Abstract
Background
Systemic therapy options are needed for women with recurrent, metastatic, or persistent cervical cancer. This systematic review and clinical practice guideline were developed to address that need, and to update a 2007 guideline from Cancer Care Ontario’s Program in Evidence-Based Care.
Methods
The literature between 2006 and April 2014 in the medline and embase databases, the Cochrane Database of Systematic Reviews (Issue 4, 2014), the Cochrane Central Register of Controlled Trials (Issue 3, 2014), relevant guideline databases, and conference proceedings of the American Society of Clinical Oncology (2007–2013) was searched. A working group developed draft guidelines and incorporated comments and feedback from internal and external reviewers.
Results
Four phase iii randomized controlled trials met the inclusion criteria for the review and provided the basis for draft recommendations. Feedback was obtained from Ontario practitioners and others abroad, which led to modifications to the draft recommendations. Three key recommendations were developed.
Conclusions
The working group concluded that all patients should be offered the opportunity to participate in appropriate randomized clinical trials. Cisplatin–paclitaxel, cisplatin–vinorelbine, cisplatin–gemcitabine, and cisplatin–topotecan are recommended combinations for this patient population. The substitution of carboplatin for cisplatin in the foregoing combinations can also be recommended because carboplatin is associated with fewer adverse effects and greater ease of administration. Selection of combination chemotherapy will depend on the toxicity profile, patient preference, and other factors. Finally, bevacizumab in combination with cisplatin–paclitaxel or carboplatin–paclitaxel is recommended for a specific subset of the target population as outlined in Gynecologic Oncology Group study 0240.
Keywords: Cervical cancer, systemic therapy, chemotherapy, systematic review, practice guideline
INTRODUCTION
Approximately 610 new cases and 150 deaths from carcinoma of the cervix occurred in Ontario in 20131. The prognosis for early-stage cervical cancer is good because of effective screening practices and early treatment options; however, the 5-year survival rate for women with cancer that has spread beyond the true pelvis to adjacent organs is only 17%2. Treatment options that can improve duration of survival while maintaining quality of life are therefore needed.
In 2007, Cancer Care Ontario’s Program in Evidence-Based Care (pebc) developed a guideline about recommended chemotherapy options for patients with recurrent, metastatic, or persistent cervical cancer3. At that time, only one trial, a comparison of cisplatin with cisplatin plus topotecan, demonstrated a statistically significant and clinically meaningful improvement in median survival duration (2.9 months) in favour of combination therapy. On that basis, that guideline recommended treatment with cisplatin–topotecan over single-agent cisplatin. However, in current practice, based on the results of more recent clinical trials, clinicians in Ontario use cisplatin (or carboplatin, which has fewer side effects and is more feasible to administer) in combination with paclitaxel to treat recurrent, metastatic, or persistent cervical cancer4,5.
More recently, other researchers, motivated by conventional chemotherapy’s modest impact on the long-term survival rate, have initiated trials of novel biologic agents. Cumulative side effects and platinum resistance after initial treatment of locally advanced cervical cancer with cisplatin-based chemoradiation5 also provide a rationale for studying novel biologic agents or non-cisplatin-containing agents in this patient population6. For example, bevacizumab is a biologic agent that inhibits the growth of tumours by binding and inactivating angiogenesis-stimulating vascular endothelial growth factor, thus limiting the formation of tumour vasculature6.
The objective of the present guideline was to update the previous Cancer Care Ontario pebc guideline on chemotherapy options for women with recurrent, metastatic, or persistent cervical cancer3, and to explore whether new therapeutic options are associated with significantly improved outcomes for that patient population, or whether new options are available that could be suitable for women who have experienced cumulative side effects or who do not tolerate the currently available treatment options. The intended users of this guideline are gynecologic oncologists and oncologists treating gynecologic cancers.
METHODS
The evidence base was developed using the methods of the practice guidelines development cycle7. Evidence was selected and reviewed by a small working group comprising members of the pebc Gynecologic Cancer Disease Site Group, which includes individuals with expertise in gynecologic oncology and health research methodology.
Literature Search Strategy
The literature in medline and embase (March 2006 to April 2014), the Cochrane Database of Systematic Reviews (Issue 4 of 12, April 2014), the Cochrane Central Register of Controlled Trials (Issue 3 of 12, March 2014), the Canadian Medical Association Infobase, the U.S. National Guidelines Clearinghouse, and conference proceedings of the American Society of Clinical Oncology (2007–2013) were searched for reports of new or ongoing trials. Relevant articles and abstracts were selected and reviewed, and the reference lists in those sources and recent review articles were also searched for additional trials. Table i presents the complete search strategy.
TABLE I.
