Abstract
Background
The endpoints of progression-free survival (pfs) and time-to-progression (ttp) are frequently used to evaluate the clinical benefit of anticancer drugs. However, the surrogacy of those endpoints for overall survival (os) is not validated in all cancer settings. In the present study, we used a trial-based approach to assess the relationship between median pfs or ttp and median os in chronic lymphocytic leukemia (cll).
Methods
The pico (population, interventions, comparators, outcomes) method was used to conduct a systematic review of the literature. The population consisted of patients with cll; the interventions and comparators were standard therapies for cll; and the outcomes were median pfs, ttp, and os. Two independent reviewers screened titles, abstracts, and full papers for eligibility and then extracted data from selected studies. Correlation coefficients were calculated to assess the relationship between median pfs or ttp and median os. Subgroup correlation analyses were also conducted according to the characteristics of the selected studies (such as line of treatment and type of treatment under investigation).
Results
Of the 1263 potentially relevant articles identified during the literature search, twenty-three were included. On average, median pfs or ttp was 16.0 months (standard deviation: 12.4 months) and median os was 43.5 months (standard deviation: 31.2 months). Results of the correlation analysis indicated that median pfs or ttp is highly correlated with median os (Spearman correlation coefficient: 0.813; p ≤ 0.001). A significant correlation between median pfs or ttp and median os was observed in second- and subsequent-line therapies, but not in the first-line setting.
Conclusions
Our study demonstrates a strong correlation between median pfs or ttp and median os in previously treated cll, which reinforce the hypothesis that pfs and ttp could be adequate surrogate endpoints for os in this cancer setting.
Keywords: Progression-free survival, overall survival, chronic lymphocytic leukemia, surrogate endpoints
INTRODUCTION
Chronic lymphocytic leukemia (cll) is a lymphoproliferative disorder defined by an accumulation of incompetent clonal B lymphocytes1. There are several risk factors for cll, including family history, male sex, white race, and advanced age1. In Europe and North America, cll represents the most common form of adulthood leukemia, and it accounts for approximately one third of all leukemia cases2–4. In the United States, 15,680 new cases of cll were recorded in 2013, with 4580 deaths5. In Canada, 1345 new cases in men and 850 new cases in women were recorded in 2010, and deaths among men and women in 2011 were 372 and 228 respectively6. Median survival from a first diagnosis of cll is estimated to range from 18 months to more than 10 years depending on disease severity7. The prognosis for patients with cll depends on several factors, including clinical stage, leucocyte count at diagnosis, leucocyte doubling time, levels of serum lactate dehydrogenase, mutation status of the immunoglobulin heavy-chain variable region (IgHV) genes, and CD38 expression level3.
In oncology, an appropriate and universally recognized measure for evaluating clinical benefit is improvement in overall survival (os), which is defined as the time from randomization to the time of death from any cause. Because of its objectivity, clinical relevance, and ease of interpretation, os has historically been considered the “gold standard” for measuring the clinical efficacy of a new anticancer drug. However, trials have encountered difficulties in demonstrating clinical benefit in terms of os, because use of that endpoint is associated with several limitations. Indeed, os can be influenced by the use of subsequent-line treatments after disease progression, making it difficult to assess the impact on survival of just one treatment. Overall survival can also be affected by the confounding effect of crossover therapy, because for ethical reasons, many trials allow patients in the control arm to receive the experimental treatment after disease progression. Moreover, large sample sizes and extended periods of follow-up are required to detect a significant difference in os, often resulting in long and expensive trials8.
More recently, intermediate clinical endpoints such as progression-free survival (pfs, defined as the time from randomization to objective tumour progression or death) and time to progression (ttp, defined as the time from randomization to objective tumour progression only) have been clinically accepted for anticancer drug approvals8,9. The validity of pfs and ttp as surrogate endpoints for os has been assessed in several cancer settings, including advanced colorectal cancer, advanced breast cancer, and advanced non-small-cell lung cancer. Furthermore, the relationship of pfs or ttp with os has also been explored in the context of hematologic malignancies. More specifically, Lee et al. used a literature review to evaluate the correlation between pfs and os in non-Hodgkin lymphoma (nhl)10. Those authors concluded that improvements in 3-year pfs were highly correlated with 5-year os in aggressive nhl (r = 0.90; 95% confidence interval: 0.73 to 0.96), but that no correlation in indolent nhl was evident.
Until now, the association between these endpoints has never been assessed in the specific context of cll. The objective of the present study was therefore to use a trial-based approach to evaluate the relationship of median pfs or ttp with median os in the context of cll.
METHODS
Literature Search Strategy
A systematic review of the literature identified studies of cll therapy that reported median pfs or ttp and median os. The review question was established using the pico (population, interventions, comparators, outcomes) method11: the population consisted of patients with cll; the interventions and comparators (when applicable) were standard therapies for cll, and the outcomes were median pfs or ttp and median os. The systematic search was conducted using the electronic databases medline (1950–2011), embase (1980–2011), All EMB Reviews (including the Cochrane Database of Systematic Reviews, the American College of Physicians Journal Club, the Database of Abstracts of Reviews of Effects, the Cochrane Central Register of Controlled Trials, the Cochrane Methodology Register, the Health Technology Assessment Database, and the NHS Economic Evaluation Database) and Current Contents (1993–2011). The keywords used for the search were “B-cell chronic lymphocytic leukemia,” “survival, ” “disease progression,” “cancer survival,” “survival time,” “survival rate,” “progression,” “progression-free survival,” “event-free survival,” “cause specific survival,” and “survival analysis.” To limit the introduction of publication bias, the grey literature was also searched. More specifically, abstracts from annual meetings were searched on the Web sites of the American Society of Clinical Oncology and the American Society of Hematology. Furthermore, retrieved articles were cross-referenced to identify additional publications.
