ABSTRACT
Volatiles are small air-transmittable chemicals with diverse biological activities. In this study, we showed that volatiles produced by the bacterium Bacillus subtilis had a profound effect on biofilm formation of neighboring B. subtilis cells that grew in proximity but were physically separated. We further demonstrated that one such volatile, acetic acid, is particularly potent in stimulating biofilm formation. Multiple lines of genetic evidence based on B. subtilis mutants that are defective in either acetic acid production or transportation suggest that B. subtilis uses acetic acid as a metabolic signal to coordinate the timing of biofilm formation. Lastly, we investigated how B. subtilis cells sense and respond to acetic acid in regulating biofilm formation. We showed the possible involvement of three sets of genes (ywbHG, ysbAB, and yxaKC), all encoding putative holin-antiholin-like proteins, in cells responding to acetic acid and stimulating biofilm formation. All three sets of genes were induced by acetate. A mutant with a triple mutation of those genes showed a severe delay in biofilm formation, whereas a strain overexpressing ywbHG showed early and robust biofilm formation. Results of our studies suggest that B. subtilis and possibly other bacteria use acetic acid as a metabolic signal to regulate biofilm formation as well as a quorum-sensing-like airborne signal to coordinate the timing of biofilm formation by physically separated cells in the community.
IMPORTANCE
Volatiles are small, air-transmittable molecules produced by all kingdoms of organisms including bacteria. Volatiles possess diverse biological activities and play important roles in bacteria-bacteria and bacteria-host interactions. Although volatiles can be used as a novel and important way of cell-cell communication due to their air-transmittable nature, little is known about how the volatile-mediated signaling mechanism works. In this study, we demonstrate that the bacterium Bacillus subtilis uses one such volatile, acetic acid, as a quorum-sensing-like signal to coordinate the timing of the formation of structurally complex cell communities, also known as biofilms. We further characterized the molecular mechanisms of how B. subtilis responds to acetic acid in stimulating biofilm formation. Our study also suggests that acetic acid may be used as a volatile signal for cross-species communication.
INTRODUCTION
Bacteria are capable of forming structurally complex multicellular communities called biofilms (1–3). Bacillus subtilis is a well-established model system for biofilm studies (4, 5). In the laboratory, B. subtilis cells form either floating pellicles in the liquid-air interface (pellicle biofilm) or highly structured colonies on the solid agar surface (colony biofilm) (6). Recent studies also showed that B. subtilis is capable of forming biofilms on the root surfaces of plants, which protect plants from infections by plant pathogens (7–10). Within B. subtilis biofilms, individual cells are held together by an extracellular matrix composed mainly of the TasA and TapA proteins, made from a three-gene tapA-sipW-tasA operon, and an exopolysaccharide, synthesized by the protein products encoded by a 15-gene epsA-to-epsO (epsA-O) operon (11–13). In addition, a small hydrophobic protein called BslA was also shown to be abundantly present in the biofilm matrix of B. subtilis (14–16). BslA proteins localize to the outer surface of the biofilms and provide strong surface hydrophobicity to the biofilms (14–16).
B. subtilis biofilm formation depends in part on induction of the two matrix operons tapA-sipW-tasA and epsA-O, whose activities are controlled by a genetic circuitry consisting of an array of regulatory proteins (Fig. 1) (5). At the core of the circuitry is SinR, the transcription repressor that directly represses the two matrix operons under non-biofilm-inducing conditions (12, 17). SinR is antagonized by a small antirepressor, SinI, through protein-protein interactions (12, 18). The sinI gene is controlled by Spo0A (19, 20), a master regulator whose activity is regulated by protein phosphorylation through a phosphor relay in response to various upstream signals (Fig. 1) (21, 22). Our previous study (23) showed that SinR is also targeted by a parallel antirepressor, SlrA, in a way similar to that seen with SinI (Fig. 1). slrA expression is repressed by YwcC, a TetR-type ligand-responsive transcription repressor (Fig. 1 and 2A) (23, 24). Although the putative ligand for YwcC likely functions as a signal to activate the YwcC-SlrA pathway for derepression of expression of SinR-controlled matrix genes and, therefore, biofilm formation, the chemical nature of the putative ligand has not been characterized. Next to slrA is a two-gene operon consisting of ywcB and ywcA (ywcB-ywcA) whose function is unknown but which is predicted to encode a small-molecule transporter (Fig. 2A).
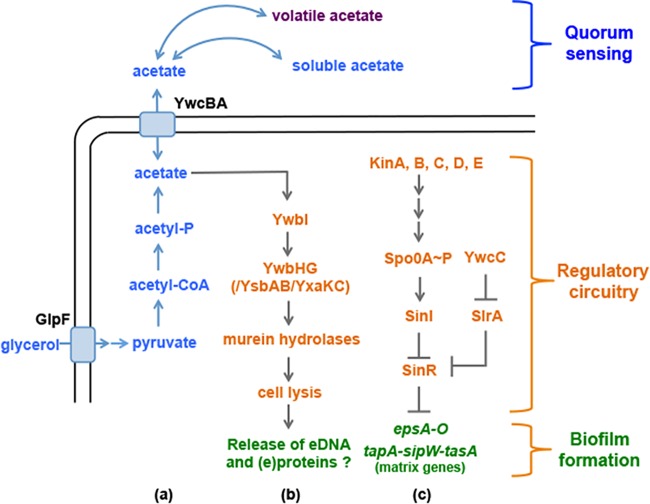
FIG 1 .
A working model for acetate-mediated biofilm induction in B. subtilis. This model integrated three different pathways: the biosynthesis and secretion pathway for acetic acid (a), the proposed targets and predicted molecular mechanisms of acetic acid in the regulation of biofilm formation (b), and the genetic circuitry that regulates biofilm formation (c). As shown in panel a, when glycerol is consumed as a carbon source, it is sequentially converted to pyruvate, acetyl-CoA, and acetyl-phosphate and finally to acetate. GlpF is the glycerol uptake facilitator protein. YwcBA is the putative acetate transporter. As shown in panel b, YwbI is the regulatory protein that activates the expression of ywbHG in response to acetate. We propose that YwbHG, YsbAB, and YxaKC collectively regulate the activity of murein hydrolases and that the latter then triggers regulated cell lysis. Extracellular DNA (and/or proteins) released from regulated cell lysis could influence biofilm formation in B. subtilis. As shown in panel c, the matrix operons (epsA-O and tapA-sipW-tasA) are repressed by SinR, whose activity is antagonized by two antirepressors SinI and SlrA.
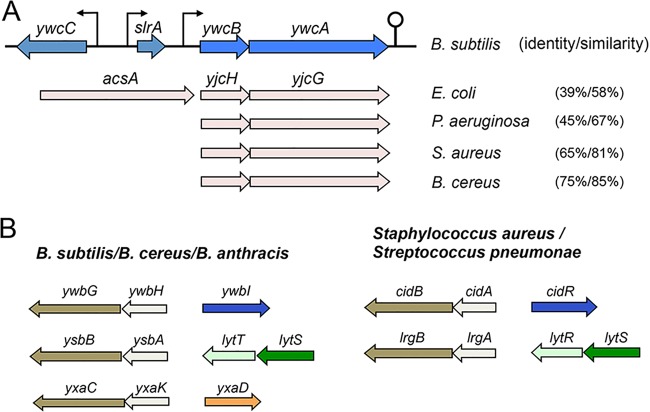
FIG 2 .
Putative genes involved in acetic acid transportation and genes encoding holin-antiholin-like proteins are highly conserved. (A) Arrangement of genes homologous to ywcBA in other bacteria. ywcC and slrA are two genes known to be involved in biofilm formation (23, 24). acsA is part of the operon with yjcH and yjcG in E. coli (54). Percentage numbers in the parentheses represent sequence identity and similarity between ywcA and the homologous genes in other bacteria. Note that, based on our BLAST research, only a few S. aureus isolates seem to contain genes homologous to ywcBA. (B) Organization of the genes encoding holin-antiholin-like proteins in Bacillus sp., S. aureus, and Streptococcus pneumoniae. Regulatory genes are also shown next to the holin-antiholin-like genes.
Biofilm formation of B. subtilis can be triggered by various environmental signals (25, 26). These putative signals have been investigated recently (8, 10, 27–31). In B. subtilis, there are five histidine kinases (KinA, KinB, KinC, KinD, and KinE) that can potentially sense those signals and activate directly or indirectly the Spo0A master regulator by protein phosphorylation (Fig. 1) (21). In one recent study, KinA and KinB were proposed to sense redox signals in response to oxygen limitation (27). KinC, on the other hand, was shown to sense structurally diverse molecules, including those present in the environment as well as surfactins produced by B. subtilis itself (29). The results of several recent studies suggest that another histidine kinase, KinD, may directly or indirectly sense environmental or host signals and activate Spo0A when triggering biofilm formation (8, 10, 30). Those putative signals include small organic acids from the plant host and plant cell wall polysaccharides (8, 10). In addition to environmental signals, bacterial cellular metabolism also plays an important role in biofilm formation. Metabolic molecules constitute an important category of signals in triggering or fine-tuning biofilm formation. As a well-studied example, in some bacteria, accumulation of the ppGpp stress response alarmone during the stringent response was shown to regulate biofilm formation (32–34). Nucleotide-based secondary messengers represent another example of metabolically derived signals that can regulate biofilm formation in a variety of bacteria (35). In a recent study (36), we showed that in B. subtilis, intracellular levels of the amino acid serine function as both a proxy for nutrient conditions and a trigger for biofilm formation when the level of serine runs low (36). Serine limitation, which coincides with the entry into biofilm formation, caused lowered protein levels of SinR, the key transcription repressor of the matrix genes, probably by reducing the translation efficiency of the sinR mRNA mediated by ribosome pausing (36).
