Abstract
Early-life stress (ELS) leads to sustained changes in gene expression and behavior, increasing the likelihood of developing a psychiatric disorder in adulthood. The neurobiological basis for the later-in-life psychopathology is relatively unknown. The current study used a mouse model of ELS, achieved by daily maternal separations during the first 2 weeks of postnatal life, to test the role of amygdalar glucocorticoid receptor (GR) function in mediating the persistent increase in risk-taking behaviors. ELS produced a decrease in GR mRNA in the brain, with a notable reduction in the amygdala that was associated with sustained alterations in anxiety, fear and sociability-like behaviors. Lentiviral-mediated restoration of the GR mRNA deficit, specifically within the adult central nucleus of the amygdala (CeA), reversed the enduring changes in anxiety and social behavior after ELS. These results provide evidence of lasting changes in CeA GR neural circuitry following ELS and suggest a mechanistic role for GR-regulated processes in the CeA in mediating the lifelong maladaptive behaviors of ELS. We demonstrate that the long-lasting behavioral effects of ELS are reversible later in life and implicate the involvement of CeA GR-dependent activity in the sustained dysregulation of emotion following ELS.
Introduction
More than 10% of children in the US are subjected to some form of maltreatment, including abuse and neglect, during early life.1 Early-life stress (ELS) in humans increases vulnerability to later-in-life psychiatric disorders, including depression, anxiety disorders, personality disorders and schizophrenia.2, 3, 4, 5, 6, 7, 8 Likewise, in rodents, early postnatal experience has profound and long-lasting effects on stress responsiveness and emotionality.9, 10, 11
Glucocorticoids are important regulators of basal and stress-related homeostasis maintained through the hypothalamic–pituitary–adrenal (HPA) axis and function as transcription factors to influence a wide array of gene expression in almost every organ and tissue.12, 13 As the critical end products of the HPA axis, glucocorticoids, cortisol in humans and corticosterone in rodents promote adaptation to stress and stress recovery through negative-feedback signaling via glucocorticoid receptors (GR).14 Alterations in GR signaling, and the subsequent dysregulation of HPA axis function, may in part underlie the later-in-life psychopathologies that result following ELS. Dysfunctional GR signaling has been implicated in the pathogenesis of psychiatric disorders, including mood disorders, posttraumatic stress disorder (PTSD), schizophrenia and bipolar disorder.13, 15, 16, 17, 18, 19
The amygdala is a critical component of the neural circuitry involved in mediating fear, social behavior and anxiety. Early-life adversity has been linked to alterations in amygdalar structure and function in both rodents and humans.20, 21, 22 This altered amygdala activity has been shown to persist into adulthood and is associated with a dysregulation in fear responsiveness and socio-emotional disturbances over the lifetime.21, 23, 24 Furthermore, amygdala abnormalities have been reported in many psychiatric disorders including depression,25 anxiety disorders,7 borderline personality disorder,26 PTSD27 and schizophrenia.28 Within the amygdala, the central nucleus (CeA) is strategically placed to mediate many aspects of fear and anxiety, as CeA neurons project to sites involved in mediating different aspects of the stress response, including the hypothalamus, basal forebrain and brainstem.29, 30 Extensive studies in rodents have examined the functional contributions of specific amygdala nuclei and demonstrate a role for the CeA in innate and learned behavioral and physiological responses to aversive stimuli.31, 32, 33, 34, 35 Glucocorticoid action in the CeA has been implicated in mediating a positive feedback loop that potentiates activity of the HPA axis, anxiety and acquisition or expression of emotionally salient memory. Taken together, these data suggest that amygdala function is programmable by ELS and this dysfunction is maintained over the lifetime.
Here, we show that ELS applied to neonatal mice results in a persistent decrease in GR mRNA expression throughout the brain with a particularly prominent reduction in the amygdala. These changes in GR expression were associated with life-long alterations in anxiety, fear and sociability-like behavior, indicative of amygdala dysfunction. Viral-mediated delivery of GR to the CeA in adult mice following ELS restored GR expression to this region and reversed the abnormalities in anxiety and social behavior. These results provide evidence for the reversibility of the later-in-life psychopathology of ELS with site-specific restoration of GR function and support a mechanistic role for GR-dependent activity in the CeA as providing a causal link between ELS and the increased risk for psychiatric disorders.
Materials and methods
Animals
Animal experiments were approved by the Cincinnati Children's Institutional Animal Care and Use Committee and procedures were carried out under strict compliance with ethical principles and guidelines of the NIH Guide for the Care and Use of Laboratory Animals. Male C57BL6/J mice were used for all the experiments. Breeding pairs of C57BL6/J wild-type mice from Jackson were set up in the colony and litters were assigned randomly to ELS (maternal separation, MS), control or delayed weaning (DLW) conditions (described below). Subsequent litters from the same dam were assigned to different treatment groups. Mice had ad libitum access to food and water in a temperature- and humidity-controlled vivarium maintained on a 12-h light/dark cycle.