Study Selection
Studies were first selected based on title and abstract; full-text articles were then reviewed using a predefined eligibility form. The included studies were randomized or nonrandomized clinical studies (phase ii or iii) or observational studies (retrospective or prospective) published in English or French between 1990 and 2011 (14 December). Each treatment arm had to include at least 30 patients, and the endpoints of median pfs or ttp and median os both had to be reported. The only definitions of the former endpoints that were accepted were these:
□ pfs: the time from study entry until objective tumour progression or death (all causes)
□ ttp: the time from study entry until objective tumour progression or death (cll-related)
Studies were excluded if full-text articles were not available, if fewer than 80% of the patients in the sample had cll, and if the treatments under investigation included surgery, radiotherapy, or hematopoietic stem-cell transplantation without a conditioning regimen. All eligibility criteria were defined a priori. To avoid bias in study selection, the selection was performed by two independent reviewers. Disagreement between the reviewers was discussed and resolved by consensus. When more than one publication was retrieved for the same trial, the most recent article was selected.
Data Extraction and Quality Assessment
The general information and outcome measures extracted from selected studies were author, year of publication, number of patients included, definitions of pfs and ttp, median pfs and ttp and median os, and possibility for patients in the control arm to cross over to the experimental arm after progression (where applicable). Patient characteristics—sex, age, median follow-up, type of treatment under investigation, line of treatment, median number of prior treatments, Eastern Cooperative Oncology Group performance status, and median time between diagnosis and study entry—were also extracted. The extraction also focused on the risk profile of the included patients and on clinical disease staging by Binet stage and Rai classification. The predefined criteria equated Rai class 0 or Binet stage A (or both) with low-risk cll; Rai class i–ii or Binet stage B (or both) with intermediate-risk cll; and Rai calss iii–iv or Binet stage C (or both) with high-risk cll12. Other prognosis factors extracted were 17p deletion, 11q deletion, 13q deletion, mutation status of the immunoglobulin heavy-chain variable region genes, CD38 expression level, zap70 deficiency, level of β2-microglobulin, and trisomy 12 syndrome.
The included studies were assessed for quality using the Jadad scale13 for randomized studies and the strobe statement14 for nonrandomized studies. The Jadad scale includes three items associated with reduction of bias (description of the methods used for randomization and for double-blinding, and description of withdrawals and dropouts). The strobe statement uses a 22-item checklist relating to the study title, abstract, and introduction, methods, results, and discussion sections to evaluate the quality of reporting in cohort, case–control, and cross-sectional studies. For validation purposes, data extraction and quality assessment were performed by two independent reviewers.
Statistical Analyses
Descriptive analyses were performed first, to illustrate the characteristics of the included studies. Correlation analyses subsequently assessed the relationship of median pfs or ttp with median os. For the latter analysis, each treatment arm provided one observation. All data were tested for normality using Kolmogorov–Smirnov test. To examine the degree of association of pfs or ttp with os, the Pearson product moment or Spearman rank correlation coefficient was calculated, depending on whether the data were or were not normally distributed. Degrees of association were defined a priori: by range, correlation coefficients were considered to represent a very weak (0.00–0.19), weak (0.20–0.39), moderate (0.40–0.59), strong (0.60–0.79), or very strong (>0.8) association15. To explore possible reasons for heterogeneity, subgroup correlation analyses were also separately conducted according to the characteristics of selected studies.
RESULTS
Trials Included in the Analysis
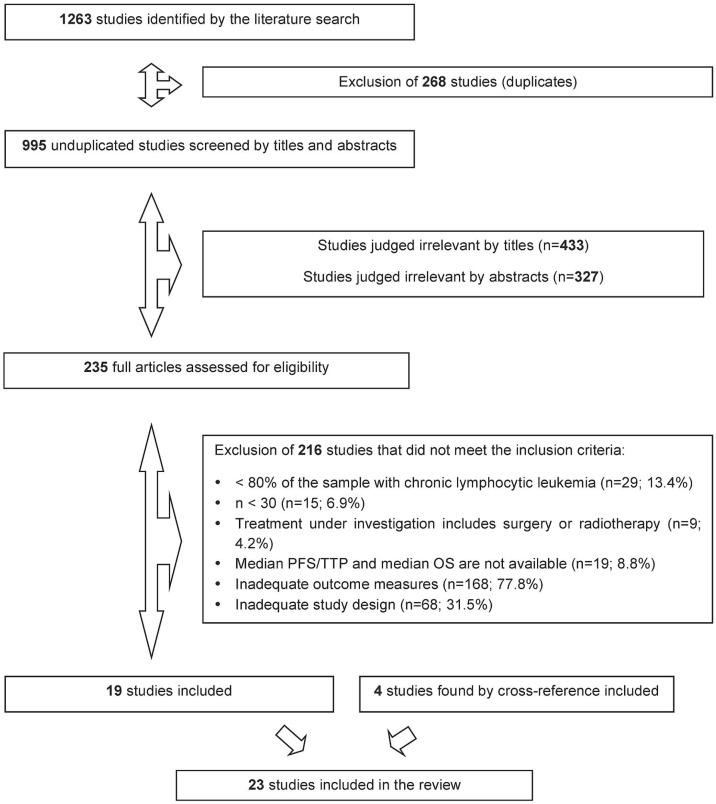
The literature search identified 1263 potentially relevant studies, with 268 duplicates that were excluded. After the screening by title and abstract, 235 full-text articles were assessed according to the eligibility criteria. The nineteen studies that met the criteria were included, and four studies found by cross-reference were added, for a total of twenty-three articles. No relevant study was found during the grey literature search (Figure 1).
