Abstract
OBJECTIVE:
The aim of this study was to summarize the experience of a tertiary center in treating hepatoblastoma for the last 21 years.
PATIENTS AND METHODS:
Fifty-eight cases were included. The tumor extent and prognosis were assessed using the PRETEXT system. The following data were analyzed: age at diagnosis, comorbidities, prematurity, treatment modalities, histopathological findings, surgical details and complications, treatment outcomes, chemotherapy schedules, side effects and complications. Treatment outcomes included the occurrence of local or distant recurrence, the duration of survival and the cause of death. The investigation methods were ultrasonography, CT scan, serum alpha-fetoprotein level measurement and needle biopsy. Chemotherapy was then planned, and the resectability of the tumor was reevaluated via another CT scan.
RESULTS:
The mean numbers of neoadjuvant cycles and postoperative cycles of chemotherapy were 6±2 and 1.5±1.7, respectively. All children except one were submitted for surgical resection, including 50 partial liver resections and 7 liver transplantations. Statistical comparisons demonstrated that long-term survival was associated with the absence of metastasis (p=0.04) and the type of surgery (resection resulted in a better outcome than transplantation) (p=0.009). No associations were found between vascular invasion, incomplete resection, histological subtype, multicentricity and survival. The overall 5-year survival rate of the operated cases was 87.7%.
CONCLUSION:
In conclusion, the experience of a Brazilian tertiary center in the management of hepatoblastoma in children demonstrates that long survival is associated with the absence of metastasis and the type of surgery. A multidisciplinary treatment involving chemotherapy, surgical resection and liver transplantation (including transplantations using tissue from living donors) led to good outcomes and survival indexes.
Keywords: Hepatoblastoma, Liver Tumors, Liver Transplantation, Treatment, Children
INTRODUCTION
Hepatoblastoma is the most common malignant liver tumor in children; it accounts for 50% of liver tumors and 1.3% of malignant tumors in children 1. Hepatoblastoma is generally sensitive to chemotherapy. Surgery combined with chemotherapy can significantly improve the remission rate, which is as high as 70% 2.
The recommended timing of chemotherapy varies. The evolution of chemotherapeutic agents, predominantly cisplatin-based treatments used in neoadjuvant and adjuvant protocols, has largely contributed to the decrease in mortality among hepatoblastoma patients over the past several decades 3,4,5.
However, surgical resection remains the cornerstone of curative treatment in all age groups with hepatoblastoma. Surgical resection to achieve extirpation of a hepatoblastoma includes segmentectomy, sectionectomy, and/or hemihepatectomy for PRETEXT I and II tumors and trisectionectomy for PRETEXT III and IV tumors. Recently, liver transplantation has been performed for unresectable disease (bilobar portal vein or hepatic vein and inferior vena cava invasion, as well as extensive loss of normal parenchyma) in cases with no evidence of distant metastasis 6,7.
In this study, we aimed to summarize our experience in treating hepatoblastoma for the last 21 years. We then analyzed the clinical therapeutic effects, complications and outcomes of chemotherapy, surgery, and liver transplantation as administered to these patients.
PATIENTS AND METHODS
The medical records of children admitted to our institution with a diagnosis of hepatoblastoma between January 1993 and January 2014 were reviewed. A total of 58 cases were included in this study. All diagnoses were confirmed by histological examination. Children were prospectively followed based on periodic outpatient reviews.
For all patients, the tumor extent and prognosis were assessed using the PRETreatment EXTent of disease (PRETEXT) system. The following data were analyzed: age at diagnosis, comorbidities, prematurity, treatment modalities, histopathological findings, surgical details, operative complications, and treatment outcomes. Chemotherapy schedules, side effects, complications and outcomes were evaluated.
The collected data related to surgery included the extent of hepatic resection, the presence or absence of free margins, and the occurrence of operative complications.
If the tumor was considered to be unresectable according to the results of the postchemotherapy CT scan, liver transplantation was considered. Before transplantation was approved, the patient underwent extensive scanning to search for distant metastases.
Treatment outcomes were evaluated according to the occurrence of local or distant recurrence, the duration of survival, and the cause of death.
Investigation methods
Ultrasonography was the first imaging tool used in all patients clinically suspected to have an abdominal mass. Once the presence of a liver tumor was confirmed by ultrasonography, a CT scan was performed to evaluate the extent of the lesion, its vascular supply, the involvement of adjacent organs and lymph nodes, and the relationship of the mass to major vessels such as the portal vein, hepatic arteries, and hepatic veins.
