Abstract
Introduction
This systematic review addresses the use of epidermal growth factor receptor (egfr) inhibitors in three populations of advanced non-small-cell lung cancer (nsclc) patients—unselected, selected, and molecularly selected—in three treatment settings: first line, second line, and maintenance.
Methods
Ninety-six randomized controlled trials found using the medline and embase databases form the basis of this review.
Results
In the first-line setting, data about the efficacy of egfr tyrosine kinase inhibitors (tkis) compared with platinum-based chemotherapy are inconsistent. Results from studies that selected patients based on clinical characteristics are also mixed. There is high-quality evidence that an egfr tki is preferred over a platinum doublet as initial therapy for patients with an activating mutation of the EGFR gene. The egfr tkis are associated with a higher likelihood of response, longer progression-free survival, and improved quality of life. Multiple trials of second-line therapy have compared an egfr tki with chemotherapy. Meta-analysis of those data demonstrates similar progression-free and overall survival. There is consequently no preferred sequence for second-line egfr tki or second-line chemotherapy. The egfr tkis have also been evaluated as switch-maintenance therapy. No molecular marker could identify patients in whom a survival benefit was not observed; however, the magnitude of the benefit was modest.
Conclusions
Determination of EGFR mutation status is essential to making appropriate treatment decisions in patients with nsclc. Patients who are EGFR mutation–positive should be treated with an egfr tki as first-line therapy. An egfr tki is still appropriate therapy in patients who are EGFR wild-type, but the selected agent should be administered as second- or third-line therapy.
Keywords: Non-small-small cell lung cancer, egfr inhibitors, mutation status, systematic review
INTRODUCTION
Lung cancer represents a major health burden. Many affected individuals present with advanced disease and are candidates for palliative systemic therapy. Historically, all patients with advanced non-small-cell lung cancer (nsclc) would receive similar therapy, in which platinum doublets were recommended as initial (first-line) therapy1,2, pemetrexed3 or docetaxel4,5 as second-line therapy, and erlotinib as second- or third-line therapy6,7.
Significant changes have taken place in the approach to the treatment of advanced nsclc since 2010. Treatment algorithms are now heavily influenced by the histologic subtype of nsclc8, and multiple trials have examined the sequence of subsequent lines of therapy [epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) vs. chemotherapy]. More importantly, the discovery of molecular abnormalities such as mutations of the EGFR gene9,10 and translocations of the ALK11 gene have identified a group of patients who appear to derive significantly greater benefit from molecularly targeted therapies.
METHODS
Four clinical members of the Program in Evidence-Based Care’s Lung Cancer Disease Site Group and one methodologist selected and reviewed evidence related to egfr tkis in nsclc. The body of evidence in this review primarily encompasses mature randomized controlled trial data.
Literature Search Strategy
The medline (2006 to March 2014), embase (2006 to March 2014), and Cochrane Library (March 2014) databases were searched for published practice guidelines, systematic reviews, and randomized clinical trials. Reference lists of papers and review articles were scanned for additional citations. The Canadian Medical Association Infobase (https://www.cma.ca/En/Pages/clinical-practice-guidelines.aspx), the U.S. National Guidelines Clearinghouse (http://www.guideline.gov/), and other Web sites were searched for existing evidence-based practice guidelines. The American Society of Clinical Oncology conference proceedings from 2007 to 2013 were also searched. Search terms indicative of nsclc, gefitinib (Iressa: AstraZeneca, Mississauga, ON), erlotinib (Tarceva: Genentech, San Francisco, CA, U.S.A.), afatinib, dacomitinib, and icotinib were used. Articles published before 2006 and included in this version of the systematic review were found using the search strategy described in the previous version of the guideline6. Only fully published articles from the previous version of this systematic review were included.
Study Selection Criteria
Publications were included in the review if they were meta-analyses or randomized trials (phase ii or iii) comparing gefitinib, erlotinib, afatinib, dacomitinib, or icotinib alone or in combination with chemotherapy with placebo, best supportive care, or chemotherapy; or comparing various doses or schedules of gefitinib, erlotinib, afatinib, dacomitinib, or icotinib; and fully published papers or published abstracts of trials in any language that reported at least one of the following outcomes by treatment group: symptom control, quality of life, tumour response rate, survival, or toxicity.
Publications were excluded from the review if they were pilot trials, dose-escalation trials, or case series (including expanded access programs); letters and editorials that reported clinical trial outcomes; or conference abstracts before 2007.
Synthesizing the Evidence
When clinically homogenous results from two or more trials were available, the data were pooled using the Review Manager software (RevMan 5.1.6) provided by the Cochrane Collaboration. Because hazard ratios (hrs), rather than the number of events at a certain time point, are the preferred statistic for pooling time-to-event outcomes12, hrs were extracted directly from the most recently reported trial results. The variances of the hr estimates were calculated from the reported confidence intervals (cis) using the methods described by Parmar et al.12. A random effects model was used for all pooling.
Statistical heterogeneity was calculated using the chi-square test for heterogeneity and the I2 percentage. A probability level for the chi-square statistic less than or equal to 10% (p ≤ 0.10) or an I2 greater than 50% (or both) were considered indicative of statistical heterogeneity. Results are expressed as hrs with 95% cis. A hr greater than 1.0 indicates that patients receiving gefitinib, erlotinib, afatinib, dacomitinib, or icotinib had a higher probability of experiencing an event; conversely, a hr less than 1.0 suggests that patients receiving erlotinib or gefitinib had a lower probability of experiencing an event.
RESULTS
Literature Search Results
Of the 3633 English and foreign-language studies identified, ninety-six randomized trials met the predefined eligibility criteria for the present systematic review. Of those trials, sixty-six were fully published reports, and thirty were in abstract form, including four updates to fully published trials. Slide presentations associated with abstract trial reports were also included if the presentations were publicly available on meeting Web sites and if they provided additional data. No relevant systematic reviews that answered our research questions were identified.
Outcomes
This report separately considers three populations of nsclc patients (unselected, clinically selected, and molecularly selected). In the unselected group, any nsclc patient was allowed to participate in the trial as long as the other trial eligibility criteria were met in the absence of molecular testing. In the clinically selected group, patients were selected based on clinical characteristics predictive of an EGFR mutation such as Asian ethnicity, adenocarcinoma histology, female sex, smoking status, or age. In the molecularly selected group, patients were included if their tumours tested positive for an EGFR mutation.
First-Line Treatment
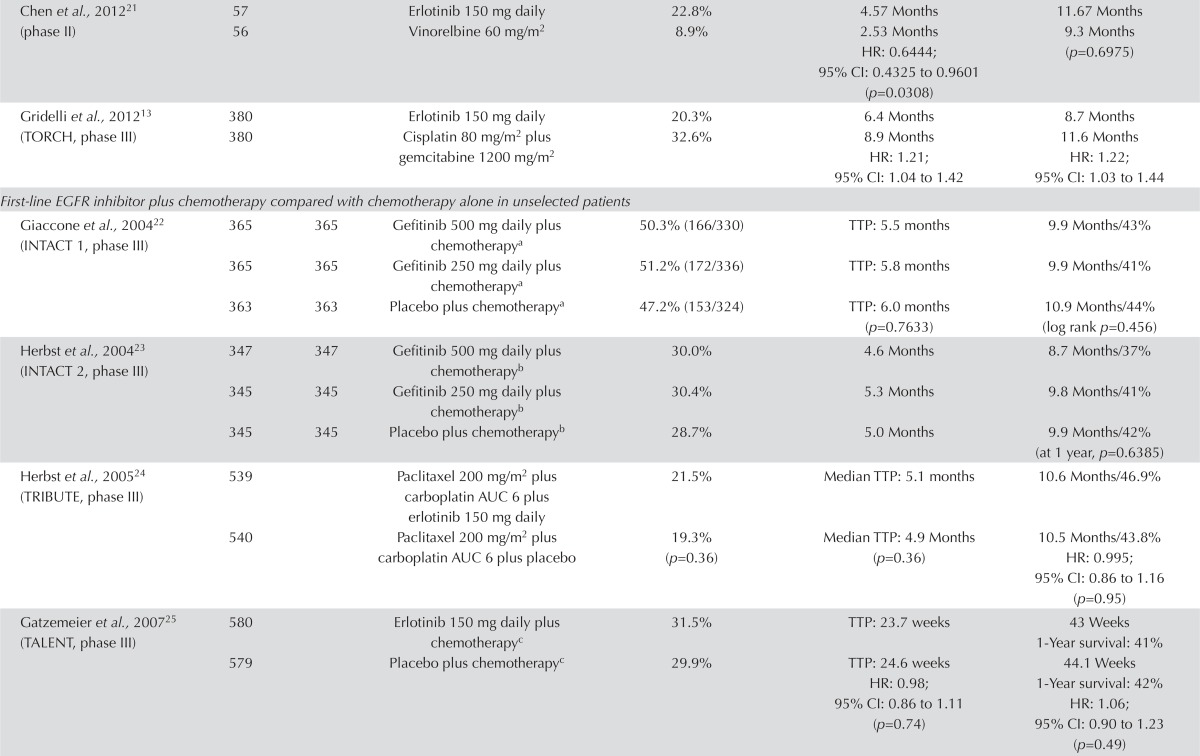
Unselected Populations: EGFR Inhibitor Compared with Chemotherapy: Six fully published papers and three abstracts compared an egfr inhibitor with platinum-based chemotherapy. Most of the trials were small, with fewer than 100 patients per arm. Only the torch trial appeared to have a sufficient number of participants to provide meaningful information on overall survival (os)13 (Table i). The findings of the trials suggest that first-line therapy with an egfr tki is inferior to chemotherapy in an unselected population of nsclc patients.
TABLE I.
First-line epidermal growth factor receptor (EGFR) inhibitors in unselected patients
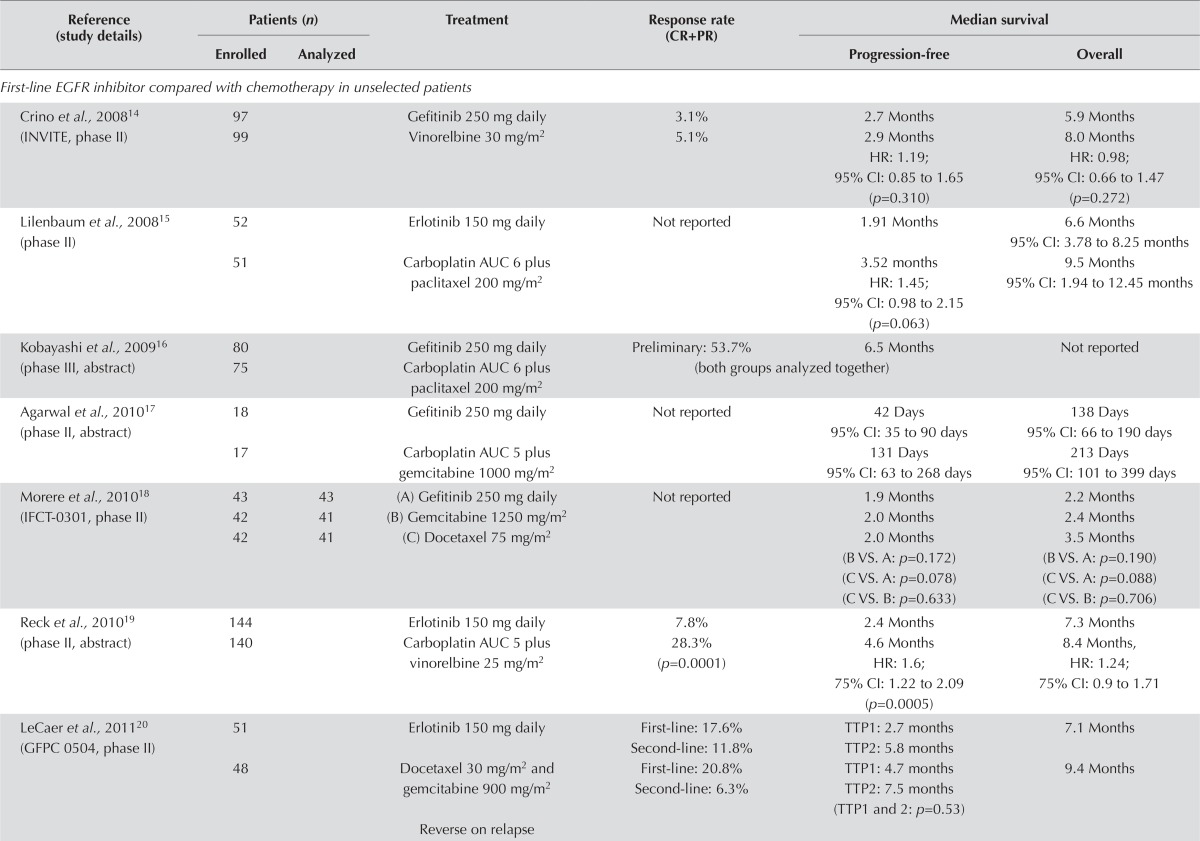
| Reference (study details) | Patients (n) | Treatment | Response rate (CR+PR) | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| First-line EGFR inhibitor compared with chemotherapy in unselected patients | ||||||
| Crino et al., 200814 (INVITE, phase II) | 97 | Gefitinib 250 mg daily | 3.1% | 2.7 Months | 5.9 Months | |
| 99 | Vinorelbine 30 mg/m2 | 5.1% | 2.9 Months HR: 1.19; 95% CI: 0.85 to 1.65 (p=0.310) |
8.0 Months HR: 0.98; 95% CI: 0.66 to 1.47 (p=0.272) |
||
| Lilenbaum et al., 200815 (phase II) | 52 | Erlotinib 150 mg daily | Not reported | 1.91 Months | 6.6 Months 95% CI: 3.78 to 8.25 months |
|
| 51 | Carboplatin AUC 6 plus paclitaxel 200 mg/m2 | 3.52 months HR: 1.45; 95% CI: 0.98 to 2.15 (p=0.063) |
9.5 Months 95% CI: 1.94 to 12.45 months |
|||
| Kobayashi et al., 200916 (phase III, abstract) | 80 | Gefitinib 250 mg daily | Preliminary: 53.7% | 6.5 Months | Not reported | |
| 75 | Carboplatin AUC 6 plus paclitaxel 200 mg/m2 | (both groups analyzed together) | ||||
| Agarwal et al., 201017 (phase II, abstract) | 18 | Gefitinib 250 mg daily | Not reported | 42 Days 95% CI: 35 to 90 days |
138 Days 95% ci: 66 to 190 days |
|
| 17 | Carboplatin AUC 5 plus gemcitabine 1000 mg/m2 | 131 Days 95% ci: 63 to 268 days |
213 Days 95% ci: 101 to 399 days |
|||
| Morere et al., 201018 (IFCT-0301, phase II) | 43 | 43 | (A) Gefitinib 250 mg daily | Not reported | 1.9 Months | 2.2 Months |
| 42 | 41 | (B) Gemcitabine 1250 mg/m2 | 2.0 Months | 2.4 Months | ||
| 42 | 41 | (C) Docetaxel 75 mg/m2 | 2.0 Months (B VS. A: p=0.172) (C VS. A: p=0.078) (C VS. B: p=0.633) |
3.5 Months (B VS. A: p=0.190) (C VS. A: p=0.088) (C VS. B: p=0.706) |
||
| Reck et al., 201019 (phase II, abstract) | 144 | Erlotinib 150 mg daily | 7.8% | 2.4 Months | 7.3 Months | |
| 140 | Carboplatin AUC 5 plus vinorelbine 25 mg/m2 | 28.3% (p=0.0001) | 4.6 Months HR: 1.6; 75% ci: 1.22 to 2.09 (p=0.0005) |
8.4 Months, HR: 1.24; 75% ci: 0.9 to 1.71 |
||
| LeCaer et al., 201120 (GFPC0504, phase II) | 51 | Erlotinib 150 mg daily | First-line: 17.6% Second-line: 11.8% |
TTP1: 2.7 months TTP2: 5.8 months |
7.1 Months | |
| 48 | Docetaxel 30 mg/m2 and gemcitabine 900 mg/m2 | First-line: 20.8% Second-line: 6.3% |
TTP1: 4.7 months TTP2: 7.5 months (TTP1 and 2: p=0.53) |
9.4 Months | ||
| Reverse on relapse | ||||||
| Chen et al., 201221 (phase II) | 57 | Erlotinib 150 mg daily | 22.8% | 4.57 Months | 11.67 Months | |
| 56 | Vinorelbine 60 mg/m2 | 8.9% | 2.53 Months HR: 0.6444; 95% CI: 0.4325 to 0.9601 (p=0.0308) |
9.3 Months (p=0.6975) | ||
| Gridelli et al., 201213 (TORCH, phase III) | 380 | Erlotinib 150 mg daily | 20.3% | 6.4 Months | 8.7 Months | |
| 380 | Cisplatin 80 mg/m2 plus gemcitabine 1200 mg/m2 | 32.6% | 8.9 Months HR: 1.21; 95% CI: 1.04 to 1.42 |
11.6 Months HR: 1.22; 95% CI: 1.03 to 1.44 |
||
| First-line EGFR inhibitor plus chemotherapy compared with chemotherapy alone in unselected patients | ||||||
| Giaccone et al., 200422 (INTACT 1, phase III) | 365 | 365 | Gefitinib 500 mg daily plus chemotherapya | 50.3% (166/330) | TTP: 5.5 months | 9.9 Months/43% |
| 365 | 365 | Gefitinib 250 mg daily plus chemotherapya | 51.2% (172/336) | TTP: 5.8 months | 9.9 Months/41% | |
| 363 | 363 | Placebo plus chemotherapya | 47.2% (153/324) | TTP: 6.0 months (p=0.7633) | 10.9 Months/44% (log rank p=0.456) | |
| Herbst et al., 200423 (INTACT 2, phase III) | 347 | 347 | Gefitinib 500 mg daily plus chemotherapyb | 30.0% | 4.6 Months | 8.7 Months/37% |
| 345 | 345 | Gefitinib 250 mg daily plus chemotherapyb | 30.4% | 5.3 Months | 9.8 Months/41% | |
| 345 | 345 | Placebo plus chemotherapyb | 28.7% | 5.0 Months | 9.9 Months/42% (at 1 year, p=0.6385) | |
| Herbst et al., 200524 (TRIBUTE, phase III) | 539 | Paclitaxel 200 mg/m2 plus carboplatin AUC 6 plus erlotinib 150 mg daily | 21.5% | Median TTP: 5.1 months | 10.6 Months/46.9% | |