FIGURE 1.
Flow chart of studies included in the systematic review of the literature. PFS = progression-free survival; TTP = time to progression; OS = overall survival.
Descriptive Analyses
Table i details the characteristics of the twenty-three included studies. Of those studies, seventeen were non-randomized16–32 and six were randomized33–38. The studies included a total of 27 treatment arms and a mean of 118 patients (minimum 30, maximum 724). On average, the median age of the patients was 63.0 years [standard deviation (sd): 3.6 years], the median follow-up period was 40.0 months (sd: 18.3 months), and the median time between diagnosis and study entry was 44.3 months (sd: 26.2 months). Most included studies used pfs rather than ttp as their primary or secondary outcome. In some cases, studies that used ttp as an outcome also included all-cause mortality, which by definition should accompany only a pfs outcome. Considering the heterogeneity of reported definitions, pfs and ttp outcomes were thus combined as pfs/ttp for analysis. Furthermore, the averages of the median pfs/ttp and the median os were, respectively, 16.0 months (sd: 12.4 months) and 43.5 months (sd: 31.2). The treatment under investigation in most of the studies was chemotherapy—generally second- and subsequent-line therapies. Most of the included studies were conducted in patients with high-risk or intermediate-risk cll, according to Rai class and Binet stage. Other prognostic factors such as gene expression, mutation, and deletion were mostly not reported. Among the randomized studies, 1 (16.7%) had a Jadad quality score of 1/5, 2 (33.3%) had a score of 2/5, and 3 (50%) of 3/5 or more. Among the nonrandomized studies, 6 (35.4%) had a strobe score of 17/22 or less, and 11 (64.8%) had a score of 17/22 or more.
TABLE I.
Characteristics of the selected studies, including 27 treatment arms
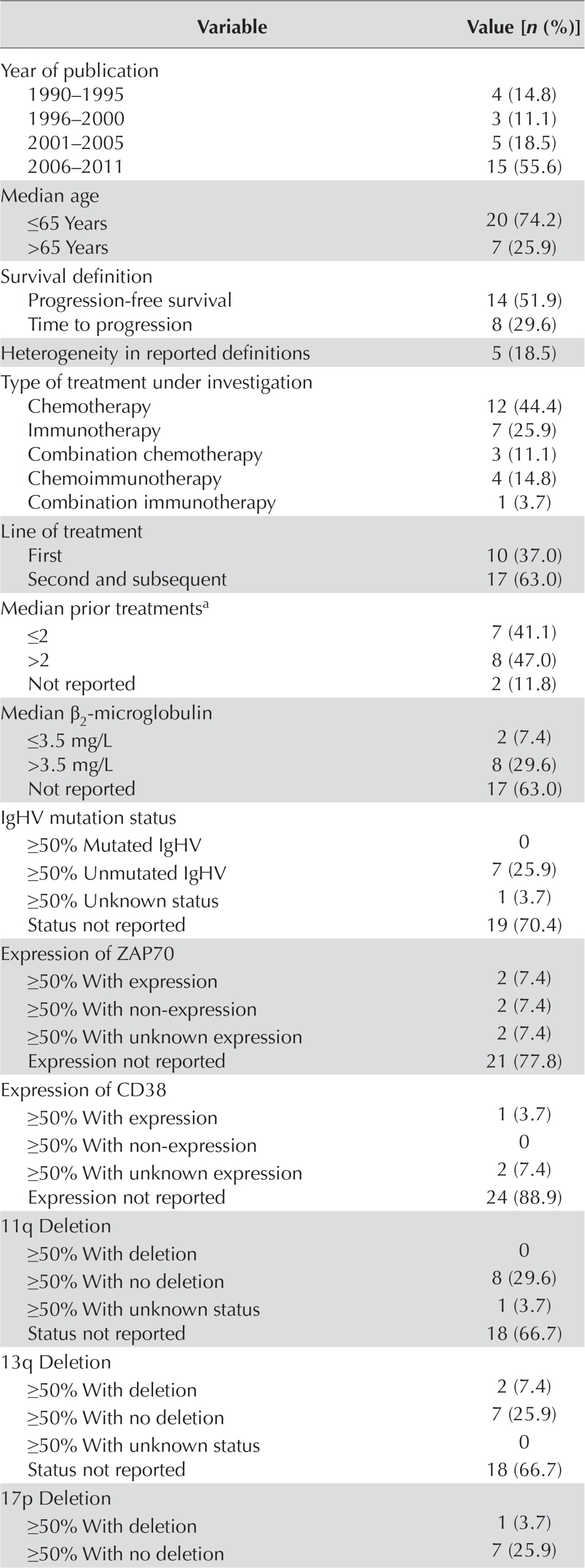
| Variable | Value [n (%)] |
|---|---|
| Year of publication | |
| 1990–1995 | 4 (14.8) |
| 1996–2000 | 3 (11.1) |
| 2001–2005 | 5 (18.5) |
| 2006–2011 | 15 (55.6) |
| Median age | |
| ≤65 Years | 20 (74.2) |
| >65 Years | 7 (25.9) |
| Survival definition | |
| Progression-free survival | 14 (51.9) |
| Time to progression | 8 (29.6) |
| Heterogeneity in reported definitions | 5 (18.5) |
| Type of treatment under investigation | |
| Chemotherapy | 12 (44.4) |
| Immunotherapy | 7 (25.9) |
| Combination chemotherapy | 3 (11.1) |
| Chemoimmunotherapy | 4 (14.8) |
| Combination immunotherapy | 1 (3.7) |
| Line of treatment | |
| First | 10 (37.0) |
| Second and subsequent | 17 (63.0) |
| Median prior treatmentsa | |
| ≤2 | 7 (41.1) |
| >2 | 8 (47.0) |
| Not reported | 2 (11.8) |