Holin-antiholin proteins were initially characterized as phage proteins that cause host cell lysis by stimulating the activity of murein hydrolases (37). It was later shown that some bacterial genomes include similar genes that resemble the phage counterparts (38). In Staphylococcus aureus, two sets of genes, cidAB and lrgAB, were shown to encode holin-antiholin-like proteins (Fig. 2B) (38, 39). These proteins were shown to be involved in programmed cell death and biofilm formation (38–41). Expression of cidAB is induced by acetic acid (39). The induction is mediated by an acetate-responsive, LysR-type transcription regulator, CidR, whose gene is transcribed divergently from cidAB (Fig. 2B) (42). lrgAB is regulated by the LytS-LytR two-component system, which is proposed to sense changes in membrane potential in response to environmental stresses such as those represented by cationic antimicrobial peptides (42). Our search indicated that there are at least three sets of genes, ywbHG, ysbAB, and yxaKC, encoding putative holin-antiholin-like proteins in the genome of B. subtilis (Y. Chai, personal observations) (Fig. 2B). Among them, the ywbHG genes highly resemble cidAB in S. aureus, whereas the ysbAB genes share strong sequence similarity with lrgAB. Finally, the yxaKC genes seem to be uniquely present in Bacillus species. All three sets of genes are also conserved in B. cereus and B. anthracis (Y. Chai, personal observations), although a recent report suggested that the B. anthracis genome contained a fourth pair of such genes (43). In B. subtilis, the function and regulation of these genes are unknown.
A number of recent studies have shown that bacteria use volatile molecules to communicate with each other and to regulate bacterium-bacterium and bacterium-host interactions (44–47). For example, in one study (44), it was shown that both a B. subtilis strain and a B. amyloliquefaciens strain produced a blend of volatile chemicals, including 2,3-butanediol and acetoin, to promote growth of Arabidopsis thaliana. Another study demonstrated that a B. nematocida strain was able to lure Caenorhabditis elegans by emitting a mixture of volatile chemicals, including benzaldehyde, chloromethyl, 2-pentanone, etc. (47). Bacterial volatiles were also shown to significantly alter the physiology of the neighboring cells from different species, as a way to mediate cross-species interactions (46). B. subtilis is a strong volatile producer. Several previous studies already identified a collection of volatiles produced by B. subtilis cells under different conditions that showed diverse activities in bacterium-bacterium and bacterium-host interactions (44, 47).
In this work, we show that biofilm formation by B. subtilis can be stimulated by bacterial volatiles and that acetic acid is a volatile that is potent in triggering early biofilm formation. We also present evidence that three pairs of genes, ywbHG, ysbAB, and yxaKC, encoding holin-antiholin-like proteins, may be involved in cells responding to acetic acid and stimulating biofilm formation. Our investigations on B. subtilis as well as our preliminary results from S. aureus implicate acetic acid in a broader role as a signal for bacterial multicellularity.
RESULTS
Bacterial volatiles induce early biofilm formation in B. subtilis.
In our studies of biofilm formation in B. subtilis, we occasionally observed that the timing of robust biofilm formation by B. subtilis could be influenced by the presence of neighboring B. subtilis cells even when the two populations of cells were physically separated from each other. In one such experiment (Fig. 3A), when B. subtilis cells were surrounded by physically separated neighboring cells (Fig. 3A, lower right-hand panel, showing 1 well of cells surrounded by 11 wells of cells), biofilm induction was earlier and more robust than when there were no surrounding cells (lower left-hand panel, showing 1 well of cells surrounded by 11 wells of media only). One possible explanation for this phenomenon is that neighboring cells produced unknown chemicals that influenced, via air transmission, the timing of biofilm formation of B. subtilis cells that grew in proximity. To further test whether there are such volatiles that can influence biofilm formation by B. subtilis, we developed a simple assay as illustrated in Fig. 3B. To describe the assay briefly, in a 6-well polyvinyl plate (VWR), well a (top and side views in Fig. 3B) was used to preinoculate B. subtilis cells on day 1 to allow growth and volatile production. On day 2, a distant well (well b) was inoculated with fresh wild-type B. subtilis cells for pellicle biofilm development. The plate was sealed and incubated at 30°C for one more day. The result of pellicle formation in well b was recorded on day 3. A detailed description of the volatile assay is provided in Materials and Methods.
FIG 3 .
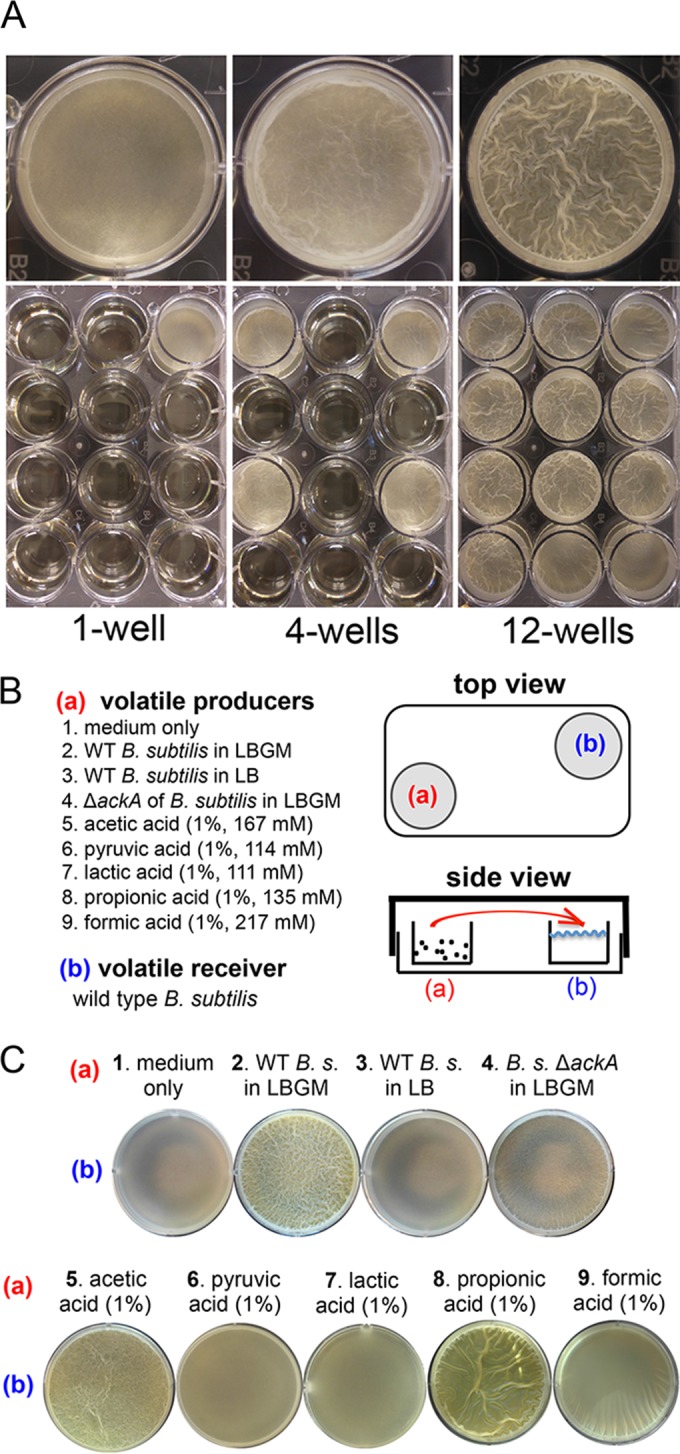
Bacterial volatiles induce early biofilm formation in B. subtilis. (A) Comparisons of the timing and robustness of pellicle biofilm formation by strain 3610 cells inoculated into 1, 4, or 12 wells of LBGM medium in the 12-well plate. The upper panels contain enlarged images of the representative corner wells at the bottom panels. (B) A cartoon illustration of the volatile assays performed in this study. As shown in both the top and side views, well a was preinoculated with either wild-type cells (strain 3610) or ackA mutant cells (YC1220) in LBGM medium or LB or pure volatile chemicals (acetic acid, pyruvic acid, lactic acid, propionic acid, or formic acid) were added. Pure acids were supplemented at a final concentration of 1% in LBGM medium. Note that the molar concentrations for each individual acid (at 1%) differed as indicated. Well b was inoculated with wild-type B. subtilis cells in LBGM medium as the volatile receiver 1 day later to examine the effect of volatiles on pellicle formation. All plates were sealed with Parafilm and incubated at 30°C for one more day before pellicle formation in well b was recorded. (C) Comparisons of pellicle formation by the wild-type B. subtilis (B. s.) cells in LBGM medium in well b when different volatile sources were provided in well a. It was clear that volatiles produced by the wild-type B. subtilis cells grown in LBGM medium (no. 2), pure acetic acid (no. 5), or pure propionic acid (no. 8) stimulated early pellicle formation of the B. subtilis cells in well b.
The results from our volatile assays showed that when well a was filled with medium only as a control, the wild-type B. subtilis cells in well b had just started to initiate pellicle formation on day 3 (Fig. 3C, no. 1). Interestingly, when well a was inoculated with B. subtilis cells, it clearly stimulated early pellicle formation of the cells in well b on day 3 (Fig. 3C, compare no. 1 and no. 2). A plausible explanation for the potent observation described above is that B. subtilis cells in well a produced volatiles that induced early pellicle formation by cells in well b. We point out that under both conditions, cells in well b eventually formed pellicle biofilms that were similarly robust (on day 4; data not shown), suggesting that the key difference between the two different conditions is likely the timing of biofilm induction.