MEDLINE literature search strategy
| 1. | exp cervix neoplasms/ |
| 2. | (cerv$ and (neoplasm$ or cancer$ or carcin$ or tumo$ or malig$)).tw. |
| 3. | 1 or 2 |
| 4. | (advance$ or metasta$ or recur$ or persistent).tw. |
| 5. | 3 and 4 |
| 6. | exp drug therapy/ |
| 7. | exp drug therapy combination/ |
| 8. | exp chemotherapy/ |
| 9. | chemothera$.tw. |
| 10. | or/ 6–9 |
| 11. | 5 and 10 |
| 12. | meta-analysis as topic/ |
| 13. | meta analysis.pt. |
| 14. | (meta analy$ or metaanaly$).tw. |
| 15. | (systematic review$ or pooled analy$ or statistical pooling or mathematical pooling or statistical summar$ or mathematical summar$ or quantitative synthes?s or quantitative overview).tw. |
| 16. | (systematic adj (review$ or overview?)).tw. |
| 17. | (exp Review Literature as topic/or review.pt or exp review/) and systematic.tw. |
| 18. | or/ 12–17 |
| 19. | (cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or chinhal or science citation index or scisearch or bids or sigle or cancerlit).ab. |
| 20. | (reference list$ or bibliograph$ or hand-search$ or relevant journals or manual search$).ab. |
| 21. | (selection criteria or data extraction or quality assessment or jadad scale or methodological quality).ab. |
| 22. | (study adj selection).ab. |
| 23. | 21 or 22 |
| 24. | review.pt. |
| 25 | 23 and 24 |
| 26. | exp randomized controlled trials as topic/ or exp clinical trials, phase iii as topic/ or exp clinical trials, phase IV as topic/ |
| 27. | (randomized controlled trial or clinical trial, phase iii or clinical trial, phase IV).pt. |
| 28. | random allocation/ or double blind method/ or single blind method/ |
| 29. | (randomi$ control$ trial? or rct or phase iii or phase IV or phase 3 or phase 4).tw. |
| 30. | or/ 26–29 |
| 31. | (phase II or phase 2).tw. or exp clinical trial/ or exp clinical trial as topic/ |
| 32. | (clinical trial or clinical trial, phase II or controlled clinical trial).pt. |
| 33. | (31 or 32) and random$.tw. |
| 34. | (clinic$ adj trial$1).tw. |
| 35. | ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3 or dummy)).tw. |
| 36. | placebos/ |
| 37. | (placebo? or random allocation or randomly allocated or allocated randomly).tw. |
| 38. | (allocated adj2 random).tw. |
| 39. | or/ 34–38 |
| 40. | 18 or 19 or 20 or 25 or 30 or 33 or 39 |
| 41. | 11 and 40 |
| 42. | (comment or letter or editorial or note or erratum or short survey or news or newspaper article or patient education handout or case report or historical article).pt. |
| 43. | 41 not 42 |
| 44. | limit 43 to English |
| 45. | Animal/ |
| 46. | Human/ |
| 47. | 45 not 46 |
| 48. | 44 not 47 |
| 49. | (200602$ or 200603$ or 200604$ or 200605$ or 200606$ or 200607$ or 200608$ or 200609$ or 200610$ or 200611$ or 200612$ or 2007$ or 2008$ or 2009$ or 2010$ or 2011$ or 2012$ or 2013$).ed. |
| 50. | 48 and 49 |
Study Selection Criteria
Inclusion Criteria
Articles were included in the systematic review of the evidence if they were fully published reports or abstracts and met these criteria:
□ They were systematic reviews based on randomized controlled trials (rcts).
□ They were phase iii rcts comparing chemotherapy with other systemic therapy agents or with no further treatment for recurrent, metastatic, or persistent cervical cancer, and they reported at least one of these outcomes: complete or partial response rate, overall or progression-free survival rate, adverse effects, or health-related quality of life (rcts reporting on heterogeneous populations—for example, women at a range of disease stages—were included if results were given separately for patients with recurrent, metastatic, or persistent cervical cancer).
The search was limited to phase iii trials because the Working Group determined that this level of evidence would be the minimum necessary to create new recommendations for clinical practice.
Exclusion Criteria
Articles were excluded if they were
□ non-English-language publications,
□ studies evaluating the role of radiotherapy administered with chemotherapy, or
□ second- or subsequent-line therapy options.
Synthesizing the Evidence
The Working Group decided that a meta-analysis of phase iii trials would be conducted if more than one study was found that compared the same patient populations and treatment regimens.
Internal Review
The draft document underwent review by the pebc Gynecologic Cancer Disease Site Group, which acted as the Expert Panel for this report, and the pebc Report Approval Panel, a three-person panel with methodology and clinical expertise. Formal approval by those panels was required, and panel members were also invited to provide comments. The Working Group was responsible for incorporating feedback and changes as required.
