Abstract
Background
The importance of histologic classification in selecting the appropriate systemic therapy for non-small-cell lung cancer (nsclc) came to attention in 2007. In British Columbia, that information was communicated through international and national meetings, our centralized cancer care program, and to the medical community at large in multidisciplinary forums. We examined the effects of those education programs on the categorization of nsclc and associated systemic treatment practices.
Methods
The BC Cancer Agency provides cancer care to 4.6 million residents of British Columbia. A retrospective review of all stage iiib and iv nsclc patients referred in 2007 and 2011 collected baseline characteristics, treatment, and outcomes. Histology was classified using the International Classification of Diseases for Oncology, 3rd edition, for the Canadian Cancer Registry.
Results
In 2007, 671 patients were referred, and 170 received chemotherapy; in 2011, the relevant figures were 680 and 197 respectively. Baseline characteristics in the cohorts were not statistically significantly different in 2007 and 2011. Histologic classifications in 2007 were 41% nonsquamous, 13% squamous, and 46% not otherwise specified (nos); in 2011, they were 63%, 17%, and 20% respectively. Exposure to pemetrexed in any line of therapy in 2007 was 22% for nonsquamous, 17% for squamous, and 10% for nos; in 2011, exposure was 39%, 3%, and 37% respectively. Exposure to epidermal growth factor receptor tyrosine kinase inhibitor (egfr tki) in 2007 was 36%, 22%, and 33%; in 2011, it was 64%, 60%, and 63%. Median overall survival duration, 2007 versus 2011, was 3.25 months versus 3.57 months with best supportive care, and 11.31 months versus 11.54 months with chemotherapy.
Conclusions
The specificity of nsclc histologic categorization improved in 2011 compared with 2007, with a reduction of 26 percentage points in the rate of nos disease. The proportion of patients treated with chemotherapy over time remained the same, but the use of pemetrexed and egfr tki increased. The increased accuracy in histologic classification resulted in more appropriate utilization of systemic drugs.
Keywords: Histology, systemic therapy, squamous, nonsquamous, lung cancer, nos
INTRODUCTION
In 2014 in Canada, an estimated 26,100 people were diagnosed with lung cancer, and 20,500 died from the disease1. Most patients are diagnosed with non-small-cell carcinoma (nsclc) at advanced stages for which systemic treatment is the standard of care. For many years, a platinum doublet was the accepted therapy backbone in all histologic nsclc subtypes, and although histologic subtype was often mentioned in studies, the choice of treatment did not depend on subtype2,3.
As the understanding of nsclc has improved and more therapies have become available, more accurate histologic subclassification has become essential for guiding systemic therapy decisions. Differentiating between adenocarcinoma and squamous cell carcinoma has now become a critical step in selecting the best therapeutic option for patients. Studies involving pemetrexed, bevacizumab, the epidermal growth factor tyrosine kinase inhibitors (egfr tkis), and the anaplastic lymphoma kinase inhibitors all favoured drug administration or molecular screening for nonsquamous histologies4–11.
Although clinical decision-making depends on the correct histologic classification, such classification is not always technically feasible because lung cancer is often diagnosed on limited tissue samples. With small biopsy or cytology specimens, classification of tumours can be difficult, and tissue might be too scant for ancillary processes such as immunohistochemical stains that could help in this regard. Algorithms and guidelines for handling and interpreting small samples have been developed to facilitate more accurate diagnoses12. Staining for thyroid transcription factor 1 and p63 to differentiate between adenocarcinoma and squamous cell carcinoma respectively is advocated as the first step. Although additional stains, including cytokeratin 7 for adenocarcinoma and cytokeratins 5/6 for squamous cell carcinoma can also be used, a conservative immunohistochemical panel is advocated. Implementation of those standardized pathways helps to preserve tissue for the molecular testing that is essential in guiding systemic therapy.