To provide further evidence that the volatile effect can be better described as stimulating earlier and more-robust biofilm induction, and to more quantitatively compare the levels of pellicle biofilm development in the presence and absence of strong volatiles, we measured both the induction of the matrix genes and the accumulation of biofilm biomass at various time points during pellicle formation under the two conditions described above. Induction of the matrix genes was assayed by using cells bearing a promoter reporter for the epsA-O operon (PepsA-lacZ), whereas biofilm biomass was assessed by measuring cell optical density (see Text S1 in the supplemental material). Our results show that the induction of expression of matrix genes in the presence of strong volatiles (conditions similar to those described for Fig. 3C, no. 2) (see diamond symbols in red in Fig. S1A) was stronger than the induction seen in the absence of strong volatiles (conditions similar to those represented in Fig. 3C, no. 1) (see Fig. S1A, square symbols in blue). In parallel, the measurement of cell optical density suggested that the number of cells in the pellicle biofilms modestly increased in the presence of strong volatiles (see Fig. S1B, diamond symbols in red) compared to the level seen in their absence (see Fig. S1B, square symbols in blue) at most given time points.
LBGM medium promotes strong production of biofilm-stimulating volatiles.
In performing the volatile assays, we also learned that the types of media used to inoculate B. subtilis cells in well a were important. It appears that LBGM (lysogenic broth [LB] [10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter broth] plus 1% [vol/vol] glycerol and 0.1 mM MnSO4) medium, previously characterized as a biofilm-inducing medium for B. subtilis (30), is an excellent medium for strong production of biofilm-stimulating volatiles (Fig. 3C, no. 2). In contrast, when LB medium was used, the biofilm-stimulating effect largely disappeared (Fig. 3C, no. 3). In our previous study (30), we showed that supplementation of glycerol and manganese converted LB, a biofilm-inert medium, to a medium that strongly induces biofilm formation for B. subtilis as well as several other Bacillus species. Additional features associated with growing B. subtilis cells in LBGM medium include strong pigment production and (perhaps) strong volatile production (judging by the presence of strong smells) (30). Results of our unpublished studies based on genome-wide transcriptomic analysis suggest that the presence of glycerol caused a major metabolic shift in that several dozens of metabolic genes involved in fermentation and production of small volatile products (such as lactic acid, acetic acid, acetoin, 2,3-butanediol, etc.) were strongly induced in the cells (Y. Chai, unpublished data). We are currently exploring the molecular basis for this “glycerol effect.” Our findings presented here further explain why LBGM medium is a medium that strongly induces biofilm formation, since the yet-to-be-identified volatile(s) may function as a self-stimulating signal for biofilm formation.
We decided to further look into the LBGM medium, trying to understand how it promotes strong production of biofilm-stimulating volatiles. Wild-type B. subtilis cells growing in LBGM medium showed little difference in doubling time from the cells growing in LB (Fig. 4A). However, a clear difference was seen in the changes of the pH values of the media over time during growth (Fig. 4B); when B. subtilis cells were grown in LBGM medium, the medium became acidic and the pH dropped to as low as 5.2 after 8 h of growth (squares in Fig. 4B), whereas the pH fluctuated only mildly over time when B. subtilis cells were grown in LB (diamonds in Fig. 4B). This indicates that more acids were being produced when cells were grown in LBGM medium.
FIG 4 .
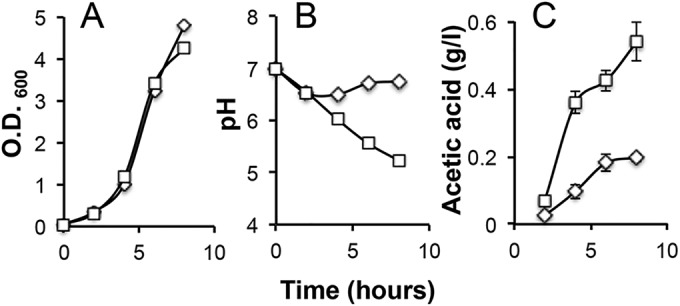
LBGM medium promotes strong production of biofilm-stimulating volatiles and higher production of acetic acid. (A) Wild-type B. subtilis cells (strain 3610) had similar generation times when grown in LBGM medium (squares) and in LB medium (diamonds). (B) Strong acid production was seen when wild-type B. subtilis cells grew in LBGM medium (squares), evidenced by the drop of the pH values of the spent media, but not when cells were grown in LB medium (diamonds). (C) A higher accumulation of acetate in the medium was seen when B. subtilis cells grew in LBGM medium (~0.55 g/liter at h 8, squares) than when they grew in LB (~0.18 g/liter at h 8, diamonds).
One of the acids predicted to result from overflow metabolism when B. subtilis cells are grown in LB plus glycerol is acetic acid (48). We thus compared levels of acetate accumulation in B. subtilis cells grown in either LBGM medium or LB (see Materials and Methods). As expected, our results confirmed that acetic acid accumulation was much higher when B. subtilis cells were grown in LBGM medium (reaching ~0.55 g/liter after 8 h) (squares in Fig. 4C) than when they were grown in LB (reaching ~0.18 g/liter after 8 h) (diamonds in Fig. 4C). Since more acetic acid was being produced in LBGM medium and since acetic acid is a known volatile, we infer that more acetic acid appears in the volatile mixture when B. subtilis cells grow in LBGM medium. Our investigations led us to conclude that LBGM medium promotes strong production of biofilm-stimulating volatiles and, coincidently, strong production of volatile acetic acid. Interestingly, two other biofilm-inducing media, MSgg (5 mM potassium phosphate [pH 7], 100 mM morpholinepropanesulfonic acid [pH 7], 2 mM MgCl2, 700 μM CaCl2, 50 μM MnCl2, 50 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 0.5% glycerol, 0.5% glutamate, 50 μg ml−1 tryptophan, 50 μg ml−1 phenylalanine, 50 μg ml−1 threonine) and 2× SGG {2× SG [0.05% MgSO4 ⋅ 7H2O, 0.2% KCl, 1.6% Difco nutrient broth, 0.1% glucose, 0.0001 mM FeSO4, 0.1 mM MnCl2 ⋅ H2O, 1 mM Ca(NO3)2] medium supplemented with 1% glycerol]}, frequently used in biofilm studies of B. subtilis, also contain significant amounts of glycerol (0.5% [vol/vol]). Volatiles produced from cells growing in those two biofilm media similarly stimulated early pellicle formation (see Fig. S2 in the supplemental material).
Acetic acid is a potent volatile in stimulating biofilm formation.
On the basis of the results described above, we further speculated that, within the hypothetical volatile mixture, acetic acid could be one such potent molecule stimulating biofilm formation. To explore this idea, we repeated the volatile experiment by testing whether the activity of pure acetic acid is sufficient to mimic the biofilm-stimulating activity of the volatile mixture produced by B. subtilis. We added 1% pure acetic acid to well a. As shown in Fig. 3C (no. 5), acetic acid exerted a biofilm-stimulating effect similar to what was seen with the hypothetical volatile mixture (Fig. 3C, compare no. 2 and 5). To test the specificity of this effect, we also applied other volatile acids as controls, including pyruvic acid, lactic acid, propionic acid, and formic acid. The same amounts of pyruvic acid and lactic acid did not show similar stimulating effects (Fig. 3C, no. 6 and 7). Formic acid had a mild biofilm-stimulating affect. Interestingly, propionic acid showed a biofilm-stimulating effect that was at least as strong as that of acetic acid (Fig. 3C, compare no. 5 and 8). We do not yet know whether LBGM medium also promotes production of propionic acid. In toto, our results suggest that volatiles produced by B. subtilis cells have a stimulating effect on biofilm formation and that acetic acid is one such volatile, being produced at high levels and capable of biofilm stimulation.
To provide further genetic evidence, we constructed a B. subtilis ΔackA mutant. The ackA gene encodes an enzyme, acetate kinase, which converts acetyl-phosphate to acetate and plays a key role in acetate production (Fig. 5A) (49). The ΔackA mutant is thought to be largely deficient in acetic acid production. By using an assay to measure acetate accumulation (see Materials and Methods), we were able to confirm that the ΔackA mutant failed to produce and accumulate detectable levels of acetate (Fig. 5B). We next applied the ΔackA mutant to the volatile experiment and found that the volatiles produced by the ΔackA mutant stimulated early pellicle formation only very mildly (Fig. 3C, compare to no. 4). The weak biofilm-stimulating activity of the volatiles produced by the ΔackA mutant could have been due to the presence of other volatile molecules that either had lower stimulating activity or were produced at lower levels. This result further supported the idea that acetic acid functions as a strong volatile signal to stimulate biofilm formation.
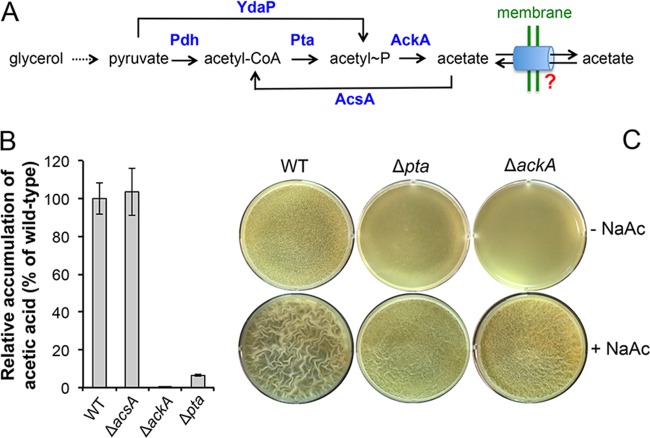
FIG 5 .
