Abstract
The genetic diversity of Mycobacterium tuberculosis isolates among patients from Sweden was determined by a combination of two PCR-based techniques (spoligotyping and variable number of tandem repeats analysis). It resulted in a clustering of 23.6% of the isolates and a rate of recent transmission of 14.1%. The clustered isolates mainly belonged to the Haarlem family (23.2%), followed by the Beijing (9.8%), Latin American and Mediterranean (LAM; 8%), and East African-Indian (EAI; 6.2%) families. A comparison of the spoligotypes with those in the international spoligotyping database showed that 62.5% of the clustered isolates and 36.6% of all isolates typed were grouped into six major shared types. A comparison of the spoligotypes with those in databases for Scandinavian countries showed that 33% of the isolates belonged to an ill-defined T family, followed by the EAI (22%), Haarlem (20%), LAM (11%), Central Asian (5%), X (5%), and Beijing (4%) families. Both the highest number of cases and the proportion of clustered cases were observed in patients ages 15 to 39 years. Nearly 10% of the isolates were resistant to one or more drugs (essentially limited to isoniazid monoresistance). However, none of the strains were multidrug resistant. Data on the geographic origins of the patients showed that more than two-thirds of the clustered patients with tuberculosis were foreign-born individuals or refugees. These results are explained on the basis of both the historical links within specific countries and recently imported cases of tuberculosis into Sweden.
About 2 million humans die from tuberculosis (TB) each year, and it is estimated that about one-third of the world's population is infected with Mycobacterium tuberculosis (20). The disease is most common in developing countries and is spreading fast because of the human immunodeficiency virus pandemic. In Western European countries, the rates of TB, determined from rates of notification to public health authorities, have increased recently, particularly because of cases among immigrants from countries with a high incidence of TB (2). In Eastern European countries, the rates of mortality and morbidity from TB have also increased dramatically because of socioeconomic difficulties or problems with TB control programs (2). Despite a low reported TB incidence of 5.2/100,000 inhabitants in Sweden, there remains a risk of spread of multidrug-resistant (MDR) TB due to migration from the countries of Eastern Europe, where the incidence of MDR TB is alarming (6).
During the last decade, molecular epidemiological methods have been developed with the aim of revealing epidemiological features of the disease, surveying its spread, and identifying risk factors associated with the dissemination of MDR strains (18). Although the “gold standard” method for studying the epidemiology of TB is IS6110-based restriction fragment length polymorphism analysis, it remains a cumbersome method. It may be successfully replaced by easier PCR-based methods, such as spoligotyping and variable number of tandem DNA repeats (VNTR) analysis, which have high discriminatory indices and reproducibilities when they are used in combination (9, 18). The aim of this study was to determine the genetic diversity of M. tuberculosis isolates from Swedish patients by using this combination of PCR-based methods and to compare the data obtained with those from neighboring countries to highlight the dissemination of major phylogenetic clades of TB within Scandinavia.
MATERIALS AND METHODS
Strains and clinical specimens.
The 220 M. tuberculosis complex strains obtained were systematically cultured from pathological samples from June 1999 to December 2002 in Göteborg, Sweden. Testing for susceptibility to rifampin (RIF), pyrazinamide (PZA), isoniazid (INH), streptomycin (SM), and ethambutol (EMB) was performed by the BACTEC 460 radiometric method (21).
Molecular typing.
Bacterial DNA was prepared by the cetyltrimethylammonium bromide (Merck, Darmstadt, Germany) method, as described previously (28). The DNA pellet was dried at room temperature, resuspended in TE buffer (10 mM Tris, 1 mM EDTA [pH 8]), and stored at 4°C.
Spoligotyping was performed with primers DRa and DRb, with primer DRa biotinylated at the 5′ end in order to amplify the whole direct repeat (DR) region, as described previously (14). Detection of hybridizing DNA was done by enhanced chemiluminescence (ECL; Enhanced Chemo-Luminescence Detection kit; Amersham, Little Chalfont, England), followed by exposure to X-ray film (Hyperfilm ECL; Amersham), in accordance with the instructions of the manufacturer. The X-ray film was developed with standard photochemicals after 2 h of exposure.