After monitoring the initial alpha-fetoprotein level, a needle biopsy was performed to histologically diagnose the tumor. Biopsies were echo-guided and were resected through the adjacent normal parenchyma to avoid spillage into the peritoneum. Neoadjuvant chemotherapy was planned. The impact of chemotherapy and the resectability of the tumor were evaluated via another CT scan.
Standard clinical staging
According to the International Society of Pediatric Oncology (SIOP) 8, pre-operative hepatoblastoma (PRETEXT) is divided into four stages, ranging from stage I to stage IV.
Treatment programs
In most cases, the chemotherapy schedules included cisplatin and doxorubicin. Four patients received cisplatin plus etoposide, four received only cisplatin, and five received cisplatin plus carboplatin plus doxorubicin. The mean number of chemotherapy courses before surgery was five. Except for one case in which the histological assessment revealed 100% necrotic cells in the liver tumor, all patients also received postoperative chemotherapy.
Surgical treatment
Liver resection
A bilateral subcostal incision was preferred, as it enabled good exposure of the operative field and an acceptable cosmetic appearance. As tumor extension was carefully assessed preoperatively via a CT scan performed a few days before the surgery, intraoperative ultrasound was not performed.
The liver was fully mobilized by detaching all anterior and posterior ligamentous attachments to the abdominal wall and to the diaphragm. The liver was then reflected upward to expose the inferior surface and enable hilar structure identification. The extent of the tumor and the possibility of resection could then be assessed. The anatomical site, extension, and vascular involvement of the tumor were carefully analyzed, and the abdomen was thoroughly examined for the presence of any metastases. After the type of resection was determined, the related vascular and biliary structures were identified and isolated.
As lymph node extension is very rare in cases of hepatoblastoma, hilar structures and arterial and portal venous branches were dissected close to the parenchyma to avoid hilar vessel lesions on the other side of the organ. In most cases, the biliary duct was transected and ligated during parenchymal sectioning. The suprahepatic inferior vena cava was exposed to enable easy control of hemorrhaging, if necessary. The hepatic veins were identified, and the right, middle, or left hepatic vein was isolated and secured depending on the type and the extent of resection. In cases of right hepatectomy, all of the small branches arising from the right lobe to the inferior vena cava were ligated and sectioned. During this dissection, the venous infusion was decreased by the anesthetic team to reduce central venous pressure and potential bleeding from the hepatic veins and the vena cava. Parenchymal division near the hilar region was directed toward the lobe that would be removed, thereby avoiding common bile duct lesions.
The parenchymal transection was performed using the Cavitron ultrasonic aspirator (CUSA, Valley Lab, Boulder, CO) or a LigaSure electrocautery held by the surgeon and a bipolar electrocautery held by the first assistant. Peritoneal drainage was routinely performed using a Jackson-Pratts drain near the raw surface of the liver.
Liver transplantation
In 7 cases, living related donor liver transplantation was performed. In contrast to liver transplantations for benign diseases, the procedure began with the recipient laparotomy and a comprehensive analysis of tumor extension and peritoneal or other abdominal organ involvement. The donor procedure was then initiated.
All of the donors were operated on by the same team of pediatric surgeons, and the recipient procedures were performed by one surgeon. The donor and recipient procedures were simultaneously performed in the university pediatric hospital in two connected operating rooms that were specifically adapted for these procedures.
In two cases, the liver grafts were procured from adult cadaveric donors. A graft reduction was performed with preservation of the left lateral segment, which was implanted into the recipient.
Statistical analysis
Multiple statistical comparisons were performed using binary logistic regression analyses, and Fisher's exact test was used with the aid of Statistical Package for Social Sciences (SPSS) for Windows, version 15.0. The following variables were analyzed: the presence of metastasis at the time of diagnosis, the type of resection (complete or incomplete), the presence of vascular invasion, multicentricity, the type of surgical treatment (resection or transplantation), histopathological findings, and the PRETEXT stage. These variables were compared with the final survival outcome to test for significance.
RESULTS
The baseline characteristics of the patients at diagnosis are summarized in Table 1. Considering patients within the last 5 years (2009 to 2014), 25% were premature at birth. In the previous period, prematurity occurred in only 5% of cases. Primary tumor data such as histology, PRETEXT stage, multifocality, vascular involvement and the presence of metastasis at diagnosis are presented in Table 2.
Table 1.
