ABSTRACT
Burkholderia cenocepacia causes opportunistic infections in plants, insects, animals, and humans, suggesting that “virulence” depends on the host and its innate susceptibility to infection. We hypothesized that modifications in key bacterial molecules recognized by the innate immune system modulate host responses to B. cenocepacia. Indeed, modification of lipopolysaccharide (LPS) with 4-amino-4-deoxy-l-arabinose and flagellin glycosylation attenuates B. cenocepacia infection in Arabidopsis thaliana and Galleria mellonella insect larvae. However, B. cenocepacia LPS and flagellin triggered rapid bursts of nitric oxide and reactive oxygen species in A. thaliana leading to activation of the PR-1 defense gene. These responses were drastically reduced in plants with fls2 (flagellin FLS2 host receptor kinase), Atnoa1 (nitric oxide-associated protein 1), and dnd1-1 (reduced production of nitric oxide) null mutations. Together, our results indicate that LPS modification and flagellin glycosylation do not affect recognition by plant receptors but are required for bacteria to establish overt infection.
IMPORTANCE
Virulence and pathogenicity are properties ascribed to microbes, which actually require careful consideration of the host. Using the term “pathogen” to define a microbe without considering its host has recently been debated, since the microbe’s capacity to establish a niche in a given host is a critical feature associated with infection. Opportunistic bacteria are a perfect example of microbes whose ability to cause disease is intimately related to the host’s ability to recognize and respond to the infection. Here, we use the opportunistic bacterium Burkholderia cenocepacia and the host plant Arabidopsis thaliana to investigate the role of bacterial surface molecules, namely, lipopolysaccharide and flagellin, in contributing to infection and also in eliciting a host response. We reveal that both molecules can be modified by glycosylation, and although the modifications are critical for the bacteria to establish an infection, they do not impact the host’s ability to recognize the pathogen.
INTRODUCTION
Burkholderia cepacia complex (BCC) denotes a group of ubiquitous Gram-negative bacterial species isolated from water, soil, plants, insects, industrial settings, and hospital environments (1). Some BCC strains have beneficial traits, as they utilize complex carbon sources to degrade toxic compounds in pesticides and herbicides, while others serve as bioremediation agents (2). However, BCC strains are opportunistic pathogens causing serious infections in immunocompromised humans, including chronic lung infection in cystic fibrosis (CF) patients (3). The most common BCC clinical isolates are B. cenocepacia and B. multivorans. In particular, B. cenocepacia has gained notoriety for its ability to cause lethal necrotizing pneumonia and its transmissibility among CF patients (3).
The opportunistic nature of infections by B. cenocepacia makes it difficult to study the pathogenic mechanisms of this bacterium, since “virulence” depends on the host’s context and its innate susceptibility to infection. Several infection models for B. cenocepacia have been established, which include mice and rats (4), the zebrafish embryo (5), the nematode Caenorhabditis elegans (6), Galleria mellonella moth larvae (7, 8), and plants like alfalfa (4) and duckweed (9). Plants have evolved sophisticated mechanisms to perceive and respond to nearly constant attacks from potential pathogens (10). These mechanisms involve passive protection, such as that provided by the waxy cuticular skin layer, and innate immune responses. Like the innate immune system in mammals and insects, the plant immune system also perceives and responds to various elicitor molecules, which are conserved microbial structures referred to as microbe- or pathogen-associated molecular patterns (PAMPs). These include bacterial flagellin, lipopolysaccharide (LPS), and lipoteichoic acid and fungal chitin and ergosterol (11). LPS consists of lipid A, embedded in the outer leaflet of the outer membrane, which is linked to core oligosaccharide and polymeric O antigen (12), while flagellin is the building block of the flagellar filament essential for bacterial motility. LPS and flagellin are typical PAMPs that stimulate plant cells and tissues to generate reactive oxygen species (ROS), alkalinization of the extracellular medium, callose deposition, and nitric oxide (NO) burst (13–15). These responses lead to programmed cell death localized to the tissue surrounding the infection site, which is known as the hypersensitive response (11, 16).
Arabidopsis thaliana is an established model organism for plant biology, but it is also a tool to understand molecular mechanisms of human diseases (17, 18), particularly those concerning the initial stages of infection (10, 19). Previous work by others reported a rapid burst of NO after treatment of A. thaliana cultured cells and leaves with B. cepacia lipid A preparations, which was associated with the transcriptional activation of defense-related genes (20). Further, treatment with B. cepacia purified lipid A and core oligosaccharide LPS components resulted in different plant transcriptional responses (21), suggesting that plant cells perceive LPS components differently. Lipid A structural modifications alter recognition and responses by the innate immune system in mammals (22) and in plants (23). LPS contributes to reduce the permeability of the outer membrane of Gram-negative bacteria, acting as a barrier for plant-derived antimicrobial substances (24). B. cenocepacia exhibits extraordinary intrinsic resistance to antimicrobial peptides and other antibiotics (25). We have shown that the B. cenocepacia lipid A and one of the sugars of the inner core oligosaccharide are modified by the incorporation of l-Ara4N, and this modification not only is essential for bacterial survival (26) but also is the major determinant of resistance to antimicrobial peptides (27). The essentiality of the LPS modification by l-Ara4N suggests the possibility that this amino sugar could also contribute to modulate innate immune responses.
Flagellin is a major activator of innate immunity in animals, where is recognized by the Toll-like receptor 5 (TLR5) (28), and in plants (29, 30). Recognition of flagellin by plants activates disease resistance mechanisms through an oxidative burst during early stages of infection (13, 15). The A. thaliana flagellin sensing 2 (FLS2) protein is a PAMP receptor kinase with an extracellular domain containing 28 leucine-rich repeats, a transmembrane domain, and an intracellular serine/threonine kinase domain (31, 32). These interactions activate production of ROS, triggering a complex defense response, including induction of pathogenesis-related genes (15). We have recently shown that the B. cenocepacia flagellin is glycosylated, and its glycosylation status modulates innate immune responses in mammalian cells (33), but the role of flagellin glycosylation in plant immune responses to B. cenocepacia infection has not been examined.
In this work, we investigated the pathogenicity of B. cenocepacia in A. thaliana and evaluated the significance of LPS modification with l-Ara4N and flagellin glycosylation in virulence and in Arabidopsis-B. cenocepacia interactions by studying innate immune responses by wild-type and mutant plants. Further, we compared bacterial virulence in planta and in the G. mellonella infection model to clarify the global role of flagellin glycosylation and LPS modification on pathogenesis. We demonstrate that flagellin glycosylation and LPS modification with l-Ara4N play a significant role in bacterial survival upon infection but do not alter the perception of these molecules by the plant innate immune receptors, indicating these modifications are only critical to establish infection.
RESULTS
Pathogenicity of B. cenocepacia in A. thaliana and tobacco seedlings.