Typing by VNTR analysis was performed as described previously (9, 10), with slight modifications. PCR was performed in a GeneAmp PCR system 9600 (Perkin-Elmer, Roche Diagnostic Systems). An aliquot of 30 μl from the reaction tubes was run on a 3% Metaphor gel (FMC Bioproducts, Rockland, Maine). Molecular size standards (100-bp ladder; AP-Biotech, Uppsala, Sweden) were run every seven lanes. Determination of the molecular sizes of the PCR fragments was performed with Taxotron software (P. A. D. Grimont, Taxolab, Institut Pasteur, Paris, France) with images digitized with the Video-Copy system (Bioprobe, Montreuil, France). Once the lengths of the PCR fragments were calculated precisely, the number of copies for each exact tandem repeat (ETR) was deduced by using an Excel spreadsheet program (Microsoft, Cupertino, Calif.) and data published previously (10). The data were documented as five-digit numbers representing allele profiles ETR-A to ETR-E.
RESULTS
Molecular typing data.
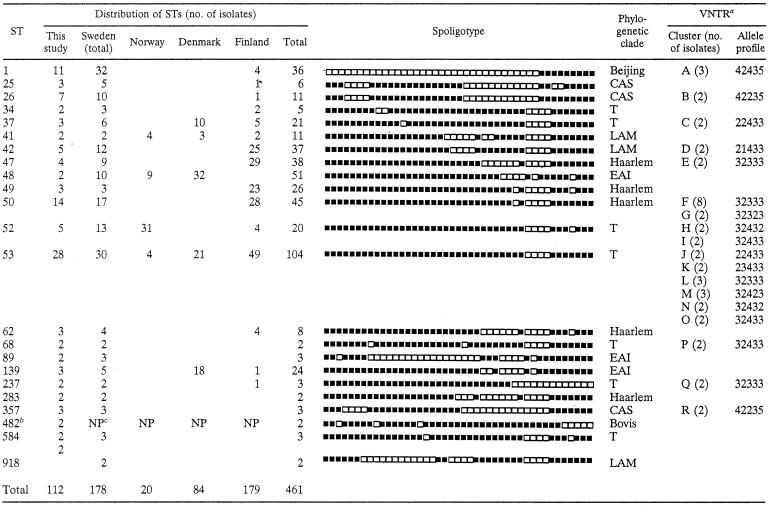
Of the 220 M. tuberculosis complex isolates available, 29 isolates could not be typed satisfactorily due to poor DNA quality. Thus, reproducible results were obtained for 191 isolates; 58.6% of the isolates were clustered by spoligotyping (22 clusters containing 112 strains, with the number of strains in each cluster ranging from 2 to 28; Table 1). Major spoligotypes were defined as types that contained five or more isolates each among the Swedish isolates. The results were also compared with those in the international spoligotyping database of the Institut Pasteur de Guadeloupe, which has defined major and minor rules for designating major spoligotyping families or clades (8). Its most recent version (version SpolDB4, which is under development) contained patterns for 26,371 clinical isolates, split into 1,527 shared types (STs; a common pattern shared by two or more isolates) and 2,446 unique (orphan) types at the time of the comparison. Thirty (15.7%) isolates from the present study were orphan types, whereas the remaining 84.3% of the spoligotypes were already present in the database. Among the clustered isolates in this study (excluding the two M. bovis BCG isolates, which were not retained for phylogenetic analysis), there were six major spoligotypes (STs 1, 26, 42, 50, 52, and 53), which consisted of 62.5% of the clustered isolates and 36.6% of all isolates typed. Sixteen minor spoligotypes, which contained less than five isolates each, were also identified and represented 22% of all isolates.
TABLE 1.
Clusters of M. tuberculosis strains determined by various typing methods and distribution of spoligotyping-defined STs found in the present study compared with those reported among Scandinavian countries in an international database
A total of 45 strains in 18 clusters were detected.
Two M. bovis BCG strains were not retained for comparative analysis, which was limited to M. tuberculosis.
NP, not performed, as the analysis was limited to M. tuberculosis only.