-Main patient characteristics at the time of hepatoblastoma diagnosis.
| Variable | Median or number | Percent or range | ||
|---|---|---|---|---|
| Gender | ||||
| Male | 30 | 51.7 | ||
| Female | 28 | 48.3 | ||
| Age | 1 y 10 ms | 2 ms–12 ys | ||
| Prematurity | 11 | 18.9 | ||
Table 2.
-Tumor characteristics.
| Number of patients (%) | |
| Histological subtype (main component) | |
| Epithelial fetal | 17 (29.3) |
| Epithelial embryonal | 7 (12.1) |
| Mixed epithelial and mesenchymal with teratoid features | 9 (15.5) |
| Mixed epithelial and mesenchymal without teratoid features | 24 (41.4) |
| Small cell undifferentiated (SCUD) | 1 (1.7) |
| PRETEXT stage | |
| I | 2 (3.4) |
| II | 12 (20.6) |
| III | 27 (46.6) |
| IV | 17 (29.3) |
| Tumor focality | |
| Unifocal | 51 (88) |
| Multifocal | 7 (12) |
| Vascular involvement (microscopic or macroscopic) | 16 (27.6) |
| Metastasis | 5 (8.6) |
The mean numbers of neoadjuvant cycles and postoperative cycles of chemotherapy were 6±2 and was 1.5±1.7, respectively. A total of 10% of the patients required hospitalization for febrile neutropenia, and 3.4% presented with cardiac dysfunction that was controlled clinically or with medications. However, no deaths related to chemotherapy complications occurred.
Surgical resections included 19 right hepatectomies, 12 right trisectionectomies, 11 left hepatectomies, 3 monosegment (segment IV a and b) resections, 3 left trisectionectomies and 2 left segmentectomies. Seven children underwent liver transplantation: 4 surgeries were primary transplants, and 3 were rescue transplants after liver recurrence. One child was not submitted to surgical treatment due to the presence of peritoneal carcinomatosis and an unresectable tumor.
The following postoperative complications occurred in 8 children (13.8%): biliary leakage on the cut surface (2), bowel subocclusion (2), early postoperative bleeding (1), surgical infection (1), and transitory hypoglycemia (1). Repeated operations were necessary in 3 cases (5.17%) because of biliary leakage, early intestinal adhesions, and hemorrhage.
Free microscopic margins were obtained in 45 of the 50 patients submitted for surgical resections. In 3 children (including 2 incomplete resection cases), the disease recurred locally, and one of these children also had distant metastasis.
The main data related to surgical resections are shown in Table 3. One early death due to sepsis occurred after a left trisectionectomy. Among the children submitted to liver transplantation, 4 of them remain alive with no disease recurrence. The other 3 died due to tumor relapse; all of them relapsed during the first year after left trisectionectomy. The overall survival rate of the operated cases was 87.7%, and 85.7% of postoperative deaths were related to the recurrence of malignant disease (all cases were PRETEXT IV tumors). The median follow-up duration was 2.3 years (range, 6 months-23 years).
Table 3.
-Main results of children submitted for surgical resection of hepatoblastoma.
| Surgical resections | 19 right hepatectomies |
| 12 right trisectionectomies | |
| 11 left hepatectomies | |
| 3 monosegment (segment IV a and IVb) resections | |
| 3 left trisectionectomies | |
| 2 left segmentectomies | |
| Postoperative complications | 2 biliary leakage on cut surface |
| 2 bowel subocclusion | |
| 1 early postoperative bleeding | |
| 1 surgical infection | |
| 1 transitory hypoglycemia | |
| Free microscopic margins | 45 |
| Reoperations | 3 |
The statistical comparisons using Fisher's exact test revealed a strong association between the absence of metastasis and survival (p=0.04). A correlation was also observed between the type of surgery (resection compared with transplantation) and survival (p=0.009). No significant associations were found between vascular invasion, incomplete resection, histological subtype, multicentricity and survival. As the total number of deaths was relatively low, no significant associations were observed based on logistic regression analyses.
DISCUSSION
Most liver tumors in children are metastatic lesions of neuroblastoma, Wilms' tumor or lymphoma, and the incidence of primary liver tumors in children is low 9,10. Thus, it takes many years for a pediatric surgeon to gain enough experience to properly perform a hepatic resection. The current series of patients was treated in a major university reference center staffed by an experienced group of pediatric clinical oncologists and pediatric surgeons with expertise in surgical oncology and pediatric liver transplantation. All of these accumulated experiences were responsible for the good outcomes reported herein.