External Review by Ontario Clinicians and Other Experts
The pebc external review process is two-pronged. It includes a targeted peer review that is intended to obtain direct feedback on the draft report from a small number of specified content experts, and a professional consultation that is intended to facilitate dissemination of the final guidance report to practitioners. After approval of the document by the internal review panels, the draft document with recommendations modified as suggested by the reviewers was circulated to external review participants for review and feedback.
Several weeks before completion of the draft report, targeted peer review nominees were contacted by e-mail and asked to serve as reviewers. Three reviewers agreed, and the draft report and a questionnaire were sent to them by e-mail. The questionnaire consisted of items evaluating the methods, presentation, and clinical soundness of the recommendations, and the completeness of reporting. Written comments were invited. The questionnaire and draft document were first sent on 22 September 2014. Follow-up reminders were sent at 2 weeks (e-mail) and at 4 weeks (telephone call) where necessary.
Professional consultation feedback was obtained through a brief online survey of health care professionals who are the intended users of the guideline. Participants were asked to rate the overall quality of the guideline and whether they would use or recommend it. Written comments were invited. Participants were contacted by e-mail and directed to the survey Web site, where they were provided with access to the survey, the guideline recommendations, and the evidentiary base. The notification email was sent 22 September 2014. The consultation period ended 24 October 2014.
LITERATURE SEARCH RESULTS
Systematic Reviews
No systematic reviews that met the inclusion criteria for the guideline were located.
Primary Literature
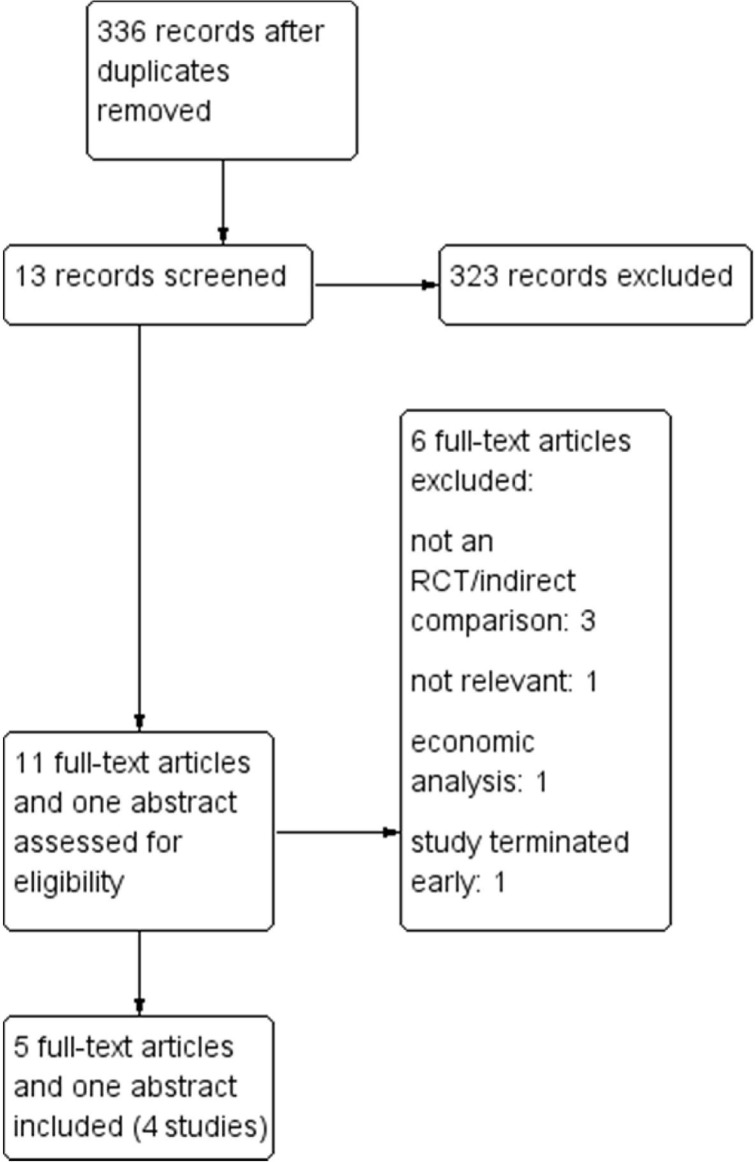
Four phase iii rcts met the inclusion criteria for the review (Figure 1), including
□ a trial of cisplatin–paclitaxel (reference arm) compared with three other cisplatin combinations [Gynecologic Oncology Group (gog)-0204]5.
□ a 2×2 factorial design comparing two types of combination chemotherapy with and without addition of the biologic agent bevacizumab (gog-0240)9.
□ a trial to assess the noninferiority of carboplatin–paclitaxel compared with cisplatin–paclitaxel [Japan Clinical Oncology Group (jcog)-0505]4.
□ a trial of cisplatin compared with mvac (methotrexate– vinblastine–doxorubicin–cisplatin)10 that was discontinued early because of 4 deaths among 64 patients in the mvac arm. The trial did not meet its accrual objectives and had inadequate power for statistical comparisons. It was therefore excluded from the analysis.