Lentiviral injections
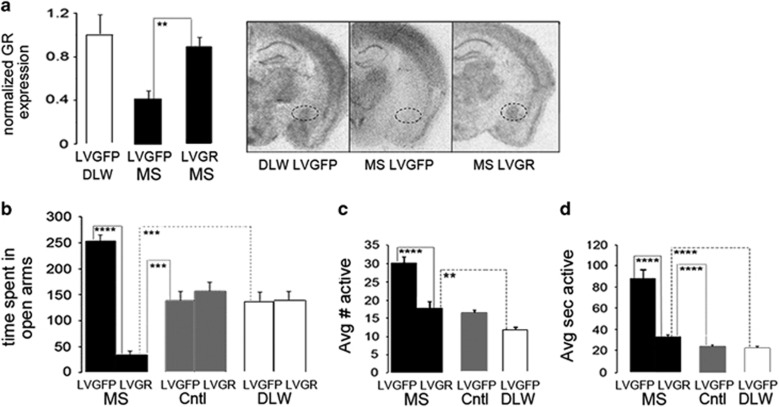
At 6–8 weeks of age, MS, control and DLW mice were stereotaxically injected with lentivirus expressing GR (LVGR) or green fluorescent protein (LVGFP). Injections were performed as previously described36 by injecting 1 μl of LVGR (7.4 × 108 IU ml−1 (integration units)) or LVGFP (3 × 108 TU ml−1 (transduction units)) into the adult bilateral CeA. All behavioral testing occurred at least 2 weeks after recovery from surgery. Correct targeting of LVGR to the CeA was verified by in situ hybridization (Figure 1a).
Figure 1.
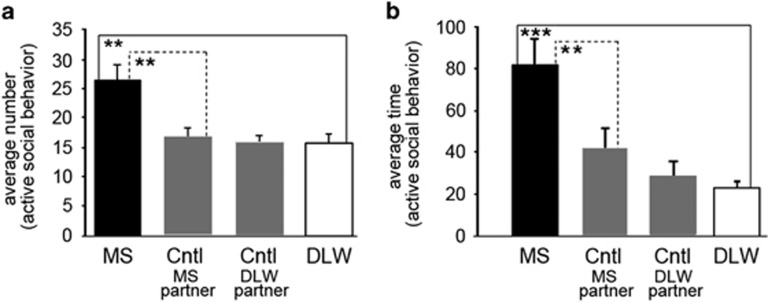
(a) LVGR restores GR expression in the adult CeA after early-life stress. Representative images show accurate targeting of LVGR to the CeA (a). Quantification of GR expression indicated that LVGR was efficiently able to express GR in vivo as indicated by the restoration of GR mRNA in the CeA of adult MS mice following ELS (one-way analysis of variance (ANOVA), P=0.019, a). LVGR stereotaxic injections significantly increased GR mRNA in the bilateral CeA of adult MS mice (MS LVGR) above MS LVGFP mice (P=0.008, a) as measured by in situ hybridization. LVGR restored GR mRNA to nearly control levels (MS LVGR CeA versus DLW LVGFP, P=0.55, a). Two bilateral injected CeA sections per animal were measured with N=3 animals per group. (b) LVGR reverses the ELS dysregulation of anxiety in the EZM. Bilateral stereotaxic injections of LVGR in the adult CeA of MS mice (N=7) produced a significant decrease in the time spent (seconds) in the open arms of the EZM (P=1.49 × 10−9, one-way ANOVA, compared with MS LVGFP (P=5.65 × 10−10, N=9), control LVGFP (P=0.0002, N=7) and DLW LVGFP (P=0.0005, N=9) mice. (c and d) LVGR reverses the social disinhibition of ELS. Stereotaxic LVGR injections in the adult amygdala of MS mice (MS LVGR, N=30) reverses the abnormal social function observed after early-life stress by significantly decreasing the number of active social behaviors (P=1.88 × 10−16 one-way ANOVA; MS LVGFP, P=7.37 × 10−7, N=32 animals, c) and the duration of time spent engaged in performing active social behaviors (P=7.15 × 10−19 one-way ANOVA; MS LVGFP, P=1.30 × 10−8, N=25, d), and restores sociability to levels similar to that exhibited by control LVGFP and DLW LVGFP mice in the number of active social behaviors (control LVGFP, P=0.52; DLW LVGFP, P=0.003, c) and the duration of active social behaviors (control LVGFP, P=0.0003; DLW LVGFP, P=9.5 × 10−6, d). CeA, central nucleus of the amygdala; DLW, delayed weaning; ELS, early-life stress; EZM, elevated zero maze; GFP, green fluorescent protein; GR, glucocorticoid receptor; LVGFP, lentivirus expressing GFP; LVGR, lentivirus expressing GR; MS, maternal separation. **P≤0.01, ***P≤0.001, ****P≤0.0001.