The approach to the investigation and treatment of hepatoblastoma in children has evolved in the last 20 years; at present, children with this disease are diagnosed and treated with chemotherapy and surgery according to universally established protocols. This standardization has resulted in excellent survival rates of 70%-85% according to various reports 11,12. In addition to the chemotherapy protocols, refinements in the surgical techniques for liver resections and adequate immediate postoperative treatment in pediatric intensive care units have contributed to this progress.
For proper diagnosis, we utilized an investigation protocol that is in accordance with the recommendations in the literature for the treatment of liver tumors. An initial ultrasound examination followed by a CT scan provides useful information about the nature of the tumor. In all cases, a diagnosis of suspected hepatoblastoma was confirmed by a needle biopsy, which additionally revealed the pathological type of the tumor. No complications related to biopsies occurred.
All cases in the present series were submitted to cycles of chemotherapy prior to surgical resection. This timing is important because chemotherapy reduces the tumor volume and because there is a strong correlation between a good response to chemotherapy and a higher survival rate, despite the presence of more advanced disease 13. We have observed that previous chemotherapy enables resectability in cases of tumors that were previously considered to be unresectable. Despite the need for hospitalization due to chemotherapy (primarily due to febrile neutropenia) in some cases, the morbidity was relatively low, and the survival benefits are incontestable.
Interestingly, prematurity and low birth weight have been associated with an increased risk of hepatoblastoma 14,15,16. In fact, we found a high percentage of a history of prematurity in our patients, and this percentage has increased in recent years (25%). A case-control study developed by the Childreńs Oncology Group aimed to elucidate the reasons for these associations 17. Children diagnosed with hepatoblastoma had a significantly longer exposure to respiratory support, greater exposure to antibiotics and more radiographic exams than controls. Although other studies demonstrated increased risk for hepatoblastoma among children with low or very low birth weight, with preterm birth <33 weeks, with small size for gestational age, and from multiple birth pregnancies 17, the actual explanation for these observations remains unknown.
The present study showed a low surgical complication rate and satisfactory treatment outcomes. To obtain this excellent result, we utilized certain surgical techniques that were learned from our extensive experience with liver transplantation surgeries 9. Surgical refinements of all steps of this complex procedure (including donor - living or cadaveric - hepatectomy, back-table surgery, recipient hepatectomy and graft implant techniques) may be applied to all liver surgeries, especially hepatic resections for neoplastic lesions.
Similar to the conditions of recipient resection of the native liver, in situations of liver transplantation during a surgery for hepatoblastoma resection, the hilar structures of the hepatic lobe to be resected are dissected very close to the parenchyma to avoid injury to any vessels of the other lobe. By following this principle, we did not have any complications related to damage to the vasculature of the remaining lobe.
Regarding the type of resection, 45 cases had free microscopic margins. Fortunately, with the current use of adequate adjuvant chemotherapy, potential microscopic residues or microscopically incomplete resection do not negatively impact survival 18,19. However, a wide margin of normal parenchyma lacking tumor cells does not prevent recurrence in cases of poor histological prognosis 20. Our policy was to maintain the resection line far from the tumor whenever possible, although in some cases, we had to perform the resection very close to the tumor to avoid damage to the remaining liver lobe.
The influence of histological subtype on hepatoblastoma prognosis remains unclear. In contrast to other publications that reported that the epithelial subtype was the most frequent 21,22, in our case series, mixed epithelial and mesenchymal hepatoblastoma without teratoid features represented 41.4% of all cases. Pure fetal histology was thought to be associated with a better prognosis 23. In fact, it has been advocated that complete surgical resection is curative for children with hepatoblastoma with a pure fetal histology and that chemotherapy should be minimized in these cases 24. However, the small cell undifferentiated histological subtype has been associated with reduced patient survival 24,25. However, in our case series, there was no association between the histopathological findings and prognosis. The relatively small number of cases of each histological subtype may have limited the statistical analysis; thus, more comprehensive studies, such as nationwide retrospective or cooperative studies with pediatric oncology groups, would lead to more meaningful results.
Vascular invasion has been considered as an important prognostic factor for hepatoblastoma, although there this issue remains under debate in the literature 12,26. In fact, in our series, we did not detect any correlation between survival and vascular invasion. However, a strong correlation was observed between the absence of metastasis and survival, and the patients submitted for transplantation had poorer survival outcomes than those who underwent liver resection. Among the 7 patients who underwent a liver transplantation, 4 are alive and free of disease. All of these patients had tumors in the four sectors of the liver, which precluded liver resection; thus, total hepatectomy with a liver transplant was the only treatment option.
