Abstract
We recently described the capsule null locus (cnl) of constitutively unencapsulated Neisseria meningitidis clonal lineages. cnl meningococci were recovered from healthy carriers at high frequency. We here report on the first case of invasive disease caused by cnl meningococci in a severely immunosuppressed patient with chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. The sequence type 845 strain was extensively typed and, furthermore, shown to be sensitive to serum bactericidal activity.
Neisseria meningitidis is a leading cause of sepsis and meningitis in toddlers and adolescents (16). The organism also is part of the human nasopharyngeal flora. Population biology studies revealed that clonal lineages responsible for most cases of invasive disease are rarely encountered in healthy carriers (1, 8). The capsular polysaccharide is the dominant virulence factor (24). Invasive disease is usually caused by the serogroups A, B, C, W-135, and Y. Other serogroups, e.g., 29E, X, and Z, are mostly found among healthy carriers. We recently demonstrated that a significant proportion (i.e., 16.4%) of strains in healthy carriers in Bavaria, Germany, lack the genes for capsule synthesis, modification, and transport (2). Instead, the organization of the chromosomal locus harboring those genes resembled that of constitutively unencapsulated neisserial species such as N. gonorrhoeae and N. lactamica. Meningococci harboring the capsule null locus (cnl) were found to be highly specific to few clonal lineages. Those lineages might therefore be considered true commensals.
In most cases of meningococcal disease, there is no obvious underlying immunological disorder. Nevertheless, immunodeficiency may render the host susceptible to disease. Deficiencies in the terminal complex and the factor properdin of the complement system (3, 5), as well as functional or anatomic asplenia, have been described as a risk factors for meningococcal disease (13). There are case reports of patients with Waldenstrom's disease (18), AIDS (11), and hypogammaglobinemia (17) suffering from meningococcal infection. In a 5-year population-based analysis of sporadic meningococcal disease in adults in Atlanta, Ga., an area with low incidence during the study period, Stephens et al. found that two-thirds of those aged 24 years or older showed one of a variety of disorders, including human immunodeficiency virus infection, corticosteroid treatment, diabetes, chronic renal failure, and bone marrow transplantation (19). It is unclear whether these findings also hold true for regions with a high incidence. Nevertheless, it was interesting that meningococcal isolates from 6 of the 44 adult patients did not belong to serogroup B, C, W-135, or Y. In line with this observation, serogroup 29E meningococci have once been reported to cause meningococcal disease in a patient with multiple myeloma, suggesting that otherwise purely commensal meningococci can be responsible for opportunistic infection in the immunocompromised host (26). Until now, cnl meningococci to our knowledge have not been described as a cause of disease. We here report the first case of cnl meningococcal bacteremia in a severely immunocompromised patient. This is another example of unusual organisms causing invasive infections in this cohort of patients (4, 20).
CASE REPORT
In January 2001, a 42-year-old patient at the University Hospital Ulm was diagnosed with Philadelphia chromosome-positive (bcr-abl+) common acute lymphatic leukemia with a delayed response to chemotherapy. A peripheral blood stem cell transplantation from an HLA-identical sibling was performed (in April 2001) after a myeloablative conditioning regimen. A second HLA-identical sibling peripheral blood stem cell transplantation without T-cell depletion was performed in February 2003 due to a relapse. The clinical course was complicated by chronic graft-versus-host disease.
In September 2003, the patient was readmitted to the hospital due to chronic progressive diarrhea. The immunosuppression was intensified. Mucositis was not observed. No infectious cause for enteritis could be identified. During hospitalization, clinical signs of sepsis developed, with acute fever (39°C) and chills, which were associated neither with a rash nor with petechiae. The white blood cell count was 6.7/nl. C-reactive protein (34.8 mg/liter) and interleukin-8 (3,279 U/liter) levels were increased. Blood cultures were taken, and an empirical antibacterial therapy was initiated (3 g of piperacillin-combactam three times a day intravenously). The clinical symptoms of sepsis disappeared a few hours after initiation of the antibacterial therapy. After microbiological diagnosis of a penicillin-sensitive meningococcal bacteremia, the antibacterial therapy was continued with penicillin for the following 4 weeks.