Of the 191 M. tuberculosis complex isolates studied by spoligotyping, 112 clustered strains were further typed by VNTR analysis. Spoligotyping and VNTR analysis combined resulted in 18 clusters containing 45 strains, with each cluster containing from 2 to 8 strains (Table 1). The rate of recent transmission was calculated by the formula [T(c) − N(c)]/T(a), where T(c) is the total number of clustered isolates, N(c) is the number of clusters, and T(a) is the total number of isolates (22). On the basis of our data, this rate was 14.1%.
A determination of predominating clades by spoligotyping showed that isolates mainly belonged to the Beijing, Central Asian (CAS), East African-Indian (EAI), Haarlem, Latin American, and Mediterranean (LAM), and T families. A major proportion of clustered strains (23.2%) belonged to the Haarlem family (STs 47, 49, 50, 62, and 283). The proportions of clustered strains that belonged to the remaining families were as follows: 9.8% to the Beijing family (ST 1), 8% to the LAM family (STs 41, 42, and 918), and 6.2% to the EAI family (STs 48, 89, and 139). The remaining isolates belonged to either the CAS family or an ill-defined T group of families (Table 1).
To see how the families observed in this study agreed with those present throughout Scandinavia, the data were also compared to the data in the SpolDB4 database. Two different analyses were performed: the first one focused on the STs observed in the present study compared with the same STs observed throughout Scandinavia (n = 461) (Table 1), whereas the second one compared all the STs, according to their classifications in the seven major families, present among the four countries studied (n = 898) (data not shown). Among the four Scandinavian countries, strains belonging to STs 25, 62, 68, 89, 283, 357, 584, and 918 were limited to Sweden; and among these STs, STs 68, 283, 357, and 918 were exclusively found during the present investigation (Table 1). Most of the STs found in this study were present in Finland; the exception was ST 48. Although the STs in the database may not be representative of the M. tuberculosis population structures present in each country, a global comparison of the STs for all the Scandinavian countries was also performed. As many as 33% of the isolates belonged to an ill-defined T family, followed by the EAI (22%), Haarlem (20%), LAM (11%), CAS (5%), X (5%), and Beijing (4%) families. Strains of some families were predominant in a given country, e.g., 90% of all Beijing isolates recorded for Scandinavia in the database were from Sweden, 70% of Haarlem isolates were from Finland, and 60% each of X and EAI isolates were from Denmark. On the other hand, CAS family strains were nearly equally split between Sweden and Norway. These differences either may underline historical links with specific countries or may represent recently imported TB cases, as detailed in the Discussion.
Patient characteristics and drug resistance patterns.
The sex ratio of the population was 1, and the age range of the patients was 1 to 89 years. The proportion of cases clustered in various age groups is summarized in Table 2. Patients in the group aged 15 to 39 years had the highest number (45.4%) of isolates, followed by those ages 65 years or older, with 30.5% of the isolates. Interestingly, the proportion of clustered cases was higher among the group aged 15 to 39 years than among those aged 65 years or older (52.7 and 22.7%, respectively, by spoligotyping alone and 48.9 and 26.7%, respectively, by spoligotyping plus VNTR analysis). Clustering by spoligotyping alone showed broad relationships among strains (clades) rather than epidemiological links, whereas clustering by combined spoligotyping and VNTR analysis may be considered to reflect the clonality of the isolates. These results could indirectly suggest that most of the cases among patients aged 15 to 39 years represented recently transmitted disease, whereas cases among elderly patients represented reactivation; nonetheless, these differences between the two age groups were not statistically significant.
TABLE 2.