With recognition of the importance of histology for choosing systemic therapy in nsclc and optimization of tools to classify tumours, multiple efforts have been made to disseminate that information. At a global level, the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society collaborated to publish an international multi-disciplinary classification of lung adenocarcinoma and addressed limited specimen characterization and preservation12. National platforms for information-sharing included Canadian thoracic oncology and pathology meetings.
Beginning in 2007 in British Columbia, the BC Cancer Agency (bcca) centralized cancer system was used to circulate information about histology to physicians involved directly in lung oncology care, including data that identified the futility of using pemetrexed in squamous cell carcinoma4,5. To facilitate communication with the wider group of health care providers, including community respirologists, surgeons, radiologists, and pathologists, multidisciplinary round-table programs were held throughout the province.
We set out to examine the effects of disseminating this knowledge about histology by comparing the proportions of all patients diagnosed with advanced nsclc in 2007 and 2011 who were assigned a pathology subclassification. The year 2007 was chosen as a comparator because pemetrexed was available for treatment in that year, but the information on the importance of histology for directing the choice of pemetrexed was not publicized; by 2011, the connection between histology and pemetrexed was well known in the academic medical oncology community. We further proposed to evaluate the effect of that change on systemic patterns of practice.
METHODS
The bcca provides cancer care to 4.6 million residents of the province and maintains a cancer registry that allows for population-based analyses. Of all patients diagnosed with advanced lung cancer in the province, 80% are referred for care to one of the 6 regional bcca cancer centres or to an affiliated community oncology network site. A provincial pharmacy database tracks all chemotherapy delivered in the province. Updates to practice guidelines issued by the bcca are communicated to oncology providers in tumour group quarterly meetings and monthly pharmacy updates. Information-sharing with medical professionals outside of the bcca umbrella are conducted nationally during the annual Canadian Lung Cancer Conference (hosted in Vancouver, BC) and the Canadian Association of Pathologists meeting, as well as locally in hospital-based continuing medical education programs throughout the province. The content of several of the larger continuing medical education programs was determined in online needs assessments.
We conducted a retrospective chart review of all patients referred to the bcca with stage iiib or iv nsclc according to the American Joint Committee on Cancer staging manual, version 6. Patients experiencing a malignancy within the preceding 5 years were excluded. Patients diagnosed between 1 January and 31 December 2007 and between 1 January and 31 December 2011 were compared.
The Outcomes and Surveillance Integration System was used to collect baseline patient characteristics such age, sex, smoking status, ethnicity, and pathology. Ethnicity was defined as “Asian” or “non-Asian.” Asian ethnicity was defined as having common ancestry from any country in Asia and was determined using multiple factors: comparison with a previously validated Asian surname list, clinician’s dictated consultations, or requirement for an Asian language translator. All other patients constituted the non-Asian cohort.
Pathology was coded according to Canadian Cancer Registry requirements using the World Health Organization’s International Classification of Diseases for Oncology, 3rd edition. Data on the method of tissue acquisition or the use of immunohistochemical staining were not collected.
Systemic treatment details, including the type of chemotherapy and the number of cycles, were collected. Outcomes including overall survival (os) from time of diagnosis to death were recorded.
Ethics approval for the study was obtained from the governing research ethics board.
Univariate analyses were conducted using chi-square tests. Multivariate analysis was conducted using logistic regression analysis. Survival was compared using the Kaplan–Meier method and the log rank test. Statistical analyses were completed using the SPSS software application (version 14.0: SPSS, Chicago, IL, U.S.A.).
RESULTS
The 1351 patients identified included 671 in the 2007 cohort and 680 in the 2011 cohort. Baseline characteristics (Table i) were as follows:
□ 2007: 47% women; median age: 68 years; smoking status: never 13%, former 42%, current 43%, unknown 2%; ethnicity: 11% Asian, 89% non-Asian
□ 2011: 49% women; median age: 69 years; smoking status: never 12%, former 41%, current 44%, unknown 3%; ethnicity: 12% Asian, 88% non-Asian
TABLE I.
