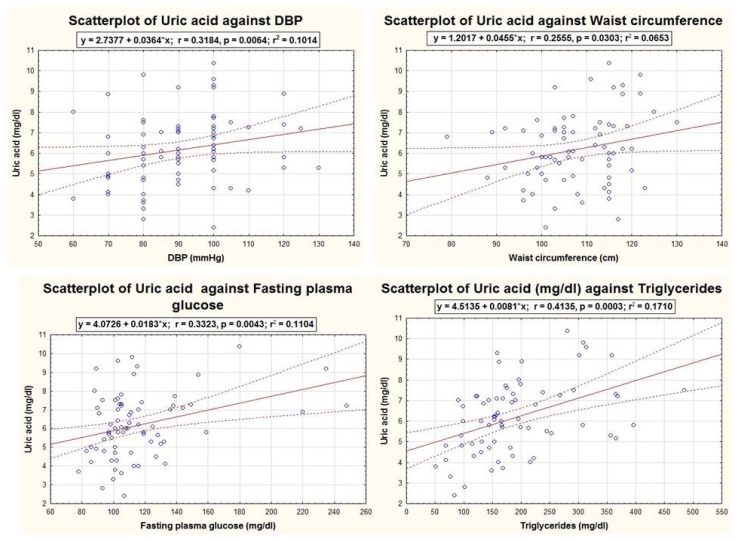
UA levels were directly and positively correlated to DBP (r=0.318, p=0.006), waist circumference (r=0.255, p=0.03), fasting glucose (r=0.332, p=0.004) and triglycerides (r=0.413, p<0.001) as are presented in Figure 2. The relationship of UA with SBP (r=0.184, p=0.12) and HDL-C (r=0.094, p=0.43) was not statistically significant.
Figure 2.
Graphic representations of the significant correlations of UA with MetS components, within the MetS group (n=72).
GPx levels were inversely and significantly correlated with SBP (r=−0.274, p=0.021) (Figure 3), but the correlations to the other MetS components were not statistically significant: DBP: r=−0.084, p=0.488; waist circumference: r=−0.004, p=0.968; TGL: r=−0.007, p=0.944; HDL-C: r=0.132, p=0.91; fasting glucose: r=−0.0620, p=0.95). Only GPx was influenced by the number of MetS components (Figure 4).
Figure 3.
Graphic representations of the significant correlations of GPx with MetS components (SBP), within the MetS group (n=72).
Figure 4.
The influence of the number of MetS components on GPx concentrations.
UA exhibited significant negative correlations with GPx (r=−0.216, p=0.018) (Table III). The ORs of MetS was calculated based on the levels of OS markers. Subjects with a lower level of GPx had a significantly greater risk of MetS (OR 0.85) (Table IV).
Table III.
The relationship among the studied biomarkers in patients with MetS.
| Variables | 8iso-PGF2α | UA | GPx | |
|---|---|---|---|---|
| 8iso-PGF2α | r | 1.000 | 0.028 | −0.137 |
| p | 0.798 | 0.211 | ||
| UA | r | 0.028 | 1.000 | −0.260 |
| p | 0.798 | 0.018 | ||
| GPx | r | −0.137 | −0.260 | 1.000 |
| p | 0.211 | 0.018 |
Pearson’s correlations test; p<0.05 is statistically significant.
Table IV.
The odds ratios for MetS calculated from logistic regression models based on the levels of OS biomarkers.
| Variables | dF | P | OR | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
|
| |||||
| UA (mg/dl) | 1 | 0.261 | 0.565 | 0.209 | 1.530 |
|
| |||||
| GPx (nmol/min/ml) | 1 | 0.031 | 0.853 | 0.739 | 0.985 |
OR: odd ratio; CI - Confidence interval; p<0.05 is statistically significant.
Disscussion
In the present study the concentrations of OS biomarkers together with powerful antioxidant enzyme activity were simultaneously evaluated in patients with MetS. The results obtained indicate that the increase of OS biomarkers and decrease of antioxidant capacity are associated with the presence of MetS. These data are in agreement with others demonstrating that MetS is associated with proinflammatory state and OS as part of a very complex mechanism underlying cardiometabolic disorders [18,19,20,21,22].