Patient characteristics and drug resistance patterns for M. tuberculosis clinical isolates
| Characteristic | Culture-positive patients (n = 220) | N-typed isolates (n = 191) | Molecular typing results (n = 191)
|
||
|---|---|---|---|---|---|
| Unclustered (n = 79) | Clustered by:
|
||||
| Spoligotyping alone (n = 112) | Spoligotyping and VNTR analysis (n = 45) | ||||
| Sex ratioa | 1 | 1.1 | 1.1 | 1.1 | 1.4 |
| No. (%) of patients of the following age (yr): | |||||
| 1-14 | 9 (4.2)b | 7 (3.7) | 5 (6.3) | 2 (1.8) | 1 (2.2) |
| 15-39 | 98 (45.4) | 85 (45.0) | 27 (34.2) | 58 (52.7) | 22 (48.9) |
| 40-64 | 43 (19.9) | 40 (21.2) | 15 (19) | 25 (22.7) | 10 (22.2) |
| ≥65 | 66 (30.5) | 57 (30.1) | 32 (40.5) | 25 (22.7) | 12 (26.7) |
| Unknown | 4 (1.8) | 2 (1.1) | 0 | 2 (1.8) | 0 |
| No. (%) of patient isolates with drug resistance | |||||
| Total drug resistance | 19 (9.0) | 15 (7.8) | 2 (2.5) | 13 (11.7)c | 3 (6.7) |
| Resistance data not available | 7 (3.2) | 1 (0.5) | 0 | 1 (0.9) | 0 |
| Resistance to one drug | 15 (7.0) | 12 (6.3) | 1 (1.3) | 11 (9.9) | 3 (6.7) |
| No. of men/no. of women | 7/8 | 5/7 | 0/1 | 5/6 | 1/2 |
| Resistance to two or more drugs | 4 (1.9) | 3 (1.6) | 1 (1.3) | 2 (1.8) | 0 |
| No. of men/no. of women | 0/4 | 0/3 | 0/1 | 0/2 | |
Number of males/number of females.
Note that for all percentages calculations did not take into account patients for whom characteristics (age or drug resistance data) were unknown.
P = 0.017.
Among the clustered strains obtained by spoligotyping, 37 (33%) were from ethnic Swedish individuals and 75 (67%) were from either foreign-born individuals or refugees. They originated from Afghanistan (n = 4), Bosnia (n = 5), Ethiopia (n = 3), Finland (n = 3), Iran (n = 2), Kosovo (n = 6), Somalia (n = 16), Turkey (n = 3), Vietnam (n = 9), Yugoslavia (n = 6), and 14 other countries (n = 18). Among the clusters obtained by the two methods (Table 3), we noticed that 14 (31.1%) patients were from Sweden, whereas 31 (68.9%) were foreign-born individuals. Among the 18 clusters defined by a combination of spoligotyping and VNTR analysis, the available epidemiological data, including the ethnicities of the patients, suggested potential links for patients in 12 clusters (clusters A, D, F, H, I, J, L, M, N, O, P, and R). All except three strains (two strains in cluster H monoresistant to INH and one strain in cluster D resistant to SM) were pansusceptible. These data suggest that clustering (with the exception of isolates in cluster H) was not associated with drug resistance.
TABLE 3.
Data on patient ethnicity, age, and residence for 18 M. tuberculosis clusters
| Cluster (no. of isolates) | Geographic origin (age or age range [yr]) of patients:
|
|
|---|---|---|
| Living in the same location | Living in different locations | |
| A (3) | Ethiopia (14), Somalia (32), and Vietnam (32) (Göteborg)a | |
| B (2) | Afghanistan (30) and Somalia (50) | |
| C (2) | Somalia (49) and Sweden (78) | |
| D (2) | Somalia (19 and 21) (Göteborg) | |
| E (2) | Sweden (59 and 84) | |
| F (8) | Bosnia (15 and 38), Spain (50), and Sweden (66-72 [3 patients]) | Sweden (two patients, both 83) |
| G (2) | Bosnia (44) and Yugoslavia (38) | |
| H (2)b | Yugoslavia (27 and 28) (Bohuslän) | |
| I (2) | Somalia (15) and Sweden (79) (Göteborg) | |
| J (2) | Portugal (52) and Syria (62) (Göteborg) | |
| K (2) | Finland (64) and Kosovo (37) | |
| L (3) | Kosovo (32 and 33) (Bohuslän) | Not Swedish (35) |
| M (3) | Marocco (43), Somalia (41) (Göteborg) | Sweden (83) |
| N (2) | Sweden (80 and 81) (Göteborg) | |
| O (2) | Somalia (18) and Turkey (24) (Göteborg) | |
| P (2) | Kosovo (28 and 38) (Borås) | |
| Q (2) | Sweden (86) and Yugoslavia (39) | |
| R (2) | Russia (24) and Sweden (27) (Göteborg) | |
The cities in parentheses are those cities in Sweden where the patients lived at the time of the study.