Acetic acid acts as a strong volatile for biofilm induction. (A) A schematic drawing of the metabolic pathway for acetate production in B. subtilis. Pdh, pyruvate dehydrogenase; Pta, phosphotransacetylase; AckA, acetate kinase; AcsA, acetyl-CoA synthetase; YdaP, putative pyruvate oxidase. A hypothetical transportation system for acetate crossing the cell membrane in B. subtilis was shown and is highlighted by the question mark. (B) Comparison of levels of acetate accumulation by the wild type (3610), the Δpta mutant (YC1230), the ΔackA mutant (YC1220), and the ΔacsA mutant (YC1218). Cells were grown in LBGM medium to an OD600 of 4, and acetate accumulation in spent media was measured following the protocol described in Materials and Methods. (C) Pellicle formation by the wild type, the Δpta mutant (YC1230), and the ΔackA mutant (YC1220) in LBGM medium in the absence or presence of 0.2% NaAc after 24 h of incubation at 30°C.
In addition to being a volatile molecule that acts on cells that are physically separated (but growing in proximity) in the community as we have shown above, acetic acid may also function as a canonical quorum-sensing-like molecule that is produced by cells during growth, is accumulated in the environment, and activates particular targets when its concentration reaches a threshold. If this hypothesis is correct, we should be able to predict not only that genetic mutants deficient in acetic acid production would be unable to stimulate early biofilm formation through volatiles, as shown in Fig. 3C (no. 4, ΔackA cells), but also that those mutants might themselves show a delay in biofilm formation. To test that, we constructed another B. subtilis mutant, the Δpta strain, which is mutated for the gene encoding the enzyme phosphotransacetylase (Pta) that converts acetyl-coenzyme A (CoA) to acetyl-phosphate and is therefore blocked in acetic acid production (Fig. 5A) (49). Similarly to the ΔackA mutant results, we were able to confirm that the Δpta mutant is also largely deficient in acetic acid production (Fig. 5B). The residual levels of acetic acid detected in the Δpta mutant could have been due to generation of acetyl~P (and thus acetate) from pyruvate by pyruvate oxidase (YdaP in B. subtilis resembles pyruvate oxidase; Y. Chai, personal observation), a reaction that is independent of Pta activity (Fig. 5A) (50, 51). We next compared the levels of pellicle biofilm formation by the wild type, the Δpta mutant, and the ΔackA mutant (Fig. 5C). Our results showed that the Δpta mutant and the ΔackA mutant showed similar delays in pellicle formation compared to the wild type (upper panels, Fig. 5C). For both the Δpta mutant and the ΔackA mutant, the delayed pellicle formation was able to be rescued by complementation of the mutation by a wild-type copy of the gene integrated at the ectopic amyE locus (see Fig. S3 in the supplemental material). More interestingly, when 0.2% sodium acetate (NaAc) was added to the medium, it largely rescued the delay of pellicle formation by the two mutants as well (lower panels, Fig. 5C). Rescues were not seen when either 0.2% sodium lactate or 0.2% sodium pyruvate was added to the media as a control (see Fig. S4). These results reinforced the idea that accumulation of acetic acid signals the timing of biofilm induction in B. subtilis. Lastly, another acetic acid metabolic mutant, the ΔacsA mutant, which is blocked in the conversion from acetate to acetyl-CoA (Fig. 5A), did not show any loss of acetic acid production or any biofilm phenotype (Fig. 5B and data not shown).
Acetate enhances expression of the matrix genes.
We showed that addition of 0.2% NaAc to the medium was largely able to rescue the delay of pellicle formation by the ΔackA and Δpta mutants (Fig. 5C). In the same experiment, we also noticed that adding 0.2% NaAc to the wild-type B. subtilis cells in LBGM medium stimulated early and robust biofilm formation (Fig. 5C and 6A). As a control, adding the same amount of sodium chloride (NaCl) did not show any stimulating effect (Fig. 6A), indicating that the stimulating effect was due to acetate. We also tested other types of acetate salts (e.g., potassium acetate). They showed similar biofilm-stimulating activities (data not shown). These results suggest that acetate salts can directly stimulate early and robust biofilm formation.
FIG 6 .
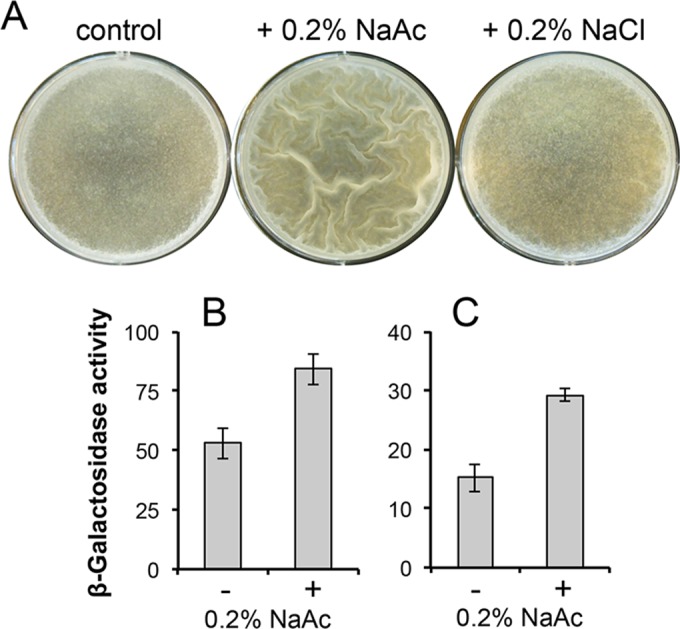
Acetate salts stimulate early biofilm formation by enhancing expression of the matrix genes. (A) Pellicle formation by wild-type B. subtilis cells (strain 3610) in LBGM medium or LBGM medium plus 0.2% NaAc or LBGM medium plus 0.2% NaCl, after 24 h of incubation at 30°C. Early pellicle formation was seen for cells growing in LBGM medium plus 0.2% NaAc. (B and C) Assays of β-galactosidase activities by B. subtilis cells harboring either the PtapA-lacZ (YC121) (B) or the PepsA-lacZ (YC110) (C) transcriptional reporter. Cells were grown in LB shaking culture in the presence or absence of 0.2% NaAc and were harvested when the OD600 of the cultures reached 2.0.
To investigate whether the biofilm-stimulating effect shown by acetate salts was due to some indirect effect or was a result of acetate promoting expression of biofilm-specific genes, we examined the effect of acetate salts on expression of the matrix genes. To do so, we assayed the activities of the B. subtilis cells harboring a transcriptional reporter for either the tapA operon (PtapA-lacZ, Fig. 6B) or the epsA-O operon (PepsA-lacZ, Fig. 6C) in the absence or presence of 0.2% NaAc. Cells were grown in LB to an optical density at 600 nm (OD600) of 2 and harvested, and β-galactosidase activities of the cells were measured accordingly. As shown in Fig. 6B and C, the two transcriptional reporters showed activities that were ~55% (Fig. 6B) and ~80% (Fig. 6C) higher in the presence of 0.2% NaAc than in its absence, suggesting that acetic acid stimulates biofilm formation in part by directly enhancing the expression of the matrix genes. To conclude, our results suggest that acetic acid can function as a canonical metabolic signal to induce biofilm formation and can also act as an airborne signal to coordinate the timing of biofilm formation of cells that live in close proximity in the same environment but are physically separated.
The ywcB-ywcA operon, predicted to encode a putative acetic acid transporter, is involved in biofilm formation.
Since mutations blocking acetic acid production can cause a delay in biofilm formation in B. subtilis (Fig. 5C), one may presume that mutations blocking acetic acid transportation may alter acetic acid accumulation and therefore the timing of biofilm formation as well. Unfortunately, although acetic acid metabolism has been well studied in some bacteria (49), the dedicated transportation system for acetic acid has not been identified except that Gimenez et al. reported that, in Escherichia coli, the yjcHG genes encode a sodium-dependent acetic acid symporter (Fig. 2A) (52). We performed a BLAST search of the B. subtilis genome, looking for genes homologous to yjcHG, and identified the ywcBA genes in B. subtilis that share strong sequence similarity with yjcHG in E. coli (39% sequence identity and 58% similarity between ywcA and yjcG, Fig. 2A). Our search also revealed that these two genes are highly conserved in different bacteria, including important pathogens such as Pseudomonas aeruginosa and S. aureus (for S. aureus, the homologous genes are present in only a few clinical isolates; Y. Chai, unpublished observations) (Fig. 2A). We hoped to point out that in B. subtilis, the ywcBA genes lie next to slrA and ywcC, whose protein products constitute the YwcC-SlrA pathway for derepression of SinR-controlled matrix genes and thereby biofilm formation (Fig. 1 and 2A) (23).
If the ywcBA genes indeed encode an acetic acid transporter, we would predict that mutations in the ywcBA genes might alter acetic acid accumulation in the cells and thus result in an altered biofilm phenotype. To test that, we constructed a B. subtilis mutant with an insertional deletion mutation in the ywcBA genes and compared the levels of pellicle formation by the wild type and the ΔywcBA mutant. Interestingly, the mutant did show early pellicle formation in both LBGM medium and another biofilm-inducing medium, MSgg (Fig. 7A). To test whether early pellicle formation was due to some indirect effect or was due to an alteration in matrix gene expression, we again introduced the two transcriptional reporters for the matrix operons into the wild type and the ΔywcBA mutant and compared the levels of expression of the matrix genes in the two strains by measuring the activities of the two transcriptional fusions (PtapA-lacZ [Fig. 7B] and PepsA-lacZ [Fig. 7C]). Our results showed that both reporters were expressed at higher levels in the ΔywcBA mutant than in the wild type, suggesting that early pellicle formation was an outcome of elevated expression of the matrix genes (Fig. 7B and C).