| Median β2-microglobulin | |
| ≤3.5 mg/L | 2 (7.4) |
| >3.5 mg/L | 8 (29.6) |
| Not reported | 17 (63.0) |
| IgHV mutation status | |
| ≥50% Mutated IgHV | 0 |
| ≥50% Unmutated IgHV | 7 (25.9) |
| ≥50% Unknown status | 1 (3.7) |
| Status not reported | 19 (70.4) |
| Expression of ZAP70 | |
| ≥50% With expression | 2 (7.4) |
| ≥50% With non-expression | 2 (7.4) |
| ≥50% With unknown expression | 2 (7.4) |
| Expression not reported | 21 (77.8) |
| Expression of CD38 | |
| ≥50% With expression | 1 (3.7) |
| ≥50% With non-expression | 0 |
| ≥50% With unknown expression | 2 (7.4) |
| Expression not reported | 24 (88.9) |
| 11q Deletion | |
| ≥50% With deletion | 0 |
| ≥50% With no deletion | 8 (29.6) |
| ≥50% With unknown status | 1 (3.7) |
| Status not reported | 18 (66.7) |
| 13q Deletion | |
| ≥50% With deletion | 2 (7.4) |
| ≥50% With no deletion | 7 (25.9) |
| ≥50% With unknown status | 0 |
| Status not reported | 18 (66.7) |
| 17p Deletion | |
| ≥50% With deletion | 1 (3.7) |
| ≥50% With no deletion | 7 (25.9) |
| ≥50% With unknown status | 1 (3.7) |
| Status not reported | 18 (66.7) |
| Trisomy 12 | |
| ≥50% With trisomy | 0 |
| ≥50% With no trisomy | 9 (33.3) |
| ≥50% With unknown status | 0 |
| Status not reported | 18 (66.7) |
| ECOG performance status | |
| ≥50% ECOG 0 | 5 (18.5) |
| ≥50% ECOG 1 | 6 (22.2) |
| ≥50% ECOG 0–1 | 4 (14.8) |
| ECOG status not reported | 12 (44.4) |
| Binet stage | |
| ≥50% Binet A | 2 (7.4) |
| ≥50% Binet B | 6 (22.2) |
| ≥50% Binet C | 3 (11.1) |
| Binet stage not reported | 16 (59.3) |
| Rai classification | |
| ≥50% Rai 0 | 2 (7.4) |
| ≥50% Rai IV | 5 (18.5) |
| ≥50% Rai I–II | 7 (25.9) |
| ≥50% Rai III–IV | 5 (18.5) |
| Classification not reported | 8 (29.6) |
| Risk profileb | |
| ≥50% Low risk | 2 (7.4) |
| ≥50% Intermediate risk | 9 (33.3) |
| ≥50% High risk | 13 (48.1) |
| Profile not reported | 3 (11.1) |
For patients receiving second- and subsequent-line treatments.
Based on Binet stage and Rai classification. Rai class 0 or Binet stage A, or both, corresponds with low-risk disease; Rai class I–II or Binet stage B, or both, corresponds with intermediate-risk disease; Rai class III–IV or Binet stage C, or both, corresponds with high-risk disease.
IgHV = immunoglobulin heavy-chain variable region; ZAP70 = zeta-chain-associated protein kinase 70; ECOG = Eastern Cooperative Oncology Group.
Correlation Analyses of Median PFS/TTP with Median OS
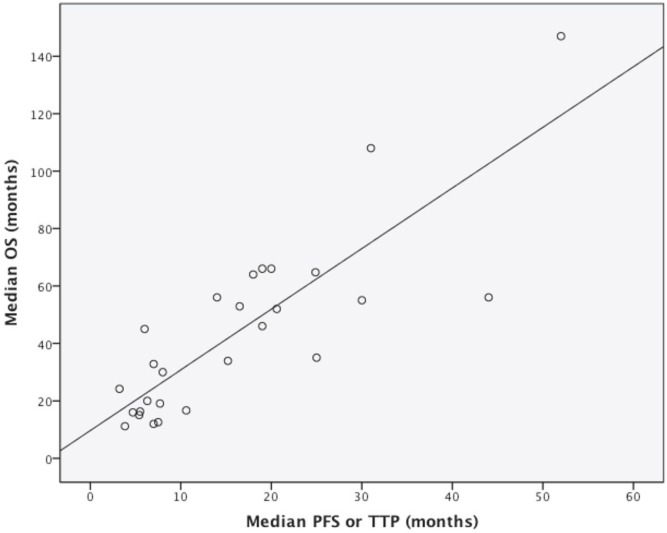
The Spearman correlation coefficient was used to evaluate the relationship between median pfs/ttp and median os. The estimated coefficient of 0.813 (p ≤ 0.001) represents a very strong association according to the pre-defined criteria (Figure 2).