| 540 | Paclitaxel 200 mg/m2 plus carboplatin AUC 6 plus placebo | 19.3% (p=0.36) | Median TTP: 4.9 Months (p=0.36) | 10.5 Months/43.8% HR: 0.995; 95% CI: 0.86 to 1.16 (p=0.95) |
||
| Gatzemeier et al., 200725 (TALENT, phase III) | 580 | Erlotinib 150 mg daily plus chemotherapyc | 31.5% | TTP: 23.7 weeks | 43 Weeks 1-Year survival: 41% |
|
| 579 | Placebo plus chemotherapyc | 29.9% | TTP: 24.6 weeks HR: 0.98; 95% CI: 0.86 to 1.11 (p=0.74) |
44.1 Weeks 1-Year survival: 42% HR: 1.06; 95% CI: 0.90 to 1.23 (p=0.49) |
||
| Nokikara et al., 200826 (phase II, abstract) | 49 | Carboplatin AUC 6 plus paclitaxel 200 mg/m2 plus gefitinib 250 mg daily | Not reported | 18.8 Months | 1 Year/61.2% | |
| 48 | Gefitinib 250 mg daily until disease progression, followed by carboplatin AUC 6 plus paclitaxel 200 mg/m2 | Not reported | 17.2 Months | 1 Year/68.1% | ||
| Mok et al., 200927 (phase II) | 76 | Erlotinib 150 mg daily plus gemcitabine 1250 mg/m2 and either cisplatin 75 mg/m2 or carboplatin AUC 5 | 35.5% | 29.4 Weeks | 74.1 Weeks | |
| 78 | Placebo plus gemcitabine 1250 mg/m2 and either cisplatin 75 mg/m2 or carboplatin AUC 5 | 24.4% | 23.4 Weeks HR: 0.47; 95% CI: 0.33 to 0.6 (p=0.0002) |
75.7 Weeks HR: 1.09; 95% CI: 0.70 to 1.69 (log rank p=0.42) |
||
| Riely et al., 200928 (phase II) | 28 | Erlotinib 150 mg daily on days 1 and 2 followed by carboplatin AUC 6 plus paclitaxel 200 mg/m2 on day 3 | 18% 95% CI: 6% to 37% |
TTP: 4 months 95% CI: 3 to 5 |
10 Months 95% CI: 8 to 16 months 1-Year survival: 49% 2-Year survival: 25% |
|
| 29 | Erlotinib 1500 mg daily on days 1 and 2 followed by carboplatin AUC 6 plus paclitaxel 200 mg/m2 on day 3 | 34% 95% CI: 18% to 54% |
TTP: 4 months 95% CI: 3 to 6 |
15 Months 95% CI: 8 months to nr 1-Year survival: 63% 2-Year survival: 42% |
||
| 29 | Carboplatin AUC 6 plus paclitaxel 200 mg/m2 on day 1 followed by erlotinib 1500 mg daily on days 2 and 3 | 28% 95% CI: 13% to 47% |
TTP: 5 months 95% CI: 3 to 8 |
10 Months 95% CI: 5 to 16 months 1-Year survival: 48% 2-Year survival: 26% |
||
| Mok et al., 201229 (FASTACT-II, phase III, abstract) | 226 | Chemotherapyd with inter-calculated erlotinib 150 mg daily, days 15–28 | 42.9% | 7.6 Months | 18.3 Months | |
| 225 | Chemotherapyd with placebo | 17.8% | 6 Months HR: 0.57; 95% CI: 0.46 to 0.70 (p<0.0001) |
14.9 Months HR: 0.78; 95% CI: 0.60 to 1.02 (p=0.069) |
||
| Other first-line trials in unselected patients | ||||||
| Goss et al., 200930 (phase II) | 100 | Gefitinib 250 mg daily plus BSC | 6.0% | 43 Days | 3.7 Months | |
| 101 | Placebo plus BSC | 1.0% | 41 Days HR: 0.82; 95% CI: 0.60 to 1.12 (p=0.217) |
2.8 Months HR: 0.84; 95% CI: 0.62 to 1.15 (p=0.272) |
||
| Gridelli et al., 201131 (phase II) | 29 | Sorafenib 800 mg daily plus erlotinib 150 mg daily | 10.3% 95% CI: 2.2% to 27.4% |
TTP: 12.7 weeks 95% CI: 2.0 to 69.4 weeks |
12.6 Months 51.9% (1-year) 95% CI: 36.0% to 74.8% |
|
| 31 | Sorafenib 800 mg daily plus gemcitabine 1200 mg/m2 | 6.5% 95% CI: 0.8% to 21.4% |
TTP: 8.1 weeks 95% CI: 1.0 to 65.0 weeks |
6.55 Months 35.2% (1-year) 95% CI: 21.4% to 57.7% |
||
| Stinchcombe et al., 201132 (phase II) | 44 | Gemcitabine 1200 mg/m2 (after disease progression, patients were offered erlotinib 150 mg daily) | 7% | 3.7 Months 95% CI: 2.3 to 4.7 months 6–22 Months 95% CI: 11 to 35 months |
6.8 Months 95% CI: 4.8 to 8.5 months |
|
| 51 | Erlotinib 150 mg daily | 0% | 2.8 Months 95% CI: 1.4 to 3.4 months 6–24 Months 95% CI: 13 to 36 months |
5.8 Months 95% CI: 3.0 to 8.3 months |
||
| 51 | Erlotinib 100 mg daily plus gemcitabine 1000 mg/m2 | 21% | 1.1 Months 95% CI2.4 to 5.0 months 6–25 Months 95% CI: 15 to 38 months |
5.6 Months 95% CI: 3.5 to 8.4 months |
||
| Thomas et al., 201133 (phase II, abstract) | 111 | Erlotinib 150 mg daily plus bevacizumab 15 mg/kg daily | 12.6% | 3.7 Months 95% CI: 2.8 to 4.3 months |
12.6 Months 95% CI: 10.3 to 16.2 months |
|
| 113 | Gemcitabine 1250 mg/m2 and cisplatin 80 mg/m2 plus bevacizumab 15 mg/kg daily | 33.6% | 7.2 Months 95% CI: 6.0 to 8.9 months |
15.7 Months 95% CI: 11.9 to 21.7 months |
||
| Boutsikou et al., 201334 (phase III) | 61 | Docetaxel 100 mg/m2 plus carboplatin AUC 5.5 | 11% | TTP: 2.23 months | 15.3 Months | |
| 52 | Docetaxel 100 mg/m2 plus carboplatin AUC 5.5 plus erlotinib 150 mg daily | 27% | TTP: 6.0 months | 16.4 Months | ||
| 56 | Bevacizumab 7.5 mg/kg plus docetaxel 100 mg/m2 plus carboplatin AUC 5.5 | 23% | TTP: 6.0 months | 19.1 Months | ||
| 60 | Docetaxel 100 mg/m2 plus carboplatin AUC 5.5 plus erlotinib 150 mg daily plus bevacizumab 7.5 mg/kg | 20% | TTP: 7.3 months (Significant for combination: p=0.001) | 22.1 Months (Did not differ between the four groups: p=0.381) | ||
| Lee et al., 201435 (TOPICAL, phase III) | 350 | Erlotinib 150 mg daily plus BSC | Not reported | 2.8 Months | 3.7 Months | |
| 320 | Placebo plus BSC | 2.6 Months HR: 0.83; 95% CI: 0.71 to 0.97 (p=0.019) |
3.6 Months HR: 0.94; 95% CI: 0.81 to 1.10 (p=0.46) |
|||
Gemcitabine 1250 mg/m2 plus cisplatin 80 mg/m2.
Paclitaxel 225 mg/m2 plus carboplatin AUC 6.
Gemcitabine 1250 mg/m2 plus cisplatin 80 mg/m2.
Gemcitabine 1250 mg/m2 plus carboplatin 5×AUC or cisplatin 75 mg/m2.
CR = complete response; PR = partial response; HR = hazard ratio; CI = confidence interval; AUC = area under curve; TTP = time to progression.
Response rate was not reported in three studies. In one study, the response rate favoured the egfr inhibitor21, and in four studies, it favoured chemotherapy13,14,19–21. The study by Reck et al.19 found a significantly higher response rate in patients randomized to chemotherapy (p = 0.0001).
The results show improved progression-free survival (pfs) for patients randomized to chemotherapy. Median pfs was similar in two trials14,18. In one trial, pfs was longer in the egfr inhibitor group: 4.57 months for erlotinib versus 2.53 months for vinorelbine (hr: 0.6444; 95% ci: 0.4325 to 0.9601; p = 0.0308)21. In five trials, pfs was longer in the chemotherapy group13,15,17,19,20. Several of the trials found that pfs significantly favoured chemotherapy13,15,19. One trial examined time to progression and found that it was longer with chemotherapy, but not significantly so20.
One trial reported nonsignificant improvements in os in the egfr inhibitor group21. In seven trials, os was prolonged with chemotherapy13–15,17–20. In the largest trial (torch), os was significantly worse for patients randomized to erlotinib13. Those findings suggest that initial therapy with an egfr tki in an unselected population of patients with advanced nsclc could be inferior treatment.
Quality of life and symptom control were discussed in three trials14,17,21. In the trial by Crino et al.14, the gefitinib group scored higher on all four of the quality of life assessment tools. The trials by Agarwal et al.17 and Chen et al.21 found no difference in quality of life, although the patients in the erlotinib group in the Chen et al. trial reported significantly better physical well-being.
The most significant toxicities from egfr inhibitors are diarrhea and rash. Most other adverse effects were mild and occurred at similar rates in all trials, with the exception of neutropenia, which occurred more commonly in the chemotherapy arm.
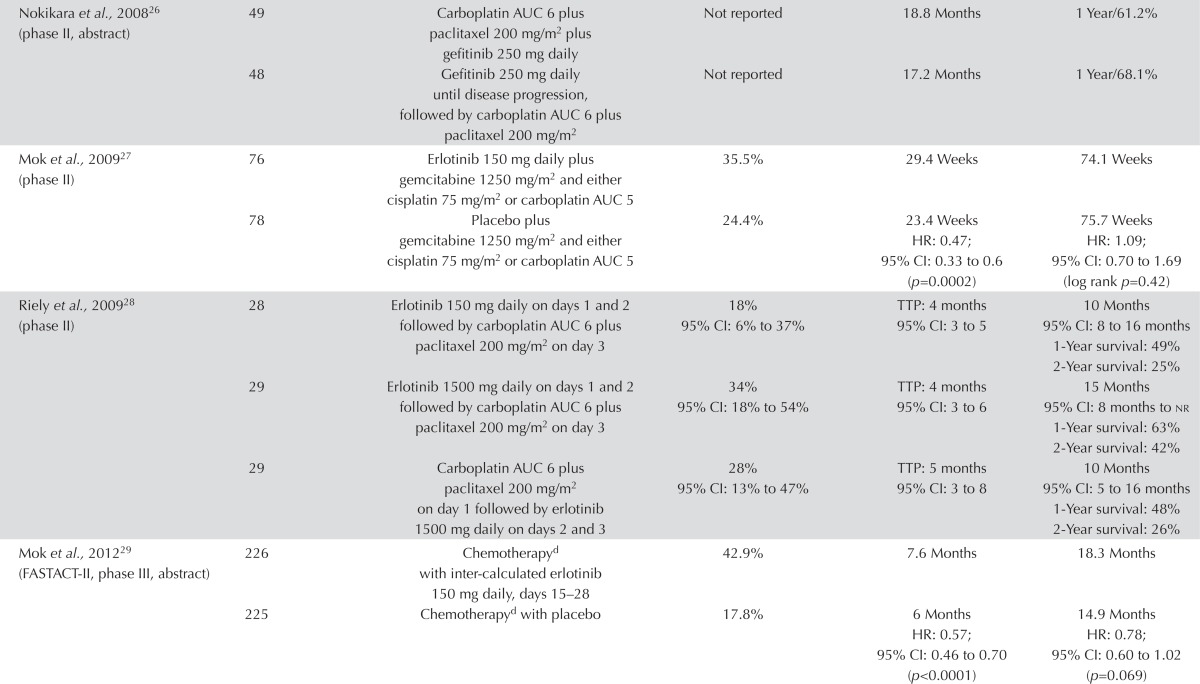
EGFR Inhibitor Plus Chemotherapy Compared with Chemo-therapy Alone: Eight trials examined the use of a first-line egfr inhibitor plus chemotherapy compared with chemotherapy alone in unselected patients. Four trials evaluated continuous egfr tki plus chemotherapy, three trials evaluated intermittent egfr tki (intercalated), and one trial evaluated combination chemotherapy plus an egfr tki compared with sequential egfr tki followed by chemotherapy.
The data showed no benefit for the addition of an egfr tki to first-line chemotherapy, although the trial of intercalated egfr tki showed an improvement in pfs. No significant differences in the response rate were observed in four trials involving more than 4000 patients22–25 (Table i). In three additional trials, the response rate favoured the egfr inhibitor group22–25,27–29. In the trial by Riely et al.28, the response rate was the highest (34%) for erlotinib 1500 mg daily, followed by paclitaxel and carboplatin chemotherapy. The response rate was 18% in the arm in which the dose of erlotinib was 150 mg, and 28% in the arm in which paclitaxel and carboplatin was followed by erlotinib 1500 mg daily.
Three trials reported pfs, with all reporting a longer pfs in the combined egfr inhibitor and chemotherapy groups23,27,29. Statistical significance was reported in two of the trials, which both favoured the egfr plus chemotherapy groups27,29. Four trials reported time to progression22,24,25,28,34. The intact 1 and 2, tribute, and talent trials all showed no significant difference in time to progression across all arms22,24,25. The trial by Riely et al.28 did not show an increase in time to progression when erlotinib daily doses of 150 mg and 1500 mg were compared (both followed by paclitaxel and carboplatin): in both groups, time to progression was 4 months. The combination of paclitaxel and carboplatin followed by erlotinib 1500 mg daily showed a 1-month increase in time to progression. An unplanned subgroup analysis by mutation status for patients in the tribute trial with available tissue showed an increase in time to progression for erlotinib plus paclitaxel and carboplatin (12.5 months) compared with chemotherapy alone (6.6 months), but that difference did not reach significance (p = 0.092)24.
There was no clear improvement in os with the addition of an egfr tki to chemotherapy. Statistical significance was not reached in any trial. In the trial by Riely et al.28, survival was greatest with erlotinib 1500 mg daily followed by paclitaxel and carboplatin: 15 months compared with 10 months for both erlotinib 150 mg daily followed by paclitaxel and carboplatin, and paclitaxel and carboplatin followed by erlotinib 1500 mg daily. The fast-act ii trial observed a trend toward longer os favouring the chemotherapy plus erlotinib arm (hr: 0.78; 95% ci: 0.60 to 1.02; p = 0.069)29. Those results do not support the addition of an egfr tki to platinum-based chemotherapy. Toxicities were similar between the groups, with the exception of diarrhea and skin disorders, which occurred more frequently in the egfr inhibitor groups.
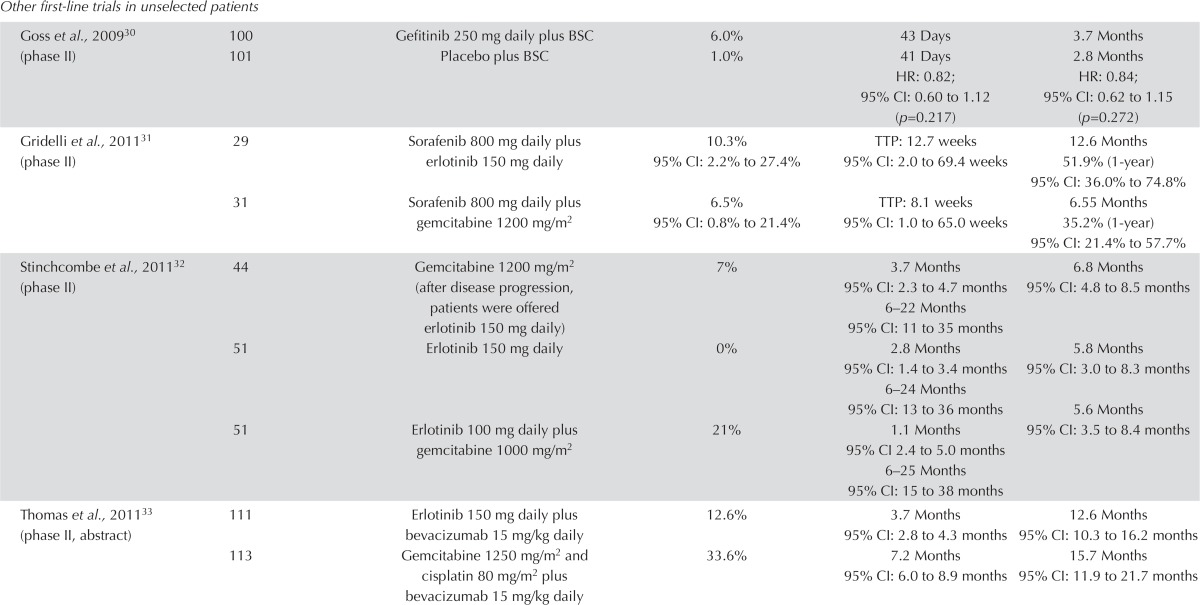
Other First-Line Trials: Six additional trials evaluating various approaches of egfr tki and chemotherapy were identified; none showed evidence of improved os. In two trials evaluating an egfr tki compared with placebo in patients not suitable for chemotherapy, no clear differences in pfs or os were observed (Table i). Statistical significance was reached in the trial by Lee et al.35 for pfs, but neither trial showed a difference in os30,35. Quality of life in the Goss et al.30 trial was not different between the two arms. For gefitinib, the rates of improvement in quality of life were 21.1% [by the Functional Assessment of Cancer Therapy–Lung (fact-L)], 15.8% (by the Trial Outcome Index), 32.9% (by the lung cancer subscale of the fact-L), and 28.3% (by the Pulmonary Symptom Improvement test); for placebo, the corresponding rates were 20%, 13.8%, 30.89%, and 28.3% respectively.
In the 3-arm trial by Stinchcombe et al.32, sequential and concurrent gemcitabine plus erlotinib both led to higher response rates and longer pfs than did erlotinib alone, although the differences were not statistically significant. The longest os was observed in patients receiving sequential chemotherapy followed by erlotinib. No clear difference in quality of life was evident using the Trial Outcome Index (p = 0.76), the lung cancer subscale of the fact-L (p = 0.85), or the fact-L (p = 0.57).
The two trials that compared an egfr inhibitor plus a targeted agent with a targeted agent and chemotherapy showed mixed results31,33. The trial by Boutsikou et al.34 used a factorial design to evaluate the addition of erlotinib and bevacizumab to cisplatin and docetaxel. No significant improvement in os was observed, although the response rate was highest in the chemotherapy plus erlotinib arm. Time to progression was significant and longest in the combination arm (p = 0.001).