FIGURE 1.
Literature search flow diagram. The search strategy in Table I was applied for the period 2006 to April 2014.
A meta-analysis was not considered because considerable heterogeneity was evident with respect to the therapy combinations under assessment.
Study Design and Quality
Two studies were fully published, and one was available as an abstract (Table ii). On the whole, the aggregate risk of bias in the body of evidence was considered to be high because of a lack of information about the randomization method, sequence generation, and allocation concealment. Table ii sets out those and other study characteristics and quality indicators.
TABLE II.
Study design and quality
| Reference (study name) | Phase, randomization method, and allocation concealment | Blinding | Selective outcome reporting | ITT analysis planned | Funding source | Power calculation described | Baseline characteristics | Comments |
|---|---|---|---|---|---|---|---|---|
| Monk et al.,20095 (GOG-0204) | Phase III multicentre study Randomization method not described Allocation concealment not described |
Not mentioned | No | No, but “a full ITT analysis including ineligible patients also gave similar results” | U.S. NIH | Yes | Balanced for disease status and performance status for analysis of primary outcome (OS); not clear for other outcomes because 41 patients from an earlier study were included and not described | None |
| Kitagawa et al.,20124,8 (JCOG0505, abstract) | Phase III, minimization method with institution, performance status, histology, and tumour sites as balancing factors at the data centre Allocation concealment not mentioned |
Not mentioned | No | Unknown | Ministry of Health, Labour and Welfare of Japan | Yes | Unknown | Results published in an abstract |
| Tewari et al.,20149 (GOG-0240) | Phase III 2×2 factorial design Randomization method not described Allocation concealment not described 15 Patients crossed over to bevacizumab and 33 patients on bevacizumab crossed over to other salvage therapy |
Open-label | No | Yes | U.S. NCI and Genentech | Yes | Balanced | Second interim analysis not mentioned in study protocol |
ITT = intention-to-treat; NIH = National Institutes of Health; OS = overall survival rate; NCI = National Cancer Institute.
Study Characteristics
Table iii presents the characteristics of the included studies. In gog-0204, the overall survival (os) rate was the primary outcome, and response rate, progression-free survival rate, adverse effects, and quality of life were secondary objectives5. In gog-0240, primary outcomes were the os rate and the frequency and severity of adverse effects; secondary outcomes were the progression-free survival rate and tumour response9. All patients in both studies had an Eastern Cooperative Oncology Group performance status of 0 or 1, meaning that they were at least ambulatory and able to complete work of a light or sedentary nature11. Prior surgery was not reported for either study. In one study5, patients were ineligible if they had received prior chemotherapy for metastatic disease. In another9, they were ineligible if they had received prior chemotherapy for recurrence of cancer. Prior platinum chemoradiation therapy was common in patients in both groups, ranging from 64% to 73% in the gog-0204 study arms5, and from 71% to 77% in the gog-0240 study arms9. In all arms of both studies, the percentage of patients with stage ivb cancer (advanced: cancer has spread to parts of the body away from the cervix, such as the liver, intestines, lungs, or bones) was 16%–18%. More than two thirds of the participants had recurrent cancer (range in the study arms: 69%–78%), and the prevalence of persistent cancer ranged from 6% to 14%. The percentage of patients with disease confined to the pelvis was not reported in gog-02045; in gog-0240, 54% of patients in the entire study group had disease confined to the pelvis9. Table iii presents the dose and scheduling of the systemic therapy options.
TABLE III.
Study characteristics
| Reference (study name) | Pts (n) | Agent, dose, and schedule (all schedules used 21-day cycles) | Prior platinum radiation therapy (%) | Stage IVB (%) | Recurrent (%) | Persistent (%) |
|---|---|---|---|---|---|---|
| Monk et al., 20095 (GOG-0204) | 118 | Cisplatin: 50 mg/m2 on day 2 Paclitaxel: 135 mg/m2 on day 1 over 24 h |
68 | 17 | 72 | 12 |
| 117 | Cisplatin: 50 mg/m2 on day 1 Vinorelbine: 30 mg/m2 on days 1 and 8 |
73 | 16 | 71 | 13 | |
| 119 | Cisplatin: 50 mg/m2 on day 1 Gemcitabine: 1000 mg/m2 on days 1 and 8 |
64 | 18 | 71 | 11 | |
| 118 | Cisplatin: 50 mg/m2 on day 1 Topotecan: 0.75 mg/m2 on days 1, 2, and 3 |
73 | 18 | 69 | 13 | |
| Kitagawa et al., 20128 (JCOG0505, abstract) | 253 | Cisplatin: 50 mg/m2 on day 2 over 2 h Paclitaxel: 135 mg/m2 on day 1 over 24 h Carboplatin: AUC 5 on day 1 over 1 h Paclitaxel: 175 mg/m2 on day 1 over 3 h |
Pts had received 0 to 1 prior platinum agents (percentages not reported) | 100 | 100 | 0 |
| Tewari et al. 20149,a (GOG-0240) | 114 | Cisplatin: 50 mg/m2 Paclitaxel: 135 mg/m2 or 175 mg/m2 on day 1 |
75 | 16 | 78 | 6 |
| 111 | Topotecan: 0.75 mg/m2 on days 1–3 Paclitaxel: 175 mg/m2 on day 1 |
73 | 17 | 69 | 14 | |
| 115 | Cisplatin: 50 mg/m2 Paclitaxel: 135 mg/m2 or 175 mg/m2on day 1 Bevacizumab: 15 mg/kg on day 1 |
77 | 17 | 71 | 12 | |
| 112 | Topotecan: 0.75 mg/m2 on days 1–3 Paclitaxel: 175 mg/m2 on day 1 Bevacizumab: 15 mg/kg on day 1 |
74 | 18 | 70 | 13 |
Three options for the administration of paclitaxel were available, used at the discretion of the investigator.