FIGURE 2.
Scatterplot of the association between the combination of median progression-free survival (PFS) or time to progression (TTP) and median overall survival (OS). Each circle corresponds to a treatment arm. The Spearman correlation coefficient was estimated to be 0.813 (p ≤ 0.001).
Results of the subgroup analyses indicate a higher correlation in studies with patients whose median age exceeded 65 years (r = 0.964, p ≤ 0.001) and with a median follow-up of 30 months or less (r = 0.917, p = 0.001, Table ii). Analysis of the potential effect of line of treatment showed a statistically significant association of pfs/ttp with os in studies assessing second- and subsequent-line therapies. However, no statistically significant correlation was observed in studies of previously untreated patients receiving a first-line treatment. Type of therapy under investigation also had a significant effect on correlation between the endpoints. A statistically significant correlation was observed in studies assessing chemotherapy and immunotherapy agents, but no such correlation was found in studies assessing combinations of treatments. That observation might be the result of a lack of statistical power.
TABLE II.
Correlation analyses according to the characteristics of the selected studies
| Variable | Observations (n) | Spearman correlation coefficient | p Value |
|---|---|---|---|
| Median age | |||
| ≤65 Years | 20 | 0.753 | ≤0.001 |
| >65 Years | 7 | 0.964 | ≤0.001 |
| Median follow-up | |||
| ≤30 Months | 9 | 0.917 | 0.001 |
| >30 Months | 9 | 0.619 | NS |
| Chemotherapy | 12 | 0.781 | 0.003 |
| Immunotherapy | 7 | 0.786 | 0.036 |
| Combination chemotherapy | 3 | −0.500 | NS |
| Combination chemoimmunotherapy | 4 | 1.000 | NS |
| Line of treatment | |||
| First | 10 | 0.388 | NS |
| Second and subsequent | 17 | 0.589 | 0.013 |
| Median prior treatments | |||
| ≤2 | 17 | 0.664 | 0.004 |
| >2 | 8 | 0.738 | 0.037 |
| Median β2-microglobulin > 3.5 mg/L | 8 | 0.643 | NS |
| ≥50% of population with | |||
| Unmutated IgHV | 7 | 0.857 | 0.014 |
| ECOG PS | |||
| 0 | 5 | −0.667 | NS |
| 1 | 6 | 0.771 | NS |
| 0–1 | 4 | 0.800 | NS |
| Binet stage | |||
| B | 6 | 0.543 | NS |
| C | 3 | 1.000 | NS |
| Rai classification | |||
| IV | 5 | 0.900 | 0.037 |
| I–II | 7 | 0.536 | NS |
| III–IV | 5 | −0.100 | NS |
| Riska | |||
| Intermediate | 9 | 0.441 | NS |
| High | 13 | 0.465 | NS |
| Intermediate or high | 22 | 0.797 | ≤0.001 |
| Median time, diagnosis to study entry | |||
| ≤30 Months | 4 | 0.400 | NS |
| >30 Months | 7 | 0.536 | NS |
Based on Binet stage and Rai classification. Rai class 0 or Binet stage A, or both, corresponds with low-risk disease; Rai class I–II or Binet stage B, or both, corresponds with intermediate-risk disease; Rai class III–IV or Binet stage C, or both, corresponds with high-risk disease.
NS = statistically nonsignificant; ECOG PS = Eastern Cooperative Oncology Group performance status.
Subgroup correlation analyses according to cll stage showed that the relationship of pfs/ttp with os applied specifically to studies in patients with intermediate- or high-risk profiles (r = 0.797, p ≤ 0.001). Moreover, a very strong correlation was observed for studies in which 50% or more of the included patients had Rai class iv cll (r = 0.900, p = 0.037).
Because most prognostic factors were not reported in the included studies, correlation analyses involving only a few of those variables were performed. One factor that could be evaluated was median β2-microglobulin (>3.5 mg/L), which did not demonstrate a statistically significant correlation. However, a very strong correlation was observed for studies in which 50% or more of patients had unmutated IgHV (r = 0.857, p = 0.014, Table ii).
DISCUSSION
The objective of the present study was to use a trial-based approach to evaluate the relationship of median pfs/ttp with median os in the context of cll. The results demonstrated that pfs/ttp is highly correlated with os (correlation coefficient: 0.813, p ≤ 0.001). Moreover, age, a median follow-up period of 30 months or less, studies in which 50% or more of the patients had unmutated IgHV or Rai class iv disease, use of chemotherapy and immunotherapy agents, median number of prior treatments, and second-and subsequent-line therapies were determinants of a statistically significant relationship. Correlation was statistically significant only in studies in which second- and subsequent-line therapies were being investigated and not in studies assessing a first-line treatment.