Early-life stress
Beginning on P1, P0 designated as day of birth, and continuing daily through P14, pups were removed from the dam in ELS litters and placed in pre-warmed, individual plastic cups and maintained at 37 °C maintained at 50% humidity for 8 h. At the end of the 8-h maternal-separation period, pups were reunited with the dam. ELS pups were weaned at P28 and housed in standard laboratory cages. Control and DLW assigned litters remained undisturbed with the dam from birth until weaning at P21 or P28, respectively. Our MS procedure produced a significant reduction in body weight of pups at P14 compared with controls (P=0.023, Supplementary Figure 1). This failure to thrive and grow, as the control pups do, is evidence that our ELS paradigm produces a stressful environment for the pups during the first 2 weeks of postnatal life. This decrease in body weight was not present in adulthood (12 weeks postnatal, P=0.488).
Corticosterone RIA
Mice were single housed for a week, and submandibular bleeds were performed in mice at circadian nadir (2 h after lights on) and peak (1 h before lights off) time points. The blood was centrifuged at 14 000 r.p.m. for 6 min, and the plasma was removed and stored at −80 °C until an RIA was performed. RIA was performed using the Corticosterone Double Antibody-125I RIA Kit (MP Biomedicals, Santa Ana, CA, USA).
Behavior
Behavior testing began at 8–10 weeks of age and testing proceeded from least to most stressful (elevated zero maze (EZM), open field (OF), direct social interaction (DSI) and fear conditioning) with 2 days between each test.
Elevated zero maze
The EZM was utilized as a measure to assess anxiety-like and motor behavior of mice. The task and methods utilized in this 5-min task have been previously described.37 Increased time spent in the open arms indicate reduced anxiety-like behavior.
Open field
Anxiety-like and motor behavior of mice was also assessed in the OF tasks as previously described.37 The total distance traveled in the EZM and OF tests can be used as an index of general motor behavior.
Direct social interaction
At 8–10 weeks of age, mice were housed in partitioned cages with age- and weight-matched partners from an opposite treatment group. Three days later, the DSI task was conducted as previously described.38
Fear conditioning
Conditioning capabilities were evaluated using a test of Pavlovian fear conditioning that included CS/US training and contextual and auditory cued components as previously described.36
In situ hybridization
Brains were collected under basal conditions from MS, control and DLW mice at 10–12 weeks of age and processed as previously described to evaluate GR mRNA expression. Densitometric analysis of in situ signal was performed using NIH Image J software for three brains per treatment group measuring bilateral nuclei (amygdala (central and basolateral), hippocampus (CA1) and paraventricular nucleus (PVN) of the hypothalamus) in two sections per brain.
Statistical analysis
Statistical significance was determined using a one-way analysis of variance (ANOVA) followed by an unpaired two-tailed Student's t-test, if the ANOVA resulted in an overall significant difference between groups. Differences were considered significant at P≤0.05.
Results
ELS produces a significant decrease in brain GR expression
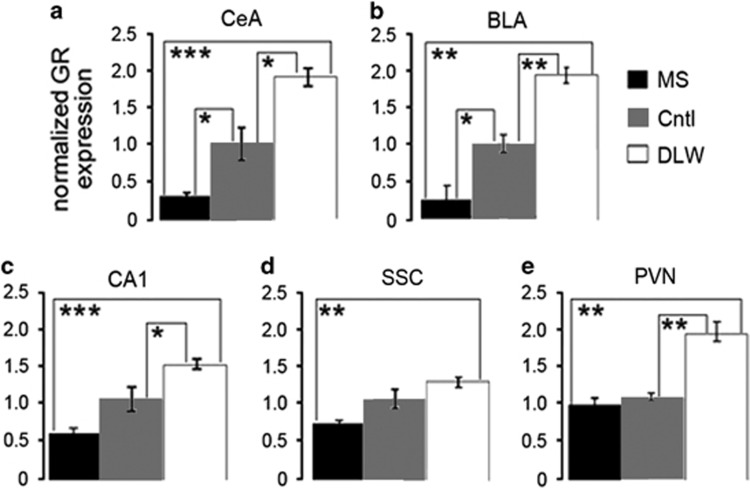
To evaluate the effect of ELS on GR expression in the brain, we measured GR mRNA in adult mice (8–10 weeks of age) following ELS using in situ hybridization (Figures 2a and e, representative images, Supplementary Figure 2). A one-way ANOVA revealed a significant difference in GR expression between MS, control and DLW mice in the CeA (P=0.005), basolateral nucleus of the amygdala (BLA, P=0.002), CA1 (P=0.015) and the PVN (P=0.007) but not in the somatosensory cortex (SSC, P=0.084). We found that ELS reduced GR mRNA in the CeA (P=0.037, P=0.0003, Figure 2a), BLA (P=0.030, P=0.006, Figure 2b), CA1 (P=0.060, P=0.0009, Figure 2c), SSC (P=0.070, P=0.006, Figure 2d) and the PVN (P=0.336, P=0.005, Figure 2e) compared with control and DLW mice, respectively. In addition, we found that control mice express less GR mRNA in the CeA (P=0.023, Figure 2a), BLA (P=0.008, Figure 2b), CA1 (P=0.053, Figure 2c) and the PVN (P=0.005, Figure 2e) compared with DLW mice. These data suggest a direct relationship between early-life rearing conditions and GR expression in the amygdala (CeA and BLA), hippocampus (CA1) and PVN.