Pts = patients; AUC = area under the curve.
In jcog-05054, the percentage of patients who had received prior platinum was not reported, and all patients had stage ivb or recurrent disease (Table iii).
Table v presents adverse event rates from the trials.
TABLE V.
Adverse events (grades 3 and 4, unless otherwise noted)
| Reference | Pts (n) | Treatment arm | Adverse events [% (p Value)]a | Deaths (n) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Neutropeniab | Febrile neutropenia | Thrombocytopenia | Thromboembolism | Leucopenia | Anemia | Hypertensionc | Neuropathy | Infection | GI or GU fistula | Nausea | Vomiting | Paind | ||||
| Monk et al.,20095 (GOG-0204) | 118 | Cisplatin–paclitaxel | 78 | 13 | 7 | 5 | 63 | 17 | NR | 2 | 13 | 16 | 14 | 20 | 10 | 2 |
| 117 | Cisplatin–vinorelbine | 78 | 14 | 8 | 6 | 68 | 29 | 3 | 8 | 13 | 12 | 13 | 10 | 4 | ||
| 119 | Cisplatin–gemcitabine | 42 | 6 | 42 | 0 | 43 | 34 | 1 | 9 | 10 | 6 | 10 | 13 | 2 | ||
| 118 | Cisplatin–topotecan | 83 (<0.0001) |
10 | 83 (<0.0001) |
6 | 71 (<0.0001) |
35 (0.02) |
5 | 5 (0.04) |
10 | 8 | 8 | 6 | 3 | ||
| Kitagawa et al.,20128 (JCOG0505, abstract) | 253 | Cisplatin–paclitaxel | 85.1 | 16 | 3.3 | NR | NR | NR | NR | Motor: 0.8 Sensory: 0 |
NR | NR | NR | NR | NR | NR |
| Carboplatin–paclitaxel | 76.4 | 7.3 | 23.6 | Motor: 2.4 Sensory: 4.9 |
||||||||||||
| Tewari et al.,20149 (GOG-0240) | 219 | Chemotherapy | 26a | 5 | NR | 1 | NR | NR | 2 | NR | NR | <1 | NR | NR | NS | 5 |
| 220 | Chemotherapy plus bevacizumab | 35 (0.04) |
5 (1.00) |
8 (0.001) |
25 (<0.001) |
6 (0.002) |
6 | |||||||||
| 114 | Cisplatin–paclitaxel (no bevacizumab) | 48 | NS | NR | NR | 36 | NR | NR | 9 | NS | NR | 8 | 5 | NR | 1 | |
| 111 | Topotecan–paclitaxel (no bevacizumab) | 64 | 53 | 2 | 3 | 3 | 4 | |||||||||
Values in boldface type are statistically significant (p < 0.05).
Grade 4 or higher.
Grade 2 or higher, defined as recurrent or continuous hypertension for a period of more than 24 hours.
Grade 2 or higher.
Pts = patients; GI = gastrointestinal; GU = genitourinary; NR = not reported; NS = nonsignificant.
TARGETED PEER REVIEW AND PROFESSIONAL CONSULTATION
The three reviewers who provided responses during the targeted peer review were located in the Canadian provinces of British Columbia, Quebec, and Alberta. The professional consultation resulted in 18 replies from Ontario, Quebec, and Nova Scotia.
The participants in both processes rated the guideline highly on methods, presentation, recommendations, completeness of reporting, information included, and quality. As in the internal review, the cost-effectiveness of bevacizumab was cited as a concern, as were the increase in adverse events and the high discontinuation rates associated with that option. Access to bevacizumab and funding approval were considered potential barriers to implementation of the recommendations. The initial draft recommendations contained an endorsement of carboplatin rather than cisplatin as the preferred agent in combination chemotherapy. Reviewers raised significant concerns about the recommendation for carboplatin rather than cisplatin in the bevacizumab combination, especially considering that the recommendation was based on data published in an abstract. In response, the Working Group further emphasized that the triplet would be considered an option, rather the preferred option.