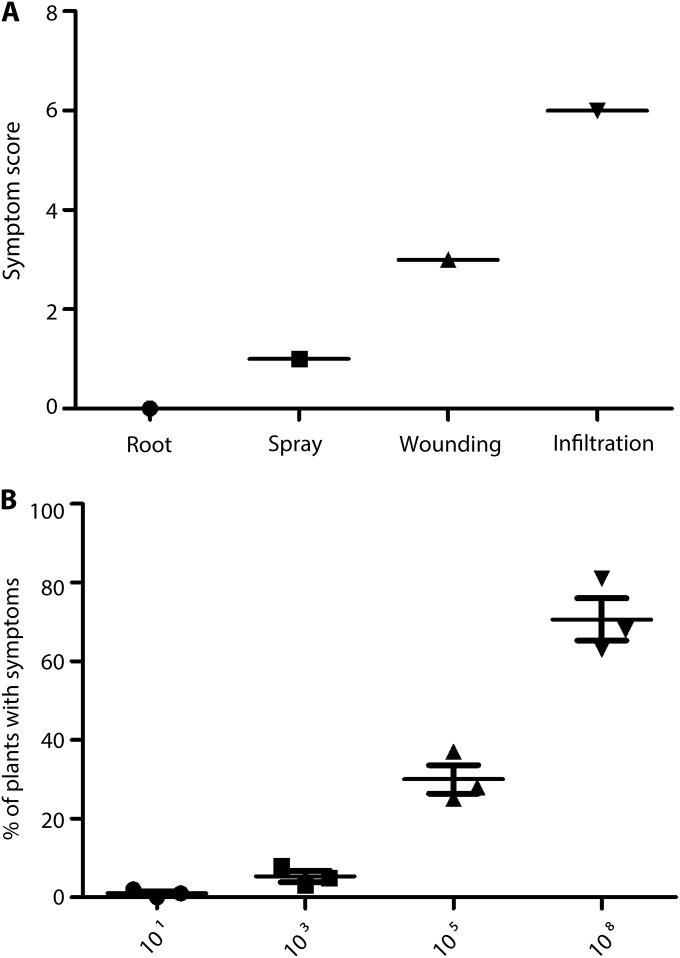
We investigated the ability of B. cenocepacia K56-2, a CF clinical isolate, to induce a hypersensitive response (HR) in A. thaliana and tobacco. Seedlings from both species were inoculated by different methods with 101 to 108 bacteria and examined for symptoms over 1 week. A simple infection model was developed in which leaves were wounded with a needle and then inoculated with dilute suspensions of B. cenocepacia. Disease was scored for every seedling on an index of 1 to 6 based on the extent of HR symptoms and tissue necrosis using a standard HR scoring system (34). Depending on the bacterial dose, infection of seedlings via wound inoculation or syringe infiltration led to necrosis (brown areas on leaves at higher doses) or chlorosis (yellowing of the leaf tissue), which were clearly visible after day 4 postinfection. In contrast, infections by soil drainage or surface spray gave few symptoms even at day 7 postinfection (Fig. 1A). There was a direct correlation between inoculum size and HR symptoms (Fig. 1B). Similar results were observed in tobacco plants, where infection via roots or leaf spraying did not cause distinguishable symptoms in plantlets (see Fig. S1 in the supplemental material). Since several mutants in innate immunity pathways and pathogen receptors are available for A. thaliana ecotype Colombia Col-0, we used only seedlings from this species for the remainder of this study, which were inoculated with 108 CFU via wound inoculation or syringe infiltration.
FIG 1 .
HR in A. thaliana leaves using various inoculation methods. (A) Quantitative scoring of HR in leaves of wild-type Arabidopsis inoculated with K56-2 by different inoculation methods. Symptom scores were assigned using an index from 1 to 6 based on the extent of tissue collapse and color change, where 0 is no symptom, 1 is random yellow or dark spots, 2 is chlorosis over the inoculation site, 3 is chlorosis and mild tissue collapse at and around the inoculation site, 4 is more than 50% collapse at and around the inoculation site, 5 is 100% collapse at the inoculation site, and 6 is 100% collapse beyond the inoculation site, as observed in day 7 postinfection. The average score was calculated based on a minimum of 45 leaves per treatment cumulative from 3 individual repeats. (B) Effect of inoculum size on the incidence of infection. Values indicate the percentage ± standard error (SE) of seedlings showing disease symptoms at 7 days postinfection after inoculation with doses ranging from 101 to 108 CFU; 15 plants per infection dose were tested. Data represent the average from three experiments.
l-Ara4N modification of LPS and flagellin glycosylation are required to cause pathology in A. thaliana.
B. cenocepacia requires l-Ara4N modification of LPS for viability and resistance to antimicrobial peptides (27). However, a suppressor mutation in the lptG gene, which encodes an essential protein for the export of LPS to the outer membrane, restores viability in the absence of l-Ara4N LPS modification (27). We therefore investigated if lack of l-Ara4N in the LPS of this mutant (MH55; ΔarnT lptGD31H) (Table 1) could affect B. cenocepacia survival in A. thaliana. Infection of A. thaliana seedlings with the ΔarnT lptGD31H mutant caused mild symptoms of chlorosis in 27% of inoculated plants (Fig. 2A and C); very few bacteria were recovered from leaves, compared to plants infected with the K56-2 parental strain (Fig. 2B). These data demonstrate that l-Ara4N modification of LPS is critical for B. cenocepacia pathogenicity. The poor infectivity of the suppressor mutant is most likely due to the lack of l-Ara4N in the LPS of the ΔarnT lptGD31H mutant, as revealed by mass spectrometry of purified lipid A (see Fig. S2 in the supplemental material), which makes it extremely sensitive to naturally produced antimicrobial peptides in plants, as we previously demonstrated with polymyxin B (27).
TABLE 1 .
Bacterial strains used in this study
| Strain | Characteristic(s) | Source or reference |
|---|---|---|
| K56-2 | B. cenocepacia ET12 clone related to J2315, CF clinical isolate | BCRRCa |
| K56-2 ΔatsR | K56-2 carrying an unmarked deletion of atsR gene | 37 |
| MH55 | K56-2, ΔarnT-arnBC+ lptGD31H (lptGS) | 27 |
| ΔBCAL0111 mutant | K56-2, unmarked deletion of flmQ (BCAL0111) | 33 |
| MSS25 | K56-2, fliC::pSM62, insertional inactivation of fliC | 35 |
BCRRC, B. cepacia Research and Referral Repository for Canadian CF Clinics.
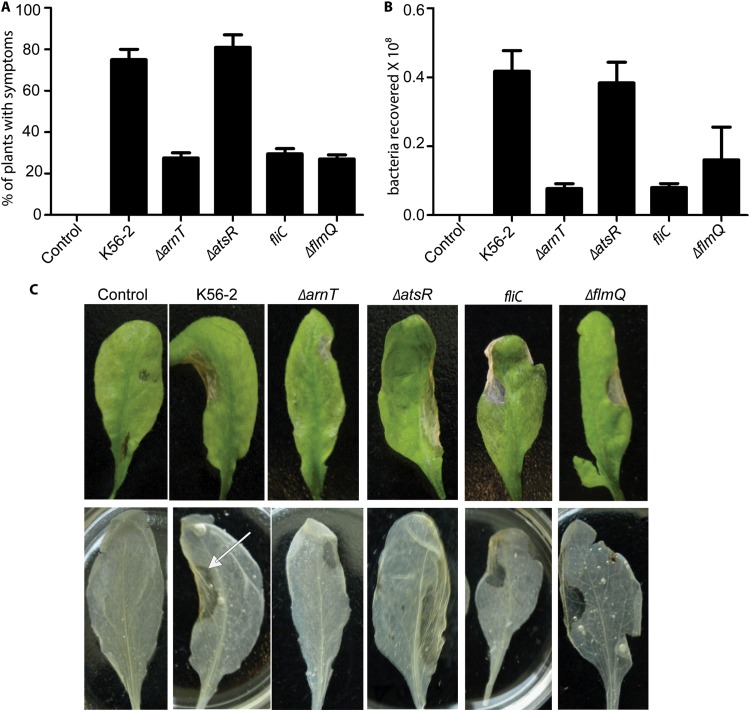
FIG 2 .
Infection and replication of B. cenocepacia strains in Arabidopsis seedlings. (A) Values show the percentages ± SE of the seedlings showing disease symptoms visible on day 7 postinfection after wound inoculation with 108 CFU of each B. cenocepacia strain; 15 plants per infection dose were tested. Data are the average from three repeats. (B) The graph represents bacterial counts on day 7 postinfection from Arabidopsis leaves infected with B. cenocepacia by wound inoculation. (C) Photographs of representative leaves (from three replicate treatments) from Arabidopsis plants inoculated with B. cenocepacia strains by infiltration (upper row). Leaves were removed from plants and bleached in ethanol (lower row). An arrow indicates the inoculated region, which is more transparent and flattened, undergoing tissue collapse, compared with the rest of the leaf and the wild-type control treated with buffer. Similar results were observed with wound-inoculated leaves (see Fig. S4 in the supplemental material).