Liver transplantation for primary malignant hepatic tumors has been reported to have an incidence of approximately 2% of all pediatric transplantations 27. In fact, in our series of 590 children submitted for liver transplantation, 7 (1.19%) had hepatoblastoma. We prefer to perform these transplants using grafts obtained from a living related donor due to the scarcity of cadaveric donors and the large number of patients on the waiting list for a liver transplant.
In conclusion, our current report demonstrates that multidisciplinary treatment of children with hepatoblastoma is becoming more successful, with excellent survival rates. This mode of treatment includes the use of tumor-specific chemotherapy, adequate planning of surgical treatment to ensure the removal of the entire tumor via partial resection of the liver or, if necessary, total removal of the liver and immediate liver transplantation (using tissue from living or deceased donors).
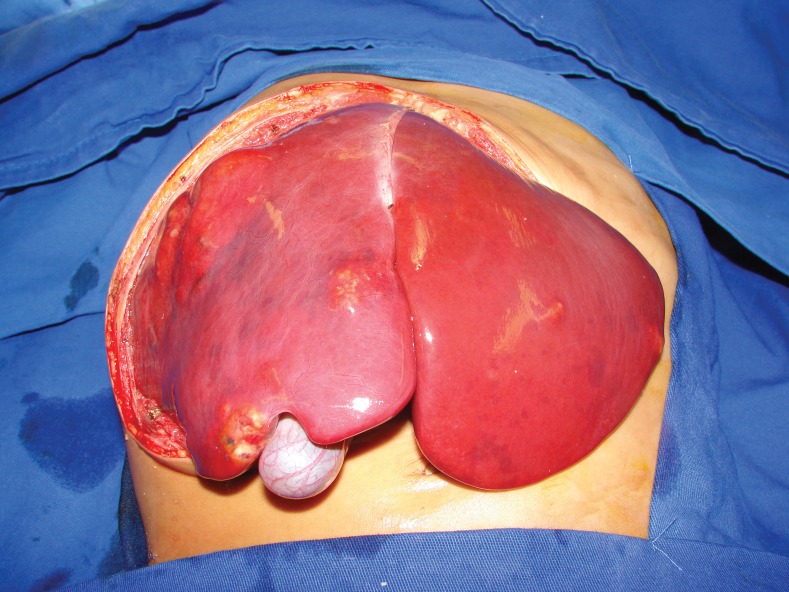
Figure 1.
Surgical aspect of a patient with multifocal hepatoblastoma. Note the multiple nodes affecting all of the liver parenchyma (PRETEX IV). The patient underwent total hepatectomy and living donor liver transplantation.
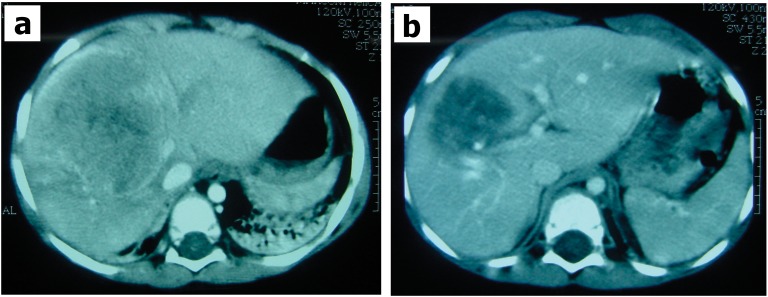
Figure 2.