In the past, the surrogacy of pfs for os has been assessed in various advanced cancer settings, including advanced colorectal cancer, advanced breast cancer, advanced non-small-cell lung cancer, advanced ovarian cancer, advanced gastric cancer, glioblastoma multiforme, and metastatic prostate cancer39. In the context of hematologic malignancies, Lee et al.10 combined thirty-eight randomized controlled studies with at least 100 patients per arm to evaluate the relationship of pfs with os in nhl, finding a statistically significant correlation in aggressive nhl (correlation coefficient: 0.90; 95% confidence interval: 0.73 to 0.96). However, in the same evaluation, a combination of twenty studies did not show a statistically significant correlation in indolent nhl.
The present study differs from the former one in several respects. For instance, the study by Lee et al. included trials from 1978 to 2005; it also included event-free survival as an endpoint. Findings from the study by Lee et al.10 support the tendency of pfs to correlate with os in the context of advanced or high-risk cancers. In the present analysis, most of the studies fulfilling the eligibility criteria were conducted in patients with refractory or progressive cll. The correlation between pfs and os found in our study would thus especially apply to advanced forms of cll. Accordingly, subgroup analyses using studies that included patients with intermediate- or high-risk profiles showed a statistically significant correlation between those endpoints. Even if the results of analyses by risk profile did not lead to statistically significant correlation coefficients, the discrepancy might be a result of a lack of statistical power only.
According to Broglio et al.40, who evaluated the impact of post-progression survival on the surrogacy of pfs for os, the correlation between pfs and os becomes less reliable as post-progression survival lengthens. The availability of effective treatments subsequent to disease progression therefore plays an important role in the association between endpoints, because a long post-progression period adds randomness that attenuates the ability to detect os benefits. In the context of cll, studies in previously untreated patients receiving a first-line treatment often show a statistically significant improvement of pfs, but not of os37,41–48. In fact, in studies assessing first-line treatment, the time from first therapy to final endpoint is often long enough to introduce confounding factors such as crossover and subsequent-line therapies, leading to a statistically nonsignificant difference in os. Because the time from second- or subsequent-line therapy to the final endpoint is shorter, the probability of assessing the true effect of a treatment on os, without misinterpretation, is higher. That effect was observed in the present analysis as a statistically significant correlation of pfs/ttp with os in studies assessing second- and subsequent-line therapies, but not in studies assessing a first-line treatment.
The method used to reach the results presented here was an exhaustive and rigorous systematic literature review that provided an adequate and transparent overview of the relationship between median pfs/ttp and median os. However, our study has some limitations, such as reduced statistical power in the analyses. Indeed, just twenty-three studies were included in the review, which limited the ability to conduct further analyses. Another limitation is the inclusion of nonrandomized studies. Indeed, according to the Guidance for Industry prepared by the U.S. Food and Drug Administration8, time-to-event endpoints (such as pfs and ttp) should be evaluated in randomized trials. Such measures are considered rarely to be reliable for historical data or single-arm trials, which were included in the present study. Because the analyses included only a small number of studies with two treatment arms, it was therefore impossible to evaluate the correlation of treatment effect on pfs/ttp with treatment effect on os. That limitation is important because the “demonstration across randomized comparisons that differences in the effect of randomized treatments on the surrogate endpoint are associated with the corresponding differences in the effects on the clinical endpoint of interest” is essential to validate a surrogate49. Nevertheless, despite the small number of included studies, a significant relationship between the endpoints was observed, suggesting that the observed association is real.
Moreover, definitions of pfs and ttp were not consistent throughout the included publications. For instance, some authors defined pfs or ttp as the time from response to objective tumour progression. Studies with inconsistent definitions of pfs and ttp were excluded from our literature review, which could have affected external validity. In addition, because the present analysis included only studies reporting both the endpoints of median pfs/ttp and median os, several large and well-designed randomized trials were excluded. Indeed, many main trials in the cll field reported only 3-year or 5-year survival rates, without reporting median pfs or median os, and were therefore not included in our study.
Another limitation is that the literature search included the keywords “B-cell lymphocytic leukemia,” because that term is the most frequent in the Western world. “T-cell cll” was not clearly included in the keywords of the literature search even given that T-cell cll is prevalent in Asia50.
Overall, the quality of included studies was good. Applying the strobe statement, most of the included non-randomized studies were of acceptable quality. However, the strobe statement is limited to an evaluation of the quality of reporting; it does not address the quality of the study itself. That approach might lead to a misperception of quality, because a study can be well performed, but not well written. Moreover, of the six randomized studies included, only three (50%) had a Jadad score of 3/5 or better, which might partly be a result of the choice of instrument. Indeed, the Jadad quality assessment scale can be disadvantageous for research areas in which blinding is rarely feasible, such as in oncology.
CONCLUSIONS
The present results demonstrate a very strong correlation of median pfs/ttp with median os in the context of second-and subsequent-line therapies in cll, which reinforces the hypothesis that pfs or ttp can be an adequate surrogate endpoint for os in this cancer setting.
ACKNOWLEDGMENTS
We thank Véronique Lambert-Obry and Audrey Miron for their substantial contributions to the various steps of the literature review. This study was funded by Lundbeck Canada. The funding entity did not influence any aspects of the study or the writing of the manuscript.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: JL, CB, and MEL received honoraria from Lundbeck Canada to conduct this study and to write the manuscript. FA is an employee of Lundbeck Canada. JBJ received an honorarium from Lundbeck Canada. The authors declare that they have no other financial or nonfinancial competing interests.