Next, we evaluated the significance of flagellin in A. thaliana pathogenicity. For these experiments, we used an fliC mutant that is nonmotile and unable to make flagellin (35) and the hypermotile, flagellated ΔatsR mutant (Table 1). The atsR gene encodes a global regulatory repressor protein that controls motility and the expression of various virulence factors (36–38). There were no statistically significant differences in disease symptoms between plants infected with the ΔatsR mutant and K56-2 at day 7 postinfection (Fig. 2). However, symptoms appeared 2 days earlier in ΔatsR strain-infected plants, and these results were reproducible in each experimental replica. These observations agree with the current model for the regulatory role of AtsR (37, 38) indicating that removal of inhibitory regulation on motility as well as other AtsR-controlled factors increases bacterial pathogenicity. Therefore, it is not surprising that the ΔatsR mutant provoked lesions similar to those found with the parental strain K56-2 (Fig. 2C). In contrast, loss of flagellin in the fliC mutant resulted in a strain that induced only mild HR symptoms in less than 30% of plants infected by wound inoculation (Fig. 2A), revealing the critical role of flagella in virulence and also suggesting that flagellar driven motility is important for infection. The wound inoculation method mimics entry of microbes into host plants through natural wounds, which results in bacterial spreading to intercellular spaces. In contrast, infiltration of bacteria with a syringe into the apoplast could bypass the first steps of the natural infection process and, therefore, the requirement for bacterial motility. To probe this notion, we infected Arabidopsis seedlings by syringe infiltration into leaf abaxial surfaces. Under these conditions, the fliC mutant elicited similar HR symptoms to those induced by parental K56-2 (Fig. 2C). HR also correlated with higher numbers of bacteria recovered from leaves, a difference that was particularly pronounced compared with wound inoculation (see Fig. S3 in the supplemental material).
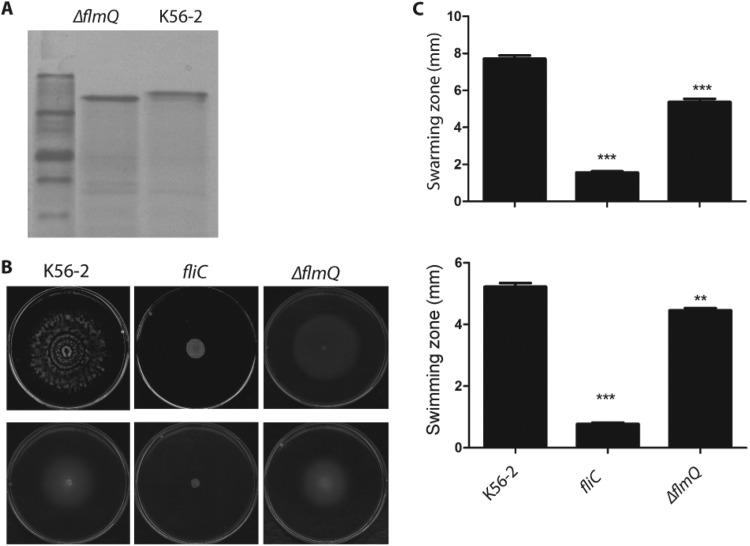
Since posttranslational modification of flagellin by glycosylation affects the virulence of Pseudomonas syringae in tobacco plants (39, 40), we compared the abilities of the parental and ΔflmQ (Δbcal0111) B. cenocepacia strains (Fig. 2A) to cause disease in A. thaliana. The flmQ gene encodes a predicted flagellin glycosyltransferase in B. cenocepacia (33). In comparison to the parental strain, the ΔflmQ mutant was less pathogenic for A. thaliana (Fig. 2A) and exhibited reduced bacterial survival (Fig. 2B). Further, the ΔflmQ mutant caused significantly less pathology on the leaves, with less than 30% of plants having disease symptoms, although slight chlorosis was observable (Fig. 2C), suggesting that nonglycosylated flagellin retains its elicitor activity. The reduced virulence of the ΔflmQ mutant on A. thaliana leaves also correlated with bacterial defects in swarming and swimming (Fig. 3B and C), consistent with our previous report indicating that glycosylation affects bacterial motility (33). Together, these experiments demonstrate that both l-Ara4N modification of LPS and flagellin glycosylation are important for infectivity in A. thaliana.
FIG 3 .
Characterization of flagellin mutants. (A) SDS-PAGE analysis of purified flagellin from the WT and K56-2 ΔflmQ mutant strain of B. cenocepacia. (B) Swarming (upper row) and swimming (lower row) motility on soft agar plates of B. cenocepacia strains. The data represent three independent experiments. (C) Quantification of the motility zone. Statistical analysis was performed by paired t test using two-tailed P values. Significant differences in comparison with B. cenocepacia parental strain (WT) as the control are indicated by *** (P < 0.005) and ** (P < 0.027).
LPS induces robust and rapid nitric oxide burst in A. thaliana.
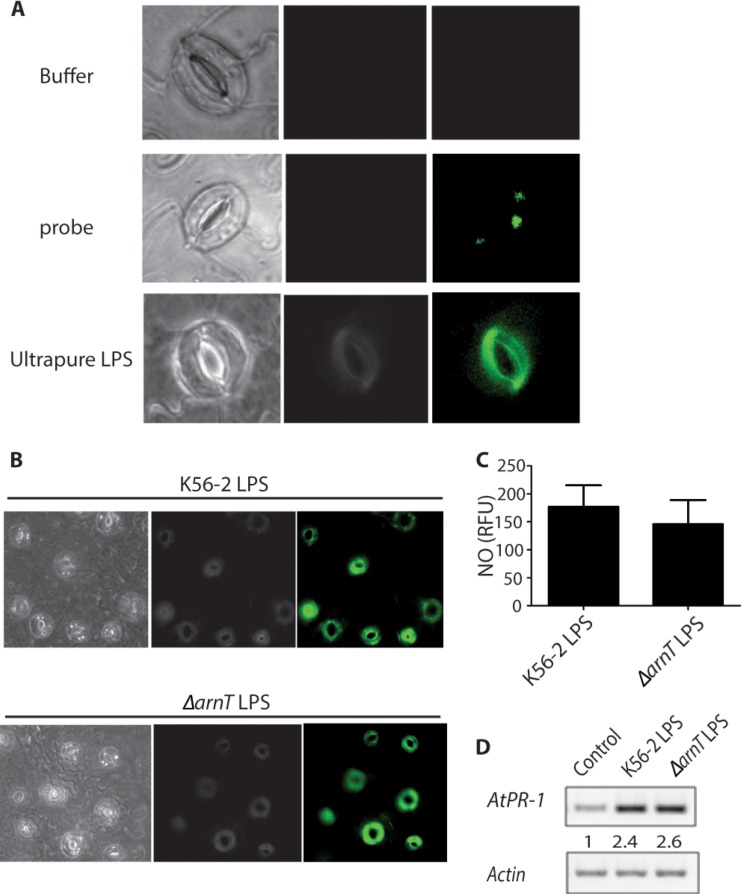
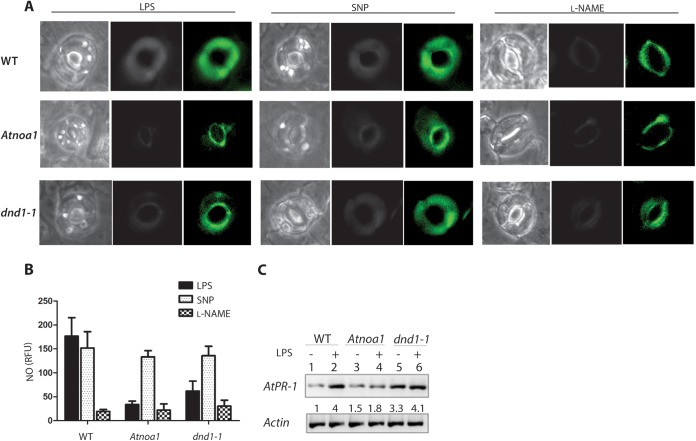
The reduced pathogenicity of the ΔarnT lptGD31H mutant could be due to reduced bacterial survival during the initial stages of infection or, alternatively, poor recognition of LPS devoid of l-Ara4N by the plant innate immune system. We therefore investigated the levels of NO production in A. thaliana guard cells upon exposure to B. cenocepacia LPS from mutant and parental sources. Guard cells are specialized epidermal cells in the leaf epidermis surrounding stomatal pores that come into close contact with bacterial pathogens. LPS typically induces NO generation in A. thaliana (20, 34). An NO burst was detected in vivo using the NO-specific fluorescent dye diaminofluorescein diacetate (DAF-2DA). Treatment of guard cells with the ultrapure Escherichia coli LPS control resulted in a rapid burst of green fluorescence, indicative of NO production (Fig. 4A). Robust LPS induction of NO in intact guard cells could be reproduced using phenol-extracted LPS from both wild-type (WT) K56-2 and a mutant strain lacking l-Ara4N (Fig. 4B). Quantification of the NO burst using a spectrofluorometric assay that detects NO accumulation indicated that LPS devoid of l-Ara4N has similar levels of elicitor activity, suggesting that LPS modification does not affect the innate immune response in Arabidopsis in vivo. Further, we compared LPS-induced NO