Figure 2.
GR mRNA is decreased in adult mouse brain following early-life stress. In situ hybridization was used to measure GR expression in adult 8–10-week-old MS, Cntl and DLW mice. A one-way analysis of variance revealed a significant difference in GR expression between MS, control and DLW mice in the CeA (P=0.005, a), BLA (P=0.002, b), CA1 (P=0.015, c) and the PVN (P=0.007, e) but not in the SSC (P=0.084, d). Next, a Student's t-test was used to determine significant differences between groups in each brain nuclei. ELS reduced GR mRNA in the CeA (P=0.037, P=0.0003, a), BLA (P=0.030, P=0.006, b), CA1 (P=0.060, P=0.0009, c), SSC (P=0.070, P=0.006, d) and the PVN (P=0.336, P=0.005, e) compared with control and DLW mice, respectively. In addition, we found that control mice express less GR mRNA in the CeA (P=0.023, a), BLA (P=0.008, b), CA1 (P=0.053, c) and the PVN (P=0.005, e) compared with DLW mice. GR mRNA was measured in two sections per brain for N=3 animals per group. BLA, basolateral nucleus of the amygdala; CA1, hippocampus; CeA, central nucleus of the amygdala; Cntl, control; DLW, delayed weaning; ELS, early-life stress; GR, glucocorticoid receptor; mRNA, messenger RNA; MS, maternal separation; PVN, paraventricular nucleus; SSC, somatosensory cortex. *P≤0.05, **P≤0.01, ***P≤0.001.
To measure the effect of the lifelong decrease in GR expression as a result of ELS, we measured CRH gene expression and plasma corticosterone levels as a measure of HPA axis regulation in adult mice. Under basal conditions, we found no difference in CRH mRNA between treatment groups using in situ hybridization (P=0.754, Supplementary Figure 3). In addition, we measured plasma corticosterone levels in adult mice. We found no difference in corticosterone levels between treatment groups at circadian nadir (P=0.839) or peak (P=0.121) time points (Supplementary Figure 4).
Loss of GR mRNA following ELS is associated with persistent alterations in anxiety-related behavior
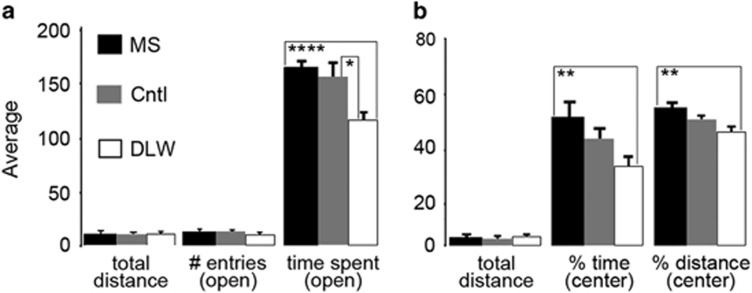
To determine the lifelong effects of ELS on anxiety-related behavior, adult mice performed the EZM and OF tasks. In the EZM, a one-way ANOVA revealed a significant difference in the time spent in the open arms of the EZM between MS, control and DLW adult (8–10 weeks) mice (P=0.005). ELS produced a significant increase in the amount of time spent in the open arms compared with adult DLW mice (P=0.0001, Figure 3a). We also found a significant effect of early-life treatment on time spent in the open arms of the EZM with control mice spending more time in the open arms compared with DLW mice (P=0.030) (Figure 3a). In the OF, a one-way ANOVA revealed a significant difference in the time spent in the center of the OF between MS, control and DLW adult (8–10 weeks) mice (P=0.030). The OF data reflect similar findings as the EZM showing a significant increase in the time spent exploring (P=0.006) and the distance traveled (P=0.005) in the center zone by MS mice compared with the DLW treatment group, respectively (Figure 3b). No changes were found in locomotor parameters (total distance traveled) measured in both the EZM and OF tests between treatment groups (P=0.319 (EZM); P=0.836 (OF), Figures 3a and b, respectively).
Figure 3.
Early-life stress decreases anxiety-like behavior in the elevated zero maze (EZM) and open field (OF) tasks. A one-way analysis of variance (ANOVA) revealed a significant difference in the time spent in the open arms of the EZM between MS, Cntl and DLW adult (8–10 weeks) mice (P=0.005). MS mice show a significant increase in the time spent (seconds) in the open arms of the EZM compared with DLW mice (P=0.0001, a). Similarly, control mice spent significantly more time in the open arms compared with DLW mice (P=0.030, a). In the OF, a one-way ANOVA revealed a significant difference in the time spent in the center zone of the OF between groups (P=0.030). MS mice spent a significantly greater total percentage of time (seconds, P=0.006) and traveled a significantly greater distance (meters) in the open center zone (P=0.005) compared with DLW mice (b). No changes were found in locomotor parameters (total distance traveled) measured in both the EZM and OF tests between treatment groups (P=0.319 (EZM); P=0.836 (OF)), N=13 mice per group. Cntl, control; DLW, delayed weaning; MS, maternal separation. *P≤0.05, **P≤0.01, ****P≤0.0001.