REFERENCES
- 1.Fausel CA, Kiel PJ. Chronic leukemias. In: DiPiro JT, Talbert RL, Yee GC, Matzke GA, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw–Hill Medical; 2011. p. 2559. [Google Scholar]
- 2.American Cancer Society . Learn About Cancer > Leukemia – Chronic Lymphocytic (CLL) > Detailed Guide > What are the key statistics for chronic lymphocytic leukemia? Atlanta, GA: American Cancer Society; 2013. [Current version available at: http://www.cancer.org/cancer/leukemia-chroniclymphocyticcll/detailedguide/leukemia-chronic-lymphocytic-key-statistics; cited 16 September 2013] [Google Scholar]
- 3.Weinberg JB, Volkheimer AD, Chen Y, et al. Clinical and molecular predictors of disease severity and survival in chronic lymphocytic leukemia. Am J Hematol. 2007;82:1063–70. doi: 10.1002/ajh.20987. [DOI] [PubMed] [Google Scholar]
- 4.Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–29. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- 5.United States, National Institutes of Health, National Cancer Institute (nci). Chronic Lymphocytic Leukemia Treatment (PDQ) [Web page] Bethesda, MD: NCI; 2013. [Current version available at: http://www.cancer.gov/cancertopics/pdq/treatment/CLL/healthprofessional/page1; cited 16 September 2013] [Google Scholar]
- 6.Canadian Cancer Society. Home >Cancer information > Cancer type > Leukemia – Chronic lymphocytic (CLL) > Statistics > Chronic lymphocytic leukemia statistics [Web page] Toronto, ON: Canadian Cancer Society; 2015. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics/?region=on; cited 8 April 2015] [Google Scholar]
- 7.Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M, on behalf of the esmo Guidelines Working Group Chronic lymphocytic leukemia: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6):vi50–4. doi: 10.1093/annonc/mdr377. [DOI] [PubMed] [Google Scholar]
- 8.United States, Department of Health and Human Services, Food and Drug Administration (fda), Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research . Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Rockville, MD: FDA; 2007. [Google Scholar]
- 9.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 10.Lee L, Wang L, Crump M. Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin’s lymphoma: correlation of complete response, time-to-event and overall survival end points. Ann Oncol. 2011;22:1392–403. doi: 10.1093/annonc/mdq615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgings JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Ver. 5.1.0. [Available online at: http://www.cochrane-handbook.org; cited 8 April 2015] [Google Scholar]
- 12.Pinilla-Ibarz J, McQuary A. Chronic lymphocytic leukemia: putting new treatment options into perspective. Cancer Control. 2010;17(suppl):4–15. doi: 10.1177/1073274810017002S03. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, on behalf of the strobe Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (strobe) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Aapro M, Leonard RC, Barnadas A, et al. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer–Anemia and the Value of Erythropoietin (brave) study. J Clin Oncol. 2008;26:592–8. doi: 10.1200/JCO.2007.11.5378. [DOI] [PubMed] [Google Scholar]
- 16.Badoux XC, Keating MJ, Wang X, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011;118:2085–93. doi: 10.1182/blood-2011-03-341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd JC, Peterson BL, Gabrilove J, et al. on behalf of the Cancer and Leukemia Group B Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from cancer and leukemia group B study 19805. Clin Cancer Res. 2005;11:4176–81. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 18.Byrd JC, Peterson BL, Rai KR, et al. Fludarabine followed by alemtuzumab consolidation for previously untreated chronic lymphocytic leukemia: final report of Cancer and Leukemia Group B study 19901. Leuk Lymphoma. 2009;50:1589–96. doi: 10.1080/10428190903150839. [DOI] [PubMed] [Google Scholar]
- 19.Cortelezzi A, Pasquini MC, Gardellini A, et al. Low-dose subcutaneous alemtuzumab in refractory chronic lymphocytic leukaemia (cll): results of a prospective, single-arm multicentre study. Leukemia. 2009;23:2027–33. doi: 10.1038/leu.2009.148. [DOI] [PubMed] [Google Scholar]
- 20.Faderl S, Ferrajoli A, Wierda W, O’Brien S, Lerner S, Keating MJ. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–5. doi: 10.1002/cncr.25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenchel K, Bergmann L, Wijermans P, et al. Clinical experience with fludarabine and its immunosuppressive effects in pretreated chronic lymphocytic leukemias and low-grade lymphomas. Leuk Lymphoma. 1995;18:485–92. doi: 10.3109/10428199509059649. [DOI] [PubMed] [Google Scholar]
- 22.Fiegl M, Erdel M, Tinhofer I, et al. on behalf of the Austrian Collaborative Study Group on Alemtuzumab in Chronic Lymphocytic Leukemia, in cooperation with The Czech Leukemia Study Group for Life Clinical outcome of pretreated B-cell chronic lymphocytic leukemia following alemtuzumab therapy: a retrospective study on various cytogenetic risk categories. Ann Oncol. 2010;21:2410–19. doi: 10.1093/annonc/mdq236. [DOI] [PubMed] [Google Scholar]
- 23.Fiegl M, Falkner F, Steurer M, et al. on behalf of the Austrian Collaborative Study Group on Alemtuzumab in Chronic Lymphocytic Leukemia and the Czech Leukemia Study Group for Life Successful alemtuzumab retreatment in progressive B-cell chronic lymphocytic leukemia: a multicenter survey in 30 patients. Ann Hematol. 2011;90:1083–91. doi: 10.1007/s00277-011-1192-5. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase ii trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–66. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 25.Hui D, Lam W, Toze C, et al. Alemtuzumab in clinical practice: a British Columbia experience. Leuk Lymphoma. 2008;49:218–26. doi: 10.1080/10428190701760029. [DOI] [PubMed] [Google Scholar]