Baseline characteristics of patients with stage IIIB/IV non-small-cell lung cancer, 2007 and 2011
| Characteristic | 2007 | 2011 | p Value |
|---|---|---|---|
| Patients (n) | 671 | 680 | |
| Sex [n (%)] | 0.491 | ||
| Women | 318 (47) | 335 (49) | |
| Men | 353 (53) | 345 (51) | |
| Age (years) | 0.592 | ||
| Median | 68 | 69 | |
| Range | 38–94 | 33–93 | |
| Smoking status [n (%)] | 0.932 | ||
| Never | 81 (12) | 85 (13) | |
| Former | 284 (42) | 277 (41) | |
| Current | 289 (43) | 302 (44) | |
| Unknown | 17 (3) | 16 (2) | |
| Ethnicity [n (%)] | 0.074 | ||
| Asian | 71 (11) | 85 (12) | |
| Other | 600 (89) | 595 (88) | |
| Histology | <0.001 | ||
| Nonsquamous | 277 (41) | 426 (63) | |
| Squamous | 84 (13) | 115 (17) | |
| Not otherwise specified | 310 (46) | 139 (20) |
There were no statistically significant differences in baseline characteristics between the cohorts.
Histologic classification was 41% nonsquamous, 13% squamous, and 46% not otherwise specified (nos) in 2007, compared with 63%, 17%, 20% respectively in 2011 (Table ii), for an absolute reduction of 26 percentage points in the rate of nos. In 2007 within the nonsquamous group, adenocarcinoma accounted for 87% of cases, large-cell carcinoma for 11%, bronchioalveolar carcinoma for 1%, and others for 1%. In 2011, within the nonsquamous group, adenocarcinoma accounted for 96% of cases, with adenosquamous, bronchioalveolar, large-cell carcinoma, and others accounting for 1% each.
TABLE II.
Detailed histologic classification for non-small-cell lung cancer diagnosed in 2007 and 2011
| Class | Patients [n (%)] | |
|---|---|---|
|
| ||
| 2007 | 2011 | |
| Nonsquamous cell carcinoma | 277 (41) | 426 (63) |
| Adenocarcinoma | 240 (87) | 406 (96) |
| Adenosquamous carcinoma | 0 | 5 (1) |
| Bronchioalveolar carcinoma | 4 (1) | 5 (1) |
| Large-cell carcinoma | 30 (11) | 5 (1) |
| Other | 3 (1) | 5 (1) |
| Squamous cell carcinoma | 84 (13) | 115 (17) |
| Not otherwise specified | 310 (46) | 139 (20) |
| TOTAL | 671 | 680 |
The number of cases diagnosed as large-cell carcinoma declined from 2007 (n = 30, 11%) to 2011 (n = 5, 1%). That difference was paralleled by increases in adenocarcinoma diagnoses, whose proportion rose to 94% in 2011 from 84% in 2007, and in squamous cell carcinoma diagnoses (rose to 17% from 13%).
In 2007, 30% of nonsquamous, 21% of squamous, and 22% of nos patients received first-line chemotherapy (Table iii). Second-line therapy was delivered to 42% of nonsquamous, 56% of squamous, and 42% of nos patients. Third-line therapy was administered in 40% of nonsquamous, 20% of squamous, and 51% of nos patients. Exposure to pemetrexed in all histologies and in any line of therapy was 22%, 17%, and 14% respectively. Exposure to an egfr tki in all histologies and in any line of therapy was 36%, 22%, and 33% respectively.
TABLE III.