ELS is associated with attenuation of the auditory-cued fear response
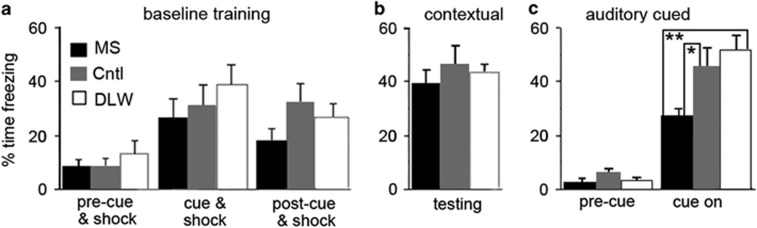
We measured Pavlovian fear conditioning to determine the effect of ELS on lifelong changes in fear behavior in our mouse model of ELS. ELS did not produce any significant changes in freezing behavior between groups during training (pre-cue and shock (P=0.391), cue and shock (P=0.505) and postshock (P=0.201, Figure 4a) or contextual testing (P=0.709, Figure 4b). There was a significant difference in the time spent freezing between groups, in the presence of the auditory cue, in auditory-cued conditioning (pre-cue (P=0.702) and cue on (P=0.022)). ELS produced a significant deficit in the freezing response in the presence of the auditory cue (cue on; P=0.03 and P=0.004) compared with control and DLW mice, respectively (Figure 4c). Previous reports have reported a similar decrease in both contextual and cued fear conditioning following ELS in rodents.39, 40 C57Bl/6 mice have been reported to show hearing impairments.41 The acoustic startle response has been used previously to examine hearing abilities in mice. We observed no difference in startle response in the acoustic startle response behavior task between treatment groups in adult mice (P=0.991; one-way ANOVA; data not shown).
Figure 4.
Early-life stress alters fear conditioning behavior. ELS did not produce any significant changes in freezing behavior between groups during training (pre-cue and shock (P =0.391), cue and shock (cue and shock (P=0.505) and postshock (P=0.201), a) or contextual testing (P=0.709, b). However, there was a significant difference in the time spent freezing between groups, in the presence of the auditory cue, in auditory cued conditioning (pre-cue (P=0.702) and cue on (P=0.022), c). MS mice showed a significant deficit in fear behavior (freezing) in the auditory-cued phase of Pavlovian fear conditioning compared with control (P=0.03) and DLW (P=0.004) mice (c). N=13 (MS), N=12 (control and DLW). Cntl, control; DLW, delayed weaning; ELS, early-life stress; MS, maternal separation. *P≤0.05, **P≤0.01.
Sociability-like behavior is altered by ELS
ELS has been shown to impair social behavior in rodents and humans.42 To determine whether ELS causes alterations in sociability-like behaviors in adulthood, mice were subjected to the DSI test at 8–10 weeks of age. A one-way ANOVA revealed a significance between the number of active counts (P=0.0002) and active duration (P=0.0002) between groups. ELS significantly increased the number of active social behaviors (P=0.003, (MS versus control); P=0.003 (MS versus DLW, Figure 5a)), defined as behavior of the subject mouse initiated toward the partner, and spent a significantly greater amount of time engaged in performing active social behaviors compared with control (P=0.01) DLW (P=0.0005, Figure 5b) mice. Passive social behavior, defined as behavior of the subject responding to behavior initiated by the partner mouse, was also scored. Passive responses included receptive behavior, fleeing and freezing. Involvement in active social behavior excluded concurrent participation in passive behavior and thus passive social behavior is an inverse reflection of the active social behavior (data not shown for passive social behavior). To verify that the behavioral responses of mice in the DSI were not dependent upon the treatment group of the paired mouse, control mice were paired with MS and DLW partners in independent tests. We found no significant effect of the partner's treatment group on the subject's social behavior (number of active social behaviors performed and duration of time spent engaged in active social behaviors by control subject paired with either MS or DLW partner, respectively (P=0.531 and P=0.324, Figures 5a and b). Non-social behaviors, including digging, self-grooming and sleeping, were also scored as well as the percentage of time spent in the original housed territory and in the partner's territory. We found no significant interaction between the number and time spent engaged in non-social behaviors, or the time spent in each territory and the treatment group (data not shown).
Figure 5.
Early-life stress alters sociability-like behavior. A one-way analysis of variance revealed a significance between the number of active counts (P=0.0002) and active duration (P=0.0002) between groups. MS mice (N=14) performed a significantly greater number of active social behaviors, that is, behaviors initiated toward the partner mouse by the subject mouse, compared with control (P=0.003, N=13) and DLW (P=0.003, N=10) mice (a). Likewise, there is a significant increase in the amount of time spent (seconds) engaged in active social behavior in MS mice in comparison with control (P=0.01) and DLW (P=0.0005) mice (b). Social behavior was also measured in control mice paired with DLW partners to determine the effect of partner treatment group on the subject's social behavior. Neither the number of active social behaviors performed (control subject paired with MS partner versus control subject paired with DLW partner, P=0.531, a) nor the time spent engaged in performing active social behaviors (control subject paired with MS partner versus control subject paired with DLW partner, P=0.343, b) differed in control mice paired with either a MS or DLW partner. Cntl, control; DLW, delayed weaning; MS, maternal separation. **P≤0.01, ***P≤0.001.