Patients harboring isolates with INH monoresistance.
Drug susceptibility results were available for 213 (96.8%) isolates (Table 2). Nineteen patients (9%; 7 men and 12 women) were infected with resistant isolates. Among the 19 isolates, 15 isolates were resistant to one drug and 4 were resistant to two drugs. Among the strains from the seven male patients, three strains were resistant to SM (patient age range, 19 to 22 years), two strains were resistant to INH (patient ages, 26 and 63 years, respectively), and two isolates were resistant to PZA (patient ages, 73 and 76 years, respectively). However, the last two strains were identified as M. bovis BCG. These were isolated from two Swedish patients, born in 1925 and 1928, respectively, with bladder cancer who had been treated with the M. bovis BCG vaccine and who became infected by the vaccine strain. Among the strains from the 12 female patients, 7 strains were resistant to INH (patient age range, 22 to 78 years), 1 was resistant to PZA (patient age, 34 years), and 4 were resistant to INH and SM (patient age range, 22 to 41 years). The clustering of isolates by spoligotyping alone was significantly associated with resistance (11.7%; P = 0.017), but clustering by spoligotyping and VNTR analysis was not (6.7%) (Table 2). This underlines the fact that the proportion of resistant isolates was higher among the most prevalent clades of M. tuberculosis, probably due to imported cases of the disease from countries with higher rates of drug resistance or reactivation among previously treated patients.
DISCUSSION
The present study gives a first outline of the diversity of the population structure of M. tuberculosis in patients from western Sweden. The sex ratio of 1 in our study is in agreement with a recent report on TB notification rates for Scandinavian countries for the years 1995 to 2000 (2). However, the sex ratio among clustered cases was higher (1.4) (Table 2), probably reflecting a higher proportion of men among foreign-born patients with TB. As the clustering of isolates is crucially dependent on the completeness of the sample (12), this study included all cultures received in Göteborg from June 1999 to December 2002. All strains were first typed by spoligotyping. Although spoligotyping is useful for a first screening, this method is known to overestimate the proportion of clustered isolates, mainly due to the presence of common STs, such as STs 42, 50, and 53, present around the world (24). Consequently, all clusters defined by spoligotyping were further typed by VNTR analysis (9). This resulted in a final clustering rate of 23.6% instead of the rate of 58.6% achieved by spoligotyping alone. This clustering rate of 23.6% for Swedish strains is lower than those reported in other studies, e.g., 37.5% in New York City (1), 40% in San Francisco, California (22), 45% in South Africa (31), and 46% in The Netherlands (27).
The rate of recent transmission in our study (14.1%) is significantly lower than that reported in The Netherlands (35%) and may represent an underestimation due to a smaller sample size (n = 191) or a relatively short study period (31 months) (27). Nonetheless, the transmission rate of 14.1% in our study is similar to that in London, England (14.4%) (17), and Norway (11%) (4) but is below the rate of 24% reported in Denmark (32). Although most of the patients in the clusters defined by a combination of spoligotyping and VNTR analysis were foreign born (Table 3), this fact does not necessarily imply that transmission was associated with the country of origin. Indeed, the fact that transmission could have occurred abroad was apparent for only 14 of 45 patients (Table 3). The clustering of strains from one patient born in Portugal and one patient born in Syria, for example, is unlikely to be the result of transmission in the country of origin (even though both patients were foreign born).