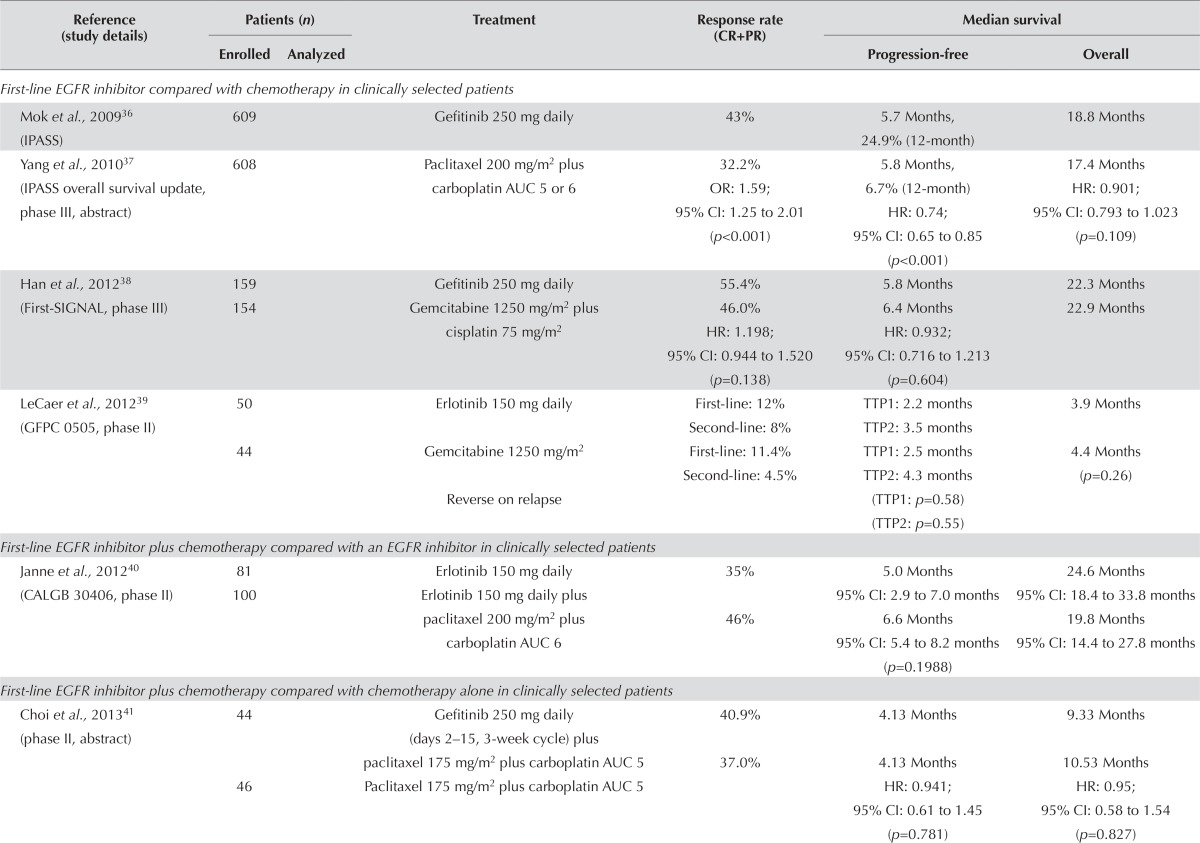
Clinically Selected Populations: Three studies that compared an egfr inhibitor with chemotherapy in clinically selected patients in the first-line setting (Table ii) were identified. A large proportion of the patients in these trials crossed over to the alternative therapy at progression. The ipass trial demonstrated significant improvements in response rate and pfs, but no difference in os36. No significant outcome differences were observed in the other two trials38,39.
TABLE II.
First-line epidermal growth factor receptor (EGFR) inhibitors in clinically selected patients
| Reference (study details) | Patients (n) | Treatment | Response rate (CR+PR) | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| First-line EGFR inhibitor compared with chemotherapy in clinically selected patients | ||||||
| Mok et al., 200936 (IPASS) | 609 | Gefitinib 250 mg daily | 43% | 5.7 Months, 24.9% (12-month) | 18.8 Months | |
| Yang et al., 201037 (IPASS overall survival update, phase III, abstract) | 608 | Paclitaxel 200 mg/m2 plus carboplatin AUC 5 or 6 | 32.2% OR: 1.59; 95% CI: 1.25 to 2.01 (p<0.001) |
5.8 Months, 6.7% (12-month) HR: 0.74; 95% CI: 0.65 to 0.85 (p<0.001) |
17.4 Months HR: 0.901; 95% CI: 0.793 to 1.023 (p=0.109) |
|
| Han et al., 201238 (First-SIGNAL, phase III) | 159 | Gefitinib 250 mg daily | 55.4% | 5.8 Months | 22.3 Months | |
| 154 | Gemcitabine 1250 mg/m2 plus cisplatin 75 mg/m2 | 46.0% HR: 1.198; 95% CI: 0.944 to 1.520 (p=0.138) |
6.4 Months HR: 0.932; 95% CI: 0.716 to 1.213 (p=0.604) |
22.9 Months | ||
| LeCaer et al., 201239 (GFPC0505, phase II) | 50 | Erlotinib 150 mg daily | First-line: 12% Second-line: 8% |
TTP1: 2.2 months TTP2: 3.5 months |
3.9 Months | |
| 44 | Gemcitabine 1250 mg/m2 | First-line: 11.4% Second-line: 4.5% |
TTP1: 2.5 months TTP2: 4.3 months |
4.4 Months (p=0.26) | ||
| Reverse on relapse | (TTP1: p=0.58) (TTP2: p=0.55) |
|||||
| First-line EGFR inhibitor plus chemotherapy compared with an EGFR inhibitor in clinically selected patients | ||||||
| Janne et al., 201240 (CALGB30406, phase II) | 81 | Erlotinib 150 mg daily | 35% | 5.0 Months | 24.6 Months | |
| 100 | Erlotinib 150 mg daily plus paclitaxel 200 mg/m2 plus carboplatin AUC 6 | 46% | 95% CI: 2.9 to 7.0 months 6.6 Months 95% CI: 5.4 to 8.2 months (p=0.1988) |
95% CI: 18.4 to 33.8 months 19.8 Months 95% CI: 14.4 to 27.8 months |
||
| First-line EGFR inhibitor plus chemotherapy compared with chemotherapy alone in clinically selected patients | ||||||
| Choi et al., 201341 (phase II, abstract) | 44 | Gefitinib 250 mg daily (days 2–15, 3-week cycle) plus paclitaxel 175 mg/m2 plus carboplatin AUC 5 | 40.9% | 4.13 Months | 9.33 Months | |
| 46 | Paclitaxel 175 mg/m2 plus carboplatin AUC 5 | 37.0% | 4.13 Months HR: 0.941; 95% CI: 0.61 to 1.45 (p=0.781) |
10.53 Months HR: 0.95; 95% CI: 0.58 to 1.54 (p=0.827) |
||
| Michael et al., 201242 (GATE, phase II, abstract) | 26 | Erlotinib 150 mg daily (days 15–28) plus gemcitabine 1000 mg/m2 | 3.8% | 10.3 Months | Not reported | |
| 28 | Gemcitabine 1000 mg/m2 | 7.1% | 8.0 Months HR: 1.3; 95% CI: 0.63 to 2.68 (p=0.4798) |
|||
| Liang et al., 201043 (phase II, abstract) | 25 | Pemetrexed 500 mg/ m2 plus cisplatin 75 mg/m2 plus gefitinib 250 mg daily | Not reported | 9.95 Months | 74.8% (12-month) 59.6% (24-month) |
|
| 24 | Pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 | 6.83 Months HR: 0.533; 95% CI: 0.272 to 1.044 (p=0.067) |
93.3% (12-month) 71.1% (24-month) |
|||
CR = complete response; PR = partial response; AUC = area under curve; OR = odds ratio; CI = confidence interval; HR = hazard ratio.
Subgroup analyses for the ipass and First-signal trials were done for patients with tumour samples available for EGFR mutation testing36,38. In the First-signal trial, EGFR mutation–positive patients treated with gefitinib (compared with those treated with gemcitabine and cisplatin) showed a higher overall response rate (84.6% vs. 37.5%, p = 0.002) and a trend toward longer pfs (hr: 0.544; 95% ci: 0.269 to 1.100; p = 0.086). The mutation-negative patients in the gemcitabine and cisplatin arm (compared with the those in the gefitinib arm) showed a trend toward a higher overall response rate (51.9% vs. 25.9%, p = 0.051) and longer pfs (hr: 1.419; 95% ci: 0.817 to 2.466; p = 0.226). The treatment arms showed no significant differences in os according to EGFR mutation status (mutation-positive subgroup hr: 1.043; 95% ci: 0.498 to 2.182; mutation-negative subgroup hr: 1.000; 95% ci: 0.523 to 1.911; and mutation-unknown subgroup hr: 0.880; 95% ci: 0.639 to 1.210)38.
Findings were similar in the ipass trial: pfs was significantly longer for patients in the mutation-positive subgroup receiving gefitinib than for those receiving carboplatin–paclitaxel (hr: 0.48; 95% ci: 0.36 to 0.64; p < 0.001). In the mutation-negative subgroup, pfs was significantly shorter in patients receiving gefitinib than in those receiving carboplatin–paclitaxel (hr: 2.85; 95% ci: 2.05 to 3.98; p < 0.001). Results in the subgroup with unknown EGFR mutation status were similar to those for the overall population. The os with gefitinib therapy trended longer in the mutation-positive subgroup (hr: 0.78; 95% ci: 0.50 to 1.20) than in the mutation-negative subgroup (hr: 1.38; 95% ci: 0.92 to 2.09) or in the mutation-unknown subgroup (hr: 0.86; 95% ci: 0.68 to 1.09)36, which suggests that the benefit of first-line therapy with an egfr tki is limited to patients with tumours known to harbour an EGFR mutation. Clinical characteristics should not be used to select patients for first-line egfr tki therapy.
One trial evaluated the combination of an egfr tki plus chemotherapy compared with an egfr tki alone in clinically selected patients. The response rate was greater in the egfr inhibitor plus chemotherapy arm; however, no significant differences in pfs (p = 0.1988) or os40 were observed. Adverse effects were consistent with those associated with chemotherapy and egfr inhibitors40.
Three additional trials compared the combination of an egfr tki plus chemotherapy with chemotherapy alone in clinically selected patients. The addition of gefitinib to cisplatin and pemetrexed resulted in a trend toward longer pfs, but no improvement in os43. No clear benefit was observed in the other two trials evaluating the addition of gefitinib to carboplatin and paclitaxel41 or of erlotinib to gemcitabine42.
Results for symptom control and quality of life were addressed in two studies. In the ipass trial, statistical and clinically relevant improvements in quality of life were associated with the use of the egfr inhibitor36. The First-signal trial found significant differences in physical (p < 0.001) and social functioning (p = 0.013) favouring gefitinib. No significant differences in emotional and cognitive functioning were observed38.
Adverse effects were consistent with those known for egfr inhibitors and chemotherapy.
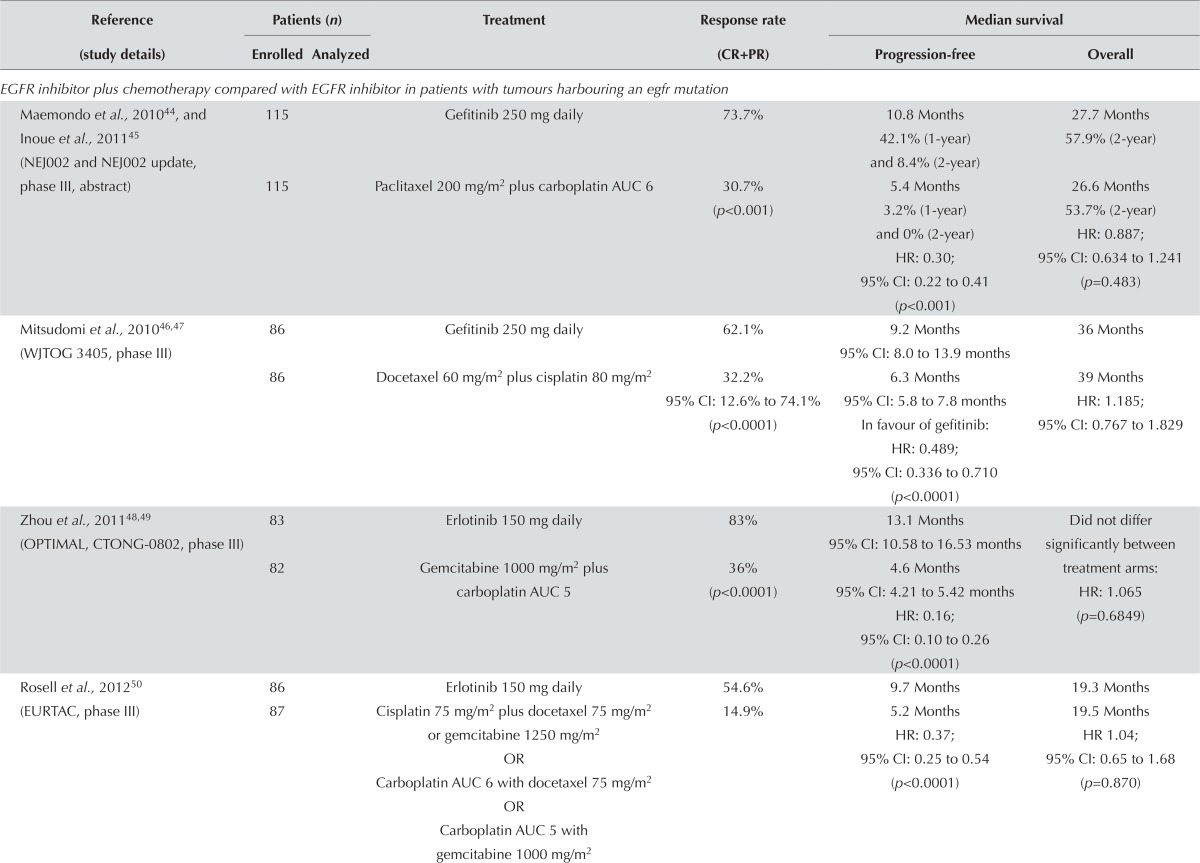
Molecularly Selected Populations: Seven trials used an egfr inhibitor in molecularly selected patients with stage iiib/iv nsclc. One trial selected patients on the basis of egfr protein overexpression (assessed by immunohistochemistry) or increased gene copy number (assessed by fluorescence in situ hybridization, Table iii). Six trials selected patients with tumours harbouring an EGFR mutation. A meta-analysis of this group of patients was performed because the patients were homogenous, and the treatment comparators were platinum-based chemotherapy regimens. All six trials observed higher response rates favouring the egfr inhibitor group. Three of the trials (Mitsudomi et al.46, Zhou et al.48 and Yang et al.51) found the results to be statistically significant (p < 0.0001).
TABLE III.
First-line epidermal growth factor receptor (EGFR) inhibitor in molecularly selected patients
| Reference (study details) | Patients (n) | Treatment | Response rate (CR+PR) | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| EGFR inhibitor plus chemotherapy compared with EGFR inhibitor in patients with tumours harbouring an egfr mutation | ||||||
| Maemondo et al., 201044, and Inoue et al., 201145 (NEJ002 and NEJ002 update, phase III, abstract) | 115 | Gefitinib 250 mg daily | 73.7% | 10.8 Months 42.1% (1-year) and 8.4% (2-year) |
27.7 Months 57.9% (2-year) |
|
| 115 | Paclitaxel 200 mg/m2 plus carboplatin AUC 6 | 30.7% (p<0.001) | 5.4 Months 3.2% (1-year) and 0% (2-year) HR: 0.30; 95% CI: 0.22 to 0.41 (p<0.001) |
26.6 Months 53.7% (2-year) HR: 0.887; 95% CI: 0.634 to 1.241 (p=0.483) |
||
| Mitsudomi et al., 201046,47 (WJTOG3405, phase III) | 86 | Gefitinib 250 mg daily | 62.1% | 9.2 Months 95% CI: 8.0 to 13.9 months |
36 Months | |
| 86 | Docetaxel 60 mg/m2 plus cisplatin 80 mg/m2 | 32.2% 95% CI: 12.6% to 74.1% (p<0.0001) |
6.3 Months 95% CI: 5.8 to 7.8 months In favour of gefitinib: HR: 0.489; 95% CI: 0.336 to 0.710 (p<0.0001) |
39 Months HR: 1.185; 95% CI: 0.767 to 1.829 |
||
| Zhou et al., 201148,49 (OPTIMAL, CTONG-0802, phase III) | 83 | Erlotinib 150 mg daily | 83% | 13.1 Months 95% CI: 10.58 to 16.53 months |
Did not differ significantly between treatment arms: | |
| 82 | Gemcitabine 1000 mg/m2 plus carboplatin AUC 5 | 36% (p<0.0001) | 4.6 Months 95% CI: 4.21 to 5.42 months HR: 0.16; 95% CI: 0.10 to 0.26 (p<0.0001) |
HR: 1.065 (p=0.6849) | ||
| Rosell et al., 201250 (EURTAC, phase III) | 86 | Erlotinib 150 mg daily | 54.6% | 9.7 Months | 19.3 Months | |
| 87 | Cisplatin 75 mg/m2 plus docetaxel 75 mg/m2 or gemcitabine 1250 mg/m2 OR Carboplatin AUC 6 with docetaxel 75 mg/m2 OR Carboplatin AUC 5 with gemcitabine 1000 mg/m2 |
14.9% | 5.2 Months HR: 0.37; 95% CI: 0.25 to 0.54 (p<0.0001) |
19.5 Months HR 1.04; 95% CI: 0.65 to 1.68 (p=0.870) |
||
| EGFR inhibitor plus chemotherapy compared with EGFR inhibitor in patients with tumours harbouring an EGFR mutation | ||||||
| Yang et al., 201251 (LUX-Lung 3, phase III, abstract) | 230 | Afatinib 40 mg daily | 56% | 11.1 Months | ||
| 115 | Pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 | 23% (p<0.0001) | 6.9 Months HR: 0.58; 95% CI: 0.43 to 0.78 (p=0.0004) |
|||
| Wu et al., 201352 (LUX-Lung 6, phase III) | 242 | Afatinib 40 mg daily | 66.9% | 11 Months | 22.1 Months | |
| 122 | Gemcitabine 1000 mg/m2 plus cisplatin 75 mg/m2 | 23.0% | 5.6 Months HR: 0.28; 95% ci:0.20 to 0.39 |
22.2 Months HR: 0.95; 95% CI: 0.68 to 1.33 (p=0.76) |
||
| EGFR inhibitor plus chemotherapy compared with EGFR inhibitor alone in patients with EGFR protein overexpression or increased gene copy number | ||||||
| Hirsch et al., 201153 (phase II) | 72 | 69 | Erlotinib 150 mg daily | 11.6% | 2.69 Months 30.7% (6-month) |
16.7 Months 59% (1-year) |
| 71 | 68 | Erlotinib 150 mg daily plus paclitaxel 200 mg/m2 plus carboplatin AUC 6 | 22.4% | 4.57 Months 26.4% (6-month) |
11.43 Months 46% (1-year) |
|
CR = complete response; PR = partial response; AUC = area under curve; HR = hazard ratio; CI = confidence interval.