Lentiviral restoration of GR following ELS
To determine whether adult replacement of GR could restore the behavioral alterations resulting from ELS, we generated and previously validated a lentiviral vector expressing full-length mouse GR in vitro.43 To establish the ability of LVGR to express GR mRNA in the CeA in vivo, we stereotaxically injected bilateral CeA nuclei of adult (8–10 weeks old) MS mice following ELS with LVGR alongside MS and DLW LVGFP-injected mice. Two weeks after viral delivery, we measured in situ hybridization GR expression. Quantification of GR expression indicated that LVGR was efficiently able to express GR in vivo as indicated by the restoration of GR mRNA in the CeA of adult MS mice following ELS (P=0.019, one-way ANOVA, Figure 1a). LVGR stereotaxic injections significantly increased GR mRNA in the bilateral CeA of adult MS mice (P=0.008, Figure 1a). CeA LVGR viral delivery increased GR expression to similar levels expressed by DLW LVGFP mice (MS LVGR CeA versus DLW LVGFP CeA, P =0.551, Figure 1a).
CeA LVGR rescues ELS alterations in anxiety in adulthood
We hypothesized that CeA GR activity was involved in mediating the lifelong alterations in anxiety after ELS and that replacing CeA GR function in adulthood would reverse these alterations and return anxiety levels to normal. Two weeks after bilateral CeA injections, mice performed the EZM to measure anxiety-like behavior. MS mice injected with LVGFP in the bilateral CeA displayed a similar deficit in anxiety-like behavior that was present initially after ELS (Figure 3a). CeA injections of LVGR after ELS (MS LVGR) produced a significant decrease in time in the open arms (P=1.49 × 10−9 one-way ANOVA, t-test compared with MS LVGFP mice, P=5.65 × 10−10, Figure 1b). Delivery of CeALVGR after ELS significantly decreased time in open arms to below that of control LVGFP (P=0.0002) and DLW LVGFP (P=0.0005) mice (Figure 1b).
CeA LVGR rescues ELS sociability alterations in adulthood
Next, we tested the hypothesis that ELS-induced modifications in CeA GR function produced the lifelong disinhibition in sociability-like behavior (Figure 5), by replacing GR in the adult CeA with LVGR stereotaxic injections. MS CeA LVGFP-injected mice showed similar patterns in increased number, and time spent, in active social behavior as we saw initially in uninjected adult MS mice following ELS (Figures 5a and b). CeA LVGR injections reversed this effect of ELS (MS LVGR) on sociability-like behavior by significantly decreasing the number of active social behaviors (P=1.88 × 10−16 one-way ANOVA; MSLVGFP, P=7.37 × 10−7, Figure 1c) and the duration of active social behavioral interactions (P=7.15 × 10−19 one-way ANOVA; MS LVGFP, P=1.30 × 10−8, Figure 1d). Similar to CeA LVGR's ability to return anxiety levels to normal after ELS (Figure 1b), delivery of CeA LVGR after ELS (MS LVGR) reduced the number of active social behaviors as well as the time spent involved in active social behaviors to below that of control (control LVGFP, P=0.52, P=0.0003) and DLW (DLW LVGFP, P=0.003, P=9.5 × 10−6) mice, respectively. These data strongly suggest a role for the involvement of CeA GR gene regulatory networks in promoting the persistent maladaptive anxiety and sociability-like behavior after ELS. After ELS, adult MS mice demonstrated a significant deficit in auditory-cued fear behavior (Figure 4c). However, after CeA LV injections, this difference was not detectable (data not shown). Thus, we could not determine rescue of this behavioral alteration.
Discussion
In the current study, we investigated the role of CeA-specific GR function in mediating the lifelong alterations in fear, anxiety and sociability-like behaviors following ELS. GR mRNA was decreased in many regions of the brain including a prominent reduction in the amygdala. This deficit in GR expression was associated with a decrease in anxiety and fear responsiveness and an increase in sociability, or an overall increase in risk-taking behavior, implicating altered amygdala function after ELS. We hypothesized that the enduring loss of GR following ELS was responsible for maintaining altered anxiety, sociability and fear responsiveness over the lifetime and that restoring the GR deficit in adulthood, specifically within amygdalar nuclei, would reverse these changes in behavior. Our hypothesis focused on the amygdala, and more specifically the CeA, because of its role in regulating emotionality and additionally, because of association with its dysfunction in major psychiatric disorders. To investigate a causal link between the persistent loss in amygdalar GR expression and the lifelong alterations in emotionality after ELS, we regionally restored GR activity to the adult CeA following ELS and measured the behavioral effects. Replacing CeA-specific GR activity in adulthood rescued the altered anxiety and social behaviors of ELS. These data support a role for CeA GR-regulated mechanism(s) in mediating maladaptive anxiety and social behavior over the lifetime after ELS and show later-in-life reversibility of these behavioral patterns established in early life.