OS damage was assessed by urinary 8-isoPGF2α which is considered the “gold-standard” for OS injury in vivo [8]. 8-isoPGF2α is an eicosanoid biosynthesized from esterified arachidonic acid mainly through non-enzymatic free radical-catalyzed reactions in vivo [7]. In general, they have short half-lives, but they are chemically stable compounds, not sensitive to dietary intake of lipids relatively stable within individuals (especially when assayed in first morning urine void), but are widely variable in human populations [23]. A substantial portion of IsoP appears to undergo β-oxidation in tissues prior to release into the plasma. Intact IsoP, together with their β-oxidized metabolites, are efficiently excreted into the urine [24]. Thus, urinary levels of 8-isoPGF2α should better reflect actual changes in total body lipid peroxidation, and are easier to determine as they are much higher (~ng/ml) than plasma levels (~pg/ml) [25]. Severe methods were developed to quantify IsoP levels: the gas chromatographic/negative ion chemical ionization mass spectrometric (GC/MS) method, liquid chromatographic (LC)/MS method and the EIA method. In the present study 8-isoPGF2α was determined by EIA [16]. A potential drawback of EIA is that limited information is currently available regarding their precision and accuracy. Despite potential limitations, EIA have expanded IsoP research due to their low cost and relative ease of use [26].
According to AHA/NHLBI/IDF 2009 definition of MetS used to conduct our study, obesity was not a mandatory criterion. In our research, hypertension proved to be the key component influencing the concentrations of all the studied biomarkers. Hypertension was present in 68 patients (94.4%) and SBP and/or DBP was related to both OS biomarkers and antioxidant status. The role of OS in the pathogenicity of hypertension is still not completely understood, explaining the need for further research regarding hypertension-induced target organ damage by OS-derived products. The current hypothesis is that endothelial cells play a major role in arterial relaxation by releasing nitric oxide (NO) [27] and thus a decrease in NO bioavailability and an increase in OS (based in general on increased levels of biomarkers of lipid peroxidation) might be responsible for the development of hypertension in human subjects [28,29,30]. Accordingly, Abdilla et al. [6]observed that the increase in OS and the reduction in activity of antioxidant enzymes in hypertensives were not affected by the presence of additional components of the MetS (high triglycerides and fasting glucose). This observation sustains the hypothesis that hypertension might be a pivotal factor for the development of OS and endothelial dysfunction [6]. Another argument to this hypothesis is represented by the correlation between GPx levels and BP. No correlation was observed between GPx and the other features of MetS, marking the importance of hypertension in our study group. Decreased antioxidant activity [31], and reduced levels of ROS scavengers (vitamin E, glutathione) [28] may contribute to OS. Activation of the renin-angiotensin system has been proposed as a mediator of ROS production [32]. Moreover, among the studied biomarkers, only GPx levels were correlated to the number of MetS components, being associated with the risk for MetS (OR 0.85). Blankenberg et al. [9] also demonstrated that GPx 1 activity was among the strongest univariate predictors of the risk of cardiovascular events, which was inversely associated with increasing quartiles of GPx1 activity (P for trend <0.001). Patients in the highest quartile of GPx 1 activity had a hazard ratio of 0.29 (95% CI, 0.15 to 0.58; P<0.001), as compared with those in the lowest quartile [9].
The positive relationship between 8-isoPGF2α and fasting plasma glucose is concordant with other studies, elevated levels of 8-isoPGF2α being found in plasma or urinary samples from type 2 diabetic patients, compared to the non-diabetic controls [33,34]. In a large cross-sectional study in elderly men (77 years), Helmersson et al. showed that the 24-h urinary level of 8-isoPGF2α was significantly higher in men with type 2 diabetes (n=101) as compared to the control group (n=585) [35]. On the other hand, there are authors that observed no associations [36] or even inverse association between IsoP levels and T2DM [37]. It is worth mentioning that 8-isoPGF2α was not significantly correlated with abdominal circumference and BMI. A positive correlation between BMI and 8-isoPGF2α levels is claimed in some studies [16,38,39], but not corroborated with others [35], leaving the subject opened for research. Interestingly, we found no correlation between 8-isoPGF2α and the other markers of OS, as expected, even though UA and GPx concentrations were equally and significantly influenced by the presence of MetS. These findings might explain the paradox of uric acid. We can only presume (as the study type does not allow us to draw ethio-pathogenetic conclusions) that a decrease in antioxidant capacity (as shown by GPx levels) may induce an increase in UA which should have a potent antioxidant activity. But as was demonstrated in previous studies, hyperuricemia induces OS through endothelial dysfunction, by reducing the bioavailability of NO [40]. Thus the UA turns from a powerful and benefic antioxidant into a pro-oxidant damaging molecule.