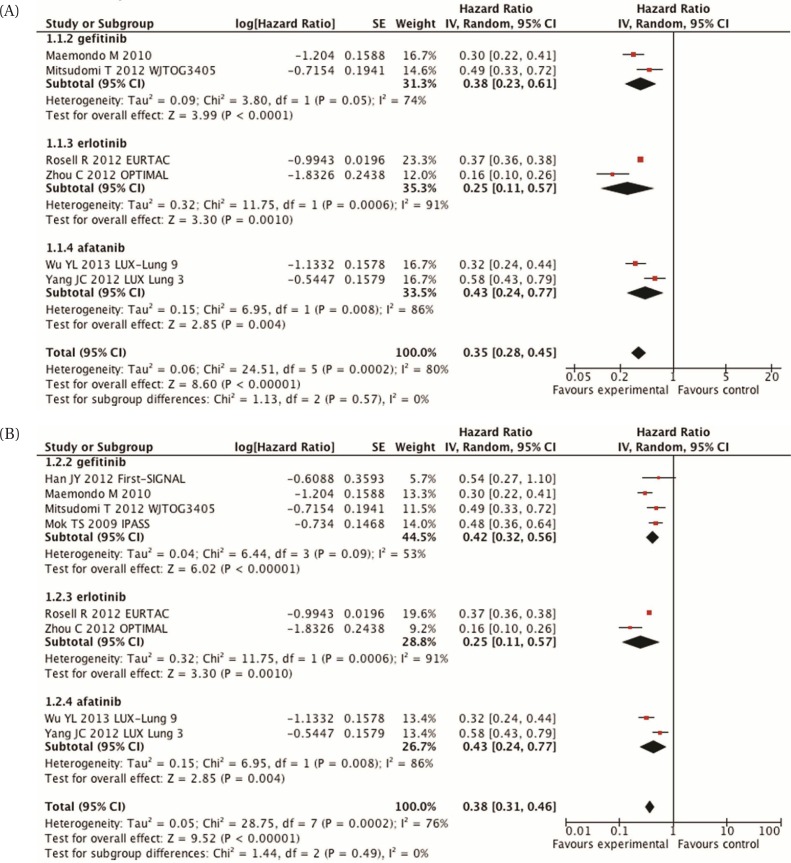
In every trial, pfs was also statistically significant and favoured the egfr inhibitor44,46,48,50–52. A meta-analysis [Figure 1(A)] demonstrated a statistically significant improvement in pfs (hr: 0.35; 95% ci: 0.28 to 0.45; p < 0.00001). However, the I2 is high at 80%, which shows considerable statistical heterogeneity. In each of the subgroup analyses (different egfr inhibitors), the I2 also remains high. The cause of the heterogeneity remains unknown at this time.
FIGURE 1.
(A) Meta-analysis of progression-free survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in molecularly selected patients. (B) Meta-analysis of progression-free survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in molecularly selected patients, including those in the IPASS and First-SIGNAL trials. SE = standard error; IV = inverse variance; CI = confidence interval.
The addition of the subgroup analyses from both the ipass and First-signal trials in patients with a known EGFR mutation status36,38 resulted in similar findings [hr: 0.38; 95% ci: 0.31 to 0.46; p < 0.00001; Figure 1(B)]. Evidence of statistical heterogeneity remains, with an I2 of 76%.
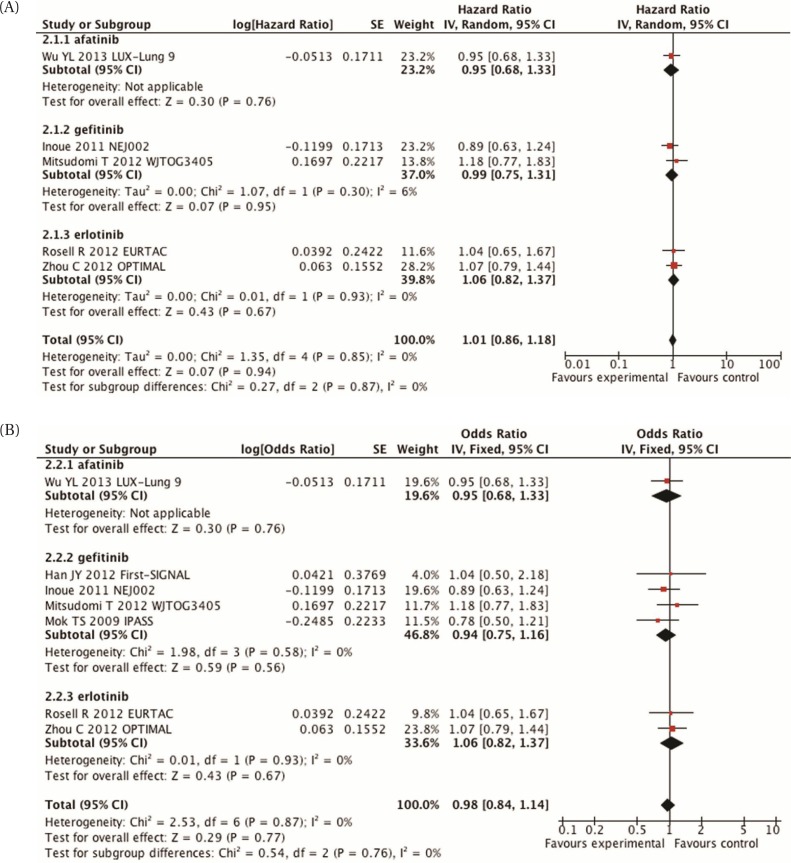
Six trials reported os. The data are difficult to interpret, because many patients are likely to have crossed over to the other treatment arm, but the actual percentages are not reported. Meta-analysis of those trials demonstrates no difference in survival between the two groups [hr: 1.01; 95% ci: 0.86 to 1.18; p = 0.94; Figure 2(A)]. Inclusion of data from the ipass and First-signal trials did not change that result [hr: 0.98; 95% ci: 0.84 to 1.14; p = 0.77; Figure 2(B)].
FIGURE 2.
(A) Meta-analysis of overall survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in molecularly selected patients. (B) Meta-analysis of overall survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in molecularly selected patients, including those in the IPASS and First-SIGNAL trials. SE = standard error; IV = inverse variance; CI = confidence interval.
One additional study compared an egfr inhibitor plus chemotherapy with an egfr inhibitor alone in patients with egfr protein overexpression or increased gene copy number53. No clear recommendation can be made from that trial. Response rate and pfs were higher in the egfr plus chemotherapy group, but os favoured the egfr-inhibitor-alone group The most significant toxicity was skin rash, which occurred in slightly higher numbers in the egfr-inhibitor-alone group53.
Symptom control and quality of life were discussed in the Yang et al.51 and Wu et al.52 studies. A significant delay in time to deterioration of the cancer-related symptoms of cough (hr: 0.60; p = 0.0072) and dyspnea (hr: 0.68; p = 0.0145) was seen with the egfr inhibitor afatinib51. A higher proportion of patients in the afatinib group experienced a significantly longer time to deterioration (hr: 0.56; 95% ci: 0.41 to 0.77; p = 0.0002)52.
The adverse effects were consistent with those found with egfr inhibitors and chemotherapy.
Second-Line Treatment
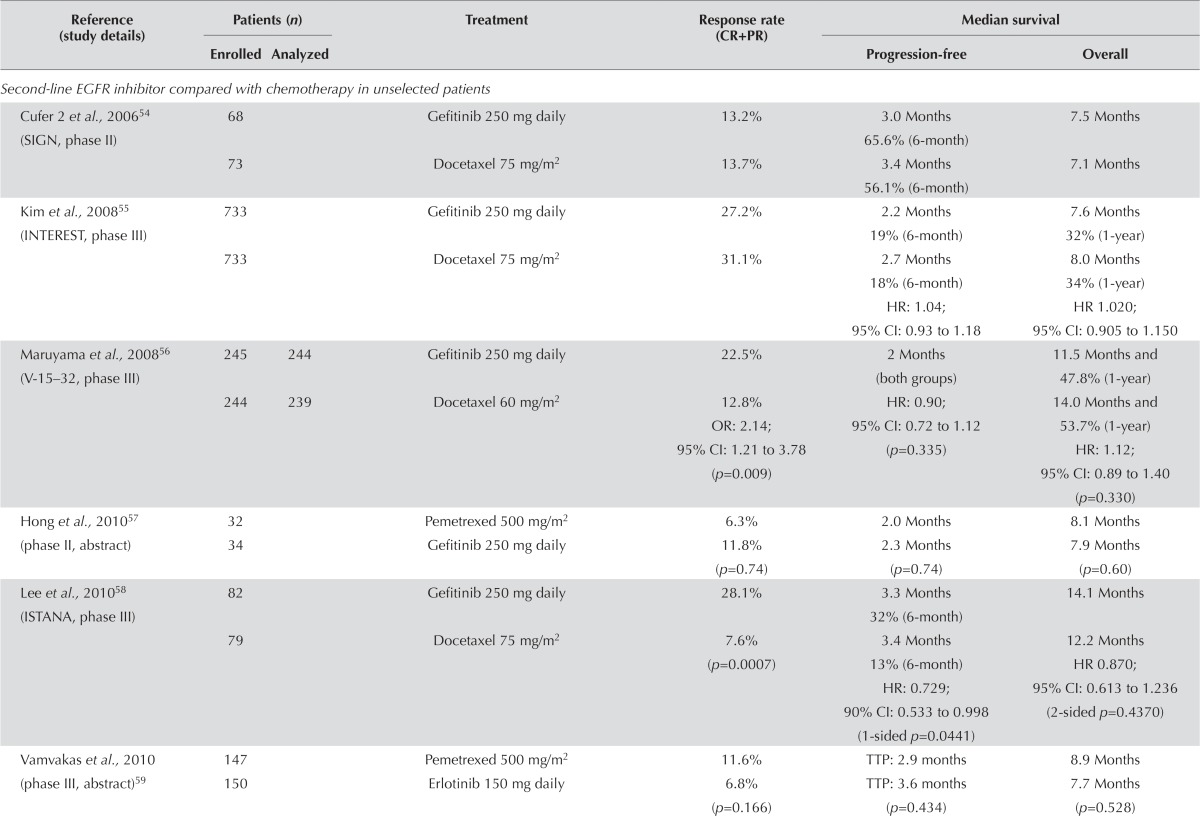
Unselected Populations: EGFR Inhibitor Compared with Chemotherapy: Ten studies54–63 compared an egfr inhibitor with chemotherapy (docetaxel or pemetrexed) in second-line treatment (Table iv). None of the trials incorporated a planned crossover to the other agent at the time of progression. However, at progression, patients were permitted to receive the alternative treatment to which they were assigned. No significant difference in response rate was observed in six of the ten studies54,55,57,59–61,63. In three of the four studies conducted in Asian populations, the egfr inhibitor was associated with a significantly higher response rate56,58,63.
TABLE IV.
Second-line epidermal growth factor receptor (EGFR) inhibitor in unselected patients
| Reference (study details) | Patients (n) | Treatment | Response rate (CR+PR) | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| Second-line EGFR inhibitor compared with chemotherapy in unselected patients | ||||||
| Cufer 2 et al., 200654 (SIGN, phase II) | 68 | Gefitinib 250 mg daily | 13.2% | 3.0 Months 65.6% (6-month) |
7.5 Months | |
| 73 | Docetaxel 75 mg/m2 | 13.7% | 3.4 Months 56.1% (6-month) |
7.1 Months | ||
| Kim et al., 200855 (INTEREST, phase III) | 733 | Gefitinib 250 mg daily | 27.2% | 2.2 Months 19% (6-month) |
7.6 Months 32% (1-year) |
|
| 733 | Docetaxel 75 mg/m2 | 31.1% | 2.7 Months 18% (6-month) HR: 1.04; 95% CI: 0.93 to 1.18 |
8.0 Months 34% (1-year) HR 1.020; 95% CI: 0.905 to 1.150 |
||
| Maruyama et al., 200856 (V-15–32, phase III) | 245 | 244 | Gefitinib 250 mg daily | 22.5% | 2 Months (both groups) | 11.5 Months and 47.8% (1-year) |
| 244 | 239 | Docetaxel 60 mg/m2 | 12.8% OR: 2.14; 95% CI: 1.21 to 3.78 (p=0.009) |
HR: 0.90; 95% CI: 0.72 to 1.12 (p=0.335) |
14.0 Months and 53.7% (1-year) HR: 1.12; 95% CI: 0.89 to 1.40 (p=0.330) |
|
| Hong et al., 201057 (phase II, abstract) | 32 | Pemetrexed 500 mg/m2 | 6.3% | 2.0 Months | 8.1 Months | |
| 34 | Gefitinib 250 mg daily | 11.8% (p=0.74) | 2.3 Months (p=0.74) | 7.9 Months (p=0.60) | ||
| Lee et al., 201058 (ISTANA, phase III) | 82 | Gefitinib 250 mg daily | 28.1% | 3.3 Months 32% (6-month) |
14.1 Months | |
| 79 | Docetaxel 75 mg/m2 | 7.6% (p=0.0007) | 3.4 Months 13% (6-month) HR: 0.729; 90% CI: 0.533 to 0.998 (1-sided p=0.0441) |
12.2 Months HR 0.870; 95% CI: 0.613 to 1.236 (2-sided p=0.4370) |
||
| Vamvakas et al., 2010 (phase III, abstract)59 | 147 | Pemetrexed 500 mg/m2 | 11.6% | TTP: 2.9 months | 8.9 Months | |
| 150 | Erlotinib 150 mg daily | 6.8% (p=0.166) | TTP: 3.6 months (p=0.434) | 7.7 Months (p=0.528) | ||
| Ciuleanu et al., 201260 (TITAN, phase III) | 203 | Erlotinib 150 mg daily | 7.9% | 6.3 Weeks | 5.3 Months | |
| 221 | Docetaxel or pemetrexed, dose determined by centre | 6.3% | 8.6 Weeks HR: 1.19; 95% CI: 0.97 to 1.46 (p=0.089) |
5.5 Months HR: 0.96; 95% CI: 0.78 to 1.19 (p=0.73) |
||
| Karampeazis et al., 201361 (phase III) | 179 | Erlotinib 150 mg daily | 9.0% | 3.6 Months | 8.2 Months | |
| 178 | Pemetrexed 500 mg/m2 | 11.4% (p=0.469) | 2.9 Months (p=0.136) | 10.1 Months (p=0.986) | ||
| Okano et al., 201362 (DELTA, phase III, abstract) | 150 | Erlotinib 150 mg daily | Not reported | 2.0 Months | 14.8 Months | |
| 151 | Docetaxel 60 mg/m2 | 3.2 Months HR: 1.22; 95% CI: 0.97 to 1.55 (p=0.092) |
12.2 Months HR: 0.91; 95% CI: 0.68 to 1.22 (p=0.527) |
|||
| Kelly et al., 201263 (phase II) | 101 | Erlotinib 150 mg daily | 7% | 2.8 Months | 7 Months | |
| 100 | Pralatrexate 190 mg/m2 | 2% | 3.4 Months HR 0.91; 95% CI: 0.63 to 1.32 |
6.7 Months HR: 0.84; 95% CI: 0.61 to 1.14 |
||
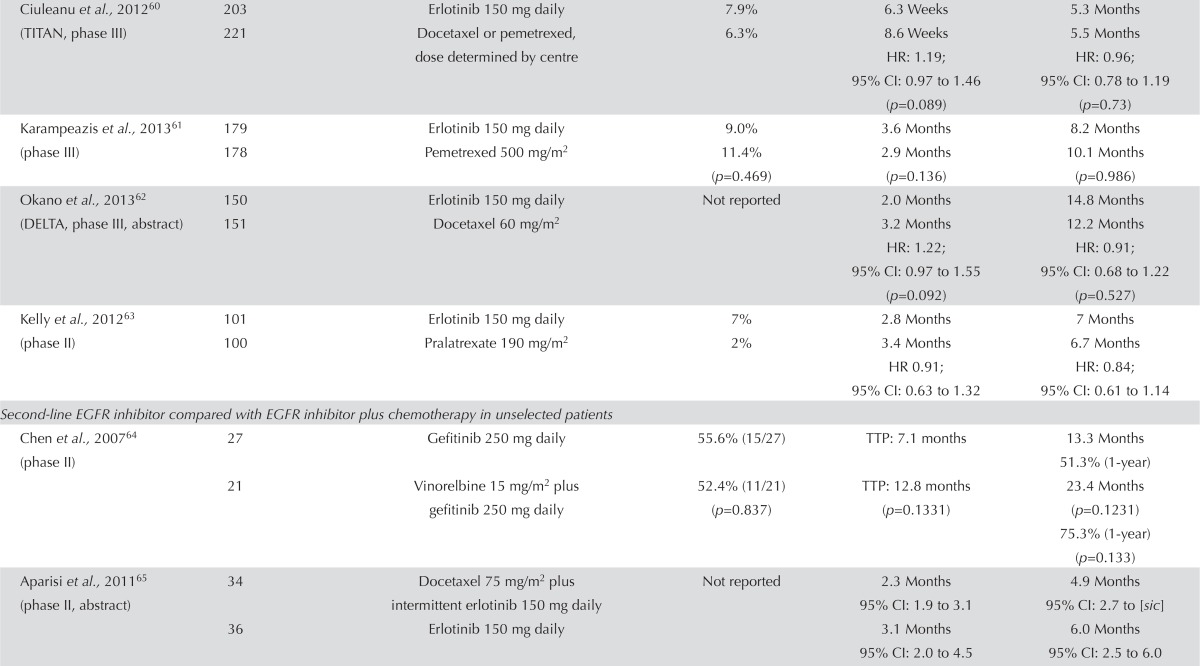
| Second-line EGFR inhibitor compared with EGFR inhibitor plus chemotherapy in unselected patients | ||||||
| Chen et al., 200764 (phase II) | 27 | Gefitinib 250 mg daily | 55.6% (15/27) | TTP: 7.1 months | 13.3 Months 51.3% (1-year) |
|
| 21 | Vinorelbine 15 mg/m2 plus gefitinib 250 mg daily | 52.4% (11/21) (p=0.837) | TTP: 12.8 months (p=0.1331) | 23.4 Months (p=0.1231) 75.3% (1-year) (p=0.133) |
||
| Aparisi et al., 201165 (phase II, abstract) | 34 | Docetaxel 75 mg/m2 plus intermittent erlotinib 150 mg daily | Not reported | 2.3 Months 95% CI: 1.9 to 3.1 |
4.9 Months 95% CI: 2.7 to [sic] |
|
| 36 | Erlotinib 150 mg daily | 3.1 Months 95% CI: 2.0 to 4.5 |
6.0 Months 95% CI: 2.5 to 6.0 |
|||
| Chen et al., 201166 (phase II) | 58 | Gefitinib 250 mg daily | 35% | 5.3 Months 18% (1-year) |
18.3 Months 64.8% (1-year) 27.7% (2-year) |
|
| 57 | Oral tegafur–uracil 1 capsule daily plus gefitinib 250 mg daily | 37% (p=0.847) | 8.3 Months 36.7% (1-year) HR: 0.65; 95% CI: 0.43 to 0.97 |
23.6 Months 68.1% (1-year) 47.1% (2-year) |
||
| Aerts et al., 201367 (NVALT-10, phase II) | 115 | Erlotinib 150 mg daily | Not reported | 4.9 Months | 5.5 Months | |
| 116 | Erlotinib 150 mg daily on days 2–16 every 21 days, plus docetaxel 75 mg/m2 for squamous disease or pemetrexed 500 mg/m2 for nonsquamous disease | 6.1 Months HR: 0.76; 95% CI: 0.58 to 1.02 (p=0.06) |
7.8 Months HR: 0.67; 95% CI: 0.49 to 0.91 (p=0.01) |
|||
CR = complete response; PR = partial response; HR = hazard ratio; CI = confidence interval; OR = odds ratio; TTP = time to progression.