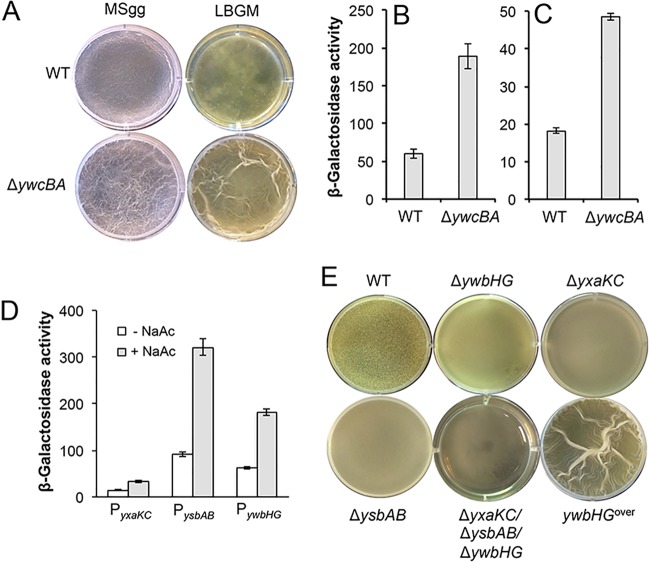
FIG 7 .
Characterization of genes involved in acetic acid-mediated biofilm formation. (A) Pellicle formation by the wild type (3610) and the ΔywcBA mutant (YC535) in MSgg and LBGM medium. Images were taken after 24 h of incubation at 30°C for LBGM medium or 48 h of incubation at 30°C for MSgg. (B and C). Assays of β-galactosidase activities of the wild-type cells and the ΔywcBA cells harboring either the PtapA-lacZ transcriptional reporter (B) or the PepsA-lacZ transcriptional reporter (C). (D) Assays of the β-galactosidase activities of the strains (FY250, FY251, and YC259) bearing one of the three promoter fusions (PyxaKC-lacZ, PysbAB-lacZ, and PywbHG-lacZ, respectively) for the three holin-antiholin operons. (E) Pellicle formation by the wild type, the three single mutants for the holin-antiholin-encoding genes (CY819 [ΔywbHG], CY881 [ΔysbAB], and CY882 [ΔyxaKC]), the triple mutant (CY886), and the strain overexpressing ywbHG (CY1250) inoculated in LBGM medium after 24 h of incubation at 30°C.
How can the early pellicle formation shown by the ΔywcBA mutant be interpreted? As we speculated above, one possibility could be that there was an early accumulation of intracellular acetic acid due to blocking of acetic acid exportation and therefore that it triggered early biofilm formation. (A second possibility could be the opposite, that is, that after glycerol depletion, import and reutilization of acetate were blocked in the ΔywcBA mutant and lack of intracellular acetate triggered early biofilm formation. The latter possibility seems less likely based on the observation that matrix genes can be induced by exogenously added acetate [Fig. 6].) To test the hypothesis presented above and to explore the putative role of YwcA-YwcB in acetic acid transportation, we conducted two specific assays. In the first assay, we compared the levels of secretion of acetic acid by the wild type and the ΔywcBA mutant by measuring acetic acid accumulation in the media during growth. Our assumption was that if acetic acid secretion were blocked due to mutations in the ywcBA genes, we would see less accumulation of extracellular acetic acid during growth of the ΔywcBA mutant. Cells of the wild type and the ΔywcBA mutant were grown in LBGM medium, and culture supernatants were collected when the OD600 of the cultures reached 3.0. Concentrations of acetic acid in the supernatants were measured following the method described in Materials and Methods. As shown in Fig. S5A in the supplemental material, we saw a modest (~19%) decrease in acetic acid accumulation in the medium with the ΔywcBA mutant compared to the wild type, indicating that YwcB-YwcA may act as an acetic acid transporter, but even so, there must be another transporter(s) involved in acetic acid secretion here.
In the second experiment, we compared the levels of importation of acetate by the wild type and the ΔywcBA mutant. We grew the wild type and the ΔywcBA mutant in a minimal medium with a limited amount of glutamate (0.5%) as both the sole carbon source and the sole nitrogen source. We then added 0.1% NaAc to the medium and measured the level of the remaining acetate in the medium periodically for 6 h. Our result showed that both the wild type and the ΔywcBA mutant were able to take up acetate, since the levels of the remaining acetate in the media dropped continuously, but that the ΔywcBA mutant showed a modestly lower uptake rate (see Fig. S5B in the supplemental material). Taken together, our results indicate that the ywcBA operon may encode a putative acetic acid transporter in B. subtilis but may not be the only one that is involved in acetic acid transportation. This finding was not a complete surprise to us, since it was previously shown in E. coli that the ΔyjcHG mutant was still able to effectively take up acetic acid, indicating the existence of another transportation system for acetic acid in E. coli (52). Subsequently, a second acetic acid transporter (YaaH) was identified in E. coli. The ability to transport acetic acid was completely abolished for the yaaH yjcGH double mutant, which did not grow on minimal media with acetate as the sole carbon source (53). Unfortunately, no gene homologous to yaaH was found in the B. subtilis genome (Y. Chai, personal observation).
Thus far, results from our investigations have tended to support the idea that YwcBA may be involved in acetic acid transportation in B. subtilis. However, strong biochemical evidence will be needed in the future to demonstrate the direct involvement of the YwcB-YwcA proteins in acetic acid transportation. In addition, in E. coli, the homologous proteins YjcH-YjcG were investigated previously only for their role in the import of acetic acid (52), whereas in our studies, we showed that YwcB-YwcA could play a role in both the import and export of acetic acid. Many studies have demonstrated the importance of similar sodium symporters in the import of substrates (often against the substrate concentration gradient and in a manner dependent on a sodium ion gradient as the energy source) (54). The importance of such symporters in the export of substrate remains less clear.
Putative holin-antiholin-encoding genes are induced by acetate and involved in biofilm formation.
The next issues we hoped to address were how B. subtilis cells sense and respond to acetic acid and how acetic acid ultimately stimulates biofilm formation. In S. aureus, two sets of genes shown to be involved in biofilm formation and induced by acetic acid are cidAB and lrgAB, both encoding putative holin-antiholin-like proteins (Fig. 2B) (39, 41, 42). Expression of cidAB is controlled by a LysR-type transcriptional regulator, CidR, in response to acetic acid (Fig. 2B) (42). As we discussed earlier, the B. subtilis genome contains three sets of genes, ywbHG, ysbAB, and yxaKC, encoding putative holin-antiholin-like proteins, and yet their function and regulation are completely unknown (Fig. 2B). In a parallel work designed to characterize genes in B. cereus that are induced under biofilm conditions by using genome-wide transcriptome analyses, we found that the genes that were most highly induced during growth of B. cereus cells in LBGM medium included three genes (B. cereus 5133 [BC5133], BC5438, and BC5439; B. cereus ATCC 14579 was used as a reference genome) that are homologous to lrgA, lrgB, and cidB, respectively (Y. Chai, unpublished results).
In light of the evidence described above, we decided to investigate the possible involvement of these putative genes in responding to acetic acid and stimulating biofilm formation in B. subtilis. We conducted two experiments. In the first experiment, we wanted to test whether any of these genes can be induced by acetic acid. For this purpose, we constructed promoter reporter fusions for each of the three operons, resulting in PyxaKC-lacZ, PywbHG-lacZ, and PysbAB-lacZ, respectively. We then grew the cells containing each of the three reporters in the media in the absence or presence of 0.2% NaAc and compared the β-galactosidase-specific activities of those cells. All three operons were found to be induced in the presence of 0.2% NaAc, with induction levels ranging from 2-fold to 3-fold (Fig. 7D). In the second experiment, we examined whether mutations in any of these genes might affect biofilm formation. We constructed several B. subtilis mutants that were mutated for each of the three sets of genes, creating mutant strains ΔywbHG, ΔysbAB, and ΔyxaKC, as well as a triple mutant with all three mutations. We performed assays of pellicle formation for those mutants and the wild type. As shown in Fig. 7E, after 24 h of incubation, each of the three single mutants showed a mild decrease in pellicle robustness. The triple mutant, however, showed a severe decrease in pellicle robustness (Fig. 7E). Note that all mutants eventually formed robust, wild type-like biofilms (data not shown). The results described above suggest that those genes encoding holin-antiholin-like proteins may be collectively involved in regulation of biofilm formation, possibly by the control of the timing for biofilm induction.
Overexpression of ywbHG stimulates early and robust biofilm formation.
In S. aureus, strong induction of the cidAB genes was shown to stimulate biofilm formation (38, 41). The ywbHG genes of B. subtilis are homologous to cidAB (Fig. 2B). The ywbHG genes sit next to ywbI, a gene homologous to cidR encoding a putative acetate-responsive, LysR-type transcription regulator (Fig. 2B and 8A). ywbHG-ywbI are also located five genes away from ywcBA (Y. Chai, personal observation) (55). We were thus interested in understanding the exact role of ywbHG in responding to acetic acid and stimulating biofilm formation. We decided to first test whether the induction of ywbHG is mediated by the YwbI LysR-type regulator, as was shown in S. aureus. To test this, the transcriptional reporter for the ywbHG genes (PywbH-lacZ) was also introduced into a strain deleted for the ywbI gene. NaAc or KAc (0.2%) was added to the shaking LB inoculated with the reporter strains (wild type and ΔywbI), and the β-galactosidase activities of the cells were measured. As shown in Fig. 8B, both NaAc and KAc significantly induced expression of the PywbH-lacZ fusion (white bars, wild type). Induction of the PywbH-lacZ reporter by acetate salts was also dependent on the presence of the YwbI regulatory protein, since no induction was observed in the ywbI mutant (gray bars, ΔywbI). These results suggest that the regulation of the ywbHG-ywbI genes in B. subtilis and that of the cidAB-cidR genes in S. aureus are similar to each other.