Systemic therapy delivered in stage IIIB/IV non-small-cell lung cancer by histology and year
| Therapy type | Patients [n (%)] treated in | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 2007 | 2011 | |||||
|
|
|
|||||
| Nonsquamous (n=277) | Squamous (n=84) | NOS (n=310) | Nonsquamous (n=426) | Squamous (n=115) | NOS (n=139) | |
| First line | 83 (30) | 18 (21) | 69 (22) | 137 (32) | 30 (26) | 30 (22) |
| Platinum doublet | 65 (78) | 17 (94) | 58 (84) | 73 (53) | 26 (87) | 21 (70) |
| Platinum–pemetrexed | 31 (23) | 4 (13) | ||||
| Single agent | 9 (11) | 1 (6) | 6 (9) | 8 (6) | 3 (10) | 2 (7) |
| EGFR TKI | 9 (11) | 5 (7) | 25 (18) | 1 (3) | 3 (10) | |
| Second line | 35 (42) | 10 (56) | 29 (42) | 86 (63) | 12 (40) | 19 (63) |
| Docetaxel | — | 1 (8) | — | |||
| Pemetrexed | 15 (43) | 3 (30) | 7 (24) | 23 (27) | — | 5 (26) |
| Maintenance | 5 | — | — | |||
| Second line | 18 | — | 5 | |||
| Erlotinib | 13 (37) | 4 (40) | 11 (38) | 48 (56) | 9 (75) | 11 (58) |
| Maintenance | 5 | 1 | 1 | |||
| Second line | 43 | 8 | 10 | |||
| Platinum doublet | 5 (14) | 7 (24) | 11 (13) | 1 (8) | 3 (16) | |
| Platinum–pemetrexed | 3 (3) | — | — | |||
| Other | 2 (6) | 3 (30) | 4 (14) | 1 (1) | 1 (8) | — |
| Third line | 14 (40) | 2 (20) | 15 (52) | 35 (41) | 4 (33) | 8 (42) |
| Docetaxel | 6 (17) | — | 1 (13) | |||
| Pemetrexed | 4 (29) | 3 (20) | 8 (23) | 1 (25) | 2 (25) | |
| Erlotinib | 9 (64) | 0 | 10 (67) | 15 (43) | 2 (50) | 5 (62) |
| Platinum doublet | 4 (11) | — | — | |||
| Platinum–pemetrexed | 1 (3) | — | — | |||
| Other | 1 (7) | 2 (100) | 2 (13) | 1 (3) | 1 (25) | |
| All lines | ||||||
| Pemetrexed | ||||||
| No | 64 (77) | 15 (83) | 59 (86) | 84 (61) | 29 (97) | 19 (63) |
| Yes | 19 (23) | 3 (17) | 10 (14) | 53 (39) | 1 (3) | 11 (37) |
| EGFR TKI | ||||||
| No | 53 (64) | 14 (78) | 46 (67) | 49 (36) | 12 (40) | 11 (37) |
| Yes | 30 (36) | 4 (22) | 23 (33) | 88 (64) | 18 (60) | 19 (63) |
nos = not otherwise specified; egfr tki = epidermal growth factor inhibitor tyrosine kinase inhibitor.
In 2011, 32% of nonsquamous, 26% of squamous, and 22% of nos patients received first-line chemotherapy (Table iii). Second-line therapy was delivered to 63% of nonsquamous, 40% of squamous, and 63% of nos patients. Third-line therapy was administered in 41% of nonsquamous, 33% of squamous, and 42% of nos patients. Exposure to pemetrexed in all histologies and in any line of therapy was 39%, 3%, and 37% respectively. Exposure to an egfr tki in all histologies and in any line of therapy was 64%, 60%, and 63% respectively.
A comparison of systemic therapy use in all histologies demonstrated that the proportion of first-line chemotherapy was similar in 2007 and 2011: 25% and 29% respectively (p = 0.133). Second-line treatment was more frequent in 2011: 59% compared with 46% in 2007 (p = 0.013). Third-line therapy rates did not differ between the cohorts: 44% in 2007 compared with 40% in 2011 (p = 0.565).