On the other hand, the spoligotyping data alone permitted detection of groups of related strains (clades), and the link with the country of origin detected in this study was consistent with those detected in other studies, possibly reflecting reactivation with strains that are predominant in the country of origin. This is certainly leading to a change in the epidemiology of TB over the years; e.g., in Norway the proportion of foreign-born patients with TB increased from 4% of all cases in the mid-1970s to 70% in 2000 (7). In Sweden, 67% of cases of TB were detected in individuals born abroad, a rate similar to that for Norway, whereas in Finland, only 9% of patients with TB were foreign born (2). For example, the strains belonging to the Beijing family in this study originated from people coming from Vietnam and the former USSR; strains belonging to the EAI family originated from people coming from Africa, Indonesia, Thailand, and Vietnam; and strains belonging to the Haarlem family essentially originated from patients of Swedish ethnicity or European descent. This finding corroborates the biogeographical specificity of most of the spoligotyping-defined clades in the SpolDB3 and SpolDB4 databases (8, 23).
During the last decade, Sweden has received between 30,000 to 70,000 immigrants per year, the majority of these being from Iraq, the former Yugoslavia, Finland, Norway, Denmark, and Somalia (www.migrationinformation.org). Although it was shown that some immigrants had been infected in the host country, the majority were infected in their own countries, e.g., 74.9% of Somalian patients living in Denmark had been infected prior to their arrival in Denmark (4, 5, 16). This could also be the case in Sweden, as evidenced by the high rate of clustering of strains from foreign-born patients in this study (68.9%) (Table 3). However, foreign-born individuals made up 67% of TB cases in Sweden, and this value is comparable to the proportions of strains from foreign-born individuals that formed clusters by spoligotyping (67%) and the combination of test methods (69%). As these values are virtually identical, they may indicate that the proportion of foreign-born patients whose strains formed clusters is the same as the overall proportion of TB cases.
By spoligotyping alone, drug-resistant (essentially INH-resistant) isolates were more prevalent among the clustered isolates (Table 2). A prevalent clone of INH-resistant M. tuberculosis was recently found among African immigrants in the Stockholm area (11). Reports from San Francisco and New York City have also shown that drug-resistant M. tuberculosis isolates are more prevalent among clustered isolates than among unclustered isolates (1, 22).
Some genotypes of M. tuberculosis have demonstrated a greater ability to be transmitted and distributed throughout the world, such as the Beijing family (29), the most prevalent spoligotype in the world (22), and the Haarlem genotype family. In our study, the majority of isolates belonged to the Haarlem family, which, according to the SpolDB4 worldwide spoligotype database, is highly prevalent in northern Europe (8). A total of 11 of 191 (5.8%) of TB cases in the present study were caused by the Beijing genotype. This prevalence is close to the 6% prevalence of Beijing genotypes in The Netherlands during 1993 and 2000 (3). On the other hand, this prevalence of 5.8% in Sweden is lower than that reported from its Eastern European neighbors Estonia and Russia (range, 30 to 50% of all cases) (15, 26). Interestingly, in contrast to Russia, where as many as 43% of the Beijing genotype isolates are characterized by MDR (i.e., simultaneous resistance to INH and RIF), none of the 11 Beijing genotype strains in our study was MDR. Indeed, only three Beijing strains showed resistance to any drug: one was resistant to INH, and two were resistant to INH and SM. This observation is not unusual, as the association among the presence of the Beijing genotype, the age of the patients, and drug resistance varies according to the country studied; e.g., the Beijing genotype is strongly associated with drug resistance in New York City, Russia, Cuba, and Estonia but not in Hong Kong (13). Finally, 7 of 11 (63.6%) of the Beijing genotype strains in our study were isolated from individuals ages 15 to 39 years (mean age, 33 years), and this age group was also characterized by the highest proportion of clustered isolates (48.9%). Whether it suggests an ongoing active transmission of the Beijing genotype or imported cases of disease among migrants from Eastern Europe and Asia (9.2 and 25.9% of all the foreign-born persons, respectively [http://www.migrationinformation.org]) remains unanswered.
Another predominant family in this study was EAI (6.2% of clustered strains by spoligotyping in our study). According to the SpolDB4 database, this family is prevalent in Southeast Asia, East Africa, and some parts of Europe. Among the Scandinavian countries, this family is overrepresented in Denmark and may be linked to recent migration from the regions where EAI is prevalent (http://www.migrationinformation.org). A study on the molecular epidemiology of TB in Denmark in 1992 showed that as many as 50% of the TB patients originated from the Indian subcontinent, Somalia, and Vietnam (32).