MATERIALS AND METHODS
Bacterial strains
Meningococci were isolated from the patient by the Institute of Microbiology and Immunology, Ulm, Germany, and were referred to the National Reference Center for Meningococci, Würzburg, Germany. The strain grew on Martin-Lewis agar, was oxidase positive, and was identified as N. meningitidis by the API NH system (BioMerieux, Marcy-Etoile, France). The serogroup B strain MC58 was isolated 1985 in the United Kingdom and belongs to the sequence type 32 (ST-32) complex. The unencapsulated derivative of strain MC58 was described recently (14).
Meningococcal typing
Multilocus sequence typing was performed as described previously (http://pubmlst.org/neisseria/) (9). Polysialyltransferase genes were analyzed by PCR as described recently (22). The capsule null locus (cnl) was identified by PCR and sequencing as described previously (2). PorA genotyping was performed by using the PorA database (http://neisseria.org/nm/typing/pora/). The FetA database was used for FetA typing (http://neisseria.org/nm/typing/feta/) (21). The cnl allele was determined as described recently (2). The presence of the lipopolysaccharide (LPS) immunotype L3,7,9 was confirmed by enzyme-linked immunosorbent assay (ELISA) with the monoclonal antibody 9-2-L379 (supplied by W. D. Zollinger). The ability of meningococci to exogenously sialylate the LPS was confirmed by ELISA as described previously, using monoclonal antibody 3F11, whose binding is blocked by terminal sialylation of the lacto-N-neotetraose structure of the LPS (10). Exogenous LPS sialylation was also confirmed by Tricine gel electrophoresis as described previously (23).
Serum killing assay
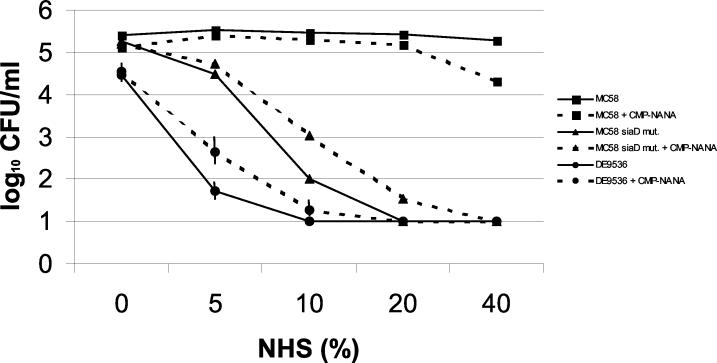
The serum killing assay was performed with normal human serum (NHS). NHS was obtained from a healthy volunteer with no history of meningococcal infection, gonorrhea, or meningococcal vaccination. The donor was selected because the serum did not kill the encapsulated strain MC58 (ST-32 complex) but did kill the unencapsulated variant thereof and therefore matched the criteria previously applied to pooled sera (23). The killing assay was performed as described previously for strain MC58, yielding results comparable to the published data (23). Meningococci were grown in the presence or absence of CMP-N-acetylneuraminic acid (CMP-NANA) (Sigma, Taufkirchen, Germany) as described previously (25). CMP-NANA is essential for exogenous sialylation of neisserial LPS, which contributes to gonococcal serum resistance (6, 15). NHS was applied for 30 min in various concentrations. The bactericidal assays were performed two (in the case of MC58) or three (in the case of DE9536) times, yielding comparable results. Extensive clumping of strain DE9536 was observed, giving rise to slightly lower colony counts in untreated bacteria than for MC58 derivatives despite of the use of identical optical densities of bacterial suspensions.