RECOMMENDATIONS, KEY EVIDENCE, AND JUSTIFICATION
Recommendation 1
It is recommended that all patients with recurrent, metastatic, or persistent cervical cancer be offered the opportunity, if available, to participate in rcts that evaluate the efficacy and adverse effects of systematic therapy regimens.
Summary of Key Evidence and Justification for Recommendation 1
Recommendation 1 is the opinion of the Working Group.
Recommendation 2
Cisplatin with paclitaxel is recommended for the target population, and cisplatin in other combinations—including cisplatin–vinorelbine, cisplatin–gemcitabine, and cisplatin–topotecan—can also be considered. The substitution of carboplatin for cisplatin in those combinations is also recommended for this target population, because carboplatin is associated with fewer adverse effects and greater ease of administration. The selection of combination chemotherapy will depend on toxicity profile, patient preference, and other factors. For example, cisplatin combinations might be preferred in cases of allergic reaction or difficulty with bone marrow suppression.
Summary of Key Evidence and Justification for Recommendation 2
The gog-0204 trial5, which included patients with a performance status of 1 or less11 (meaning that they were ambulatory, but restricted in physically strenuous activities), compared the combinations cisplatin–vinorelbine, cisplatin–gemcitabine, and cisplatin–topotecan. The reference arm was cisplatin–paclitaxel, and os was the primary endpoint. The study was terminated early because the comparator groups were unlikely to demonstrate statistical superiority for any of the combinations compared with the reference combination, thus justifying the recommendation that each combination could be considered an option for the target population.
Carboplatin (area under the curve 5 for 1 hour on day 1) in combination with paclitaxel (175 mg/m2 for 3 hours on day 1) was tested as an alternative to the standard, but more toxic, cisplatin (50 mg/m2 for 2 hours on day 2) and paclitaxel (135 mg/m2 for 24 hours on day 1) in a jcog phase iii noninferiority trial in stage ivb persistent or recurrent cervical cancer (jcog-0505)4. That study, published as an abstract, followed 253 patients for 17.4 months and demonstrated the noninferiority of carboplatin–paclitaxel compared with cisplatin–paclitaxel (os duration: 17.5 months vs. 18.3 months respectively; hazard ratio: 0.99; adjusted 90% confidence interval: 0.79 to 1.25; noninferiority p = 0.032); Table iv). Patients in the carboplatin combination group experienced lower rates of neutropenia, febrile neutropenia, rise in serum creatinine, and early treatment discontinuation because of adverse effects; they also experienced higher rates of thrombocytopenia and neuropathy. A significantly higher non-hospitalization period (a proxy for quality of life) was also observed for patients in the carboplatin–paclitaxel arm. Based on those results, and on feasibility of administration, carboplatin–paclitaxel is recommended as a treatment option for recurrent, metastatic, or persistent cervical cancer.
TABLE IV.
Study outcomes: response rates and survival ratea
| Reference (study name) | Pts (n) | Treatment arms | Response [n (%)] | Median survival (months) | Median PFS (months) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CR | PR | CR+PR | |||||
| Monk et al., 20095 (GOG-0204) | 118 | Cisplatin–paclitaxel | 3 (2.9) | 27 (26) | 30 (29.1) | 12.9 | 5.8 |
| 117 | Cisplatin–vinorelbine | 8 (7.4) | 20 (19) | 28 (25.9) | 10.0 | 4.0 | |
| 119 | Cisplatin–gemcitabine | 1 (0.9) | 24 (21) | 25 (22.3) | 10.3 | 4.7 | |
| 118 | Cisplatin-topotecan | 2 (1.8) | 24 (22) | 26 (23.4) | 10.3 (p>0.05) |
4.6 (p>0.05) |
|
| Kitagawa et al., 20128 (JCOG0505, abstract) | 253 | Cisplatin–paclitaxel | NR | NR | NR | 18.3 | 6.2 |
| Carboplatin–paclitaxel | 17.6 (noninferiority p=0.032) |
6.9 (p>0.05) |
|||||
| Tewari et al., 20149 (GOG-0240) | 229 | Cisplatin–paclitaxel ± bevacizumab | NR | NR | 89 (38.9) | 15 | 7.6 |
| 223 | Cisplatin–topotecan ± bevacizumab | NR | NR | 64 (28.7) (significance not reported) | 12.5 (1-sided p=0.88) | 5.7 (p=0.008) | |
| 225 | Cisplatin–paclitaxel or topotecan–paclitaxel | 14 (6.2) | 67 (28.8) | 36 | 13.3 | 5.9 | |
| 227 | Cisplatin–paclitaxel + bevacizumab or topotecan–paclitaxel + bevacizumab | 28 (12.3) (p=0.03) |
81 (36) | 48 (p=0.008) |
17.0 (1-sided p=0.004) |
8.2 (p=0.002) |
|
| 114 | Cisplatin–paclitaxel | 9 (7.9) | 42 (37) | 45 | 14.3 | NR | |
| 115 | Cisplatin–paclitaxel + bevacizumab | 17 (15) | 41 (35) | 50 (p=0.51) | 17.6 (1-sided p=0.04) | NR | |
| 111 | Topotecan–paclitaxel | 11 (9.9) | 19 (17) | 27 | 12.7 | NR | |
| 112 | Topotecan–paclitaxel + bevacizumab | 5 (4.5) | 48 (43) | 47 (p=0.002) | 16.2 (1-sided p=0.09) | NR | |
Statistically significant p values (<0.05) appear in boldface type. Tests are two-sided unless otherwise noted. (The one-sided p values in GOG-0240 used a significance level of 0.025.)