Our data are in line with literature suggesting that the worsening of the lipid profile is associated with an increased burden of OS biomarkers. Feri J et al. found that 8-oxo-dG, an indicator of DNA damage, was directly correlated with insulin and TGL, but inversely correlated with HDL-C [41]. OS identified by O2− production by polymorphonuclear leucocytes in hyperlipidemic subjects is raised and positively correlated with serum cholesterol, triglycerides and LDL-CL levels and negatively correlated with HDL-C levels [42].
Moreover, UA exhibited an important correlation both with MetS components, especially with HBP and the GPx levels. Our results are supported by the fact that hyperuricemia was found in 25–40% of newly diagnosed hypertensive subjects without any treatment, in 50% of those treated with diuretics, and in over 80% of those with malignant hypertension [43]. The link between hyperuricemia and HBP is not completely understood, but there are several mechanisms that try to explain it: 1) decreased renal blood flow frequently associated with HBP stimulates urate reabsorption in the proximal tubule; 2) local tissue ischaemia due to microvascular disease is responsible for the release of lactate thus blocking urate secretion in the proximal tubule and increasing UA synthesis. Tissue ischaemia leads to ATP degradation with adenosine and xanthine oxidase. Both increased xanthine and xanthine oxidase result in the increased generation of UA and oxidant (O2−) formation [44]; and 3) impaired renal UA excretion associated with enhanced sodium reabsorption in conditions such as obesity, insulin resistance, low sodium intake and diuretic therapy [45]. By contrast with observational findings, a very recent online publication has used mendelian randomization to investigate the association of plasma UA (SLC2A9) with ischaemic heart disease and hypertension and concluded that there is no strong evidence for a causal association [46]. However, evidence supports a causal effect between obesity and UA level and hyperuricaemia. This finding strongly suggests BMI as a confounder in observational associations, and suggests a role for elevated BMI or obesity in the development of UA related conditions [46]. Thus, the close association of UA concentrations and waist circumference found in our study group is of great importance. Recent findings showed that serum urate was the best predictor of ‘metabolically unhealthy obesity’ (defined as obesity associated with MetS features), with increased cardiovascular risk in adolescents and adults [47].
We found a positive and significant correlation between UA and fasting glucose. There are studies showing an association, no association, and even an inverse association, and have been reviewed recently by Li et al. [48]. The authors concluded that there is a great body of evidence to suggest that insulin resistance plays a potentially key role in the causal relationship between MetS, T2DM and hyperuricemia [48].
Limitations
The most important limitation of the present study is the small number of the subjects, mainly due to multiple exclusion criteria. Moreover, MetS patients were under drug therapy that could modify the studied pathology, but because of ethical reasons could not be interrupted. It is important to mention that the study type does not allow us to draw any conclusions regarding the causality and the pathogenetic mechanism linking OS to traditional risk factors. Larger, prospective studies with rigorous epidemiological methodology are required to establish whether OS is a cause or a consequence of MetS.
Conclusions
Elevated oxidant status and decreased antioxidant capacity are associated with MetS. The OS biomarkers are differently influenced by each component of the MetS. High blood pressure seems to be the key component linking OS to MetS. Antioxidant status is influenced by the number of MetS components with GPx being a risk factor for MetS. The decrease in antioxidant capacity might be the trigger of hyperuricemia associated with MetS explaining the UA paradox, but more experimental studies are needed to confirm these hypothesis.
Acknowledgements
Financial support from the European Social Fund through the Sectoral Operational Programme Human Resources Development 2007–2013, project number: POSDRU 107/1.5/S/78702, project title: ”Inter-universities partnership for increasing the quality and inter-disciplinarily of medical doctoral research through doctoral scholarships – DocMed.net” is acknowledged with gratitude.