production in guard cells isolated from parental A. thaliana and the Atnoa1 and dnd1 mutants. Atnoa1 is a loss-of-function mutant in nitric oxide-associated protein 1 (NOA1), which is required for arginine-dependent NO generation and involved in signal cascades responding to PAMPs (20, 41). The dnd1-1 (defense no death 1) mutant has a null mutation in the CNGC2/DND1 gene and displays reduced NO generation (34, 42). Minimal responses were detected in Atnoa1 and dnd1 guard cells compared to wild-type cells (Fig. 5A, left panel). LPS-induced NO production was reduced dramatically in cells treated with the NOS inhibitor NG-nitro-l-Arg-methyl ester (l-NAME) (Fig. 5A, right panel). Similarly, LPS-dependent NO generation was not observed in dnd1-1 or Atnoa1 mutants after treatment with l-NAME, while incubation with the NO donor sodium nitroprusside (SNP) resulted in green fluorescence in the parental A. thaliana strain and both mutants. Therefore, the reduced NO burst in Atnoa1 and dnd1-1 cells was due to a defect in NO production (Fig. 5A, middle panel). NO was quantified in LPS-treated leaves of parental and mutant plants using a fluorometric assay. LPS treatment yielded 180 relative fluorescence units (RFU) in parental leaves, compared with 78% and 66% reductions in RFU observed for the Atnoa1 and dnd1-1 mutants, respectively (Fig. 5B). LPS-induced NO production in parental leaves was repressed by 6-fold upon treatment with the NO synthase inhibitor l-NAME (Fig. 5B). In contrast, parental and mutant leaves showed similar RFU in the presence of SNP. Together, these results demonstrate that B. cenocepacia LPS triggers a robust NO burst in Arabidopsis leaves, and both intact LPS and LPS lacking l-Ara4N can be equally recognized by the plant innate immune system.
FIG 4 .
Role of l-Ara4N in NO generation in Arabidopsis guard cells. (A) Leaf epidermal peels prepared from parental (WT) plants were loaded with the buffer control (top panel) or with the NO-sensitive dye DAF-2DA (middle panel) prior to incubation with 100 µg/ml ultrapure LPS from E. coli (bottom panel). (B) Leaf epidermal peels prepared from WT plants were treated with LPS isolated from the K56-2 or K56-2 ΔarnT strains. This experiment was repeated a total of two times. In each experiment, a minimum of three epidermal peels were used as treatment replicates. (C) Fluorometric quantification of NO generated in WT Arabidopsis leaves. (D) Induction of PR-1 gene expression in Arabidopsis leaves by LPS. Arabidopsis leaves were treated with buffer (lane 1), K56-2 LPS (lane 2), or LPS isolated from the K56-2 ΔarnT strain (lane 3) for 24 h followed by RNA preparation. The actin gene was used as an internal control for RT-PCR. The numbers shown represent relative levels of PR-1 being normalized relative to the control by densitometry.
FIG 5 .
LPS activates NO production in WT and Atnoa1 and dnd1-1 mutant guard cells. (A) Leaf epidermal peels prepared from WT (top panels) or Atnoa1 (middle panels) or dnd1-1 (bottom panels) mutant plants were loaded with 5 µM DAF-2DA prior to incubation in reaction buffer alone (buffer control), 100 µg/ml LPS (left panel), 50 µM SNP (middle panel), or 200 µM l-NAME (right panel). In each case, corresponding fluorescence and bright-field images are shown; the area of the peel subjected to analysis was greater than that shown in each case. This experiment was repeated three times using at least three epidermal peels per experiment. (B) Fluorometric quantification of NO generated in leaves of WT and Atnoa1 and dnd1-1 mutant Arabidopsis seedlings. (C) Induction of PR-1 gene expression in Arabidopsis leaves by LPS. Arabidopsis leaves were treated with buffer (lanes 1, 3, and 5) or LPS (lanes 2, 4, and 6) for 24 h followed by RNA preparation. RT-PCR was performed with cDNAs for the PR-1 and actin genes. The numbers between panels represent levels of PR-1 being normalized relative to the control by densitometry.
Flagellin perception in A. thaliana occurs irrespective of the flagellin glycosylation status.
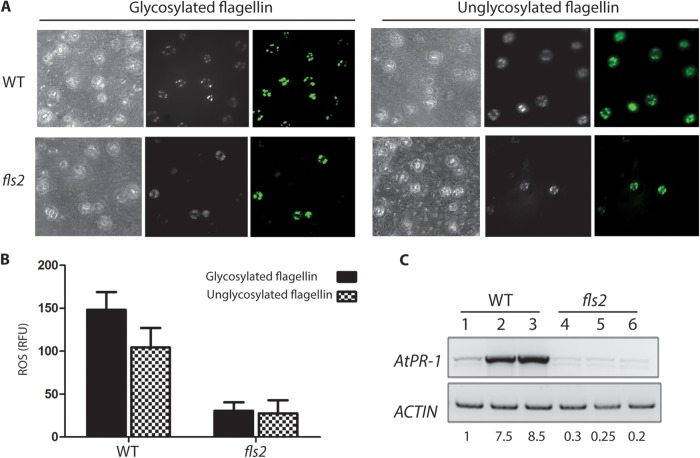
We also investigated if the glycosylation status of the B. cenocepacia flagellin alters the immunity response of A. thaliana, which involves a rapid and transient burst of ROS that is subsequently associated with programmed cell death (43). Treatment of plants with B. cenocepacia flagellin triggered ROS generation in parental A. thaliana leaves but not in leaves from the FLS2 mutant, which lacks the flagellin receptor (Fig. 6A). This result agrees with previous reports in Arabidopsis and tomato plants demonstrating the importance of flagellin perception for ROS production and disease resistance (13, 15). Fluorometric quantification of ROS in parental and fls2 mutant leaves showed a 3-fold reduction, indicating that flagellin recognition was necessary for eliciting ROS production (Fig. 6B). However, no significant differences were detected in ROS production by leaves treated with nonglycosylated flagellin, despite the fact that infection with bacteria lacking glycosylated flagellin produces fewer symptoms of infection (Fig. 2C). This result suggests that reduced pathogenicity of A. thaliana exposed to the B. cenocepacia ΔflmQ mutant is due to reduced bacterial motility rather than differential perception of glycosylated versus nonglycosylated flagellin by the FLS2 receptor.
FIG 6 .