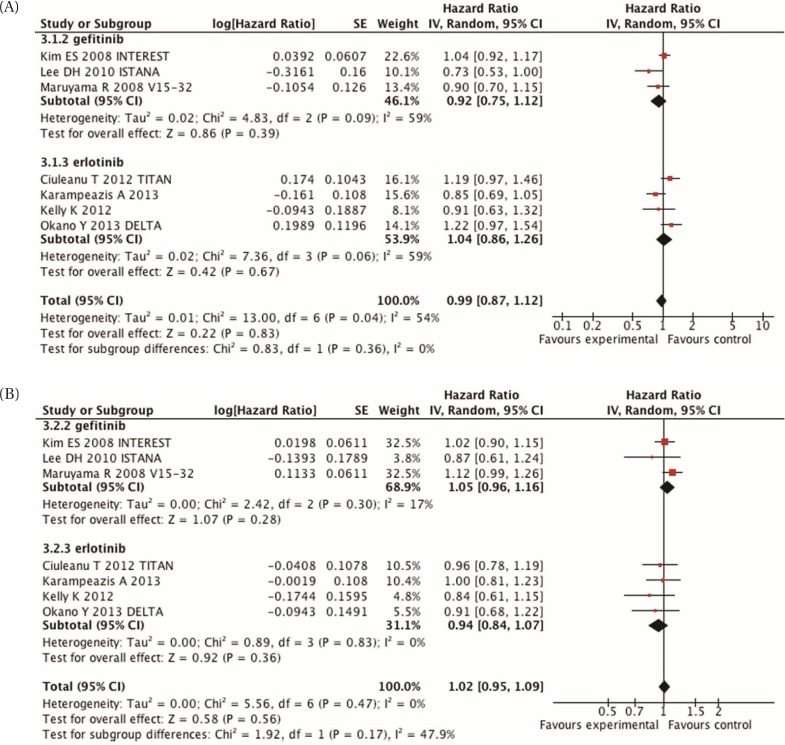
The foregoing trials underwent meta-analysis for pfs and os because they addressed similar questions and included clinically homogenous populations [Figure 3(A,B)]. (Three of the studies did not provide enough data to be included in the analysis54,57,59.) No difference in pfs was observed between egfr tki and chemotherapy (hr: 0.99; 95% ci: 0.87 to 1.312; p = 0.83). The I2 in this analysis was still high at 54%, which shows evidence of statistical heterogeneity.
FIGURE 3.
(A) Meta-analysis of progression-free survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in second-line unselected patients. (B) Meta-analysis of overall survival, comparing epidermal growth factor receptor inhibitors with chemotherapy in second-line unselected patients. SE = standard error; IV = inverse variance; CI = confidence interval.
Biomarker studies performed in the interest trial demonstrated that egfr protein expression, gene copy number, and mutation status, and KRAS mutation status were not predictive of any difference in os for either gefitinib or docetaxel68. For patients treated with gefitinib, EGFR mutation status predicted a longer pfs (hr: 0.16; 95% ci: 0.05 to 0.49; p = 0.001). However, the overall results suggest that second-line therapy with an egfr tki or with chemotherapy are both reasonable alternatives.
Similar results were observed for os. A meta-analysis showed no difference in os for second-line egfr tki or chemotherapy [hr: 1.02; 95% ci: 0.95 to 1.09; p = 0.56; Figure 3(B)]. There did not appear to be significant heterogeneity between the trials for os (I2: 0%).
Four studies evaluated symptom control and quality of life. All four found that the use of an egfr inhibitor improved both symptom control and quality of life54,56,58,60. Adverse effects were consistent with those associated with egfr inhibitors and chemotherapy.
EGFR Inhibitor Alone Compared with EGFR Inhibitor Plus Chemotherapy: Five studies compared an egfr inhibitor alone with an egfr inhibitor (concurrent or intercalated) plus chemotherapy. Three of those trials had small patient numbers64–66.
The response rate showed no clear improvement with an egfr tki combined with another agent than with an egfr tki alone (Table iv). In several trials, small improvements in pfs were noted in favour of the combination arm, but no statistically significant differences were observed64–67,69. Overall survival followed a similar pattern. All but one of the studies65 showed that os was longer with an egfr inhibitor plus another agent; in one study, the difference was statistically significant69. However, these reports come from small, inadequately powered trials, and so it is not possible to draw any real conclusions from the data.
Symptom control and quality of life were evaluated in the two studies by Chen and colleagues64,66. Using the Lung Cancer Symptom Scale, both studies found no difference in symptoms between the two groups. Adverse effects were consistent with those known for egfr inhibitors and chemotherapy.
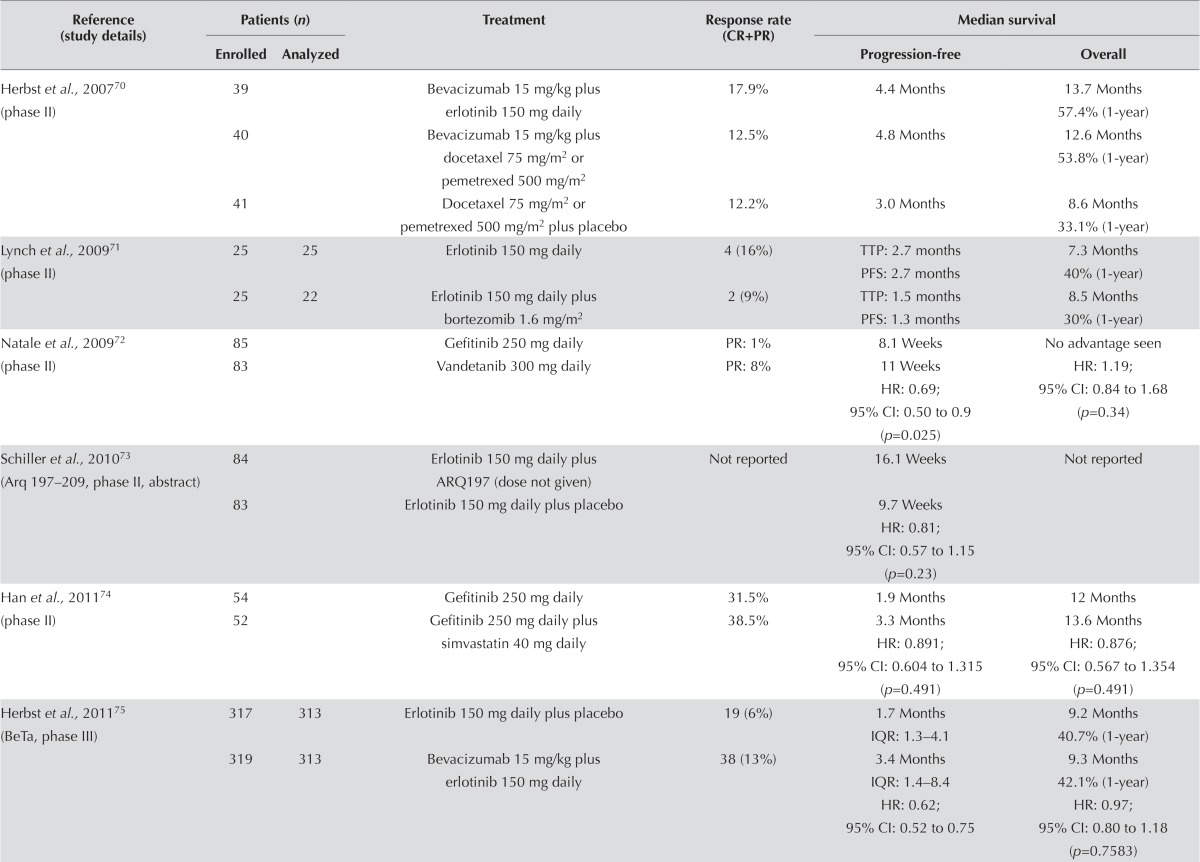
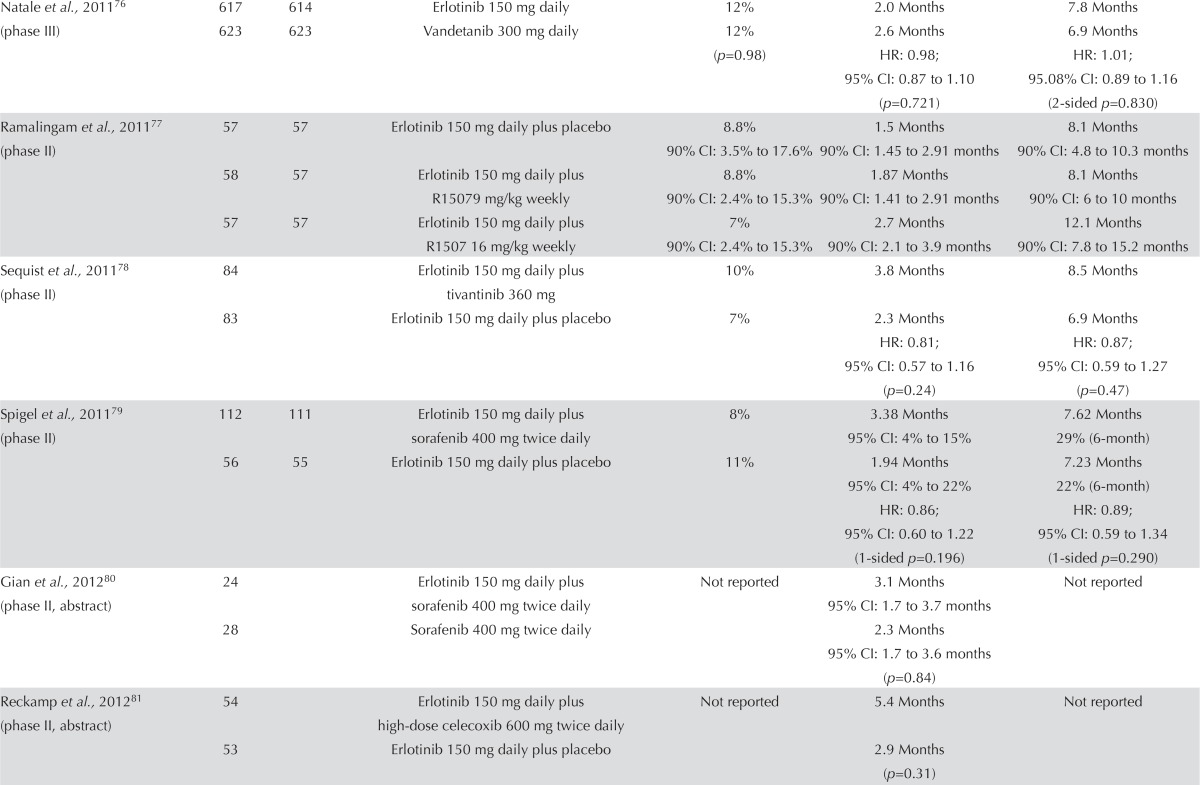
EGFR Inhibitor Alone or in Combination with a Targeted Agent: Seventeen studies examined an egfr inhibitor alone or in combination with a targeted agent. This group of trials is heterogeneous. Many are small randomized phase ii trials (Table v). Twelve studies evaluated an egfr inhibitor alone compared with an egfr inhibitor plus another targeted agent71,73–75,78,79,81–86, and five additional studies examined various combinations of egfr inhibitors and targeted agents70,72,76,77,80.
TABLE V.
Second-line epidermal growth factor receptor (EGFR) inhibitor alone or in combination with a targeted agent in unselected patients
| Reference (study details) | Patients (n) | Treatment | Response rate (CR+PR) | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| Herbst et al., 200770 (phase II) | 39 | Bevacizumab 15 mg/kg plus erlotinib 150 mg daily | 17.9% | 4.4 Months | 13.7 Months 57.4% (1-year) |
|
| 40 | Bevacizumab 15 mg/kg plus docetaxel 75 mg/m2 or pemetrexed 500 mg/m2 | 12.5% | 4.8 Months | 12.6 Months 53.8% (1-year) |
||
| 41 | Docetaxel 75 mg/m2 or pemetrexed 500 mg/m2 plus placebo | 12.2% | 3.0 Months | 8.6 Months 33.1% (1-year) |
||
| Lynch et al., 200971 (phase II) | 25 | 25 | Erlotinib 150 mg daily | 4 (16%) | TTP: 2.7 months PFS: 2.7 months |
7.3 Months 40% (1-year) |
| 25 | 22 | Erlotinib 150 mg daily plus bortezomib 1.6 mg/m2 | 2 (9%) | TTP: 1.5 months PFS: 1.3 months |
8.5 Months 30% (1-year) |
|
| Natale et al., 200972 (phase II) | 85 | Gefitinib 250 mg daily | PR: 1% | 8.1 Weeks | No advantage seen | |
| 83 | Vandetanib 300 mg daily | PR: 8% | 11 Weeks HR: 0.69; 95% CI: 0.50 to 0.9 (p=0.025) |
HR: 1.19; 95% CI: 0.84 to 1.68 (p=0.34) |
||
| Schiller et al., 201073 (Arq 197–209, phase II, abstract) | 84 | Erlotinib 150 mg daily plus ARQ197 (dose not given) |
Not reported | 16.1 Weeks | Not reported | |
| 83 | Erlotinib 150 mg daily plus placebo | 9.7 Weeks HR: 0.81; 95% CI: 0.57 to 1.15 (p=0.23) |
||||
| Han et al., 201174 (phase II) | 54 | Gefitinib 250 mg daily | 31.5% | 1.9 Months | 12 Months | |
| 52 | Gefitinib 250 mg daily plus simvastatin 40 mg daily | 38.5% | 3.3 Months HR: 0.891; 95% CI: 0.604 to 1.315 (p=0.491) |
13.6 Months HR: 0.876; 95% CI: 0.567 to 1.354 (p=0.491) |
||
| Herbst et al., 201175 (BeTa, phase III) | 317 | 313 | Erlotinib 150 mg daily plus placebo | 19 (6%) | 1.7 Months IQR: 1.3–4.1 |
9.2 Months 40.7% (1-year) |
| 319 | 313 | Bevacizumab 15 mg/kg plus erlotinib 150 mg daily | 38 (13%) | 3.4 Months IQR: 1.4–8.4 HR: 0.62; 95% CI: 0.52 to 0.75 |
9.3 Months 42.1% (1-year) HR: 0.97; 95% CI: 0.80 to 1.18 (p=0.7583) |
|
| Natale et al., 201176 (phase III) | 617 | 614 | Erlotinib 150 mg daily | 12% | 2.0 Months | 7.8 Months |
| 623 | 623 | Vandetanib 300 mg daily | 12% (p=0.98) | 2.6 Months HR: 0.98; 95% CI: 0.87 to 1.10 (p=0.721) |
6.9 Months HR: 1.01; 95.08% CI: 0.89 to 1.16 (2-sided p=0.830) |
|
| Ramalingam et al., 201177 (phase II) | 57 | 57 | Erlotinib 150 mg daily plus placebo | 8.8% 90% CI: 3.5% to 17.6% |
1.5 Months 90% CI: 1.45 to 2.91 months |
8.1 Months 90% CI: 4.8 to 10.3 months |
| 58 | 57 | Erlotinib 150 mg daily plus R15079 mg/kg weekly |
8.8% 90% CI: 2.4% to 15.3% |
1.87 Months 90% CI: 1.41 to 2.91 months |
8.1 Months 90% CI: 6 to 10 months |
|
| 57 | 57 | Erlotinib 150 mg daily plus R1507 16 mg/kg weekly |
7% 90% CI: 2.4% to 15.3% |
2.7 Months 90% CI: 2.1 to 3.9 months |
12.1 Months 90% CI: 7.8 to 15.2 months |
|
| Sequist et al., 201178 (phase II) | 84 | Erlotinib 150 mg daily plus tivantinib 360 mg | 10% | 3.8 Months | 8.5 Months | |
| 83 | Erlotinib 150 mg daily plus placebo | 7% | 2.3 Months HR: 0.81; 95% CI: 0.57 to 1.16 (p=0.24) |
6.9 Months HR: 0.87; 95% CI: 0.59 to 1.27 (p=0.47) |
||
| Spigel et al., 201179 (phase II) | 112 | 111 | Erlotinib 150 mg daily plus sorafenib 400 mg twice daily | 8% | 3.38 Months 95% CI: 4% to 15% |
7.62 Months 29% (6-month) |
| 56 | 55 | Erlotinib 150 mg daily plus placebo | 11% | 1.94 Months 95% CI: 4% to 22% HR: 0.86; 95% CI: 0.60 to 1.22 (1-sided p=0.196) |
7.23 Months 22% (6-month) HR: 0.89; 95% CI: 0.59 to 1.34 (1-sided p=0.290) |
|
| Gian et al., 201280 (phase II, abstract) | 24 | Erlotinib 150 mg daily plus sorafenib 400 mg twice daily | Not reported | 3.1 Months 95% CI: 1.7 to 3.7 months |
Not reported | |
| 28 | Sorafenib 400 mg twice daily | 2.3 Months 95% CI: 1.7 to 3.6 months (p=0.84) |
||||
| Reckamp et al., 201281 (phase II, abstract) | 54 | Erlotinib 150 mg daily plus high-dose celecoxib 600 mg twice daily | Not reported | 5.4 Months | Not reported | |
| 53 | Erlotinib 150 mg daily plus placebo | 2.9 Months (p=0.31) | ||||
| Scagliotti et al., 201282 (phase III) | 480 | Erlotinib 150 mg daily plus sunitinib 37.5 mg daily | 10.6% | 3.6 Months | 9.0 Months | |
| 480 | Erlotinib 150 mg daily plus placebo | 6.9% (p=0.0471) | 2.0 Months HR: 0.807; 95% CI: 0.695 to 0.937 |
8.5 Months HR 0.922; 95% CI: 0.797 to 1.067 (p=0.1388) |
||
| Witta et al., 201283 (phase II) | 65 | Erlotinib 150 mg daily plus placebo | 9.2% | 1.88 Months | 6.7 Months | |
| 67 | Erlotinib 150 mg daily plus entinostat 10 mg | 3.0% | 1.97 Months HR: 0.99; 95% CI: 0.68 to 1.44 (p=0.98) |
8.9 Months HR: 0.85; 95%CI: 0.59 to 1.23 (p=0.39) |
||
| Besse et al., 201384 (phase II) | 66 | Everolimus 5 mg daily plus erlotinib 150 mg daily | 12.1% | 2.9 Months 95% CI: 5.4% to 22.5% |
9.1 Months 95% CI: 2.4 to 3.9 months |
|
| 67 | Erlotinib 150 mg daily | 10.4% | 2.0 Months 95% CI: 4.3% to 20.3% |
9.7 Months 95% CI: 1.1 to 2.8 months |
||
| Groen et al., 201385 (phase II) | 65 | Sunitinib 37.5 mg daily plus erlotinib 150 mg daily | 4.6% | 2.8 Months | 8.2 Months 95% CI: 5.70 to 11.30 months |
|
| 67 | Placebo plus erlotinib 150 mg daily | 3.0% | 2.0 Months HR: 0.898; 80% CI: 0.671 to 1.203 (p=0.321) |
7.6 Months 95% CI: 5.30 to 13.40 months HR 1.066; 95% CI: 0.705 to 1.612 (p=0.617) |
||
| Spigel et al., 201386 (phase II) | 69 | Onartuzumab 15 mg/kg plus erlotinib 150 mg daily | 5.8% | 2.2 Months | 8.9 Months | |
| 68 | Erlotinib 150 mg daily plus placebo | 4.4% | 2.6 Months HR: 1.09 (p=0.69) |
7.4 Months HR: 0.80 (p=0.34) |
||
CR = complete response; PR = partial response; TTP = time to progression; PFS = progression-free survival; HR = hazard ratio; CI = confidence interval; IQR = interquartile range.