Pts = patients; CR = complete response; PR = partial response; PFS = progression-free survival; NR = not reported.
Recommendation 3
Bevacizumab in combination with cisplatin–paclitaxel is recommended for a specific subset of the target population, which includes only patients whose characteristics match those of the gog-0240 study population9. Carboplatin can be substituted for cisplatin in that patient population, based on the Summary of Key Evidence and Justification section that supports this recommendation.
The subset includes patients with primary stage ivb disease (that is, it has spread to parts of the body away from the cervix, such as liver, intestines, lungs, or bones)12 or with recurrent or persistent disease not amenable to curative treatment with surgery, radiotherapy, or both; with a performance status of 1 or less; and with adequate renal, hepatic, and bone marrow function. It excludes patients previously treated with chemotherapy for recurrence and those with nonhealing wounds, active bleeding conditions, or inadequately anticoagulated thromboembolism. In addition, gog-0240 did not include patients with stage iiib cancer (local extension to the pelvic sidewall) or iva cancer (invasion into bladder or rectum). For more details about the gog-0240 patient population, see the study details provided at ClincalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT00803062).
Contraindications to bevacizumab include uncontrolled hypertension; arterial thromboembolic events within the preceding 6 months (includes cerebrovascular accident, transient ischemic attack, and myocardial infarction); surgical procedure within 28 days; and fulldose anticoagulation.
Summary of Key Evidence and Justification for Recommendation 3
A significant os rate advantage for chemotherapy with cisplatin (50 mg/m2) plus paclitaxel (135 mg/m2 or 175 mg/ m2 on day 1) or topotecan (0.75 mg/m2 on days 1–3) plus paclitaxel (175 mg/m2 on day 1) with bevacizumab (15 mg/ kg on day 1) compared with the same chemotherapy options without bevacizumab was detected (hazard ratio: 0.71; 98% confidence interval: 0.54 to 0.95; one-sided p = 0.004). Regimens were repeated at 21-day intervals. There was also a significant difference in os for cisplatin–paclitaxel plus bevacizumab compared with cisplatin–paclitaxel without bevacizumab (median os: 17.5 months vs. 14.3 months; hazard ratio: 0.68; 95% confidence interval: 0.48 to 0.97; one-sided p = 0.04; Table IV). Patients in the bevacizumab arm experienced more hypertension of grade 2 or higher, thromboembolic events of grade 3 or higher, and gastrointestinal fistulae of grade 3 or higher; however, no significant differences in quality of life were detected. Like the gog-0204 patients, the gog-0240 patients had a performance status of 1 or less. The discontinuation rate was 25% for patients in the bevacizumab group compared with 16% for patients in the group that did not receive bevacizumab.
Although gog-0240 tested bevacizumab with cisplatin and paclitaxel, the noninferiority of carboplatin–paclitaxel as demonstrated in jcog-0505, its more favourable toxicity profile and ease of administration, and its demonstrated efficacy in other disease sites13 provide support for the recommendation for carboplatin.
Qualifying Statement for Recommendation 3
The combination of carboplatin and bevacizumab can carry a risk of thrombocytopenia. However, estimates of the level of risk for that adverse event are not available, because the combination was not tested in the patient population targeted in this guideline.
DISCUSSION
In the population of women with metastatic, recurrent, or persistent cervical cancer, incremental improvements in the duration of os are significant, and quality of life is also a primary outcome of interest for patients and their families. Based on an os rate advantage for combination chemotherapy versus cisplatin alone, the previous version of this guideline recommended cisplatin–topotecan for patients with recurrent, metastatic, or persistent cervical cancer. In the 8 years since the previous guideline was released, two new, fully published phase iii rcts and an abstract that met the inclusion criteria for this systematic review and guideline were reported. Those three studies present advances in the knowledge about effective treatments for the target population. The emergence of biologic therapy in particular represents a new frontier for treatment options.