Flagellin activates ROS generation in guard cells of WT and fls2 mutant leaves. (A) Leaf epidermal peels prepared from WT (top panels) or fls2 (bottom panels) plants were loaded with the ROS-sensitive dye DCF prior to incubation with glycosylated (left panel) or unglycosylated flagellin (right panel) from K56-2. In each case, corresponding fluorescence and bright-field images are shown; images are representative of three individual experiments. In each experiment, at least three epidermal peels were used as treatment replicates. (B) Fluorometric quantification of ROS generated in leaves of WT and fls2 mutant Arabidopsis seedlings. (C) Induction of PR-1 gene expression in Arabidopsis leaves by flagellin. Arabidopsis leaves were treated with buffer (lanes 1 and 4), WT (lanes 2 and 5), or unglycosylated flagellin (lanes 3 and 6) for 24 h followed by RNA preparation. RT-PCR was performed with cDNAs for the PR-1 and actin genes. The numbers at the bottom represent the levels of PR-1 being normalized relative to the control by densitometry.
Induction of pathogenesis-related gene 1 (PR-1) by LPS and flagellin.
Host defense responses in A. thaliana depend on ROS and NO production and induction of host defense or stress-associated genes, such as genes encoding pathogenesis-related proteins (20). We investigated PR-1 gene expression by reverse transcription-PCR (RT-PCR), since the expression of this gene is considered a hallmark of defense response in plants (20, 21). PR-1 expression was significantly increased in parental A. thaliana leaves 24 h after treatment with either intact LPS or LPS devoid of l-Ara4N LPS (Fig. 4D). In contrast, PR-1 mRNA accumulation was reduced in Atnoa1 mutant plants (Fig. 5C, lanes 3 and 4) suggesting a functional link between LPS-induced NO production and PR-1 expression. Similarly, PR-1 expression is activated by flagellin irrespective of its glycosylation status in parental A. thaliana but not in the flagellin-insensitive fls2 mutant (Fig. 6C). As expected, dnd1-1 mutant plants expressed high levels of PR-1 mRNA, which is due to the constitutively active salicylic acid signaling pathway in this mutant (44, 45). Together, our findings indicate that modified LPS and flagellin induce PR-1 transcription at levels similar to that of intact LPS and flagellin. This agrees with our results regarding induction of the oxidative burst, indicating that l-Ara4N modification of LPS and flagellin glycosylation are critical for infectivity in A. thaliana but do not alter the perception of these molecules by the plant innate immune receptors.
Comparison of Arabidopsis and Galleria infection models.
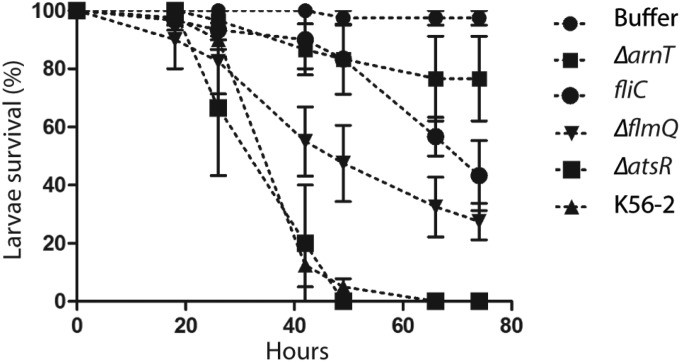
The previous results indicate that flagellin and LPS modifications alter the infectivity of B. cenocepacia in A. thaliana, but the plant can perceive these molecules irrespectively. Therefore, we tested the ability of B. cenocepacia strains utilized in the plant infection model for survival and pathogenicity in G. mellonella. The G. mellonella moth larvae have been increasingly used as an infection model for many bacterial pathogens, including several Burkholderia species (8, 46, 47). B. cenocepacia parental and mutant strains were injected into G. mellonella, and survival was monitored for over 72 h. As with A. thaliana, K56-2 ΔatsR exhibited high virulence denoted by no larvae surviving at 48 h postinjection with 104 CFU (Fig. 7), compared to the 5% ± 3% survival of larvae infected with the parental K56-2 strain. In contrast, the ΔarnT lptGD31H strain was greatly attenuated, as shown by larval survival rates of more than 80% at 48 h postinfection (Fig. 7). At infection doses of 104 CFU, similar attenuation levels were observed in the fliC mutant, while the ΔflmQ mutant showed an intermediate phenotype (50% ± 13.5% survival). At 72 h postinfection, the larval survival rates with the ΔarnT lptGD31H, fliC, and ΔflmQ strains were 77% ± 14%, 43% ± 12%, and 28% ± 7%, respectively. Control larvae injected with buffer remained viable until the end of the experiment. These results are comparable to the data obtained from the A. thaliana infections, confirming the significance of flagellin glycosylation and l-Ara4N modification of LPS in the infectivity of B. cenocepacia.
FIG 7 .
Virulence of B. cenocepacia strains in G. mellonella. Larvae were infected with 1 × 104 CFU of the indicated Burkholderia strains. Larval survival was monitored during 75 h postinfection. The data are means ± standard errors of the means (SEM). All bacteria grew at similar rates in Luria-Bertani medium (see Fig. S5 in the supplemental material).
DISCUSSION
Virulence and pathogenicity require considering both the host and the microbe; the use of these terms to define a microbe in isolation of its host has recently been debated (48, 49). Opportunistic bacteria are a classic example of microbes whose ability to cause disease is intimately related to the host’s ability to recognize and respond to the infection. However, the microbe’s capacity to establish a niche upon entering the host is a critical feature associated with infection. We show here that modifications in the LPS molecule and flagellin glycosylation do not affect recognition by plant innate immune receptors but are still required for bacteria to establish an infection. Our experiments using the B. cenocepacia K56-2–A. thaliana infection model reveal that plant seedlings sense LPS and flagellin as PAMPs and induce a burst of NO and ROS that contributes to basal resistance and eventually leads to a hypersensitive response against B. cenocepacia. Redox signaling has emerged as a main regulator of cellular function in plant pathophysiology (50). Pathogen recognition turns on a signaling cascade in which free radicals, such as NO and ROS, act as “infochemicals” for activation of various defense genes that mediate disease resistance in plants (14). Production of NO and ROS upon K56-2 LPS or flagellin treatment potentiated the expression of PR-1, which is a disease resistance marker. In contrast, PR-1 gene expression was reduced when Atnoa1 and fls2 mutant plants were treated with LPS or flagellin, indicating that LPS-responsive genes are NO inducible (20). In our work, we did not observe a distinguishable difference in the levels of expression of the PR-1 gene after treatment of leaves with intact or modified versions of LPS and flagellin.
Although we did not use purified lipid A in our study, these observations contrast with a previous report indicating that isolated lipid A of a nonpathogenic mutant of Xanthomonas campestris, consisting mainly of penta-acylated lipid A with phosphoethanolamine substitutions of its phosphate groups, was unable to elicit PR-1 expression (23). The authors concluded that the substitution of both lipid A phosphate groups with phosphoethanolamine would neutralize the net negative charge of the lipid A, which could affect binding to putative plant receptors (23). In contrast, the B. cenocepacia lipid A phosphate groups are substituted by l-Ara4N molecules, and disappearance of these residues in the ΔarnT lptGD31H mutant would expose negative charges, thus maintaining the ability of lipid A to activate PR-1 expression. Importantly, the lipid A of B. cenocepacia is tetra- and penta-acylated, but the acyl chains are longer than those of the X. campestris lipid A (51). Therefore, the fact that parental LPS and LPS devoid of l-Ara4N from B. cenocepacia induce similar levels of PR-1 gene expression suggests that the acyl chain composition may also play a role in LPS detection by plant innate immune receptors.
Irrespective of K56-2 LPS elicitor activity, B. cenocepacia mutants unable to produce l-Ara4N-modified LPS have extremely high sensitivity to polymyxin B (27), suggesting that they would also be highly sensitive to plant antimicrobial peptides. Further, we show that posttranslational modification of flagellin by glycosylation affects bacterial motility and virulence in Arabidopsis but does not significantly alter the elicitor activity of the protein. A correlation between defects in flagellin glycosylation in Pseudomonas syringae and reduced ability to induce HR in plants has been reported (39, 40), but the molecular mechanism is unknown. Our experiments indicate that reduced virulence but increased activation of plant defenses by the ΔflmQ mutant producing nonglycosylated flagellin is due to bacterial motility defects that prevent bacteria to establish infection. This could be clearly demonstrated by showing that the attenuation of the K56-2 fliC mutant strain is bypassed if bacteria are directly infiltrated into the plant’s deeper tissues. A role for flagellar motility was also reported for several plant pathogens, such as Erwinia carotovora (52), Ralstonia solanacearum (53), and several pathovars of P. syringae and Xanthomonas campestris (54–56).