FIG 8 .
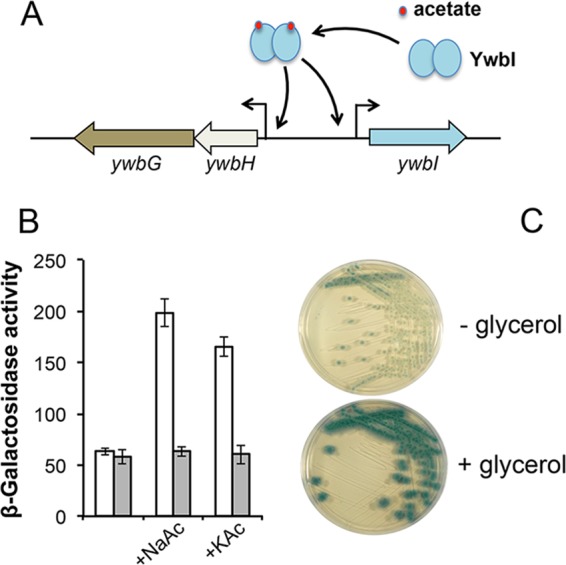
The ywbHG operon is directly induced by acetate as well as by glycerol. (A) A cartoon model showing induction of the ywbHG operon by acetate. YwbI is a LysR-type transcription factor predicted to regulate ywbHG in response to acetate. (B) Assays of the β-galactosidase activities of the wild-type cells (white bars, YC259) or the ΔywbI mutant cells (gray bars, YC1245) harboring the PywbHG-lacZ transcriptional reporter in shaking LB in the absence or presence of 0.2% NaAc or 0.2% KAc. (C) ywbHG expression was induced by glycerol. B. subtilis cells harboring the PywbHG-lacZ transcriptional fusion (YC259) were streaked out on LB agar plates plus X-Gal and without or with supplementation of 1% glycerol.
The activity of the reporter-bearing wild-type cells was also tested on LB agar plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) in the absence or presence of 1% glycerol. The result seemed to show quite clearly that glycerol also strongly induced PywbH-lacZ expression, as colonies of the reporter cells turned dark blue on the X-Gal plates in the presence of glycerol (Fig. 8C). This result suggests that when B. subtilis cells are grown in the biofilm-inducing LBGM medium, the ywbHG genes are strongly induced. Finally, to test whether induction of ywbHG by acetate is the reason that acetate can stimulate biofilm formation, we constructed a B. subtilis strain that overexpresses ywbHG under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. The resulting strain, CY1250, showed early and very robust biofilm formation upon addition of IPTG (Fig. 7E), suggesting that induction of ywbHG by acetate can at least partly explain the acetate-mediated biofilm stimulation seen in this study.
Acetate stimulates biofilm formation in S. aureus.
Finally, an issue that may have potentially broad interest is whether other bacteria also use acetic acid as a biofilm-inducing signal. We reasoned that it is possible because the putative genes involved in acetic acid metabolism and transportation and the genes encoding holin-antiholin-like proteins are highly conserved in other bacteria (Fig. 2). Furthermore, it was already shown in S. aureus that the cidAB and lrgAB genes are induced by acetic acid and are involved in programmed cell death and biofilm formation (39, 41, 42). Our unpublished results from the genome-wide transcriptome analyses (Y. Chai, unpublished results) also suggest that some of those genes in B. cereus are among the ones most highly induced under biofilm-inducing conditions (LBGM medium).
We selected S. aureus HG003, a strain capable of forming biofilms, to test whether S. aureus also uses acetate as a biofilm-inducing signal. Note that the biofilms formed by S. aureus are submerged, surface-attached biofilms (see Materials and Methods and Fig. 9) that are different from the floating pellicles formed by B. subtilis. We first tested whether acetate salts can stimulate biofilm formation in S. aureus. To do so, HG003 cells were inoculated in tryptic soy broth (TSB) supplemented with 1% glucose in 24-well polyvinyl plates (VWR) to allow formation of surface-attached biofilms (see Materials and Methods). In parallel, NaAc at different concentrations (0.2%, 0.5%, and 1%) was added to the media in order to test whether acetate stimulates biofilm formation. As shown in Fig. 9A, S. aureus cells seemed to form more surface-attached biofilms at the bottom of the wells in the presence of increasing amounts of NaAc. A semiquantitative analysis showed approximately 1.4-, 4.5-, and 10.7-fold increases in the biofilm biomass in the presence of 0.2%, 0.5%, and 1% NaAc, respectively, compared to that seen in the absence of NaAc (Fig. 9B). We concluded that acetic acid has stimulatory activity with respect to biofilm formation in S. aureus as well. This result is also consistent with findings from a recent study that suggested that production of acetic acid from overflow metabolism and weak-acid properties of acetic acid play important roles in S. aureus programmed cell death and biofilm development (51).
FIG 9 .
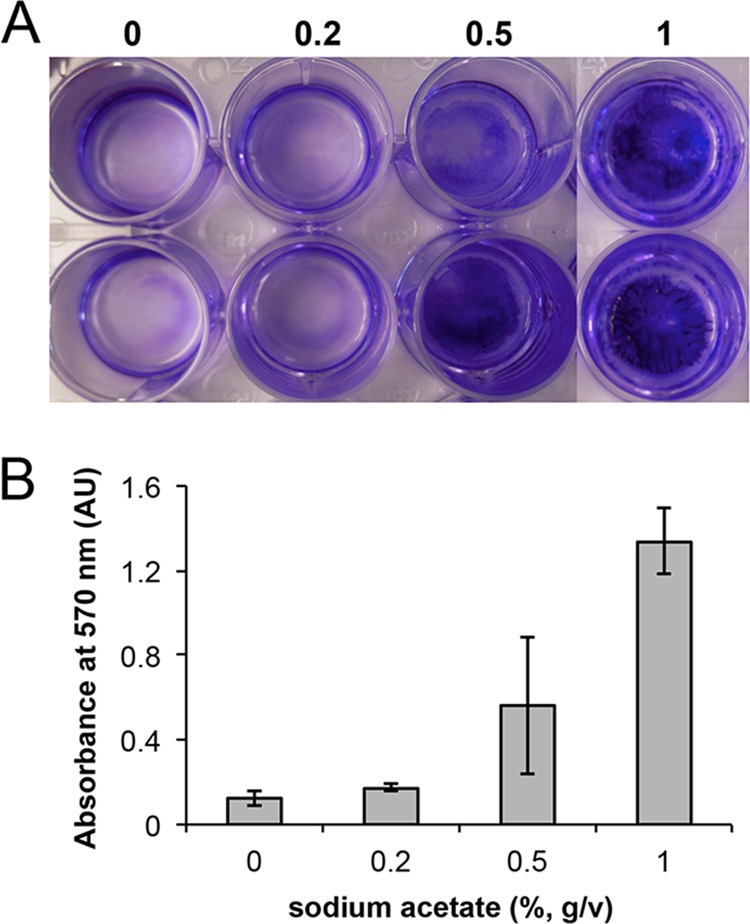
Acetate salt stimulates biofilm formation in S. aureus. (A) Formation of submerged, surface-attached biofilms by S. aureus HG003 in TSB supplemented with 1% glucose in the absence or presence of 0.2%, 0.5%, and 1% NaAc. A concentration of 1% is defined as 10 g of NaAc in 1 liter of medium. Biofilms attached to the bottom of the 24-well polyvinyl plate (VWR) were visualized by crystal violet staining. (B) Results of a semiquantitative measurement to compare the biomasses of the surface-attached biofilms described for panel A. AU, absorbance units.
DISCUSSION
In this study, we showed that B. subtilis employs self-produced volatiles as a way to coordinate multicellular development for cells that are physically separated in the community. Our results suggest that acetic acid is one such potent volatile stimulating biofilm formation in B. subtilis. We are currently investigating the amount of acetic acid in the volatile mixture and what other volatile chemicals are present in the volatile mixture under our biofilm-inducing conditions. We propose that B. subtilis cells produce and accumulate acetic acid through cell metabolism and use acetic acid as a volatile signal as well as a canonical signal to coordinate the timing of biofilm formation in the population (Fig. 1). In some ways, this is similar to the behavior seen with quorum-sensing molecules. The really interesting difference appears to be that the signal can be airborne. This also makes very good sense for bacteria during biofilm development since volatiles can provide an efficient method of cell-cell communication among discrete colonies of cells sharing the same community and since cells in natural environments do not always grow in a continuously aqueous environment that permits efficient diffusion of signal molecules.
Bacteria are known to produce and secrete acetic acid (56). Acetic acid is a common byproduct from overflow metabolism when cells grow in media supplemented with glucose or with other favorable carbon sources such as glycerol (Fig. 1) (48, 49). Acetic acid has a variety of known biological functions. In bacteria, intracellular acetic acid has also been implicated as a metabolic signal (56). However, characterizing the cellular targets of acetic acid is often challenging. In this study, we showed that acetic acid strongly induced expression of ywbHG, the genes encoding putative holin-antiholin-like proteins, in B. subtilis and that overexpression of ywbHG stimulated early biofilm formation. We suggest that enhanced expression of ywbHG in response to acetic acid may be one of the main reasons for acetic acid-induced early biofilm formation (Fig. 1). Other holin-antiholin-like genes (ysbAB and yxaKC) may also be involved in biofilm formation since the triple mutant had the most severe delay in biofilm formation in the comparisons with the individual single mutants. Our findings are also consistent with the observations from previous studies in S. aureus showing that some of the bacterial holin-antiholin-like genes (cidAB) can be induced by the metabolic signal acetic acid.