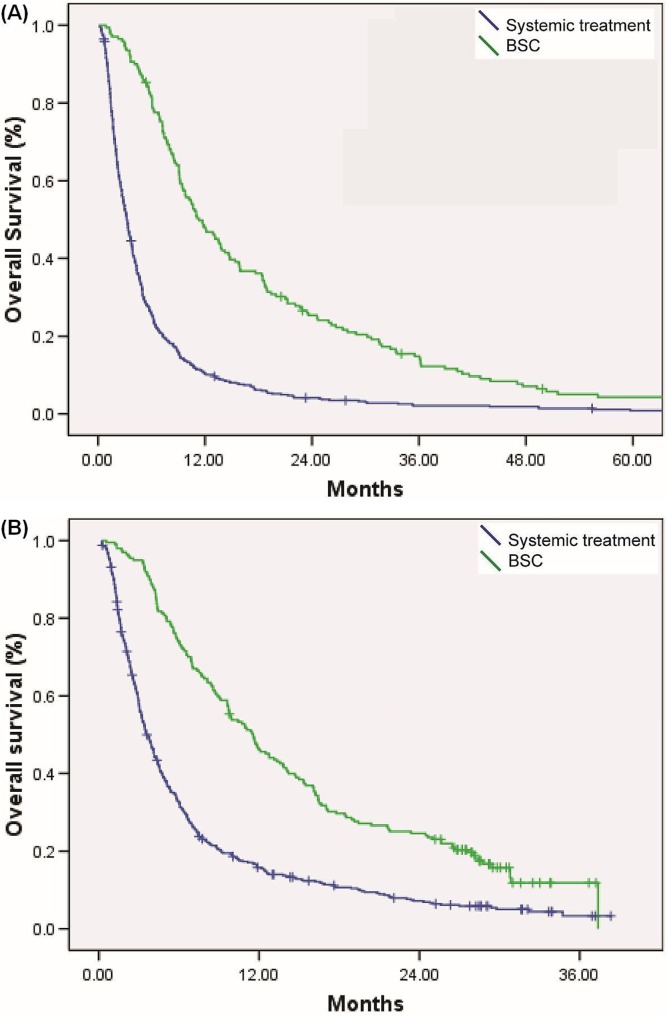
Median os duration in 2007 was 11.3 months in patients treated with systemic therapy and 3.2 months in those receiving best supportive care (bsc) alone (p < 0.001). In 2011, median os duration was 11.5 months in patients treated with systemic therapy and 3.6 months in those receiving bsc alone (p < 0.001, Figure 1). The treated and bsc groups in 2007 and 2011 were not statistically significantly different.
FIGURE 1.
Overall survival (OS) by treatment type: systemic therapy compared with best supportive care (BSC) in (A) 2007 (median OS: 11.7 months with systemic therapy vs. 3.2 months with BSC, p < 0.001) and (B) 2011 (median OS: 11.5 months with systemic therapy vs. 3.6 months with BSC, p < 0.001).
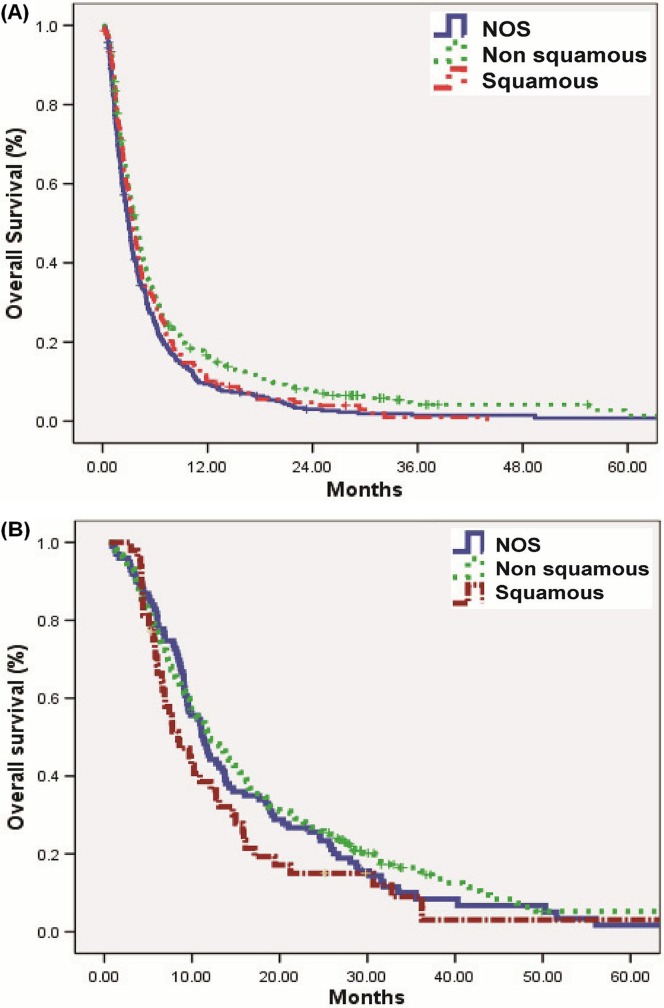
By histology, os duration for patients who received bsc was 3.8 months in the nonsquamous group, 3.5 months in the squamous group, and 3 months in the nos group (p = 0.001, Figure 2). For patients who received chemotherapy, os duration was 11.9 months in the nonsquamous group, 8.4 months in the squamous group, and 11.3 months in the nos group (p = 0.116)
FIGURE 2.
Overall survival (OS) by histology: nonsquamous compared with squamous compared with not otherwise specified (NOS) when treated with (A) best supportive care (BSC) (median OS: 3.8 months nonsquamous vs. 3.5 months squamous vs. 3.0 months NOS, p < 0.001) or (B) systemic therapy (median OS: 11.9 months nonsquamous vs. 8.4 months squamous vs. 11.3 months NOS, p < 0.116).
DISCUSSION
Our study demonstrates that knowledge communication at the international, national, and local level was effective in reducing the rate of nsclc nos by 26 percentage points. The higher rate of histologic subclassification of nsclc was also associated with improved chemotherapy decision-making and selection of appropriate therapeutic agents for patients.