Although ELS is associated with psychopathology later in life, little is known about the neurobiological changes that underlie this increased risk. Dysregulated GR function is thought to be involved, but a mechanistic role for GR has not been determined. These studies utilize a viral-mediated GR delivery approach that allowed us to isolate the role of CeA GR-dependent mechanisms in mediating the lifelong behavioral dysfunction of ELS. Although viral replacement in humans to treat psychiatric disorders is unlikely, identifying GR targets involved in modulating behavior could offer novel therapeutic approaches.
Many studies have demonstrated that ELS predicts long-term effects on behavior. However, variation in resulting phentoptes are found within these reports.44, 45 Although some studies report an increase in anxiety and depressive-like behaviors following ELS, others point to an increase in risk-taking and novelty-seeking behaviors.45 The differences between studies likely reflect variation in the MS paradigms among studies and the species used. The ELS mouse model we present here displays a consistent behavioral phenotype that correlates increased stress during early life with decreased anxiety and fear-like behavior, and increased sociability, which could represent both a resiliency to anxiety and fear behavior later in life or increased risk-taking and novelty-seeking behavior. In the initial EZM behavior, we observed a significant difference in the time spent in the open arms between control and DLW groups (Figure 3a), but this difference was not present in either the DSI task or fear conditioning (Figures 4 and 5). Following LVGR CeA injection, the difference in anxiety-like behavior between control and DLW mice is lost. Although mice recovered rapidly, we attribute this loss to consequences of the surgeries and stereotaxic injections, which still exert some longer-term stress effects.
A role for CeA GR activity has previously been established in Pavlovian fear conditioning. These studies have demonstrated that specific disruption of GR function in the adult CeA caused an attenuation in freezing (fear behavior) during both contextual and auditory cued testing.36 The studies presented here, found a similar deficit in cued fear conditioning following ELS as other studies have reported.39, 40 The difference we find in the ELS paradigm is likely due to the time course of CeA GR disruption or the degree of cellular GR reduction achieved. Prior studies that linked alterations in CeA GR activity with reduced contextual and auditory conditioning occurred alongside adult deletion, whereas reductions in CeA GR after ELS are likely constitutively present and maintained over the lifetime. Taken together, these studies, along with our present findings, support a critical role for CeA GR in mediating fear behavior and indicate the functional significance of CeA GR-dependent activity in adaptive stress responsiveness and behavior.
Emotional memory formation has been shown to be mediated in part through long-term synaptic potentiation in the BLA. Electrophysiological experiments demonstrated that acute stress changes the electrical properties of the BLA to facilitate subsequent long-term synaptic potentiation induction.46, 47 Along with β-adrenergic receptors, GR function is involved in mediating these effects. Although these studies indicated stress-induced changes in responsiveness of BLA neurons to encode for the emotional aspects of stressful events, a mechanism for CeA involvement has not previously been described. Our findings suggest that ELS produces long-lasting changes in GR-dependent processes in the CeA and provide a potential mechanism for the observed behavioral effects to persist over the lifetime.
In humans, socio-emotional development and subsequent social behavior is particularly susceptible to ELS. Children reared under institutional care exhibit low social competence,48 social impairments,48 impairments in perception of social stimuli49 and inappropriately familiar interactions with strangers.50 Likewise, in non-human animal studies, ELS has been associated with altered amygdala development and subsequent difficulties in socio-emotional behavior.10, 51, 52 Our model of ELS shows sustained impairments in sociability-like behavior that are consistent with these studies, but specific brain regions and circuits mediating these consequences have not been demonstrated previously. Within the amygdala, a specific role for the CeA has been defined as a critical component of the neural circuitry involved in the regulation of social behaviors.37, 53 However, a mechanism(s) by which ELS maintains dysfunctional social behavior over the lifetime has not been elucidated. In line with our hypothesis, replacing GR levels in the adult CeA rescued the abnormal social behavior following ELS.
Previous studies have reported that neonatal novelty exposure in rats results in functional enhancements in social dominance and reduced emotional reactivity in adulthood.54 Our MS paradigm involved daily removal of pups and placing into individual pre-warmed, plastic cups, which produced neonatal novelty exposure. Alternatively, changes in sociability-like behavior that we observe after ELS could be interpreted as increased social dominance and likewise, the decrease in anxiety and cued fear behavior interpreted a persistent reduction in emotionality. Both interpretations illustrate that ELS produces altered behavioral responses in adulthood and results in an inability to appropriately respond to environmental cues and properly assess risk.
Previous animal studies have suggested that ELS changes in neural circuitry and behavior may be irreversible even upon removal of the stressor or later development of prefrontal regulatory regions.21 In humans, the data are less clear about whether or not brain and behavioral effects of ELS can improve. From studies involving children adopted from orphanages, it can be determined that earlier adoption is associated with better outcomes, indicative of increased neuroplasticity during development. Collectively, these studies have shown that early intervention is critical to improved behavioral outcome. The studies presented here utilize later-in-life intervention aimed at rescuing programmed behaviors established in early life and maintained into adulthood, which we believe would more closely mimic the timing of potential therapeutic interventions in human populations.