Holin-antiholin proteins were originally characterized as phage proteins that are involved in host cell lysis in the lytic life cycle of phage (37). Previous studies showed that some bacteria also include holin-antiholin-like genes in their genomes. These bacterial proteins are thought to regulate the activity of murein hydrolases in a similar fashion. The biological function and regulation of these bacterial proteins are relatively well studied in S. aureus (39, 41, 42). There is genetic and molecular evidence suggesting that these proteins are involved in programmed cell death and biofilm formation in S. aureus (39, 41, 42). A link was also proposed between programmed cell death and biofilm formation in S. aureus (41, 51) in that programmed cell death leads to cell lysis in some localized regions within the biofilm and to release of extracellular DNA (eDNA) and likely proteins as well. eDNA and proteins released from cell lysis become part of the biofilm matrix and thus contribute to biofilm formation (41, 57). It seems that contributions to programmed cell death and release of eDNA and/or proteins are the main reasons that those holin-antiholin-like genes are involved in biofilm formation in S. aureus. How these putative genes contribute to biofilm formation in B. subtilis is not known. It is also not known whether localized cell death and eDNA release occur in a significant fashion during B. subtilis biofilm formation. In this study, we briefly attempted to address some of these issues by examining the presence of eDNA during B. subtilis biofilm formation. We performed a simple assay to detect and compare levels of eDNA release during biofilm development in the wild type and the triple mutant by reverse transcription-quantitative PCR (RT-qPCR) (see Text S1 in the supplemental material). Our results showed that although there were biofilm-associated eDNAs present in the samples, no significant difference in eDNA abundance (<30%) was observed between samples of the wild type and the triple mutant (see Fig. S6 in the supplemental material). Thus, it is unclear whether eDNA release is a significant feature in B. subtilis biofilm development. Further studies will be needed to address these issues.
One recent study further investigated the link between acetate-mediated programmed cell death and biofilm development in S. aureus (51). In that study, the authors showed that acetic acid production from overflow metabolism played a very important role in regulating programmed cell death and biofilm formation. The authors attempted to explicate the molecular basis of the acetate-mediated effects and came to the conclusion that the trigger of programmed cell death depends on synergistic activities of the weak-acid properties of acetate and increasing levels of reactive oxygen species (ROS). This study suggests a broader link between cell overflow metabolism and programmed cell death and biofilm formation. The importance of the weak-acid properties of acetic acid was also implied in several previous studies (57–59). In our biofilm studies, we primarily used LBGM medium, a medium with excess (1%) glycerol. Our observation of media acidification during B. subtilis biofilm development was very similar to those reported from the studies cited above. More interestingly, our unpublished results also pointed toward a strong correlation between decreasing media pH and increasing numbers of cells experiencing oxidative stress (measured by the use of a cellular reporter for detection of DNA damage) (Y. Chai, unpublished results). All these results suggest the presence of such a link between cell overflow metabolism and formation of multicellular communities in bacteria.
The metabolic pathway of acetic acid also involves intermediate products such as acetyl-CoA and acetyl-phosphate (Fig. 1 and 5A). The putative biological functions of acetyl-phosphate have been investigated recently (60–62). It was proposed previously that in some bacteria such as E. coli, acetyl-phosphate acts as an important global signal and as a phosphor/acetyl donor for protein modifications and other important regulations (60, 61). Multiple studies showed that acetyl-phosphate acts to directly modify key regulatory proteins whose activities are controlled by either protein acetylation or phosphorylation (60, 61, 63). In present report, we cannot rule out the possibility that the intermediate products of acetate metabolism, especially acetyl-phosphate, may be involved in regulating biofilm formation since activation of key regulators such as Spo0A and ComA by protein phosphorylation is critical to biofilm induction in B. subtilis (5). There was at least one study showing that acetyl-phosphate can directly regulate the activity of ComA by acting as a phosphate donor for ComA protein phosphorylation (64). ComA is the responsive regulator of the ComA-ComP two-component system, which is involved in regulating surfactin production (62). Surfactin in turn functions as a quorum-sensing molecule for biofilm formation in B. subtilis (29). In future studies, it will be interesting to investigate the possible role of acetyl-phosphate in regulating biofilm formation in B. subtilis.
MATERIALS AND METHODS
Strains and media.
Bacillus subtilis strain 3610 and its derivatives were routinely cultured in lysogenic broth (LB) (10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter broth) at 37°C. For assays of biofilm formation, three different biofilm-inducing media, LBGM medium, MSgg, and 2× SGG, were used. LBGM medium is composed of LB supplemented with 1% glycerol (vol/vol) and 100 µM MnSO4 (30). The recipes for MSgg and 2× SGG were described in previous publications (6, 23). Staphylococcus aureus was grown in brain heart infusion medium (BHI; Difco) for cell proliferation. For biofilm formation of S. aureus, tryptic soy broth (TSB; Difco) was used with the supplementation of 1% glucose. Lists of strains, plasmids, and oligonucleotides used in this work are presented in Table S1 in the supplemental material. Chemicals were purchased from Sigma. All ingredients of media were purchased from Difco. An acetate assay kit (catalog no. 10148261035) was purchased from Biopharm (a Roche company), United Kingdom.
Strain and plasmid construction.
We followed published protocols for general methods of molecular cloning (65). SSP1 phage-mediated general transduction was used to transfer antibiotic-marked DNA fragments among different strains of B. subtilis (12, 66). Long-flanking PCR mutagenesis was applied to generate insertional deletion mutations (67). The primers used to generate insertional deletion mutations of ywcBA, ywbHB, ysbAB, yxaKC, and ywbI genes and used to complement the Δpta and ΔackA mutations are described in Table S1 in the supplemental material. Details of strain constructions are described in Text S1.
The volatile assay.
In the 6-well polyvinyl plates (VWR), well a (shown in the top and side views in Fig. 3B) was used to preinoculate 106 cells of wild-type or mutant B. subtilis per ml on day 1 to allow growth. On day 2, a distant well b was freshly inoculated with 106 cells of wild-type B. subtilis per ml for pellicle development. Meanwhile, well a had already started to show robust cell growth and volatile production. Four glass beads (3 mm in diameter) were added between the lid and the bottom part of the plate (one at each corner of the plate) with the purpose of creating a headspace shared among the wells. The plate was then sealed with Parafilm (Bemis, WI) and incubated at 30°C for another day. On day 3, the plate was unsealed and pellicle formation of the wild-type B. subtilis cells in well b was recorded. For addition of the pure acids, they were directly diluted into appropriate media at a final concentration of 1% (vol/vol). In some experiments, when the defined MSgg medium was used to inoculate volatile-producing cells into well a, plates were incubated at 30°C for 2 days (instead of being incubated for 1 day as when LBGM medium, LB, or 2× SGG was used) before wild-type B. subtilis cells were inoculated into well b with the purpose of allowing robust growth and strong volatile production by cells in well a since B. subtilis tends to grow slower in MSgg. In some experiments (as shown in Fig. 3A), a slightly different assay was performed to assess the influence of volatiles from neighboring cells on pellicle formation. The 12-well polyvinyl plates (VWR) were used to inoculate B. subtilis cells into 1 or 4 or 12 wells in the plate, while the rest of the wells were filled with medium only. The plates were sealed and incubated at 30°C for 1 to 2 days. Images of the pellicle biofilms (in the corner well) were similarly taken.
Assays of pellicle formation.
Cells were grown in LB with shaking at 37°C to mid-log phase. For pellicle formation, 9 µl of the cells was mixed with 9 ml of LBGM broth and the mixture was added to the 6-well plates (VWR). Plates were incubated at 30°C for an indicated period of time. Images were taken using a Nikon CoolPix S9200 digital camera.
Biofilm assays for S. aureus.
Details of the biofilm assay for S. aureus HG003 are provided in Text S1 in the supplemental material.
Assays of β-galactosidase activities.
Details for the assay of the β-galactosidase activities are provided in Text S1 in the supplemental material.
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods. Download
Strains and plasmids used in this study.
Volatiles stimulate strong induction of matrix genes and higher biomass of the biofilms. Download
Bacterial volatiles induce early biofilm formation in B. subtilis. Download
Complementation of the delayed biofilm phenotype of the Δpta and ΔackA mutants. Download
Addition of acetate, but not addition of lactate or pyruvate, rescued the delayed biofilm phenotype of the Δpta and ΔackA mutants. Download
YwcB-YwcA may function as a putative acetic acid transporter. Download
Quantification by qPCR of eDNA release during pellicle biofilm formation by the WT (3610) and the mutant with triple holin-antiholin gene mutations (CY886). Download
ACKNOWLEDGMENTS
This work was supported by a startup grant from Northeastern University to Y. Chai. Y. Chen was supported by a grant from the National Natural Science Foundation of China (31301707) and by the Fundamental Research Funds for the Central Universities (2014QNA6018).
We thank Hussein Antar for participating in some of the work and Paul Muller for helpful discussions.
Footnotes
Citation Chen Y, Gozzi K, Yan F, Chai Y. 2015. Acetic acid acts as a volatile signal to stimulate bacterial biofilm formation. mBio 6(3):e00392-15. doi:10.1128/mBio.00392-15.