In 2007, the rate of nsclc nos was 46%; it declined to 20% in 2011. We hypothesize that the reduction in nos was likely a result of two key factors: an increase in awareness among pathologists about the importance of histologic classification, and an increase in the use of immunohistochemical stains on limited biopsy and cytology specimens to help classify tumours. In contrast, data from the California Cancer Registry showed an increase in nsclc nos from 19.9% in 1989–1994, to 25.7% in 1995–2000, and to 31.0% in 2001–200613. Similarly, analysis of the Cancer Registry of Norway for 1988–2007 demonstrated an increase in nos to 19% from 12%14. However, those cohorts were collected before the importance of histology was recognized. In a contemporary study, da Cunha Santos et al.15 examined 602 fine-needle aspirate biopsies from two periods (2001–2004 and 2005–2009) at the University Health Network in Toronto. Rates of nos improved to 24.5% from 35% at that academic centre. Our data, which reflect both academic and community hospitals and which show a significant decline in nos, confirm that communication of the importance of histology in nsclc was effective.
Another positive effect of education for pathologists was seen in the decline in the proportion of cases diagnosed as large-cell carcinoma, which in 2011 was only 1%. Those results reflect the current understanding that the group of large-cell carcinomas represents a mixture of poorly differentiated adenocarcinomas and squamous cell carcinomas, with only a small percentage of true large-cell carcinomas, which can be diagnosed only when the entire resected tumour is examined microscopically. The current recommendation is that the term “large-cell carcinoma” not be used for diagnosis in small biopsy and cytology specimens12.