The current study demonstrates a strong link between ELS-induced interruption in CeA GR-regulated mechanisms and lifelong altered regulation of anxiety and social behavior. These studies provide one mechanism, and implicate one essential neural node, involved in mediating the enduring maladaptations in anxiety and social behavior after ELS. Importantly, we find the potential for restoration of behavioral alterations arising after ELS which provides hope for future beneficial clinical interventions later in life for those suffering from psychiatric disorders contributed to by this exposure.
Acknowledgments
This work was supported in part by grant 1 R01 MH079010-01A2 to LJM.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49:109–116. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Yokouchi T, Hirai T, Kitamura T, Takahashi K. Parental loss in childhood and social support in adulthood among psychiatric patients. Group for Longitudinal Affective Disorders Study (GLADS) J Psychiatr Res. 1999;33:165–169. doi: 10.1016/s0022-3956(98)00054-5. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Sheth K, Gardner CO, Prescott CA. Childhood parental loss and risk for first-onset of major depression and alcohol dependence: the time-decay of risk and sex differences. Psychol Med. 2002;32:1187–1194. doi: 10.1017/s0033291702006219. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N Y Acad Sci. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;17:41–46. [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Webster MJ, Fullerton JM, Weickert CS. Glucocorticoid receptor mRNA and protein isoform alterations in the orbitofrontal cortex in schizophrenia and bipolar disorder. BMC Psychiatry. 2012;12:84. doi: 10.1186/1471-244X-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med. 2012;10:151–156. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci USA. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Job DE, Johnstone EC. Structural and functional abnormalities of the amygdala in schizophrenia. Ann N Y Acad Sci. 2003;985:445–460. doi: 10.1111/j.1749-6632.2003.tb07099.x. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental. Wiley-Liss: New York, NY, USA; 1992. pp. 1–67. [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Cote J, Barden N. Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinology. 1987;45:37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Hitchcock JM, Bowers MB, Berridge CW, Melia KR, Roth RH. Stress-induced activation of prefrontal cortex dopamine turnover: blockade by lesions of the amygdala. Brain Res. 1994;664:207–210. doi: 10.1016/0006-8993(94)91972-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Aghamohammadi-Sereshki A, Rezayof A, Rostami P. Nicotine-induced anxiogenic-like behaviours of rats in the elevated plus-maze: possible role of NMDA receptors of the central amygdala. J Psychopharmacol. 2012;26:555–563. doi: 10.1177/0269881111412094. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, et al. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao GS, Cheng LY, Chen LH, Tzeng WY, Cherng CG, Su CC, et al. Neonatal isolation decreases cued fear conditioning and frontal cortical histone 3 lysine 9 methylation in adult female rats. Eur J Pharmacol. 2012;697:65–72. doi: 10.1016/j.ejphar.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Przyborowska A, Makuch W, Majcher-Maslanka I, Dudys D, Wedzony K. The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behav Brain Res. 2014;264:161–172. doi: 10.1016/j.bbr.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Parham K, Willott JF. Acoustic startle response in young and aging C57BL/6J and CBA/J mice. Behav Neurosci. 1988;102:881–886. doi: 10.1037//0735-7044.102.6.881. [DOI] [PubMed] [Google Scholar]
- Kovalenko IL, Galyamina AG, Smagin DA, Michurina TV, Kudryavtseva NN, Enikolopov G. Extended effect of chronic social defeat stress in childhood on behaviors in adulthood. PLoS One. 2014;9:e91762. doi: 10.1371/journal.pone.0091762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryea G, Arnett MG, Wieczorek L, Muglia LJ. Site-specific modulation of brain glucocorticoid receptor and corticotropin-releasing hormone expression using lentiviral vectors. Mol Cell Endocrinol. 2013;371:160–165. doi: 10.1016/j.mce.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat:consistent or confusing. Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Franklin TB, Vizi S, Mansuy IM. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci. 2011;5:3. doi: 10.3389/fnbeh.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Kofink D, Karst H, de Kloet ER, Joels M. Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ss-adrenergic activity. PLoS One. 2012;7:e42143. doi: 10.1371/journal.pone.0042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. J Child Psychol Psychiatry. 1989;30:77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Moulson MC, Fox NA, Zeanah CH, Nelson CA. Early adverse experiences and the neurobiology of facial emotion processing. Dev Psychol. 2009;45:17–30. doi: 10.1037/a0014035. [DOI] [PubMed] [Google Scholar]
- Tizard B, Hodges J. The effect of early institutional rearing on the development of eight year old children. J Child Psychol Psychiatry. 1978;19:99–118. doi: 10.1111/j.1469-7610.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-inducedfearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Karom M, Pallier P, Albers HE. Role of the central amygdala in social communication in Syrian hamsters (Mesocricetus auratus) Brain Res. 1997;744:15–22. doi: 10.1016/s0006-8993(96)01061-x. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.