In conclusion, we demonstrate that flagellin glycosylation and LPS modification with l-Ara4N play a significant role in bacterial survival during the early stages upon infection but do not alter the perception of these molecules by the plant innate immune receptors, indicating these modifications are only critical to establish infection. Our experiments therefore illustrate the notion that the microbe’s perception by the host and establishment of infection are interrelated but independent events.
MATERIALS AND METHODS
Plants and growth conditions.
Seeds of Arabidopsis thaliana ecotype Columbia (Col-0) and the Atnoa1 (SALK no. CS6511), fls2 (SALK no. 121477), and dnd1-1 (SALK no. 066908C) mutants were surface sterilized by being sequentially soaked in 75% ethanol for 1 min, rinsed three times with sterile water, soaked in 20% bleach for 15 min, and rinsed five times with sterile water. Sterilized seeds were kept at 4°C in the dark for 3 days for vernalization and were planted in magenta boxes containing Murashige and Skoog basal salt mixture (Sigma-Aldrich) supplemented with 1% sucrose (wt/vol) and 0.8% agar, and placed in a growth chamber at 22°C with a light intensity of 80 µE m−2 s−1. Seedlings were grown under 16 h of light and 8 h of darkness for 21 days. Mutants were obtained from the Arabidopsis Biological Resource Center (ABRC); tobacco plants (Nicotiana benthamiana) were grown at 26°C with a 16-h photoperiod.
Bacterial strains and growth conditions.
B. cenocepacia K56-2 and its isogenic mutants (Table 1) were cultured at 37°C in Luria-Bertani (LB) broth overnight. B. cenocepacia K56-2 is a clinical isolate that belongs to the ET-12 epidemic strain lineage (57). Strain MSS25 carries a polar insertion in the fliC gene, which prevents the synthesis of the flagellin filament and also likely inactivates the downstream genes fliD1 and fliT, encoding a flagellar hook and an accessory protein, respectively (35). To analyze the growth rate of mutants, overnight cultures were diluted into fresh medium at a starting optical density at 600 nm (OD600) of 0.05. Samples were then aliquoted in a 100-well honeycomb microtiter plate, and growth rates were followed by measuring the OD600 every hour under continuous shaking for 24 h in a Bioscreen C automated growth curve analyzer (MTX Lab Systems, Vienna, VA).
Plant inoculation methods.
Five- to 6-week-old A. thaliana or Nicotiana benthamiana plants were used for the initial inoculation experiments. For soil drainage infection, plants were watered with bacterial suspensions in MES (4-morpholineethanesulfonic acid sodium salt) buffer. For the surface spraying method, bacteria from overnight cultures were suspended in 10 mM MES buffer at different CFU. For inoculation by the syringe-infiltration method, bacteria were infiltrated into the leaves using a needleless syringe. For wound inoculation, seedlings were wounded at the leaf surface by being scratched with a 20-gauge needle. Wounded seedlings were immediately inoculated with bacterial suspensions. MS agar plates containing inoculated seedlings were sealed with Parafilm and placed in growth chambers for 7 days. Plants were monitored for disease symptoms daily. At day 7, three leaves from each plantlet were excised, washed with 5% bleach for 1 min, and rinsed with sterile water. The leaf was blotted dry on sterile filter paper and imprinted on LB agar plates to determine if there were any bacteria on the surface of the leaves. The imprinted plates were incubated at 37°C for 24 h. The leaves were then weighed and macerated in 500 µl phosphate-buffered saline (PBS) with a micropestle, serially diluted, and plated on LB agar plates in triplicates. Only leaf samples that did not show any bacterial growth on the imprinted plates were counted to avoid counting contaminating bacteria from leaf surfaces. Other mutants were similarly infiltrated into leaves, and HR symptoms were visualized by bleaching the leaves in ethanol (Fig. 2C). Ethanol bleaching was used to provide enhanced visualization of HR development in leaves and facilitate comparison with the parental strain.
RNA isolation, cDNA synthesis, and RT-PCR.
Total RNA was extracted from frozen leaves of A. thaliana using the SV total RNA isolation system (Promega), and 1 µg of RNA was reverse transcribed using the QuantiTect reverse transcription kit (Qiagen). The coding sequences of the PR-1 and actin genes, available through GenBank, were used to design the following primers: forward, 5′-GATGTGCCAAAGTGAGGTG-3′, and reverse, 5′-CTGATACATATACACGTCC-3′, to amplify PR-1 and forward, 5′-TGCTCTTCCTCATGCTAT-3′, and reverse, 5′-ATCCTCCGATCCAGACACTG-3′, to amplify the actin gene. PCR was carried out with an initial denaturation step at 94°C for 4 min, followed by 30 cycles of denaturation (30 s at 94°C) for PR-1 and 21 cycles for the actin gene, annealing (30 s at 53°C), and extension (1 min at 72°C). After the last cycle, a final extension was carried out for 5 min at 72°C. PCR products were visualized on 1% agarose gels using UV light in a Gel-Doc system (Alpha Innotech). mRNA levels of PR-1 were normalized relative to the control and internal control by densitometry using ImageJ.
LPS extraction, MS, and flagellin purification.
LPS was extracted from equal biomass using the proteinase K method as previously described (58). Extracted LPS was dissolved at 1 mg/ml in water containing 2.5 mM MgCl2 plus 1 mM CaCl2 and shaken for 3 h on a rotary shaker. If not mentioned otherwise, experiments were performed with K56-2 LPS or with buffer A containing 1.0 mM CaCl2 and 2.5 mM MgCl2 (pH 7.6) as a control. Flagellin purification was performed as described earlier (33). Lipid A of strains MH55 and K56-2 (Table 1) was examined by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Cells were grown overnight in Luria-Bertani broth, centrifuged (10,000 × g), washed twice with phosphate buffer (10 mM Na2HPO4, 1.7 mM KH2PO4), and freeze-dried. Lipid A was extracted and desalted, as previously described (59). For MS analysis, dihydroxybenzoic acid (DHB) (Sigma Chemical Co., St. Louis, MO) was used as a matrix and prepared to saturation in acetonitrile–0.1% trifluoroacetic acid (1:2 vol/vol). A 2-µl sample was loaded on the target, dried, covered by 1 µl of DHB, and inserted in a Bruker Autoflex MALDI-TOF spectrometer. Data were acquired and analyzed using the Flex Analysis software.
In vivo NO and ROS analysis.
NO was measured in guard cells of leaf epidermal peels prepared from leaves of 3-week-old plants (grown as described above) using diaminofluorescein diacetate (DAF-2DA) fluorescence as described by Guo et al. (60), with the following modifications. The epidermal peels were submerged in buffer (pH 5.7) containing 5 mM MES-KOH, 0.25 mM KCl, and 1 mM CaCl2 with 5 µM DAF-2DA and transferred either to reaction buffer containing 100 µg/ml LPS or preincubated for 10 min in reaction buffer containing 50 µM sodium nitroprusside (SNP) or 200 µM NG-nitro-l-Arg-methyl ester (l-NAME) and then for 20 min with LPS. The epidermal peels were placed underneath a coverslip on a microscope slide with several drops of reaction mixture. In a similar way, ROS was detected by 2 µM 2,7-dichlorofluorescein diacetate (DCF) and flagellin treatment following the incubation of leaf epidermal leaves in MES-KOH buffer for 2 h.
Microscopy.
Fluorescence and phase-contrast images were acquired using a QImaging Retiga-SRV camera on an Axioscope 2 (Carl Zeiss).
Fluorometric quantification of NO and ROS.