Histologic classification has been identified by medical oncologists as one of the key influences on informed decision-making16. Although chemotherapy utilization rates in our study appear low (25% in 2007 and 29% in 2011), they are consistent with rates reported in studies using data from the U.S. Surveillance, Epidemiology, and End Results database and Ontario databases17,18. They are notably lower than rates in studies conducted at a single institution the United States and a U.S.-based health maintenance organization; however, the latter studies also noted a correlation between socioeconomic status or access to insurance and rates of treatment19,20. Our low rates of systemic therapy could reflect the population-based nature of the study, involving both community and academic practice and including elderly patients, those with a poor performance status, and those with significant comorbidities who all had equal access to medical care through the Canadian health care system. In our study, overall rates of pemetrexed use increased over time, which is consistent with changes in the standard of care, including the introduction of first-line pemetrexed with platinum doublets and pemetrexed in maintenance therapy21–23. In 2007, Scagliotti et al.5 and Peterson et al.4 demonstrated that survival was inferior for patients with squamous cell carcinoma treated with pemetrexed than with other standard therapies. Accordingly, we observed that the use of pemetrexed in squamous cell carcinoma declined to 3% in 2011 from 17% in 2007.
Rates of egfr tki use almost doubled from 2007 to 2011 across all histologic subtypes. In that 6-year interval, multiple practice-changing trials were published, including use of first-line egfr tkis in EGFR mutation–positive patients, and use of erlotinib in maintenance therapy8–10,24. Testing for EGFR mutation and egfr tki administration is restricted to nonsquamous histology in first-line treatment. However, the clinical trials of maintenance and second- or third-line erlotinib were conducted in unrestricted populations and have approval for use in nonsquamous and squamous nsclc24–26. Consequently, we observed no change in the rates of egfr tki use by histologic classification. The proportion of patients with squamous cell tumours who received egfr tkis had risen by 2011. Previous studies in all histologies with unknown EGFR mutation status have suggested that outcomes with second-line egfr tkis and with chemotherapy (docetaxel and pemetrexed) are similar; practice in British Columbia likely reflected the knowledge of the time27,28. However, later data (published in 2013) compared docetaxel with erlotinib in all-histology, known EGFR wild-type nsclc and found progression-free survival and os to be superior with docetaxel29. Those data suggest that second-line chemotherapy is more appropriate for patients with wild-type EGFR.
Although the patterns of chemotherapy use changed from 2007 to 2011, the proportion of patients treated with first-line chemotherapy remained the same, and no difference in os duration was observed. The increase in second-line rates over time might reflect a pattern of practice change with the approval and funding of second-line and maintenance pemetrexed. The patient subgroups by year, histology, and systemic treatment were too small to draw any conclusions about os. A retrospective study of histology in patients treated with a platinum doublet on the Eastern Cooperative Oncology Group’s E1594 trial noted no significant difference in outcome based on histology alone30. Although an improvement in the nonsquamous group would be anticipated because of the EGFR mutation–positive group, those patients comprise only 10% of the North American population, and the effect is diluted by their counterparts without mutation8–10.
The strength of the present study is its population-based nature: the data include not only academic centres, but also community-based hospitals. The study also has several limitations. Data about the type of specimen (cytology or histology) was not collected, and the use of light microscopy alone rather than in combination with immunohistochemistry was not detailed. Although many patients were included in the overall study, the proportion that received systemic treatment was small.
CONCLUSIONS
We observed a significant reduction in the rate of nsclc nos from 2007 to 2011. That finding demonstrates the effectiveness of multidisciplinary education programs in disseminating information about advances in lung cancer care. The improved accuracy and specificity of the histologic categorization of nsclc will result in more appropriate use of resources for molecular testing, better systemic treatment, and better decision-making for patient care.
ACKNOWLEDGMENTS
The Outcomes and Surveillance Integration System database was developed with the financial assistance of Boehringer Ingelheim, Eli Lilly, and Roche. Our research was generously supported by the Eleni Skalbania Endowment Fund for Lung Cancer Research and the BC Cancer Foundation.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: DNI and JL have received speaker’s fees from Eli Lilly Canada for hospital-based continuing medical education programs throughout the province (content determined by consultants).