References
- 1.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 2.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the Pathophysiology of Obesity-Associated Cardiovascular Disease. Circulation. 2002;105(24):2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]
- 6.Abdilla N, Tormo MC, Fabia MJ, Chaves FJ, Saez G, Redon J. Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. J Hum Hypertens. 2007;21(1):68–75. doi: 10.1038/sj.jhh.1002105. [DOI] [PubMed] [Google Scholar]
- 7.Basu S. Bioactive eicosanoids: role of prostaglandin F(2α) and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells. 2010;30(5):383–391. doi: 10.1007/s10059-010-0157-1. [DOI] [PubMed] [Google Scholar]
- 8.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RJ, Kang D-H, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Obesity. Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity, Geneva; 3–5 June; Geneva. 1998. [PubMed] [Google Scholar]
- 13.U.S. National Institutes of Health. Clinical Guidelines for the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda: 1998. [PubMed] [Google Scholar]
- 14.Klein S, Allison DB, Heymsfield SB, et al. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity. 2007;15(5):1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 16.Keaney JF, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 17.Paglia D, Valentine W. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 18.Helmersson J, Arnlöv J, Larsson A, Basu S. Low dietary intake of beta-carotene, alpha-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Brit J Nutr. 2009;101(12):1775–1782. doi: 10.1017/S0007114508147377. [DOI] [PubMed] [Google Scholar]
- 19.Wohlin M, Helmersson J, Sundström J, et al. Both cyclooxygenase- and cytokine-mediated inflammation are associated with carotid intima-media thickness. Cytokine. 2007;38(3):130–136. doi: 10.1016/j.cyto.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21–22):705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Comes L, Dejica D. Stresul oxidativ în bolile cardiovasculare. In: Dejica D, editor. Stresul oxidativ în bolile interne. Casa Cărţii de Ştiinţă Cluj-Napoca; 2000. pp. 246–336. [Google Scholar]
- 22.Chen S-J, Yen C-H, Huang Y-C, Lee B-J, Hsia S, Lin P-T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7(9):e45693. doi: 10.1371/journal.pone.0045693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Il’yasova D, Scarbrough P, Spasojevic I. Urinary biomarkers of oxidative status. Clin Chem Acta. 2012;413(19–20):1446–1453. doi: 10.1016/j.cca.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7(1):221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 25.Davies SS, Roberts LJ., II F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50(5):559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts LJ, Milne GL. Isoprostanes. J Lipid Res. 2009;50:S219–S223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31(Suppl 2)(24):S181–S184. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- 28.Redón J, Oliva MR, Tormos C, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 29.Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R. Increased Oxidative Stress and Platelet Activation in Patients With Hypertension and Renovascular Disease. Circulation. 2002;106(22):2800–2805. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- 30.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55(7):928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Mocan M, Blaga SN, Mureşan A. Stresul oxidativ la bolnavii cu sindrom metabolic. Clujul Medical. 2010;83(4):605–610. [Google Scholar]
- 32.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44(3):248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 33.Davi G, Ciabattoni G, Consoli A, et al. In Vivo Formation of 8-Iso-Prostaglandin F2 and Platelet Activation in Diabetes Mellitus: Effects of Improved Metabolic Control and Vitamin E Supplementation. Circulation. 1999;99(2):224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 34.Gopaul NK, Manraj MD, Hébé A, et al. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia. 2001;44(6):706–712. doi: 10.1007/s001250051679. [DOI] [PubMed] [Google Scholar]
- 35.Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109(14):1729–1734. doi: 10.1161/01.CIR.0000124718.99562.91. [DOI] [PubMed] [Google Scholar]
- 36.Il’yasova D, Morrow JD, Wagenknecht LE. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes Res. 2005;13(9):1638–1644. doi: 10.1038/oby.2005.201. [DOI] [PubMed] [Google Scholar]
- 37.Il’yasova D, Spasojevic I, Base K, et al. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care. 2012;35(1):173–174. doi: 10.2337/dc11-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block G. Factors Associated with Oxidative Stress in Human Populations. Am J Epidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 39.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 40.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 41.Ferri J, Martínez-Hervás S, Espinosa O, et al. 8-oxo-7,8-dihydro-2′-deoxyguanosine as a marker of DNA oxidative stress in individuals with combined familiar hyperlipidemia. Med Clin (Barc) 2008;131(1):1–4. doi: 10.1157/13123034. [DOI] [PubMed] [Google Scholar]
- 42.Mazor R, Shurtz-Swirski R, Farah R, et al. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis. 2008;197(2):937–943. doi: 10.1016/j.atherosclerosis.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Berbari AE. European Society of Hypertension Scientific Newsletter: Update on Hypertension Management:The role of uric acid in hypertension, cardiovascular events, and chronic kidney disease. Via Medica. 2010;11(49) [Google Scholar]
- 44.Feig D, Kang D, Johnson RJ, Haig A, Davis NS. Uric Acid and Cardiovascular Risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17(6):409–414. doi: 10.1016/j.numecd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Palmer TM, Nordestgaard BG, Benn M, et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation. BMJ. 2013;4262:1–10. doi: 10.1136/bmj.f4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangge H, Zelzer S, Puerstner P. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. Obesity (Silver Spring) 2013;21(1):20061. doi: 10.1002/oby.20061. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Hsieh M, Chang S. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):23370374. doi: 10.1097/BOR.0b013e32835d951e. [DOI] [PubMed] [Google Scholar]