To monitor NO accumulation in Arabidopsis leaf peels, the DAF-2DA fluorescence was measured at excitation of 480 nm and emission of 521 nm, using a Cary Eclipse fluorescence spectrophotometer (Varian, Inc., Mississauga, Ontario, Canada). The plate was rocked for 20 s before measuring. Similarly, to determine accumulation of ROS, DCF was added to a final concentration of 2 µM. Fluorescence was measured at excitation of 480 nm and emission of 521 nm in 96-well white plates. The background fluorescence of each probe in the buffer control was subtracted. Autofluorescence of the buffer, without adding the probes, was measured and corrected by subtraction from the fluorescence signals. The suspensions were protected from light throughout the assays to avoid photo-oxidation.
Bacterial swarming and swimming motility assays.
Swarming motility assays were performed as described previously (37). Assays were done in triplicate and repeated independently three times. Bacterial swimming motility was analyzed on soft agar plates (1% Bacto tryptone in 0.3% agar). The OD600 of overnight cultures was adjusted to 1, and 2 µl of culture was inoculated in the center of the agar plate. The diameter of the growth zone was measured after 24 h of incubation at 37°C.
Galleria mellonella infection model.
G. mellonella larvae were obtained from Recorp and stored in the dark at 4°C. Injections of 10 µl containing 1 × 104 CFU diluted in 10 mM MgSO·7H2O supplemented with 100 µg ampicillin µl−1 were given to the larvae into the hemocoel through the hindmost right proleg. Infected larvae were incubated at 30°C, and larval survival was monitored up to 75 h as judged based lack of movement in response to stimuli. Control larvae were injected with 10 µl of the same buffer without bacteria. Ten larvae were used for each condition, and the experiment was repeated on three independent occasions.
SUPPLEMENTAL MATERIAL
B. cenocepacia infection in tobacco plants. Representative images of 5-week-old tobacco plants inoculated with a dose of 108 CFU of B. cenocepacia by different inoculation methods are shown. Arrows indicate mild symptoms that are barely visible resulting from spraying bacteria on leaves. Download
MALDI-TOF spectra of purified lipid A produced by the parental B. cenocepacia strain K56-2 and the ΔarnT lptGs mutant strain MH55 (Table 1). Lipid A samples were prepared and processed as indicated in Materials and Methods. The profiles represented were obtained using the negative-ion mode. Ion peaks are color coded to indicate ions corresponding to species containing (red) versus those lacking (blue) l-Ara4N. Only ions denoting species without l-Ara4N are found in the MH55 lipid A spectrum. Download
Infection of A. thaliana by the infiltration method. The graph represents bacterial counts on day 7 postinfection recovered from Arabidopsis leaves inoculated with B. cenocepacia by infiltration. Download
Infection of Arabidopsis seedlings by the inoculation method. Representative images of leaves inoculated with a dose of 108 CFU of the various strains used in this study are shown. Download
Growth rate of parental B. cenocepacia strain K56-2 and its isogenic mutants in LB medium. Data points represent the mean of 6 replicas. Download
ACKNOWLEDGMENTS
This work was supported by grants to M.A.V. from the Natural Sciences and Engineering Research Council of Canada, Cystic Fibrosis Canada, and the UK Cystic Fibrosis Trust. We gratefully thank Vicky Lightfoot from the Department of Biology at Western University for providing us access to the growth chambers. We also thank Vojislava Grbic for the gift of wild-type Arabidopsis thaliana seeds, Katie Bain for assistance with protein purification, Hoda Yaghmaeian for seed sterilization, medium and soil preparation for plant experiments, and José A. Bengoechea for advice on MALDI-TOF spectrometry.
Footnotes
Citation Khodai-Kalaki M, Andrade A, Fathy Mohamed Y, Valvano MA. 2015. Burkholderia cenocepacia lipopolysaccharide modification and flagellin glycosylation affect virulence but not innate immune recognition in plants. mBio 6(3):e00679-15. doi:10.1128/mBio.00679-15.
REFERENCES
- 1.Mahenthiralingam E, Baldwin A, Dowson CG. 2008. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- 2.Parke JL, Gurian-Sherman D. 2001. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39:225–258. doi: 10.1146/annurev.phyto.39.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Drevinek P, Mahenthiralingam E. 2010. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect 16:821–830. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernier SP, Silo-Suh L, Woods DE, Ohman DE, Sokol PA. 2003. Comparative analysis of plant and animal models for characterization of Burkholderia cepacia virulence. Infect Immun 71:5306–5313. doi: 10.1128/IAI.71.9.5306-5313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergunst AC, Meijer AH, Renshaw SA, O’Callaghan D. 2010. Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect Immun 78:1495–1508. doi: 10.1128/IAI.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber B, Feldmann F, Köthe M, Vandamme P, Wopperer J, Riedel K, Eberl L. 2004. Identification of a novel virulence factor in Burkholderia cenocepacia H111 required for efficient slow killing of Caenorhabditis elegans. Infect Immun 72:7220–7230. doi: 10.1128/IAI.72.12.7220-7230.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uehlinger S, Schwager S, Bernier SP, Riedel K, Nguyen DT, Sokol PA, Eberl L. 2009. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect Immun 77:4102–4110. doi: 10.1128/IAI.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seed KD, Dennis JJ. 2008. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect Immun 76:1267–1275. doi: 10.1128/IAI.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson EL, Dennis JJ. 2013. Common duckweed (Lemna minor) is a versatile high-throughput infection model for the Burkholderia cepacia complex and other pathogenic bacteria. PLoS One 8:e80102. doi: 10.1371/journal.pone.0080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura MT, Dangl JL. 2010. Arabidopsis and the plant immune system. Plant J 61:1053–1066. doi: 10.1111/j.1365-313X.2010.04131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman MA, Sundelin T, Nielsen JT, Erbs G. 2013. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front Plant Sci 4:139. doi: 10.3389/fpls.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitfield C, Trent MS. 2014. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 13.Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18:265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 14.Torres MA, Jones JD, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18:277–284. doi: 10.1046/j.1365-313X.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones JD, Dangl JL. 2006. The plant immune system. Nature 444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 17.Xu XM, Møller SG. 2011. The value of Arabidopsis research in understanding human disease states. Curr Opin Biotechnol 22:300–307. doi: 10.1016/j.copbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Koornneef M, Meinke D. 2010. The development of Arabidopsis as a model plant. Plant J 61:909–921. doi: 10.1111/j.1365-313X.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel FM, Katagiri F, Mindrinos M, Glazebrook J. 1995. Use of Arabidopsis thaliana defense-related mutants to dissect the plant response to pathogens. Proc Natl Acad Sci U S A 92:4189–4196. doi: 10.1073/pnas.92.10.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J. 2004. Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci U S A 101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madala NE, Molinaro A, Dubery IA. 2012. Distinct carbohydrate and lipid-based molecular patterns within lipopolysaccharides from Burkholderia cepacia contribute to defense-associated differential gene expression in Arabidopsis thaliana. Innate Immun 18:140–154. doi: 10.1177/1753425910392609. [DOI] [PubMed] [Google Scholar]
- 22.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A 110:1464–1469. doi: 10.1073/pnas.1218080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silipo A, Sturiale L, Garozzo D, Erbs G, Jensen TT, Lanzetta R, Dow JM, Parrilli M, Newman MA, Molinaro A. 2008. The acylation and phosphorylation pattern of lipid A from Xanthomonas campestris strongly influence its ability to trigger the innate immune response in Arabidopsis. Chembiochem 9:896–904. doi: 10.1002/cbic.200700693. [DOI] [PubMed] [Google Scholar]
- 24.Newman MA, Dow JM, Molinaro A, Parrilli M. 2007. Priming, induction and modulation of plant defence responses by bacterial lipopolysaccharides. J Endotoxin Res 13:69–84. doi: 10.1177/0968051907079399. [DOI] [PubMed] [Google Scholar]