CT scan of a patient with hepatoblastoma in the right hepatic lobe before (a) and after (b) chemotherapy. The tumor size decreased, and the patient underwent right hepatectomy with surgical margins that were free of tumor cells.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Zhang Y, Zhang WL, Huang DS, Hong L, Wang YZ, Zhu X, et al. Clinical effectiveness of multimodality treatment on advanced pediatric hepatoblastoma. Eur Rev Med Pharmacol Sci. 2014;18((7)):1018–26. [PubMed] [Google Scholar]
- 2.Goedeke J, Haeberle B, Schmid I, Von Schweinitz D. AFP negative cystic liver lesion in a child should let one think of hepatoblastoma. J Pediatr Hematol Oncol. 2011;33((6)):e245–7. doi: 10.1097/MPH.0b013e3181f466ec. [DOI] [PubMed] [Google Scholar]
- 3.Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, et al. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36((11)):1418–25. doi: 10.1016/S0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 4.Douglass EC, Reynolds M, Finegold M, Cantor AB, Glicksman A. Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1993;11((1)):96–9. doi: 10.1200/JCO.1993.11.1.96. [DOI] [PubMed] [Google Scholar]
- 5.Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–70. doi: 10.1056/NEJMoa0810613. [DOI] [PubMed] [Google Scholar]
- 6.Browne M, Sher D, Grant D, Deluca E, Alonso E, Whitington PF, et al. Survival after liver transplantation for hepatoblastoma: a 2-center experience. J Pediatr Surg. 2008;43((11)):1973–81. doi: 10.1016/j.jpedsurg.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr. 2014;26((1)):29–36. doi: 10.1097/MOP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 8.Schnater JM, Aronson DC, Plaschkes J, Perilongo G, Brown J, Otte JB, et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer. 2002;94((4)):1111–20. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 9.Tannuri AC, Tannuri U, Gibelli NE, Romão RL. Surgical treatment of hepatic tumors in children: lessons learned from liver transplantation. J Pediatr Surg. 2009;44((11)):2083–7. doi: 10.1016/j.jpedsurg.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13((7)):812–20. doi: 10.1634/theoncologist.2008-0011. [DOI] [PubMed] [Google Scholar]
- 11.Kremer N, Walther AE, Tiao GM. Management of hepatoblastoma: an update. Curr Opin Pediatr. 2014;26((3)):362–9. doi: 10.1097/MOP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 12.Qiao GL, Li L, Cheng W, Ge J, Zhang Z, Wei Y. Predictors of survival after resection of children with hepatoblastoma: A single Asian center experience. Eur J Surg Oncol. 2014;40((11)):1533–9. doi: 10.1016/j.ejso.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18((22)):3819–28. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 14.Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, et al. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998;58((14)):3032–5. [PubMed] [Google Scholar]
- 15.Reynolds P Urayama KY, Von Behren J, Feusner J. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004;100((5)):1070–6. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda H, Matsuyama S, Tanimura M. Association between hepatoblastoma and very low birth weight: a trend or a chance. J Pediatr. 1997;130((4)):557–60. doi: 10.1016/S0022-3476(97)70239-7. [DOI] [PubMed] [Google Scholar]
- 17.Heck JE, Meyers TJ, Lombardi C, Park AS, Cockburn M, Reynolds P, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37((4)):390–5. doi: 10.1016/j.canep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies JQ, de la Hall PM, Kaschula RO, Sinclair-Smith CC, Hartley P, Rode H, et al. Hepatoblastoma--evolution of management and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases. J Pediatr Surg. 2004;39((9)):1321–7. doi: 10.1016/j.jpedsurg.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 19.von Schweinitz D, Hecker H, Schmidt-von-Arndt G, Harms D. Prognostic factors and staging systems in childhood hepatoblastoma. Int J Cancer. 1997;74((6)):593–9. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 20.Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;53((6)):1016–22. doi: 10.1002/pbc.v53:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon SB, Shin HB, Seo JM, Lee SK. Hepatoblastoma: 15-year experience and role of surgical treatment. J Korean Surg Soc. 2011;81((2)):134–40. doi: 10.4174/jkss.2011.81.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail H, Broniszczak D, Kaliciński P, Dembowska-Bagińska B, Perek D, Teisseyre J, et al. Changing treatment and outcome of children with hepatoblastoma: analysis of a single center experience over the last 20 years. J Pediatr Surg. 2012;47((7)):1331–9. doi: 10.1016/j.jpedsurg.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 23.Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26((1)):19–28. doi: 10.1097/MOP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 24.Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:3301–6. doi: 10.1200/JCO.2010.29.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas JE, Feusner J, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer. 2001;92:3130–4. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 26.Maibach R, Roebuck D, Brugieres L, Capra M, Brock P, Dall'Igna P, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer. 2012;48((10)):1543–9. doi: 10.1016/j.ejca.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Tannuri AC, Gibelli NE, Ricardi LR, Santos MM, Maksoud-Filho JG, Pinho-Apezzato ML, et al. Living related donor liver transplantation in children. Transplant Proc. 2011;43((1)):161–4. doi: 10.1016/j.transproceed.2010.11.013. [DOI] [PubMed] [Google Scholar]