RESULTS
A meningococcal isolate was recovered from two different blood cultures from the patient. The meningococcal strain (isolate DE9536) was sent to the National Reference Laboratory for Meningococci for fine typing. The isolate did not react with monoclonal antibodies directed against the serogroup A, B, C, W-135, and Y capsules in ELISA and slide agglutination. PCR analysis demonstrated that there was no evidence for polysialyltransferase genes (siaD) specific to serogroups B, C, W-135, and Y. However, genetic analysis performed as described recently (2) revealed a lack of genes needed for capsule synthesis and transport. Furthermore, the isolate was shown by multilocus sequence typing to be ST-845, which has been shown to comprise exclusively cnl meningococci (2). The cnl allele (cnl-2) of the patient's isolate corresponded to the one found in the ST-845 complex. Furthermore, the outer membrane protein porin A of the isolate was typed as P1.18,25. This PorA type was predominantly found in ST-845 strains from the Bavarian meningococcal carriage strain collection (our unpublished observations). The FetA allele was 5-2, and the isolate expressed an LPS that was reactive with an L379 antibody. The isolate was sensitive to the killing activity of a human serum obtained from a healthy donor with no history of meningococcal or gonococcal disease or meningococcal vaccination (Fig. 1). In contrast, a virulent meningococcal serogroup B isolate was serum resistant. Addition of CMP-NANA to the growth medium did not significantly enhance the serum resistance of the patient's isolate, although the isolate's LPS could be sialylated by the addition of exogenous CMP-NANA as evidenced by 3F11 ELISA and Tricine gel electrophoresis. This finding indicated that exogenous sialylation of LPS did not render the strain serum resistant, in contrast to the case for gonococcal strains (15). In order to test whether other cnl isolates were as serum sensitive as DE9536 even after growth in the presence of CMP-NANA, we also tested an ST-53 isolate and an ST-845 isolate from healthy carriers. Both isolates yielded a rate of survival in the bactericidal assay which was comparable to that of DE9536 (data not shown).
FIG. 1.
Serum killing assay with NHS. NHS was applied to meningococci for 30 min in various concentrations. The serogroup B strain MC58 and its unencapsulated derivative MC58 siaD mut. were used as controls in addition to the test strain DE9536. Strains were tested after growth without or with an exogenous supply of CMP-NANA. The bactericidal assays were performed two (in the case of MC58) or three (in the case of DE9536) times. Mean values of log10 CFU per milliliter are shown. Vertical bars represent the standard errors of the means, which were calculated for values derived from DE9536. The limit of detection was 1.
DISCUSSION
We report here the case of a severely immunocompromised patient with septicemia due to a constitutively unencapsulated meningococcal isolate. cnl meningococci frequently colonize the nasopharynx of the human host. Disturbance of the nasopharyngeal barrier may provide access to the bloodstream for oral and nasopharyngeal bacteria. The patient did not exhibit nasopharyngeal mucositis but did show signs of a sicca syndrome caused by chronic graft-versus-host disease, which may have weakened the barrier functions of the mucosa. Unencapsulated meningococci lack resistance to opsonophagocytosis, for which the capsule is a prerequisite (24). We show here that the patient's isolate (DE9536) was even more sensitive to the killing activity of NHS than genetically engineered unencapsulated derivatives of a pathogenic serogroup B strain. In severely immunocompromised hosts, however, a systemic proliferation of unencapsulated cnl meningococcal strains may occur, as was shown in this patient. Bacteremia with the cnl meningococcal strain was associated with a benign clinical course without signs of meningitis and a rapid clinical response after initiation of antimicrobial therapy, which may support the less virulent nature of this strain.
Vaccination of bone marrow transplant recipients against meningococcal disease has been considered (12). It should be noted that the case reported here could not have been prevented by prophylactic immunization with polyvalent polysaccharide vaccines due to the lack of a capsule.
Meningococcal disease may give rise to concerns about transmission to health care workers or other patients (7). However, in severely immunocompromised patients meningococcal disease may be caused by apathogenic isolates, as demonstrated here and by others (19, 26). Rapid detection of the cnl locus and of serogroups other than A, B, C, W-135, and Y by specialized laboratories could reduce anxiety among health care workers and avoid unnecessary antibiotic prophylaxis among persons in close contact with the patient.
In conclusion, we report the first case of invasive disease due to cnl meningococci. This report provides further evidence that meningococcal disease in severely immunocompromised patients may well be considered opportunistic in some cases.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Christine Meinhardt, Gabi Heinze, and Carmen Roldan. This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria/) developed by Man-Suen Chan and Keith Jolley and sited at the University of Oxford (curator, Keith Jolley). We furthermore made use of the N. meningitidis porA and fetA typing websites (http://neisseria.org/nm/typing/), which were developed by Keith Jolley (curators, Janet Suker and Ian Feavers, respectively).
The National Reference Laboratory for Meningococci is supported by the Robert Koch-Institute (Berlin, Germany). The development of the Neisseria Multi Locus Sequence Typing website site has been funded by the Wellcome Trust and European Commission.