- 25.Loutet SA, Valvano MA. 2011. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Microbiol 2:159. doi: 10.3389/fmicb.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega XP, Cardona ST, Brown AR, Loutet SA, Flannagan RS, Campopiano DJ, Govan JR, Valvano MA. 2007. A putative gene cluster for aminoarabinose biosynthesis is essential for Burkholderia cenocepacia viability. J Bacteriol 189:3639–3644. doi: 10.1128/JB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. 2012. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol Microbiol 85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi F, Shimizu R, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. 2003. Post-translational modification of flagellin determines the specificity of HR induction. Plant Cell Physiol 44:342–349. doi: 10.1093/pcp/pcg042. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF. 2006. Within-species flagellin polymorphism in Xanthomonas campestris pv. campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18:764–779. doi: 10.1105/tpc.105.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. 2006. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18:465–476. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez-Gómez L, Boller T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5:1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 33.Hanuszkiewicz A, Pittock P, Humphries F, Moll H, Rosales AR, Molinaro A, Moynagh PN, Lajoie GA, Valvano MA. 2014. Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses. J Biol Chem 289:19231–19244. doi: 10.1074/jbc.M114.562603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. 2007. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC nucleotide GATED CHANNEL2 and innate immunity. Plant Cell 19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldías MS, Lamothe J, Wu R, Valvano MA. 2008. Burkholderia cenocepacia requires the RpoN sigma factor for biofilm formation and intracellular trafficking within macrophages. Infect Immun 76:1059–1067. doi: 10.1128/IAI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aubert DF, Flannagan RS, Valvano MA. 2008. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect Immun 76:1979–1991. doi: 10.1128/IAI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubert DF, O’Grady EP, Hamad MA, Sokol PA, Valvano MA. 2013. The Burkholderia cenocepacia sensor kinase hybrid AtsR is a global regulator modulating quorum-sensing signalling. Environ Microbiol 15:372–385. doi: 10.1111/j.1462-2920.2012.02828.x. [DOI] [PubMed] [Google Scholar]
- 38.Khodai-Kalaki M, Aubert DF, Valvano MA. 2013. Characterization of the AtsR hybrid sensor kinase phosphorelay pathway and identification of its response regulator in Burkholderia cenocepacia. J Biol Chem 288:30473–30484. doi: 10.1074/jbc.M113.489914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taguchi F, Takeuchi K, Katoh E, Murata K, Suzuki T, Marutani M, Kawasaki T, Eguchi M, Katoh S, Kaku H, Yasuda C, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. 2006. Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell Microbiol 8:923–938. doi: 10.1111/j.1462-5822.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 40.Taguchi F, Yamamoto M, Ohnishi-Kameyama M, Iwaki M, Yoshida M, Ishii T, Konishi T, Ichinose Y. 2010. Defects in flagellin glycosylation affect the virulence of Pseudomonas syringae pv. tabaci 6605. Microbiology 156:72–80. doi: 10.1099/mic.0.030700-0. [DOI] [PubMed] [Google Scholar]
- 41.Crawford NM, Guo FQ. 2005. New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci 10:195–200. doi: 10.1016/j.tplants.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK Jr, Bent AF. 2000. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci U S A 97:9323–9328. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres MA, Jones JD, Dangl JL. 2005. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37:1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- 44.Yu IC, Parker J, Bent AF. 1998. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci U S A 95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn IP. 2007. Disturbance of the Ca2+/calmodulin-dependent signalling pathway is responsible for the resistance of Arabidopsis dnd1 against Pectobacterium carotovorum infection. Mol Plant Pathol 8:747–759. doi: 10.1111/j.1364-3703.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 46.Wand ME, Müller CM, Titball RW, Michell SL. 2011. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol 11:11. doi: 10.1186/1471-2180-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmerk CL, Valvano MA. 2013. Burkholderia multivorans survival and trafficking within macrophages. J Med Microbiol 62:173–184. doi: 10.1099/jmm.0.051243-0. [DOI] [PubMed] [Google Scholar]
- 48.Casadevall A, Pirofski LA. 2014. Microbiology: ditch the term pathogen. Nature 516:165–166. doi: 10.1038/516165a. [DOI] [PubMed] [Google Scholar]
- 49.Casadevall A, Pirofski LA. 2015. What is a host? Incorporating the microbiota into the damage-response framework. Infect Immun 83:2–7. doi: 10.1128/IAI.02627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindermayr C, Sell S, Müller B, Leister D, Durner J. 2010. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22:2894–2907. doi: 10.1105/tpc.109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silipo A, Molinaro A, Ieranò T, De Soyza A, Sturiale L, Garozzo D, Aldridge C, Corris PA, Khan CM, Lanzetta R, Parrilli M. 2007. The complete structure and pro-inflammatory activity of the lipooligosaccharide of the highly epidemic and virulent Gram-negative bacterium Burkholderia cenocepacia ET-12 (strain J2315). Chemistry 13:3501–3511. doi: 10.1002/chem.200601406. [DOI] [PubMed] [Google Scholar]
- 52.Hossain MM, Shibata S, Aizawa S, Tsuyumu S. 2005. Motility is an important determinant for pathogenesis of Erwinia carotovora subsp. carotovora. Physiol Mol Plant Pathol 66:134–143. doi: 10.1016/j.pmpp.2005.06.001. [DOI] [Google Scholar]
- 53.Tans-Kersten J, Brown D, Allen C. 2004. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment. Mol Plant Microbe Interact 17:686–695. doi: 10.1094/MPMI.2004.17.6.686. [DOI] [PubMed] [Google Scholar]
- 54.Haefele DM, Lindow SE. 1987. Flagellar motility confers epiphytic fitness advantages upon Pseudomonas syringae. Appl Environ Microbiol 53:2528–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hattermann DR. 1989. Motility of Pseudomonas syringae pv. glycinea and its role in infection. Phytopathology 79:284–289. doi: 10.1094/Phyto-79-284. [DOI] [Google Scholar]
- 56.Beattie GA, Lindow SE. 1995. The secret life of foliar bacterial pathogens on leaves. Annu Rev Phytopathol 33:145–172. doi: 10.1146/annurev.py.33.090195.001045. [DOI] [PubMed] [Google Scholar]
- 57.Johnson WM, Tyler SD, Rozee KR. 1994. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J Clin Microbiol 32:924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loutet SA, Bartholdson SJ, Govan JR, Campopiano DJ, Valvano MA. 2009. Contributions of two UDP-glucose dehydrogenases to viability and polymyxin B resistance of Burkholderia cenocepacia. Microbiology 155:2029–2039. doi: 10.1099/mic.0.027607-0. [DOI] [PubMed] [Google Scholar]
- 59.El Hamidi A, Tirsoaga A, Novikov A, Hussein A, Caroff M. 2005. Microextraction of bacterial lipid A: easy and rapid method for mass spectrometric characterization. J Lipid Res 46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Guo FQ, Okamoto M, Crawford NM. 2003. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B. cenocepacia infection in tobacco plants. Representative images of 5-week-old tobacco plants inoculated with a dose of 108 CFU of B. cenocepacia by different inoculation methods are shown. Arrows indicate mild symptoms that are barely visible resulting from spraying bacteria on leaves. Download
MALDI-TOF spectra of purified lipid A produced by the parental B. cenocepacia strain K56-2 and the ΔarnT lptGs mutant strain MH55 (Table 1). Lipid A samples were prepared and processed as indicated in Materials and Methods. The profiles represented were obtained using the negative-ion mode. Ion peaks are color coded to indicate ions corresponding to species containing (red) versus those lacking (blue) l-Ara4N. Only ions denoting species without l-Ara4N are found in the MH55 lipid A spectrum. Download
Infection of A. thaliana by the infiltration method. The graph represents bacterial counts on day 7 postinfection recovered from Arabidopsis leaves inoculated with B. cenocepacia by infiltration. Download
Infection of Arabidopsis seedlings by the inoculation method. Representative images of leaves inoculated with a dose of 108 CFU of the various strains used in this study are shown. Download
Growth rate of parental B. cenocepacia strain K56-2 and its isogenic mutants in LB medium. Data points represent the mean of 6 replicas. Download