REFERENCES
- 1.Kolter R, Greenberg EP. 2006. Microbial sciences: the superficial life of microbes. Nature 441:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 2.O’Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu Rev Microbiol 54:49–80. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar C, Vlamakis H, Losick R, Kolter R. 2007. Thinking about Bacillus subtilis as a multicellular organism. Curr Opin Microbiol 10:638–643. doi: 10.1016/j.mib.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. 2013. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH. 2013. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864. doi: 10.1111/j.1462-2920.2012.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo JH, Losick R. 2012. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol 85:418–430. doi: 10.1111/j.1365-2958.2012.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bais HP, Fall R, Vivanco JM. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. 2013. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A 110:E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 12.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. 2005. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol 55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 13.Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A 107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobley L, Ostrowski A, Rao FV, Bromley KM, Porter M, Prescott AR, MacPhee CE, van Aalten DM, Stanley-Wall NR. 2013. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc Natl Acad Sci U S A 110:13600–13605. doi: 10.1073/pnas.1306390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi K, Iwano M. 2012. BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol Microbiol 85:51–66. doi: 10.1111/j.1365-2958.2012.08094.x. [DOI] [PubMed] [Google Scholar]
- 16.Kovács AT, van Gestel J, Kuipers OP. 2012. The protective layer of biofilm: a repellent function for a new class of amphiphilic proteins. Mol Microbiol 85:8–11. doi: 10.1111/j.1365-2958.2012.08101.x. [DOI] [PubMed] [Google Scholar]
- 17.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol 59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RJ, Brannigan JA, Smith I, Wilkinson AJ. 1996. Crystallisation of the Bacillus subtilis sporulation inhibitor SinR, complexed with its antagonist, Sinl. FEBS Lett 378:98–100. doi: 10.1016/0014-5793(95)01432-2. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, González-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai Y, Norman T, Kolter R, Losick R. 2011. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. EMBO J 30:1402–1413. doi: 10.1038/emboj.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang M, Shao W, Perego M, Hoch JA. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol 38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 22.McLoon AL, Kolodkin-Gal I, Rubinstein SM, Kolter R, Losick R. 2011. Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis. J Bacteriol 193:679–685. doi: 10.1128/JB.01186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai Y, Kolter R, Losick R. 2009. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol Microbiol 74:876–887. doi: 10.1111/j.1365-2958.2009.06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K. 2008. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol Microbiol 69:1399–1410. doi: 10.1111/j.1365-2958.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- 25.Shank EA, Kolter R. 2011. Extracellular signaling and multicellularity in Bacillus subtilis. Curr Opin Microbiol 14:741–747. doi: 10.1016/j.mib.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López D, Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev 34:134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolodkin-Gal I, Elsholz AK, Muth C, Girguis PR, Kolter R, Losick R. 2013. Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev 27:887–899. doi: 10.1101/gad.215244.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shank EA, Klepac-Ceraj V, Collado-Torres L, Powers GE, Losick R, Kolter R. 2011. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc Natl Acad Sci U S A 108:E1236–E1243. doi: 10.1073/pnas.1103630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López D, Fischbach MA, Chu F, Losick R, Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A 106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shemesh M, Chai Y. 2013. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J Bacteriol 195:2747–2754. doi: 10.1128/JB.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shemesh M, Kolter R, Losick R. 2010. The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J Bacteriol 192:6352–6356. doi: 10.1128/JB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chávez de Paz LE, Lemos JA, Wickström C, Sedgley CM. 2012. Role of (p)ppGpp in biofilm formation by Enterococcus faecalis. Appl Environ Microbiol 78:1627–1630. doi: 10.1128/AEM.07036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugisaki K, Hanawa T, Yonezawa H, Osaki T, Fukutomi T, Kawakami H, Yamamoto T, Kamiya S. 2013. Role of (p)ppGpp in biofilm formation and expression of filamentous structures in Bordetella pertussis. Microbiology 159:1379–1389. doi: 10.1099/mic.0.066597-0. [DOI] [PubMed] [Google Scholar]
- 34.He H, Cooper JN, Mishra A, Raskin DM. 2012. Stringent response regulation of biofilm formation in Vibrio cholerae. J Bacteriol 194:2962–2972. doi: 10.1128/JB.00014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomelsky M. 2011. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol 79:562–565. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramaniam AR, DeLoughery A, Bradshaw N, Chen Y, O’Shea E, Losick R, Chai Y. 2013. A serine sensor for multicellularity in a bacterium. Elife 2:e01501. doi: 10.7554/eLife.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol Rev 56:430–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice KC, Firek BA, Nelson JB, Yang S-J, Patton TG, Bayles KW. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J Bacteriol 185:2635–2643. doi: 10.1128/JB.185.8.2635-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groicher KH, Firek BA, Fujimoto DF, Bayles KW. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J Bacteriol 182:1794–1801. doi: 10.1128/JB.182.7.1794-1801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma-Kuinkel BK, Mann EE, Ahn J-S, Kuechenmeister LJ, Dunman PM, Bayles KW. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J Bacteriol 191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S-J, Rice KC, Brown RJ, Patton TG, Liou LE, Park YH, Bayles KW. 2005. A LysR-type regulator, CidR, is required for induction of the Staphylococcus aureus cidABC operon. J Bacteriol 187:5893–5900. doi: 10.1128/JB.187.17.5893-5900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandramohan L, Ahn J-S, Weaver KE, Bayles KW. 2009. An overlap between the control of programmed cell death in Bacillus anthracis and sporulation. J Bacteriol 191:4103–4110. doi: 10.1128/JB.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW. 2003. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L. 2011. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058. doi: 10.1111/j.1462-2920.2011.02582.x. [DOI] [PubMed] [Google Scholar]
- 46.Létoffé S, Audrain B, Bernier SP, Delepierre M, Ghigo J-M. 2014. Aerial exposure to the bacterial volatile compound trimethylamine modifies antibiotic resistance of physically separated bacteria by raising culture medium pH. mBio 5:e00944-13. doi: 10.1128/mBio.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu Q, Huang X, Zhang L, Xu J, Yang D, Wei K, Niu X, An Z, Bennett JW, Zou C, Yang J, Zhang K-. 2010. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc Natl Acad Sci 107:16631–16636. doi: 10.1073/pnas.1007276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz Ramos H, Hoffmann T, Marino M, Nedjari H, Presecan-Siedel E, Dreesen O, Glaser P, Jahn D. 2000. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J Bacteriol 182:3072–3080. doi: 10.1128/JB.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol 181:6889–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorquet F, Goffin P, Muscariello L, Baudry J-B, Ladero V, Sacco M, Kleerebezem M, Hols P. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J Bacteriol 186:3749–3759. doi: 10.1128/JB.186.12.3749-3759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas VC, Sadykov MR, Chaudhari SS, Jones J, Endres JL, Widhelm TJ, Ahn J-S, Jawa RS, Zimmerman MC, Bayles KW. 2014. A central role for carbon-overflow pathways in the modulation of bacterial cell death. PLoS Pathog 10:e1004205. doi: 10.1371/journal.ppat.1004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gimenez R, Nuñez MF, Badia J, Aguilar J, Baldoma L. 2003. The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J Bacteriol 185:6448–6455. doi: 10.1128/JB.185.21.6448-6455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sá-Pessoa J, Paiva S, Ribas D, Silva IJ, Viegas SC, Arraiano CM, Casal M. 2013. SATP (YaaH), a succinate-acetate transporter protein in Escherichia coli. Biochem J 454:585–595. doi: 10.1042/BJ20130412. [DOI] [PubMed] [Google Scholar]
- 54.Jung H. 2001. Towards the molecular mechanism of Na+/solute symport in prokaryotes. Biochim Biophys Acta 1505:131–143. doi: 10.1016/S0005-2728(00)00283-8. [DOI] [PubMed] [Google Scholar]
- 55.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foulston L, Elsholz AK, DeFrancesco AS, Losick R. 2014. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 5:e01667-14. doi: 10.1128/mBio.01667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schellhorn HE, Stones VL. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol 174:4769–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukhopadhyay S, Schellhorn HE. 1994. Induction of Escherichia coli hydroperoxidase I by acetate and other weak acids. J Bacteriol 176:2300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ. 2007. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol 189:5574–5581. doi: 10.1128/JB.00564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinert BT, Iesmantavicius V, Wagner SA, Schölz C, Gummesson B, Beli P, Nyström T, Choudhary C. 2013. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell 51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gherardini F, Wolfe AJ, Yang XF. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog 6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehman MK, Bose JL, Sharma-Kuinkel BK, Moormeier DE, Endres JL, Sadykov MR, Biswas I, Bayles KW. 2015. Identification of the amino acids essential for LytSR-mediated signal transduction in Staphylococcus aureus and their roles in biofilm-specific gene expression. Mol Microbiol 95:723–737. doi: 10.1111/mmi.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SB, Shin BS, Choi SK, Kim CK, Park SH. 2001. Involvement of acetyl phosphate in the in vivo activation of the response regulator ComA in Bacillus subtilis. FEMS Microbiol Lett 195:179–183. doi: 10.1111/j.1574-6968.2001.tb10518.x. [DOI] [PubMed] [Google Scholar]
- 65.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 66.Yasbin RE, Young FE. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol 14:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in Saccharomyces cerevisiae. Yeast 12:259–265. doi:. [DOI] [PubMed] [Google Scholar]
- 68.Barbe V, Cruveiller S, Kunst F, Lenoble P, Meurice G, Sekowska A, Vallenet D, Wang T, Moszer I, Médigue C, Danchin A. 2009. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155:1758–1775. doi: 10.1099/mic.0.027839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Download
Strains and plasmids used in this study.
Volatiles stimulate strong induction of matrix genes and higher biomass of the biofilms. Download
Bacterial volatiles induce early biofilm formation in B. subtilis. Download
Complementation of the delayed biofilm phenotype of the Δpta and ΔackA mutants. Download
Addition of acetate, but not addition of lactate or pyruvate, rescued the delayed biofilm phenotype of the Δpta and ΔackA mutants. Download
YwcB-YwcA may function as a putative acetic acid transporter. Download
Quantification by qPCR of eDNA release during pellicle biofilm formation by the WT (3610) and the mutant with triple holin-antiholin gene mutations (CY886). Download