REFERENCES
- 1.Caugant, D. A., B. E. Kristiansen, L. O. Froholm, K. Bovre, and R. K. Selander. 1988. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect. Immun. 56:2060-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claus, H., M. C. Maiden, R. Maag, M. Frosch, and U. Vogel. 2002. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148:1813-1819. [DOI] [PubMed] [Google Scholar]
- 3.Densen, P., J. M. Weiler, J. M. Griffiss, and L. G. Hoffmann. 1987. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N. Engl. J. Med. 316:922-926. [DOI] [PubMed] [Google Scholar]
- 4.Escudier, E., C. Cordonnier, and J. Poirier. 1986. Infections of the central nervous system in malignant hemopathies. Rev. Neurol. (Paris) 142:116-125. [PubMed] [Google Scholar]
- 5.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox, A. J., A. Curry, D. M. Jones, R. Demarco de Hormaeche, N. J. Parsons, J. A. Cole, and H. Smith. 1991. The surface structure seen on gonococci after treatment with CMP-NANA is due to sialylation of surface lipopolysaccharide previously described as a ‘capsule.’ Microb. Pathog. 11:199-210. [DOI] [PubMed] [Google Scholar]
- 7.Gehanno, J. F., L. Kohen-Couderc, J. F. Lemeland, and J. Leroy. 1999. Nosocomial meningococcemia in a physician. Infect. Control Hosp. Epidemiol. 20:564-565. [DOI] [PubMed] [Google Scholar]
- 8.Jolley, K. A., J. Kalmusova, E. J. Feil, S. Gupta, M. Musilek, P. Kriz, and M. C. Maiden. 2000. Carried meningococci in the Czech Republic: a diverse recombining population. J. Clin. Microbiol. 38:4492-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandrell, R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173:2823-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morla, N., M. Guibourdenche, and J. Y. Riou. 1992. Neisseria spp. and AIDS. J. Clin. Microbiol. 30:2290-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkkali, T., H. Kayhty, H. Lehtonen, T. Ruutu, L. Volin, J. Eskola, and P. Ruutu. 2001. Tetravalent meningococcal polysaccharide vaccine is immunogenic in adult allogeneic BMT recipients. Bone Marrow Transplant. 27:79-84. [DOI] [PubMed] [Google Scholar]
- 13.Pearson, H. A. 1977. Sickle cell anemia and severe infections due to encapsulated bacteria. J. Infect. Dis. 136(Suppl.):S25-S30. [DOI] [PubMed] [Google Scholar]
- 14.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853-50862. [DOI] [PubMed] [Google Scholar]
- 15.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 17.Salit, I. E. 1981. Meningococcemia caused by serogroup W135. Association with hypogammaglobulinemia. Arch. Intern. Med. 141:664-665. [PubMed] [Google Scholar]
- 18.Singwe-Ngandeu, M., N. Buchs, P. Rohner, and C. Gabay. 2001. Waldenstrom's disease complicated by recurrent meningococcal arthritis. J. Clin. Microbiol. 39:3013-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens, D. S., R. A. Hajjeh, W. S. Baughman, R. C. Harvey, J. D. Wenger, and M. M. Farley. 1995. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann. Intern. Med. 123:937-940. [DOI] [PubMed] [Google Scholar]
- 20.Stratton, C. W. 1994. Blood cultures and immunocompromised patients. Clin. Lab Med. 14:31-49. [PubMed] [Google Scholar]
- 21.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849-1858. [DOI] [PubMed] [Google Scholar]
- 22.Vogel, U., H. Claus, and M. Frosch. 2001. Capsular operons, p. 187-201. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 23.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1999. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect. Immun. 76:954-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 32:1133-1139. [DOI] [PubMed] [Google Scholar]
- 25.Vogel, U., S. Hammerschmidt, and M. Frosch. 1996. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. Berlin 185:81-87. [DOI] [PubMed] [Google Scholar]
- 26.Wachter, E., A. E. Brown, T. E. Kiehn, B. J. Lee, and D. Armstrong. 1985. Neisseria meningitidis serogroup 29E (Z′) septicemia in a patient with far advanced multiple myeloma (plasma cell leukemia). J. Clin. Microbiol. 21:464-466. [DOI] [PMC free article] [PubMed] [Google Scholar]