Strains of the CAS family are essentially present in Central and Middle Eastern Asia (8) and have also been designated the Delhi type (30). Like the EAI and Beijing families, the CAS family also belongs to major genetic group I of Sreevatsan et al. (25) and may represent the founding strains of the human M. tuberculosis bacillus in Central and Middle Eastern Asia. The CAS family could have been among one of the possible ancestors of the Beijing family.
Compared to the families belonging to major genetic group I mentioned above, this study also underlines the presence of relatively “modern” strains of major genetic group II (25), i.e., the Haarlem family of European origin; the LAM family, which is prevalent in Latin America and the Mediterranean region; and the X family, which is known to contain strains with low IS6110 copy numbers, probably of Anglo-Saxon origin (8). Strains of the Haarlem (23%) and LAM (8%) families were also commonly found in Finland (19). These may reflect the strong migratory links between Finland and Sweden (http://www.migrationinformation.org). The remaining strains belonged to the ill-defined T family (in which spacers 33 to 36 are absent).
In conclusion, this study gives a first snapshot of the M. tuberculosis strains circulating in Sweden and the distributions of the major phylogenetic families in Sweden and its neighboring countries. It contributes to a better understanding of the current trend of TB transmission in a low-incidence country like Sweden. This study will be continued and further extended to other Scandinavian countries to provide a better estimation of the groups at risk for the development TB in this region.
Acknowledgments
This work was supported by grants from the Réseau International des Instituts Pasteur et Instituts Associés, Institut Pasteur, Paris, France, and EU Project QLK2-CT-2000-630, entitled New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. K.B. was cofunded by the Institut Pasteur and European Social Funds, provided through the Regional Council of Guadeloupe.
REFERENCES
- 1.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Drucker, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2002. Surveillance of tuberculosis in Europe. Report on tuberculosis cases notified in 2000. EuroTB, Saint-Maurice Cedex, France.
- 3.Borgdorff, M. W., P. de Haas, K. Kremer, and D. van Soolingen. 2003. Mycobacterium tuberculosis Beijing genotype, The Netherlands. Emerg. Infect. Dis. 9:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahle, U. R., P. Sandven, E. Heldal, and D. A. Caugant. 2003. Continued low rates of transmission of Mycobacterium tuberculosis in Norway. J. Clin. Microbiol. 41:2968-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahle, U. R., P. Sandven, E. Heldal, T. Mannsaaker, and D. A Caugant. 2003. Deciphering an outbreak of drug-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 41:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinal, M. A., A. Laszlo, L. Simonsen, F. Boulahbal, S. J. Kim, A. Reniero, S. Hoffner, H. L. Rieder, N. Binkin, C. Dye, R. Williams, M. C. Raviglione, et al. 2001. Global trends in resistance to antituberculosis drugs. N. Engl. J. Med. 344:1294-1303. [DOI] [PubMed] [Google Scholar]
- 7.Farah, M. G., A. Tverdal, R. Selmer, E. Heldal, and G. Bjune. 2003. Tuberculosis in Norway by country of birth, 1986-1999. Int. J. Tuberc. Lung Dis. 7:232-235. [PubMed] [Google Scholar]
- 8.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. D. Anh, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, H. M. Ly, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 41:1963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filliol, I., S. Ferdinand, L. Negroni, C. Sola, and N. Rastogi. 2000. Molecular typing of Mycobacterium tuberculosis based on variable number of tandem DNA repeats used alone and in association with spoligotyping. J. Clin. Microbiol. 38:2520-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frothingham, R., and W. A. Meeker-O'Connell. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144:1189-1196. [DOI] [PubMed] [Google Scholar]