Two studies of platinum-containing combination therapy established the non-superiority of three different platinum-containing chemotherapy doublets over cisplatin–paclitaxel, and the noninferiority of carboplatin–paclitaxel, which is also associated with fewer adverse events than is cisplatin–paclitaxel. The choice of combination therapy should be guided by patient and clinician preference, the toxicity profile of the therapy combination, and ease of administration.
In a recently published study, the addition of the biologic agent bevacizumab to cisplatin or topotecan combined with paclitaxel resulted in a statistically significant improvement in the os rate. In that trial, although self-reported health-related quality of life was not significantly lower in patients in the bevacizumab group, a higher rate of adverse events was observed, including more gastrointestinal and genitourinary fistulae, which is a concern. As a result, the addition of bevacizumab is recommended only for the specific subset of the population that is relatively healthy (performance status 0–1) and has the other characteristics detailed in the report recommendations. In addition, the consultation and approval process for this guideline elicited concerns that the cost and the increase in adverse effects associated with the addition of bevacizumab might not be worth the small potential change in outcome, given the ultimately dismal prognosis for this group of patients as a whole.
Despite the positive results with the addition of bevacizumab to chemotherapy, the prognosis for our target patient population remains poor, and alternatives to conventional therapy such as exploitation of the genetic diversity of cervical cancer and potential immunotherapeutic approaches are still needed14. Accruing enough patients to obtain sufficient power to test novel strategies is a challenge, given a small prevalent population and cost concerns; however, more research is needed to improve efficacy and reduce the adverse effects associated with treatment of recurrent, metastatic, or persistent cervical cancer.
ACKNOWLEDGMENTS
The pebc Gynecologic Cancer Disease Site Group and the working group thank the following individuals for providing feedback on draft versions of this guideline: Melissa Brouwers, Sheila McNair, Hans Messersmith, Eric Winquist, and Rebecca Wong. They also thank Elizabeth Chan for conducting a data audit.
The pebc is a provincial initiative of Cancer Care Ontario supported by the Ontario Ministry of Health and Long-Term Care. All work produced by the pebc is editorially independent from the Ontario Ministry of Health and Long-Term Care.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interest: LE has authored an editorial, commentary, or other opinion regarding the objects of study. The remaining authors did not report any conflicts of interest.
REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics. Toronto, ON: Canadian Cancer Society; 2013. 2013. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Hirte HW, Strychowsky JE, Oliver T, Fung-Kee-Fung M, Elit L, Oza AM. Chemotherapy for recurrent, metastatic, or persistent cervical cancer: a systematic review. Int J Gynecol Cancer. 2007;17:1194–204. doi: 10.1111/j.1525-1438.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 4.Kitagawa R, Katsumata N, Ando M, et al. A multi-institutional phase ii trial of paclitaxel and carboplatin in the treatment of advanced or recurrent cervical cancer. Gynecol Oncol. 2012;125:307–11. doi: 10.1016/j.ygyno.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Sill MW, McMeekin DS, et al. Phase iii trial of four cisplatin-containing doublet combinations in stage ivb, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–55. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Study Protocol for:Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer N Engl J Med 2014370734–43.[Additional details available online at: http://www.nejm.org/doi/suppl/10.1056/NEJMoa1309748/suppl_file/nejmoa1309748_protocol.pdf; cited 15 April 2015] 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 8.Saito I, Kitagawa R, Fukuda H, et al. A phase iii trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage ivb, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (jcog0505) Jpn J Clin Oncol. 2010;40:90–3. doi: 10.1093/jjco/hyp117. [DOI] [PubMed] [Google Scholar]
- 9.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long HJ, 3rd, Nelimark RA, Podratz KC, et al. Phase iii comparison of methotrexate, vinblastine, doxorubicin, and cisplatin (mvac) vs. doxorubicin and cisplatin (ac) in women with advanced primary or recurrent metastatic carcinoma of the uterine endometrium. Gynecol Oncol. 2006;100:501–5. doi: 10.1016/j.ygyno.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Eastern Cooperative Oncology Group (ecog). ECOG Performance Status [Web page] Philadelphia, PA: ECOG-ACRIN Cancer Research Group; 2006. [Available at: http://www.ecog.org/general/perf_stat.html; cited 16 April 2014] [Google Scholar]
- 12.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci). Cervical Cancer Treatment (PDQ): Stages of cervical cancer [Web page] Bethesda, MD: NCI; 2014. [Available at: http://www.cancer.gov/types/cervical/patient/cervical-treatment-pdq#link/stoc_h2_1; cited 23 June 2014] [Google Scholar]
- 13.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 14.Dizon DS, Mackay HJ, Thomas GM, et al. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120:2282–8. doi: 10.1002/cncr.28722. [DOI] [PubMed] [Google Scholar]