- 26.Keating MJ, Hester JP, McGredie KB, Burgess MA, Murphy WK, Freireich EJ. Long-term results of cap therapy in chronic lymphocytic leukemia. Leuk Lymphoma. 1990;2:391–7. doi: 10.3109/10428199009069292. [DOI] [PubMed] [Google Scholar]
- 27.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.V99.10.3554. [DOI] [PubMed] [Google Scholar]
- 28.Schiavone EM, De Simone M, Palmieri S, et al. Fludarabine plus cyclophosphamide for the treatment of advanced chronic lymphocytic leukemia. Eur J Haematol. 2003;71:23–8. doi: 10.1034/j.1600-0609.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 29.Sorensen JM, Vena DA, Fallavollita A, Chun HG, Cheson BD. Treatment of refractory chronic lymphocytic leukemia with fludarabine phosphate via the group C protocol mechanism of the National Cancer Institute: five-year follow-up report. J Clin Oncol. 1997;15:458–65. doi: 10.1200/JCO.1997.15.2.458. [DOI] [PubMed] [Google Scholar]
- 30.Stilgenbauer S, Zenz T, Winkler D, et al. on behalf of the German Chronic Lymphocytic Leukemia Study Group Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the cll2h study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- 31.Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase i–ii study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 32.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A, on behalf of the Hx-CD20-406 study investigators Ofatumumab is active in patients with fludarabine-refractory cll irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118:5126–9. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brugiatelli M, Jaksic B, Planinc-Peraica A, et al. Treatment of chronic lymphocytic leukemia in early and stable phase of the disease: long-term results of a randomized trial. Eur J Haematol. 1995;55:158–63. doi: 10.1111/j.1600-0609.1995.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 34.Eichhorst BF, Busch R, Stilgenbauer S, et al. on behalf of the German cll Study Group First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–91. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 35.Elter T, Gercheva-Kyuchukova L, Pylylpenko H, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. Lancet Oncol. 2011;12:1204–13. doi: 10.1016/S1470-2045(11)70242-X. [DOI] [PubMed] [Google Scholar]
- 36.Mabed M, Aref S, Fouda M, El-Sharawy S. Chlorambucil plus theophylline vs chlorambucil alone as a front line therapy for B-cell chronic lymphatic leukemia. Leuk Lymphoma. 2004;45:2029–35. doi: 10.1080/10428190410001714061. [DOI] [PubMed] [Google Scholar]
- 37.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–7. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 38.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 39.Sherrill B, Kaye JA, Sandin R, Cappelleri JC, Chen C. Review of meta-analyses evaluating surrogate endpoints for overall survival in oncology. Onco Targets Ther. 2012;5:287–96. doi: 10.2147/OTT.S36683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catovsky D, Richards S, Matutes E, et al. on behalf of the U.K. National Cancer Research Institute (ncri) Haematological Oncology Clinical Studies Group and the ncri Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the lrf cll4 trial): a randomised controlled trial. Lancet. 2007;370:230–9. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 42.Eichhorst BF, Busch R, Hopfinger G, et al. on behalf of the German CLL Study Group Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–91. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 43.Flinn IW, Neuberg DS, Grever MR, et al. Phase iii trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: U.S. Intergroup trial E2997. J Clin Oncol. 2007;25:793–8. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 44.Leporrier M, Chevret S, Cazin B, et al. on behalf of the French Cooperative Group on Chronic Lymphocytic Leukemia. Randomized comparison of fludarabine, cap, and chop in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–25. doi: 10.1182/blood.V98.8.2319. [DOI] [PubMed] [Google Scholar]
- 45.Robak T, Blonski JZ, Gora-Tybor J, et al. on behalf of the Polish Leukemia Group Cladribine alone and in combination with cyclophosphamide or cyclophosphamide plus mitoxantrone in the treatment of progressive chronic lymphocytic leukemia: report of a prospective, multicenter, randomized trial of the Polish Adult Leukemia Group. Blood. 2006;108:473–9. doi: 10.1182/blood-2005-12-4828. [DOI] [PubMed] [Google Scholar]
- 46.Robak T, Bloński JZ, Kasznicki M, et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood. 2000;96:2723–9. [PubMed] [Google Scholar]
- 47.Steurer M, Pall G, Richards S, Schwarzer G, Bohlius J, Greil R, on behalf of the Cochrane Haematologic Malignancies Group Single-agent purine analogues for the treatment of chronic lymphocytic leukaemia: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32:377–89. doi: 10.1016/j.ctrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Q, Tan DC, Samuel M, Chan ES, Linn YC. Fludarabine in comparison to alkylator-based regimen as induction therapy for chronic lymphocytic leukemia: a systematic review and meta-analysis. Leuk Lymphoma. 2004;45:2239–45. doi: 10.1080/10428190412331283260. [DOI] [PubMed] [Google Scholar]
- 49.Hughes MD. Practical issues arising in an exploratory analysis evaluating progression-free survival as a surrogate endpoint for overall survival in advanced colorectal cancer. Stat Methods Med Res. 2008;17:487–95. doi: 10.1177/0962280207081860. [DOI] [PubMed] [Google Scholar]
- 50.Kalil N, Cheson BD. Chronic lymphocytic leukemia. Oncologist. 1999;4:352–69. [PubMed] [Google Scholar]