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Available online at: http://www.cancer.ca/~/media/cancer.ca/cw/cancer%20information/cancer%20101/canadian%20cancer%20statistics/canadian-cancer-statistics-2014-en.pdf; cited 11 October 2014] [Google Scholar]
- 2.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-Small Cell Lung Cancer Collaborative Group BMJ. 1996;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Peterson P, Fossella F, Gatzemeier U, John W, Scagliotti G. Is pemetrexed more effective in patients with non-squamous histology? A retrospective analysis of a phase iii trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (nsclc) [abstract] EJC Suppl. 2007;5:363–4. doi: 10.1016/S1359-6349(07)71349-6. [DOI] [Google Scholar]
- 5.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to nsclc histology: a review of two phase iii studies. Oncologist. 2009;14:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Reck M, von Pawel J, Zatloukal P, et al. Phase iii trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: avail. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu Y, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, et al. on behalf of the Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC, Yamamoto N, et al. Phase iii study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 31:3327–34. doi: 10.1200/JCO.2012.44.2806. 20213; [DOI] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Noguchi M, et al. on behalf of the American Thoracic Society International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou SH, Zell JA. Carcinoma nos is a common histologic diagnosis and is increasing in proportion among non–small cell lung cancer histologies. J Thorac Oncol. 2009;4:1202–11. doi: 10.1097/JTO.0b013e3181b28fb9. [DOI] [PubMed] [Google Scholar]
- 14.Sagerup CM, Småstuen M, Johannesen TB, Helland A, Brustugun OT. Increasing age and carcinoma not otherwise specified: a 20-year population study of 40,118 lung cancer patients. J Thorac Oncol. 2012;7:57–63. doi: 10.1097/JTO.0b013e3182307f7e. [DOI] [PubMed] [Google Scholar]
- 15.da Cunha Santos G, Lai SW, Saieg MA, et al. Cyto-histologic agreement in pathologic subtyping of non small cell lung carcinoma: review of 602 fine needle aspirates with follow-up surgical specimens over a nine year period and analysis of factors underlying failure to subtype. Lung Cancer. 2012;77:501–6. doi: 10.1016/j.lungcan.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 16.Schnabel PA, Smit E, Carpeno Jde C, et al. Influence of histology and biomarkers on first-line treatment of advanced non-small cell lung cancer in routine care setting: baseline results of an observational study (frame) Lung Cancer. 2012;78:263–29. doi: 10.1016/j.lungcan.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Earle CC, Neumann PJ, Gelber RD, Weinstein MC, Weeks JC. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–92. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 18.Yu JL, Simmons C, Victor JC, et al. Impact of new chemotherapeutic and targeted agents on survival in stage iv non-small cell lung cancer. Oncologist. 2011;16:1307–15. doi: 10.1634/theoncologist.2011-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5:1529–35. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritzwoller DP, Carroll NM, Delate T, et al. Patterns and predictors of first-line chemotherapy use among adults with advanced non-small cell lung cancer in the cancer research network. Lung Cancer. 2012;78:245–52. doi: 10.1016/j.lungcan.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scagliotti GV, Parikh P, von Pawel J, et al. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 22.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 23.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 24.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. on behalf of the National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 26.OSI Pharmaceuticals . Highlights of prescribing information: Tarceva (erlotinib) tablets, for oral use [2013 update] Farmingdale, NY: OSI Pharmaceuticals; 2013. [Available online at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021743s018lbl.pdf; cited 11 October 2014] [Google Scholar]
- 27.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 28.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (titan): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–8. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 29.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (tailor): a randomised controlled trial. Lancet Oncol. 2013;14:981–8. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 30.Hoang T, Dahlberg SE, Schiller JH, Johnson DH. Does histology predict survival of advanced non–small cell lung cancer patients treated with platin-based chemotherapy? An analysis of the Eastern Cooperative Oncology Group Study E1594. Lung Cancer. 2013;81:47–52. doi: 10.1016/j.lungcan.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]