No clear trend in response rate was evident. Some results favoured the egfr inhibitor alone71,79, some favoured the combination arm70,78,82–86, and some found no difference between groups76,77. Progression-free survival followed the same trend as response rate. A number of trials demonstrated improved pfs for the combination of an egfr inhibitor and a targeted agent. However, none of the trials demonstrated any statistically significant improvements in os74. Despite the heterogeneous nature of the trials, it is reasonable to conclude that no available evidence currently supports the combination of erlotinib with another targeted agent.
Symptom control and quality of life were reported in two studies. The study by Scagliotti et al.82 also found no statistical difference in the mean health index score on the EQ-5D (EuroQoL, Rotterdam, Netherlands) between treatment groups (p = 0.3373). The study by Natale et al.76 found that scores on the European Organisation for Research and Treatment of Cancer’s 30-question Quality of Life Questionnaire was similar between the groups: erlotinib 80% and vandetanib 82%. Adverse effects were in line with those commonly associated with egfr inhibitors and chemotherapy.
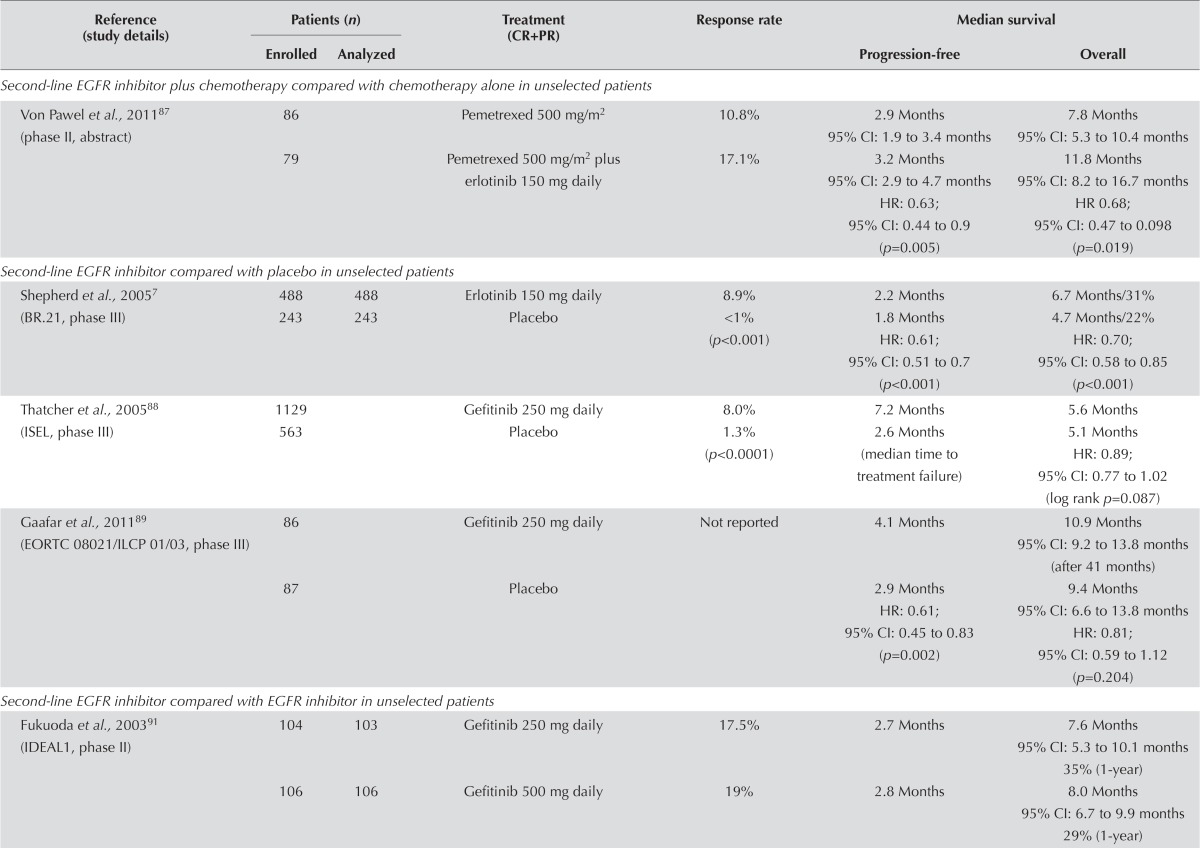
EGFR Inhibitor Plus Chemotherapy Compared with Chemotherapy Alone: One study of 165 patients examined the use of an egfr inhibitor plus chemotherapy compared with chemotherapy alone (Table vi). That study demonstrated a greater response rate and longer pfs for chemotherapy plus an egfr inhibitor. The result for pfs was significant (hr: 0.63; 95% ci: 0.44 to 0.90; p = 0.005). In addition, os was prolonged in the combination arm, and that result was significant (hr: 0.68; 95% ci: 0.47 to 0.98; p = 0.019)87. Given the small size of the trial, the evidence is insufficient to recommend the combination of an egfr tki plus chemotherapy.
TABLE VI.
Other second-line epidermal growth factor receptor (EGFR) inhibitor trials in unselected patients
| Reference (study details) | Patients (n) | Treatment (CR+PR) | Response rate | Median survival | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| Second-line EGFR inhibitor plus chemotherapy compared with chemotherapy alone in unselected patients | ||||||
| Von Pawel et al., 201187 (phase II, abstract) | 86 | Pemetrexed 500 mg/m2 | 10.8% | 2.9 Months 95% CI: 1.9 to 3.4 months |
7.8 Months 95% CI: 5.3 to 10.4 months |
|
| 79 | Pemetrexed 500 mg/m2plus erlotinib 150 mg daily | 17.1% | 3.2 Months 95% CI: 2.9 to 4.7 months HR: 0.63; 95% CI: 0.44 to 0.9 (p=0.005) |
11.8 Months 95% CI: 8.2 to 16.7 months HR0.68; 95% CI: 0.47 to 0.098 (p=0.019) |
||
| Second-line EGFR inhibitor compared with placebo in unselected patients | ||||||
| Shepherd et al., 20057 (BR.21, phase III) | 488 | 488 | Erlotinib 150 mg daily | 8.9% | 2.2 Months | 6.7 Months/31% |
| 243 | 243 | Placebo | <1% (p<0.001) | 1.8 Months HR: 0.61; 95% CI: 0.51 to 0.7 (p<0.001) |
4.7 Months/22% HR: 0.70; 95% CI: 0.58 to 0.85 (p<0.001) |
|
| Thatcher et al., 200588 (ISEL, phase III) | 1129 | Gefitinib 250 mg daily | 8.0% | 7.2 Months | 5.6 Months | |
| 563 | Placebo | 1.3% (p<0.0001) | 2.6 Months (median time to treatment failure) | 5.1 Months HR: 0.89; 95% CI: 0.77 to 1.02 (log rank p=0.087) |
||
| Gaafar et al., 201189 (EORTC08021/ILCP01/03, phase III) | 86 | Gefitinib 250 mg daily | Not reported | 4.1 Months | 10.9 Months 95% CI: 9.2 to 13.8 months (after 41 months) |
|
| 87 | Placebo | 2.9 Months HR: 0.61; 95% CI: 0.45 to 0.83 (p=0.002) |
9.4 Months 95% CI: 6.6 to 13.8 months HR: 0.81; 95% CI: 0.59 to 1.12 (p=0.204) |
|||
| Second-line EGFR inhibitor compared with EGFR inhibitor in unselected patients | ||||||
| Fukuoda et al., 200391 (IDEAL1, phase II) | 104 | 103 | Gefitinib 250 mg daily | 17.5% | 2.7 Months | 7.6 Months 95% CI: 5.3 to 10.1 months 35% (1-year) |
| 106 | 106 | Gefitinib 500 mg daily | 19% | 2.8 Months | 8.0 Months 95% CI: 6.7 to 9.9 months 29% (1-year) |
|
| Second-line EGFR inhibitor compared with EGFR inhibitor in unselected patients | ||||||
| Kris et al., 200392 (IDEAL2, phase II) | 106 | 102 | Gefitinib 250 mg daily plus placebo | 12% (12/102) 95% CI: 6% to 20% |
Not reported | 7 Months 27% (1-year projected) |
| 115 | 114 | Gefitinib 500 mg daily (2×250 mg) | 9% (10/114) 95% CI: 4% to 16% (p=0.51) |
6 Months (p=0.40) 24% (1-year projected) (p=0.54) |
||
| Ahn et al., 201093 (phase II, abstract) | 48 | Erlotinib 150 mg daily | 39.6% | 3.1 Months | Not reported | |
| 48 | Gefitinib 250 mg daily | 47.9% (p=0.411) | 4.9 Months HR: 0.81; 95% CI: 0.52 to 1.25 (p=0.336) |
|||
| Ramalingam et al., 201294 (phase II) | 94 | Dacomitinib 45 mg daily | 17.0% | 2.86 Months | 9.53 Months | |
| 94 | Erlotinib 150 mg daily | 5.3% (p=0.011) | 1.91 Months HR: 0.66; 95% CI: 0.47 to 0.91 (p=0.012) |
7.44 Months HR: 0.80; 95% CI: 0.56 to 1.1 (p=0.205) |
||
| Shi et al., 201395 (ICOGEN, phase III) | 200 | Icotinib 125 mg three times daily | ORR: 27.6% | 4.6 Months 95% CI: 3.5 to 6.3 months |
13.3 Months | |
| 199 | Gefitinib 250 mg daily | ORR: 27.2% | 3.4 Months 95% CI: 2.3 to 3.8 months HR0.84; 95% CI: 0.67 to 1.0 (p=0.13) |
13.9 Months HR: 1.02; 95% CI: 0.82 to 1.27 (p=0.57) |
||
CR = complete response; PR = partial response; CI = confidence interval; HR = hazard ratio; ORR = overall response rate.
EGFR Inhibitor Compared with Placebo: Three fully published studies compared an egfr inhibitor with placebo7,88,89. The trial by Shepherd et al. (ncic br.21, examining erlotinib versus placebo) and the isel trial of gefitinib compared with placebo both showed response rates significantly better with the egfr inhibitor than with placebo7,88. Significant improvements in pfs were also observed in both trials, as well as in the third trial of gefitinib versus placebo89. However, only the br.21 trial of erlotinib demonstrated a significant improvement in os. Erlotinib is recommended as second- or third-line therapy in patients who are not candidates for further chemotherapy.
Correlative studies from br.21, reported by Tsao et al.90, evaluated the association between os and EGFR mutation status, egfr protein expression, and EGFR gene copy number. Survival was longer in the erlotinib group than in the placebo group when egfr protein was overexpressed (hr: 0.68; 95% ci: 0.49 to 0.95; p = 0.02).
Symptom control and quality of life were addressed in two studies7,88. Time to deterioration of symptoms of cough (p = 0.04), dyspnea (p = 0.03), and pain (p = 0.04) was prolonged and significant with erlotinib in the study by Shepherd et al.7. Symptom improvement was significant with gefitinib in the study by Thatcher et al.88 (p = 0.019). Adverse effects were also in line with those associated with use of egfr inhibitor.
EGFR Inhibitor Compared with EGFR Inhibitor: Five studies compared egfr inhibitors or dosing of the same egfr inhibitor. The ideal 1 and 2 trials compared two dose levels of gefitinib and found no difference in any of the reported outcomes (Table vi). Similarly, the icogen trial comparing gefitinib with icotinib and a second trial comparing gefitinib with erlotinib reported no difference in outcomes. A randomized phase ii trial comparing dacomitinib with erlotinib demonstrated a significant improvement in response rate and pfs favouring dacomitinib and a trend toward improvement in os94. However, those findings require confirmation in a phase iii trial.
Quality of life was addressed in the two ideal studies. No differences in symptom response were evident for the different doses of gefitinib91,92. Adverse effects were consistent with those known for egfr inhibitors. The adverse effects were slightly elevated with gefitinib 500 mg daily.
Clinically Selected Populations: EGFR Inhibitor Compared with Chemotherapy: Two trials compared pemetrexed with an egfr inhibitor as second-line therapy in never-smokers (Table vii). The overall response rate was significantly higher for gefitinib (30.1% vs. 14.9%, p < 0.001)96. Progression-free survival was significantly longer for patients randomized to gefitinib (9.4 months vs. 2.9 months, p = 0.010) and also for patients randomized to a combination of erlotinib and pemetrexed (7.4 months vs. 3.8 months for erlotinib and 4.4 months for pemetrexed alone; hr: 0.57; 95% ci: 0.40 to 0.81; p = 0.002)97. However, the survival rates were nonsignificantly different (p = 0.89)96,97.
TABLE VII.
Second-line epidermal growth factor receptor (EGFR) inhibitor trials in clinically selected populations
| Reference (study details) | Patients (n) | Treatment (CR+PR) | Response rate | Survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Median pf s | Other | |||
| Second-line EGFR inhibitor compared with chemotherapy in clinically selected patients | ||||||
| Ahn et al., 201196 (KCSG-LU08–01, phase iii, abstract) | 135 (not broken down) |
Gefitinib 250 mg daily | ORR: 30.1% | 9.4 Months | 73.6% (1-year) | |
| Pemetrexed 500 mg/m2 | ORR: 14.9% (p<0.001) | 2.9 Months (p=0.010) | 70.5% (1-year) (p=0.89) | |||
| Lee et al., 201397 (phase ii) | 78 | (A) Erlotinib 150 mg daily plus pemetrexed 500 mg/m2 | 7.4 Months | 20.5 Months | ||
| 82 | (B) Erlotinib 150 mg daily | 3.8 Months | 22.8 Months | |||
| 80 | (C) Pemetrexed 500 mg/m2 | 4.4 Months | 17.7 Months | |||
| A vs. B+C: hr: 0.57; 95% CI: 0.40 to 0.81 (p=0.002) |
A vs. B+C: HR: 1.08; 95% CI: 0.69 to 1.67 (p=0.747) |
|||||
| Yang et al., 201398 (CTONG 0806, phase II, abstract) | 81 | Gefitinib 250 mg daily | 14.7% | 1.6 Months | Overall survival | |
| 76 | Pemetrexed 500 mg/m2 | 13.3% (p=0.814) | 4.8 Months HR: 0.51; 95% CI: 0.36 to 0.73 (p<0.001) |
not yet mature | ||
| Third- or fourth-line EGFR inhibitor compared with placebo in clinically selected patients | ||||||
| Miller et al., 201299 (LUX-Lung1, phase IIB/III) | 390 | Afatinib 50 mg daily plus BSC | 7% | 3.3 Months | 10.8 Months | |
| 195 | Placebo plus BSC | 0.5% | 1.1 Months HR: 0.38; 95% CI: 0.31 to 0.48 (p<0.0001) |
12.0 Months HR: 1.08; 95% CI: 0.86 to 1.35 (p=0.74) |
||
CR = complete response; PR = partial response; PFS = progression-free survival; ORR = overall response rate; HR = hazard ratio; CI = confidence interval; BSC = best supportive care.
One study examined the use of gefitinib in patients with nonsquamous disease in the second-line setting (Table vii)98. No difference in the response rate was observed; however, pfs was significantly better with pemetrexed (4.8 months vs. 1.6 months with gefitinib; hr: 0.51; 95% ci: 0.36 to 0.73; p < 0.001)98. Overall survival was not yet reached for this trial.
Third- or Fourth-Line EGFR Inhibitor Compared with Placebo: The lux-Lung 1 trial evaluated afatinib in patients who had received 1 or 2 prior chemotherapy treatments and, in a selected population of patients, also gefitinib or erlotinib (Table vii). The response rates were 7% for afatinib and 0.5% for placebo. A significant improvement in pfs was evident for patients randomized to afatinib (3.3 months vs. 1.1 months, p < 0.0001). However, no difference in the primary outcome of os was observed (10.8 months vs. 12 months, p = 0.74). Adverse effects were consistent with those associated with egfr inhibitors99. There is therefore currently no evidence that further therapy with an egfr tki in patients who have already received gefitinib or erlotinib improves os.
Molecularly Selected Populations: EGFR Inhibitor Compared with Chemotherapy: One study compared the use of an egfr inhibitor with the use of chemotherapy in patients known to be EGFR wild-type100. The trial specifically excluded crossover to the other treatment at the time of progression. Compared with erlotinib, docetaxel was associated with an improved pfs (hr: 0.71; 95% ci: 0.53 to 0.95; p = 0.02). The primary outcome in the trial was os, which was also significant for docetaxel at 8.2 months compared with 5.4 months for erlotinib (hr: 0.73; 95% ci: 0.53 to 1.00; p = 0.05; Table viii)100.
TABLE VIII.
Second-line epidermal growth factor receptor (EGFR) inhibitor trials in molecularly selected populations
| Reference (study details) | Patients (n) | Treatment (CR+PR) | Response rate | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| Second-line EGFR inhibitor compared with chemotherapy in molecularly selected patients | ||||||
| Garassino et al., 2013100 (TAILOR, phase III) | 112 | Erlotinib 150 mg daily | Not reported | 2.4 Months | 5.4 Months | |
| 110 | Docetaxel 75 mg/m2 | 2.9 Months HR: 0.71; 95% CI: 0.53 to 0.95 (p=0.02) |
8.2 Months HR: 0.73; 95% CI: 0.53 to 1.00 (p=0.05) |
|||
| Second-line EGFR inhibitor plus another agent compared with EGFR inhibitor in molecularly selected patients | ||||||
| Gitlitz et al., 2011101 (APRICOT-L, phase II, abstract) | 120 | Erlotinib 150 mg daily plus apricoxib 400 mg daily | Not reported | TTP: 2.1 months | 5.6 Months | |
| 176 | Placebo plus erlotinib 150 mg daily | TTP: 1.8 months HR: 0.5 (p=0.018) |
5.9 Months HR: 0.4 (p=0.025) |
|||
| Belani et al., 2013102 (phase II) | 18 | PF-3512676 (0.20 mg/kg) plus erlotinib 150 mg daily | Not reported | 1.6 Months | 6.4 Months | |
| 21 | Erlotinib 150 mg daily | 1.7 Months HR: 1.00; 95% CI: 0.5 to 2.0 (p=0.9335) |
4.7 Months HR: 1.3; 95% CI: 0.6 to 2.8 (p=0.4925) |
|||
| Second-line EGFR inhibitor compared with EGFR inhibitor in molecularly selected patients | ||||||
| Kim et al., 2012103 (phase II) | 48 | Gefitinib 250 mg daily | 47.9% | 4.9 Months | Not reached | |
| 48 | Erlotinib 150 mg daily | 39.6% | 3.1 Months (p=0.336) | |||
CR = complete response; PR = partial response; HR = hazard ratio; CI = confidence interval; TTP = time to progression.