- 11.Ghebremichael, S., T. Koivula, S. Hoffner, V. Romanus, B. Petrini, B. Noren, S. Sylvan, and G. Kallenius. 2002. Resistant tuberculosis is spreading in Sweden. Molecular epidemiological strain identification by “fingerprinting” can make the infection tracing easier. Lakartidningen 99:2618-2619, 2622-2623. [PubMed] [Google Scholar]
- 12.Glynn, J. R., E. Vynnycky, and P. E. Fine. 1997. Influence of incomplete case ascertainment on estimates of recent transmission of Mycobacterium tuberculosis using DNA fingerprinting techniques. Am. J. Epidemiol. 149:366-371. [DOI] [PubMed] [Google Scholar]
- 13.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 8:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruuner, A., S. E. Hoffner, H. Sillastu, M. Danilovits, K. Levina, S. B. Svenson, S. Ghebremichael, T. Koivula, and G. Kallenius. 2001. Spread of drug-resistant pulmonary tuberculosis in Estonia. J. Clin. Microbiol. 39:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillebaek, T., A. B. Andersen, J. Bauer, A. Dirksen, S. Glismann, P. de Haas, and A. Kok-Jensen. 2001. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J. Clin. Microbiol. 39:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire H., J. W. Dale, T. D. McHugh, P. D. Butcher, S. H. Gillespie, A. Costetsos, H. Al-Ghusein, R. Holland, A. Dickens, L. Marston, P. Wilson, R. Pitman, D. Strachan, F. A. Drobniewski, and D. K. Banerjee. 2002. Molecular epidemiology of tuberculosis in London 1995-7 showing low rate of active transmission. Thorax 57:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moström, P., M. Gordon, C. Sola, M. Ridell, and N. Rastogi. 2002. A survey of methods used in molecular epidemiology of tuberculosis. Clin. Microbiol. Infect. 8:694-704. [DOI] [PubMed] [Google Scholar]
- 19.Puustinen, K., M. Marjamaki, N. Rastogi, C. Sola, I. Filliol, P. Ruutu, P. Holmstrom, M. K. Viljanen, and H. Soini. 2003. Characterization of Finnish Mycobacterium tuberculosis isolates by spoligotyping. J. Clin. Microbiol. 41:1525-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastogi, N. 2003. An introduction to mycobacterial taxonomy, structure, drug resistance, and pathogenesis, p. 89-115. In D. Dionisio (ed.), Textbook-atlas of intestinal infections in AIDS. Springer-Verlag, Milan, Italy.
- 21.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 23.Sola, C., I. Filliol, M. C. Gutierrez, I. Mokrousov, V. Vincent, and N. Rastogi. 2001. Spoligotype database of Mycobacterium tuberculosis: biogeographic distribution of shared types and epidemiologic and phylogenetic perspectives. Emerg. Infect. Dis. 7:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sola, C., L. Horgen, J. Maïsetti, A. Devallois, K. S. Goh, and N. Rastogi. 1998. Spoligotyping followed by double-repetitive-element PCR as rapid alternative to IS6110 fingerprinting for epidemiological studies of tuberculosis. J. Clin. Microbiol. 36:1122-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toungoussova, O. S., P. Sandven, A. O. Mariandyshev, N. I. Nizovtseva, G. Bjune, and D. A. Caugant. 2002. Spread of drug-resistant Mycobacterium tuberculosis strains of the Beijing genotype in the Archangel Oblast, Russia. J. Clin. Microbiol. 40:1930-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Soolingen, D., M. W. Borgdorff, P. E. de Haas, M. M. Sebek, J. Veen, M. Dessens, K. Kremer, and J. D. van Embden. 1999. Molecular epidemiology of tuberculosis in The Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 180:726-736. [DOI] [PubMed] [Google Scholar]
- 28.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijaya-Bhanu, N., D. van Soolingen, J. D. van Embden, L. Dar, R. M. Pandey, and P. Seth. 2002. Predominance of a novel Mycobacterium tuberculosis genotype in the Delhi region of India. Tuberculosis (Edinburgh) 82:105-112. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson, D., M. Pillay, J. Crump, C. Lombard, G. R. Davies, and A. W. Sturm. 1997. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Trop. Med. Int. Health 2:747-753. [DOI] [PubMed] [Google Scholar]
- 32.Yang, Z. H., P. E. de Haas, C. H. Wachmann, D. van Soolingen, J. D. van Embden, and A. B. Andersen. 1995. Molecular epidemiology of tuberculosis in Denmark in 1992. J. Clin. Microbiol. 33:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]