EGFR Inhibitor Plus Another Agent Compared with an EGFR Inhibitor: Two studies examined the use of an egfr inhibitor plus another agent compared with erlotinib alone in molecularly selected patients101,102 (Table viii). Time to progression was significantly longer with erlotinib and apricoxib (p = 0.018) in the Gitlitz et al. trial101, but no different in the Belani et al. trial102. However, os favoured the erlotinib and placebo group (hr: 0.4; p = 0.025) in the Gitlitz et al. trial101. Again, no difference was seen between the groups in the Belani et al. trial102. Adverse effects were in line with those associated with egfr inhibitors.
EGFR Inhibitor Compared with EGFR Inhibitor: One study compared egfr inhibitors in molecularly selected patients103 (Table viii). Response rate and pfs were higher in the gefitinib group than in the erlotinib group. Significance was not reached for pfs (p = 0.336). Adverse effects were in line with those associated with egfr inhibitors103.
Maintenance
Unselected Populations: EGFR Inhibitors: In recent years, attempts to improve the survival of patients with advanced nsclc have led to considerable interest in evaluating maintenance therapies. Trials have evaluated continuing a drug (“continuation maintenance”) or switching to another drug (“switch maintenance”). Five studies have examined egfr inhibitors in unselected patients in the switch-maintenance setting, but none of those trials mandated the use of an egfr tki in the placebo arm at the time of disease progression.
One study compared an egfr inhibitor with chemotherapy in the maintenance setting (Table ix). Bylicki et al.107 randomized patients to maintenance therapy with erlotinib, gemcitabine, or observation. In the observation group, patients received no treatment. No clear improvement in pfs was observed for either erlotinib or gemcitabine. No significant difference in os was observed, but a trend toward improved survival was evident in both the erlotinib group (hr: 0.80; 95% ci: 0.61 to 1.05; p = 0.13) and the gemcitabine group (hr: 0.81; 95% ci: 0.61 to 1.07; p = 0.109) compared with the observation group. No outstanding adverse effects occurred in the erlotinib group107.
TABLE IX.
Epidermal growth factor receptor (EGFR) inhibitors compared with chemotherapy in the maintenance setting
| Reference (study details) | Patients (n) | Treatment (CR+PR) | Response rate | Median survival | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Enrolled | Analyzed | Progression-free | Overall | |||
| EGFR inhibitors in unselected patients in the maintenance setting | ||||||
| Cappuzzo et al., 2010104 (SATURN, phase III) | 438 | 437 | Erlotinib 150 mg day | 11.9% | 12.3 Weeks 25% (6-month) 95% CI: 21% to 29% |
12 Months |
| 451 | 447 | Placebo | 5.4% (p=0.0006) | 11.1 Weeks 15% (6-month) 95% CI: 12% to 19% HR: 0.71; 95% CI: 0.62 to 0.82 (p<0.0001) |
11 Months HR: 0.81; 95% CI: 0.70 to 0.95 (p=0.0088) |
|
| Takeda et al., 2010105 (WJTOG 0203, phase III) | 302 | 298 | Chemotherapya plus gefitinib 250 mg daily | 34.2% | 4.6 Months | 13.7 Months |
| 301 | 297 | Chemotherapya | 29.3% (p=0.20) | 4.3 Months HR: 0.68; 95% CI: 0.57 to 0.80 (p<0.001) |
12.9 Months | |
| Zhang et al., 2012106 (phase III) | 148 | Gefitinib 250 mg daily | 24% | 4.8 Months | 18.7 Months | |
| 148 | Placebo | 1% OR: 54.10 95% CI: 7.17 to 408 (p=0.0001) |
2.6 Months HR: 0.42; 95% CI: 0.33 to 0.55 (p<0.0001) |
16.9 Months HR: 0.84; 95% CI: 0.62 to 1.14 (p=0.26) |
||
| Bylicki et al., 2013107 (IFCT-GFPC 05–02, phase II) | 155 | (A) Erlotinib 150 mg daily | 14% | A vs. C: | 9.1 Months | |
| 154 | (B) Gemcitabine 1250 mg/m2 | 6% | 4.2 vs. 3.9 months; | 8.3 Months | ||
| 155 | (C) Observation | 14% | HR: 0.83; 95% CI: 0.64 to 1.09 B vs. C: 4.2 vs. 3.9 months; HR: 0.81; 95% CI: 0.62 to 1.06 |
7.5 Months A vs. C: HR: 0.80; 95% CI: 0.61 to 1.05 (p=0.13) B vs. C: HR: 0.81; 95% CI: 0.61 to 1.07 (p=0.109) |
||
| Johnson et al., 2013108 (ATLAS, phase II) | 370 | Erlotinib 150 mg daily plus bevacizumab 15 mg/kg | Not reported | 4.8 Months | 14.4 Months | |
| 373 | Bevacizumab 15 mg/kg | 3.7 Months HR: 0.708; 95% CI: 0.580 to 0.864 (p<0.001) |
13.3 Months HR: 0.917; 95% CI: 0.698 to 1.205 (p=0.5341) |
|||
| EGFR inhibitor in clinically selected patients in the maintenance setting | ||||||
| Ahn et al., 2012109 (phase II) | 25 | Gefitinib 250 mg daily | 46.2% | HR: 0.191; 95% CI: 0.074 to 0.0497 |
80.6% (6-month) 74.8% (12-month) 59.5% (24-month) |
|
| 24 | Pemetrexed 500 mg/m2 with optional cisplatin 75 mg/m2 | 35.5% OR: 1.56; 95% CI: 0.59 to 4.10 (p=0.369) |
93.3% (6-month) 93.3% (12-month) 77.4% (24-month) HR: 2.151; 95% CI: 0.826 to 5.599 |
|||
Carboplatin AUC 6 plus (paclitaxel 200 mg/m2 or cisplatin 80 mg/m2) plus (irinotecan 60 mg/m2 or cisplatin 80 mg/m2) plus (vinorelbine 25 mg/m2 or cisplatin 80 mg/m2) plus (gemcitabine 1000 mg/m2 or cisplatin 80 mg/m2) plus docetaxel 60 mg/m2.
CR = complete response; PR = partial response; AUC = area under the curve; HR = hazard ratio; CI = confidence interval; OR = odds ratio.
Four trials evaluated an egfr tki as maintenance therapy. A clear improvement in pfs was observed, but only one trial showed a significant improvement in os. One Japanese trial compared 6 cycles of a platinum doublet with 3 cycles of a platinum doublet followed by gefitinib until progression. A significant improvement in pfs was observed, but no significant improvement in os105. A second trial compared bevacizumab plus erlotinib with bevacizumab alone in patients treated with 4 cycles of carboplatin, paclitaxel, and bevacizumab. A significant improvement in pfs (4.8 months vs. 3.7 months, p < 0.001) was observed108. Two additional studies evaluated an egfr tki as maintenance therapy, comparing it with a placebo control, after 4 cycles of a platinum doublet. Both studies showed significant improvements in pfs. The saturn trial, which evaluated maintenance erlotinib, showed a significant improvement in os, although the difference in median survival was only 1 month104. In a pre-planned subgroup analysis of the saturn trial, patients with stable disease after first-line chemotherapy experienced a greater os benefit with maintenance erlotinib (median survival: 11.9 months for erlotinib vs. 9.6 months for placebo; hr: 0.72; 95% ci: 0.59 to 0.89; p = 0.0019) than did patients who experienced a previous complete or partial response (12.5 months for erlotinib vs. 12.0 months for placebo; hr: 0.94; 95% ci: 0.74 to 1.20; p = 0.618)104. Zhang et al.106 showed a similar effect on os with maintenance gefitinib, although the difference was not statistically significant (hr: 0.84; 95% ci: 0.62 to 1.14).
Quality of life and adverse effects were assessed in two studies. The saturn study showed no statistically significant difference in quality of life (fact-L questionnaire) between patients receiving erlotinib and those receiving placebo (hr for time to deterioration in quality of life: 0.96; 95% ci: 0.79 to 1.16). A post hoc analysis showed that time to pain (hr: 0.61; 95% ci: 0.42 to 0.88; p = 0.008) and time to analgesic use (hr: 0.66; 95% ci: 0.46 to 0.94; p = 0.02) were both significantly improved with erlotinib104. The Zhang et al.106 study showed that, based on the fact-L questionnaire, median time to worsening of lung cancer symptoms was 4.3 months with gefitinib and 2.3 months with placebo.
Adverse effects were consistent with what would be expected for gefitinib and erlotinib (increase in rash and diarrhea).
Clinically Selected Populations: EGFR Inhibitors: One fully published study109 examined the use of an egfr inhibitor in clinically selected patients in the maintenance setting. Table ix presents the study characteristics.
The trial randomized 49 patients to gefitinib or pemetrexed, making it underpowered to provide meaningful data on efficacy. Median pfs was associated with a hr of 0.191 (95% ci: 0.074 to 0.0497), and os was prolonged in the pemetrexed and optional-cisplatin group (hr: 2.151; 95% ci: 0.826 to 5.599). Adverse effects were consistent with those associated with egfr inhibitors and chemotherapy.
DISCUSSION AND CONCLUSIONS
Analysis of early trials evaluating egfr tkis suggested that clinical characteristics such as Asian ethnicity, female sex, non-smoking status, and adenocarcinoma were associated with a higher likelihood of response. Those characteristics were subsequently used in clinical trials to enrich the population of patients who might benefit from those drugs. However, it is now clear that the population of patients who derive the greatest benefit from egfr tkis are patients with tumours harbouring activating mutations of the EGFR gene. Nevertheless, the available data still support a more modest benefit from egfr tkis in unselected populations of nsclc patients. The present systematic review provides guidance for the use of egfr tki therapy in advanced nsclc and, in particular, whether there are subpopulations of nsclc patients in whom the sequence of therapy should be different.
In the first-line setting, data about the efficacy of egfr tkis compared with the efficacy of platinum-based chemotherapy are inconsistent. The largest trial in that setting, torch13, showed a statistically significantly inferior os for patients receiving first-line egfr tki therapy, and those agents are therefore not recommended in the first-line setting for an unselected population of nsclc patients. Studies selecting patients based on clinical characteristics such as Asian ethnicity, smoking status, and adenocarcinoma histology have also had mixed results. Although selection strategies are designed to increase the proportion of patients with an EGFR mutation, data from the ipass trial show that, when clinical characteristics are used to select patients, only 60% typically have EGFR mutations36. Significantly worse response rates and pfs are observed for patients with wild-type EGFR who are treated with first-line gefitinib. The use of clinical characteristics such as ethnicity, sex, smoking status, and histology cannot therefore be recommended in selecting patients for first-line therapy with an egfr tki. No available data support combining an egfr tki with platinum-based chemotherapy. However, high-quality evidence from multiple randomized clinical trials shows that an egfr tki is the preferred initial therapy (in preference to a platinum doublet) for patients with an activating mutation of the EGFR gene. Such treatment is associated with a higher likelihood of response, longer pfs, and improved quality of life, but with no clear difference in os. Many patients randomized in the trials to platinum-doublet chemotherapy crossed over to an egfr tki as subsequent therapy. The likely effect of that crossover was to dilute any survival difference between the groups, making comparisons of os less informative.
Cohort data from the Spanish Lung Cancer Group30 report on egfr tkis given as either first- or second-line therapy in patients with EGFR mutations. The benefit appears to be similar in both groups, so that even though the comparison was nonrandomized, the consensus is that crossover explains the difference. Although the trials show statistical heterogeneity, no available data suggest that one egfr tki is superior to another in this setting. Some trials included only patients with exon 19 deletion and exon 21 L858R point mutation; other trials such as lux-Lung 3 included other less common mutations. Those considerations might be a factor in making a choice of agent. However, the decision to use gefitinib, erlotinib, or afatinib is largely influenced by concerns about their toxicity or cost.
Data from the ncic br.21 trial of erlotinib compared with placebo demonstrate a modest improvement in survival and quality of life with erlotinib in patients who are no longer candidates for further chemotherapy7. Based on those data, erlotinib was recommended as a last line of therapy in the previous version of this guideline. However, multiple trials of second-line therapy comparing an egfr tki with chemotherapy have now been reported. A meta-analysis of the data demonstrates similar pfs and os. Level 1 evidence therefore now shows that there is no preferred sequence for second-line egfr tki or second-line chemotherapy. The findings of translational research from the interest study suggests that molecular analyses could not identify a subgroup of patients with improved os on an egfr tki or second-line chemotherapy55. It is therefore reasonable to consider an egfr tki as either second- or third-line therapy in the treatment of patients with advanced nsclc. Data from the tailor100 trial, performed only in patients with wild-type EGFR, demonstrated improved pfs and os when patients received docetaxel chemotherapy (compared with erlotinib). That trial did not allow crossover between the treatment arms, thus denying patients a previously established therapy. Those data therefore do not alter treatment recommendations at this time. The data concerning the combination of an egfr tki with either chemotherapy or another targeted agent are inconsistent. Some promising data have emerged from randomized phase ii trials, but they require confirmation in phase iii trials. Combination therapy with an egfr tki in the second- or third-line setting is therefore not recommended at this time.
Current data do not support the routine use of an egfr tki after disease progression on therapy with another egfr tki. Although data from the lux-Lung 1 trial demonstrated a significant improvement in pfs in a select subgroup of patients, that trial did not meet its primary objective of improved os99. Given the absence of improved survival, therapy with afatinib after progression on another egfr tki is not recommended.
The egfr tkis have also been evaluated as switch-maintenance therapy. The saturn trial demonstrated improved os in patients receiving maintenance erlotinib104. Interestingly, that benefit was observed whether the patients were EGFR mutation–positive or EGFR wild-type. No molecular marker could identify patients in whom a survival benefit was not observed. The magnitude of the benefit was modest, and other available maintenance therapy strategies should be considered. Nevertheless, there are data to support maintenance therapy with erlotinib after 4 cycles of platinum-based chemotherapy.
Lastly, it is evident from this review that determination of EGFR mutation status is essential to make appropriate treatment decisions. Patients who are EGFR mutation–positive should be treated with an egfr tki as first-line therapy. An egfr tki is still appropriate therapy in patients who are EGFR wild-type, but it should be administered as second- or third-line therapy.
ACKNOWLEDGMENTS
The Program in Evidence-Based Care (pebc) is a provincial initiative of Cancer Care Ontario supported by the Ontario Ministry of Health and Long-Term Care. All work produced by the pebc is editorially independent of the Ontario Ministry of Health and Long-Term Care.
The authors thank Hans Messersmith, pebc Assistant Director, Quality and Methods; Sheila McNair, pebc Assistant Director, Business Operations; Carol De Vito, Documents Manager; Hawkanwal Randhawal and Jagpreet Kaler for conducting the data audit; and Glenn Fletcher and Xiaomei Yao for internal peer review.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: PME has received consulting fees from Roche, Boehringer–Ingelheim, and Pfizer. PME is also study chair for the ncic Clinical Trials Group br.26, which is comparing dacomitinib with placebo in nsclc patients who have received prior chemotherapy and an egfr tki. RF is a member of the Roche and AstraZeneca boards. The remaining authors declared that they had no conflicts of interest.
REFERENCES
- 1.Scagliotti GV, Parikh P, von Pawel J, et al. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. on behalf of the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase iii trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The tax 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [Erratum in: J Clin Oncol 2004;22:209] [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Feld R, Sridhar SS, Shepherd FA, et al. Use of the epidermal growth factor receptor inhibitors gefitinib and erlotinib in the treatment of non-small cell lung cancer: a systematic review. J Thorac Oncol. 2006;1:367–76. doi: 10.1097/01243894-200605000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. on behalf of the National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Ellis PM, Blais N, Soulieres D, et al. A systematic review and Canadian consensus recommendations on the use of biomarkers in the treatment of non-small cell lung cancer. J Thorac Oncol. 2011;6:1379–91. doi: 10.1097/JTO.0b013e318220cb8e. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 12.Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [Erratum in: Stat Med 2004;23:1817] [DOI] [PubMed] [Google Scholar]
- 13.Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the torch randomized trial. J Clin Oncol. 2012;30:3002–11. doi: 10.1200/JCO.2011.41.2056. [DOI] [PubMed] [Google Scholar]
- 14.Crino L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (invite): a randomized, phase ii study. J Clin Oncol. 2008;26:4253–60. doi: 10.1200/JCO.2007.15.0672. [DOI] [PubMed] [Google Scholar]
- 15.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase ii trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol. 2008;26:863–9. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Inoue A, Maemondo M, et al. First-line gefitinib versus first-line chemotherapy by carboplatin (cbdca) plus paclitaxel (txl) in non-small cell lung cancer (nsclc) patients (pts) with EGFR mutations: a phase iii study by North East Japan Gefitinib Study Group [abstract 8016] J Clin Oncol. 2009;27 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/27/15S/8016; cited 29 March 2015] [Google Scholar]
- 17.Agarwal S, Hirsh V, Agulnik JS, Cohen V, Mihalcioiu CL, Whittom R. A phase ii study of gefitinib (g) versus carboplatin and gemcitabine (cg) in chemotherapy-naive patients with advanced non-small cell lung cancer (nsclc) and ecog performance status (ps) 2 [abstract e18090] J Clin Oncol. 2010;28 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/e18090; cited 29 March 2015] [Google Scholar]
- 18.Morere JF, Brechot JM, Westeel V, et al. Randomized phase ii trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2 or 3 (ifct-0301 study) Lung Cancer. 2010;70:301–7. doi: 10.1016/j.lungcan.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Von Pawel J, Fischer JR, et al. Erlotinib versus carboplatin/vinorelbine in elderly patients (age 70 or older) with advanced non-small cell lung cancer (nsclc): a randomized phase ii study of the German Thoracic Oncology Working Group [abstract 7565] J Clin Oncol. 2010;28:15s. [Available online at: http://meetinglibrary.asco.org/content/44017-74; cited 29 March 2015] [Google Scholar]
- 20.LeCaer H, Barlesi F, Corre R, et al. on behalf of the gfpc 0504 team A multicentre phase ii randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs. the reverse sequence, in elderly patients with advanced non small–cell lung cancer selected with a comprehensive geriatric assessment (the gfpc 0504 study) Br J Cancer. 2011;105:1123–30. doi: 10.1038/bjc.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YM, Tsai CM, Fan WC, et al. Phase ii randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol. 2012;7:412–18. doi: 10.1097/JTO.0b013e31823a39e8. [DOI] [PubMed] [Google Scholar]
- 22.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase iii trial—intact 1. J Clin Oncol. 2004;22:777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase iii trial—intact 2. J Clin Oncol. 2004;22:785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Prager D, Hermann R, et al. on behalf of the tribute investigator group tribute: a phase iii trial of erlotinib hydrochloride (osi-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 25.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase iii study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 26.Nokihara H, Ohe Y, Yamada K, et al. Randomized phase ii study of sequential carboplatin/paclitaxel (cp) and gefitinib (g) in chemotherapy-naive patients with advanced non-small-cell lung cancer (nsclc): final results [abstract 8069] J Clin Oncol. 2008;26 doi: 10.3892/mco.2016.1076. [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/26/15_suppl/8069; cited 29 March 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok TSK, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase ii study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:5080–7. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- 28.Riely GJ, Rizvi NA, Kris MG, et al. Randomized phase ii study of pulse erlotinib before or after carboplatin and paclitaxel in current or former smokers with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:264–70. doi: 10.1200/JCO.2008.17.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok TS, Wu YL, Thongprasert S, et al. A randomized placebo-controlled phase iii study of intercalated erlotinib with gemcitabine/platinum in first-line advanced non-small cell lung cancer (nsclc): fastact-ii [abstract 7519] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/93895-114; cited 5 April 2015] [Google Scholar]
- 30.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase ii study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. 2009;27:2253–60. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gridelli C, Morgillo F, Favaretto A, et al. Sorafenib in combination with erlotinib or with gemcitabine in elderly patients with advanced non-small-cell lung cancer: a randomized phase ii study. Ann Oncol. 2011;22:1528–34. doi: 10.1093/annonc/mdq630. [DOI] [PubMed] [Google Scholar]
- 32.Stinchcombe TE, Peterman AH, Lee CB, et al. A randomized phase ii trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age >= 70 years) with stage iiib/iv non-small cell lung cancer. J Thorac Oncol. 2011;6:1569–77. doi: 10.1097/JTO.0b013e3182210430. [DOI] [PubMed] [Google Scholar]
- 33.Thomas M, Reuss A, Fischer JR, et al. Innovations: randomized phase ii trial of erlotinib (e)/bevacizumab (b) compared with cisplatin (p)/gemcitabine (g) plus b in first-line treatment of advanced nonsquamous (ns) non–small cell lung cancer (nsclc) [abstract 7504] J Clin Oncol. 2011;29 doi: 10.1200/JCO.2010.34.1966. [Available online at: http://meeting.ascopubs.org/cgi/content/short/29/15_suppl/7504; cited 29 March 2015] [DOI] [Google Scholar]
- 34.Boutsikou E, Kontakiotis T, Zarogoulidis P, et al. Docetaxel– carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:125–34. doi: 10.2147/OTT.S42245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SM, Khan I, Upadhyay S, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (topical): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012;13:1161–70. doi: 10.1016/S1470-2045(12)70412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 37.Yang CH, Fukuoka M, Mok TS, et al. Final overall survival (os) results from a phase iii, randomised, open-label, first-line study of gefitinib (g) v carboplatin/paclitaxel (c/p) in clinically selected patients with advanced non-small cell lung cancer (nsclc) in Asia (ipass) [abstract LBA2] Ann Oncol. 2010;21(suppl 8):viii1–2. doi: 10.1093/annonc/mdp303. [DOI] [Google Scholar]
- 38.Han JY, Park K, Kim SW, et al. First-signal: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 39.LeCaer H, Greillier L, Corre R, et al. A multicenter phase ii randomized trial of gemcitabine followed by erlotinib at progression, versus the reverse sequence, in vulnerable elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the gfpc 0505 study) Lung Cancer. 2012;77:97–103. doi: 10.1016/j.lungcan.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Janne PA, Wang X, Socinski MA, et al. Randomized phase ii trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: calgb 30406 trial. J Clin Oncol. 2012;30:2063–9. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi YJ, Kim SW, Lee DH, et al. Paclitaxel/carboplatin (pc) intercalated with gefitinib or paclitaxel/carboplatin (pc) for advanced non-small cell lung cancer (nsclc) in selected population who were smoker or wild-type EGFR: randomized phase ii study [abstract e19079] J Clin Oncol. 2013;31 doi: 10.1200/JCO.2012.46.0980. [Available online at: http://meetinglibrary.asco.org/content/116000-132; cited 29 March 2015] [DOI] [Google Scholar]
- 42.Michael M, White S, Abdi E, et al. A multi-center randomized, open-label phase ii trial of Tarceva in sequential combination with gemcitabine compared to gemcitabine monotherapy as first-line therapy in elderly or ecog ps of 2 patients with advanced nsclc. J Thorac Oncol. 2012;3:S177–8. doi: 10.1111/ajco.12178. [DOI] [PubMed] [Google Scholar]
- 43.Liang J, Ahn M, Kang J, et al. First-line treatment (txt) with pemetrexed–cisplatin (pc), followed sequentially by gefitinib (g) or pemetrexed, in Asian, never-smoker (n/smkr) patients (pts) with advanced nsclc: an open-label, randomized phase ii trial [abstract 7591] J Clin Oncol. 2010;28 [Available online at: http://meetinglibrary.asco.org/content/31344-74; cited 29 March 2015] [Google Scholar]
- 44.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 45.Inoue A, Kobayashi K, Maemondo M, et al. Final overall survival results of NEJ002, a phase iii trial comparing gefitinib to carboplatin (cbdca) plus paclitaxel (txl) as the first-line treatment for advanced non-small cell lung cancer (nsclc) with EGFR mutations [abstract 7519] J Clin Oncol. 2011;29 [Available online at: http://meeting.ascopubs.org/cgi/content/short/29/15_suppl/7519; cited 30 March 2015] [Google Scholar]
- 46.Mitsudomi T, Morita S, Yatabe Y, et al. on behalf of the West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog 3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 47.Mitsudomi T, Morita S, Yatabe Y, et al. Updated overall survival results of wjtog 3405, a randomized phase iii trial comparing gefitinib (g) with cisplatin plus docetaxel (cd) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (egfr) [abstract 7521] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/93530-114; cited 30 March 2015] [Google Scholar]
- 48.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation–positive non-small-cell lung cancer (optimal, ctong-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 49.Zhou C, Wu YL, Liu X, et al. Overall survival (os) results from optimal (ctong 0802), a phase iii trial of erlotinib (e) versus carboplatin plus gemcitabine (gc) as first-line treatment for Chinese patients with EGFR mutation–positive advanced non-small cell lung cancer (nsclc) [abstract 7520] J Clin Oncol. 2012;30 doi: 10.1200/JCO.2011.41.4557. [Available online at: http://meetinglibrary.asco.org/content/96275-114; cited 30 March 2015] [DOI] [Google Scholar]
- 50.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 51.Yang JCH, Schuler MH, Yamamoto N, et al. lux-Lung 3: a randomized, open label, phase iii study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR–activating mutations [abstract LBA7500] J Clin Oncol. 2012;30 [Available online at: http://meeting.ascopubs.org/cgi/content/short/30/18_suppl/LBA7500; cited 29 March 2015] [Google Scholar]
- 52.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (lux-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch FR, Kabbinavar F, Eisen T, et al. A randomized, phase ii, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:3567–73. doi: 10.1200/JCO.2010.34.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K, on behalf of the sign study group Phase ii, open-label, randomized study (sign) of single-agent gefitinib (Iressa) or docetaxel as second-line therapy in patients with advanced (stage iiib or iv) non-small-cell lung cancer. Anticancer Drugs. 2006;17:401–9. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 55.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 56.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase iii study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–52. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 57.Hong J, Kyung SY, Lee SP, et al. Randomized phase ii study of pemetrexed versus gefitinib for patients with previously treated non-small cell lung cancer [abstract P-036] J Thorac Oncol. 2010;5(suppl 5):S401. doi: 10.1097/JTO.0b013e3181c5b198. [DOI] [Google Scholar]
- 58.Lee DH, Park K, Kim JH, et al. Randomized phase iii trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 59.Vamvakas L, Agelaki S, Kentepozidis NK, et al. Pemetrexed (mta) compared with erlotinib (erl) in pretreated patients with advanced non-small cell lung cancer (nsclc): results of a randomized phase iii Hellenic Oncology Research Group Trial [abstract 7519] J Clin Oncol. 2010;28 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/7519; cited 30 March 2015] [Google Scholar]
- 60.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (titan): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–8. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 61.Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (horg) randomized phase 3 study. Cancer. 2013;119:2754–64. doi: 10.1002/cncr.28132. [DOI] [PubMed] [Google Scholar]
- 62.Okano Y, Ando M, Asami K, et al. Randomized phase iii trial of erlotinib (e) versus docetaxel (d) as second- or third-line therapy in patients with advanced non–small cell lung cancer (nsclc) who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and Erlotinib Lung Cancer Trial (delta) [abstract 8006] J Clin Oncol. 2013;31 doi: 10.1200/JCO.2013.52.4694. [Available online at: http://meetinglibrary.asco.org/content/112718-132; cited 30 March 2015] [DOI] [PubMed] [Google Scholar]
- 63.Kelly K, Azzoli CG, Zatloukal P, et al. Randomized phase 2b study of pralatrexate versus erlotinib in patients with stage iiib/iv non-small-cell lung cancer (nsclc) after failure of prior platinum-based therapy. J Thorac Oncol. 2012;7:1041–8. doi: 10.1097/JTO.0b013e31824cc66c. [DOI] [PubMed] [Google Scholar]
- 64.Chen YM, Liu JM, Chou TY, Perng RP, Tsai CM, Whang-Peng J. Phase ii randomized study of daily gefitinib treatment alone or with vinorelbine every 2 weeks in patients with adenocarcinoma of the lung who failed at least 2 regimens of chemotherapy. Cancer. 2007;109:1821–8. doi: 10.1002/cncr.22616. [DOI] [PubMed] [Google Scholar]
- 65.Aparisi F, Garcia Sanchez J, Sanchez-Hernandez A, et al. A multicenter, open, randomized, phase ii study to investigate the sequential administration of docetaxel and intermittent erlotinib versus erlotinib as a second-line therapy for advanced non-small cell lung cancer (nsclc) [abstract e18036] J Clin Oncol. 2011;29 [Available online at: http://meetinglibrary.asco.org/content/84426-102; cited 30 March 2015] [Google Scholar]
- 66.Chen YM, Fan WC, Tsai CM, et al. A phase ii randomized trial of gefitinib alone or with tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. J Thorac Oncol. 2011;6:1110–16. doi: 10.1097/JTO.0b013e3182121c09. [DOI] [PubMed] [Google Scholar]
- 67.Aerts JG, Codrington H, Lankheet NA, et al. on behalf of the nvalt study group A randomized phase ii study comparing erlotinib versus erlotinib with alternating chemotherapy in relapsed non-small-cell lung cancer patients: the nvalt-10 study. Ann Oncol. 2013;24:2860–5. doi: 10.1093/annonc/mdt341. [DOI] [PubMed] [Google Scholar]
- 68.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase iii interest trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 69.Aparisi F, Sanchez-Hernandez A, Giner V, et al. Clinical benefits of sequential administration of docetaxel and intermittent erlotinib as a second-line therapy for advanced non-small cell lung cancer (nsclc): a phase ii randomized study [abstract e18049] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/96702-114; cited 30 March 2015] [Google Scholar]
- 70.Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase ii study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small–cell lung cancer. J Clin Oncol. 2007;25:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 71.Lynch TJ, Fenton D, Hirsh V, et al. A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:1002–9. doi: 10.1097/JTO.0b013e3181aba89f. [DOI] [PubMed] [Google Scholar]
- 72.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii study. J Clin Oncol. 2009;27:2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 73.Schiller JH, Akerley WL, Brugger W, et al. Results from arq 197–209: a global randomized placebo-controlled phase ii clinical trial of erlotinib plus arq 197 versus erlotinib plus placebo in previously treated egfr inhibitor-naive patients with locally advanced or metastatic non-small cell lung cancer (nsclc) [abstract LBA7501] J Clin Oncol. 2010;28 doi: 10.1200/JCO.2009.24.2008. [Available online at: http://meetinglibrary.asco.org/content/51587-74; cited 30 March 2015] [DOI] [Google Scholar]
- 74.Han JY, Lee SH, Yoo NJ, et al. A randomized phase ii study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17:1553–60. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 75.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (beta): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–54. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Natale RB, Thongprasert S, Greco FA, et al. Phase iii trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:1059–66. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 77.Ramalingam SS, Spigel DR, Chen D, et al. Randomized phase ii study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2011;29:4574–80. doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sequist LV, Von Pawel J, Garmey EG, et al. Randomized phase ii study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29:3307–15. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 79.Spigel DR, Burris HA, 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase ii trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2582–9. doi: 10.1200/JCO.2010.30.7678. [DOI] [PubMed] [Google Scholar]
- 80.Gian V, Rubin MS, Shipley D, et al. Sorafenib and continued erlotinib or sorafenib alone in patients with advanced non-small cell lung cancer progressing on erlotinib: a randomized phase ii study of the Sarah Cannon Research institute (scri) [abstract 7587] J Clin Oncol. 2012;30 doi: 10.1016/j.lungcan.2017.09.007. [Available online at: http://meetinglibrary.asco.org/content/100756-114; cited 30 March 2015] [DOI] [PubMed] [Google Scholar]
- 81.Reckamp KL, Koczywas M, Cristea MC, et al. Randomized phase ii trial oferlotinib (e) plus high-dose celecoxib (hd-c) or placebo in advanced non-small cell lung cancer [abstract 7518] J Clin Oncol. 2012;30 [Available online at: http://meet-inglibrary.asco.org/content/93734-114; cited 30 March 2015] [Google Scholar]
- 82.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase iii trial. J Clin Oncol. 2012;30:2070–8. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 83.Witta SE, Jotte RM, Konduri K, et al. Randomized phase ii trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol. 2012;30:2248–55. doi: 10.1200/JCO.2011.38.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Besse B, Leighl N, Bennouna J, et al. Phase ii study of everolimus–erlotinib in previously treated patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25:409–15. doi: 10.1093/annonc/mdt536. [DOI] [PubMed] [Google Scholar]
- 85.Groen HJ, Socinski MA, Grossi F, et al. A randomized, double-blind, phase ii study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (nsclc) Ann Oncol. 2013;24:2382–9. doi: 10.1093/annonc/mdt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase ii trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:4105–14. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Von Pawel J, Papai-Szekely Z, Vinolas N, et al. A randomized phase ii study of pemetrexed versus pemetrexed plus erlotinib in second-line treatment for locally advanced or metastatic, nonsquamous nsclc [abstract 7526] J Clin Oncol. 2011;29 doi: 10.1016/j.ejca.2014.03.007. [Available online at: http://meetinglibrary.asco.org/content/76036-102; cited 30 March 2015] [DOI] [PubMed] [Google Scholar]
- 88.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 89.Gaafar RM, Surmont VF, Scagliotti GV, et al. on behalf of the eortc Lung Cancer Group and the Italian Lung Cancer Project A double-blind, randomised, placebo-controlled phase iii Intergroup study of gefitinib in patients with advanced nsclc, non-progressing after first line platinum-based chemotherapy (eortc 08021/ilcp 01/03) Eur J Cancer. 2011;47:2331–40. doi: 10.1016/j.ejca.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 90.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [Erratum in: N Engl J Med 2006;355:1746] [DOI] [PubMed] [Google Scholar]
- 91.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase ii trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the ideal 1 trial) J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [Erratum in: J Clin Oncol 2004;22:4863] [DOI] [PubMed] [Google Scholar]
- 92.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 93.Ahn J, Kim S, Ahn M, et al. Randomized phase ii study of gefitinib versus erlotinib in patients with advanced non–small cell lung cancer who failed previous chemotherapy [abstract 7551] J Clin Oncol. 2010;28 doi: 10.1016/j.lungcan.2011.05.022. [Available online at: http://meetinglibrary.asco.org/content/50243-74; cited 30 March 2015] [DOI] [PubMed] [Google Scholar]
- 94.Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase ii study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3337–44. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (icogen): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–61. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 96.Ahn M, Sun J, Ahn JS, et al. Randomized phase iii trial of gefitinib or pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (kcsg-lu08-01) [abstract 76003] J Clin Oncol. 2011;29 [Available online at: http://meetinglibrary.asco.org/content/80152-102; cited 30 March 2015] [Google Scholar]
- 97.Lee DH, Lee JS, Kim SW, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non–small cell lung cancer. Eur J Cancer. 2013;49:3111–21. doi: 10.1016/j.ejca.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 98.Yang J, Cheng Y, Zhao M, et al. A phase ii trial comparing pemetrexed with gefitinib as the second-line treatment of nonsquamous nsclc patients with wild-type egfr (ctong 0806) [abstract 8042] J Clin Oncol. 2013;31 [Available online at: http://meetinglibrary.asco.org/content/109906-132; cited 30 March 2015] [Google Scholar]
- 99.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (lux-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–38. doi: 10.1016/S1470-2045(12)70087-6. [Erratum in: Lancet Oncol 2012;13:e186] [DOI] [PubMed] [Google Scholar]
- 100.Garassino MC, Martelli O, Broggini M, et al. on behalf of the tailor trialists Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (tailor): a randomised controlled trial. Lancet Oncol. 2013;14:981–8. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 101.Gitlitz BJ, Bernstein ED, Keogh GP, et al. Apricot-i: results of a biomarker-based phase ii randomized placebo controlled study of apricoxib in combination with erlotinib in non-small cell lung cancer [abstract 7528] J Clin Oncol. 2012;29 [Available online at: http://meetinglibrary.asco.org/content/78570-102; cited 30 March 2015] [Google Scholar]
- 102.Belani CP, Nemunaitis JJ, Chachoua A, et al. Phase 2 trial of erlotinib with or without PF-3512676 (CPG 7909, a Toll-like receptor 9 agonist) in patients with advanced recurrent EGFR-positive non-small cell lung cancer. Cancer Biol Ther. 2013;14:557–63. doi: 10.4161/cbt.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim ST, Uhm JE, Lee J, et al. Randomized phase ii study of gefitinib versus erlotinib in patients with advanced non– small cell lung cancer who failed previous chemotherapy. Lung Cancer. 2012;75:82–8. doi: 10.1016/j.lungcan.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 104.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. on behalf of the saturn investigators Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 105.Takeda K, Hida T, Sato T, et al. Randomized phase iii trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (wjtog 0203) J Clin Oncol. 2010;28:753–60. doi: 10.1200/JCO.2009.23.3445. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (inform; c-tong 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012;13:466–75. doi: 10.1016/S1470-2045(12)70117-1. [DOI] [PubMed] [Google Scholar]
- 107.Bylicki O, Ferlay C, Chouaid C, et al. Efficacy of pemetrexed as second-line therapy in advanced nsclc after either treatment-free interval or maintenance therapy with gemcitabine or erlotinib in ifct-gfpc 05-02 phase iii study. J Thorac Oncol. 2013;8:906–14. doi: 10.1097/JTO.0b013e31828cb505. [DOI] [PubMed] [Google Scholar]
- 108.Johnson BE, Kabbinavar F, Fehrenbacher L, et al. atlas: randomized, double-blind, placebo-controlled, phase iiib trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:3926–34. doi: 10.1200/JCO.2012.47.3983. [DOI] [PubMed] [Google Scholar]
- 109.Ahn MJ, Yang JCH, Liang J, et al. Randomized phase ii trial of first-line treatment with pemetrexed–cisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer. 2012;77:346–52. doi: 10.1016/j.lungcan.2012.03.011. [DOI] [PubMed] [Google Scholar]