After accounting for potential immune reconstitution inflammatory syndrome effects, longer duration on boosted protease inhibitors reduced Kaposi sarcoma incidence after at least 1 year on treatment among human immunodeficiency virus-infected male veterans who received any combination antiretroviral therapy.
Keywords: human immunodeficiency virus, Kaposi sarcoma, combination antiretroviral therapy, veterans, risk assessment
Abstract
Background. Kaposi sarcoma (KS) incidence has decreased since combination antiretroviral therapy (cART). However, effects of cART type and duration on KS remain difficult to interpret secondary to KS-associated immune reconstitution inflammatory syndrome (IRIS).
Methods. We performed a retrospective study of Veterans Affairs Human Immunodeficiency Virus Clinical Case Registry data from 1985 to 2010. We analyzed the relationship between cART regimens and KS using multivariable Poisson regression, stratified or adjusted for timing around cART initiation. KS was identified by ≥1 inpatient or ≥2 outpatient International Classification of Diseases, Ninth Revision codes (176.0–9). Percent of cART on specific regimen and total duration on specific regimen were examined.
Results. There were 341 KS cases among 25 529 HIV-infected male veterans (incidence rate = 2.02/1000 person-years). Stratified by years after starting cART, every additional 10% time on boosted protease inhibitors (BPIs) was associated with reduced KS incidence in the third year of cART (incidence rate ratio [IRR] = 0.79; 95% confidence interval [CI], .69–.90). Months on BPIs was associated with lower KS incidence (P = .02). KS incidence was lower at 12–23 (IRR = 0.47; 95% CI, .23–.95) and ≥36 (IRR = 0.14; 95% CI, .02–1.00) months on BPIs compared with <6 months. Longer duration on other regimens was not associated with decreased KS incidence.
Conclusions. Lower KS incidence was observed with longer BPI use, after accounting for potential IRIS and other factors. Future research should evaluate newer cART regimens and long-term benefits of PI-based cART on KS in other cohorts and prospective studies.
The introduction of combination antiretroviral therapy (cART) revolutionized human immunodeficiency virus (HIV)-infection management, resulting in improved outcomes and survival [1]. However, compared with the general population, HIV-infected individuals continue to experience increased risk for developing AIDS-defining cancers such as Kaposi sarcoma (KS) [2]. In the United States, KS incidence has significantly decreased since widespread cART use, but KS remains the most common HIV-associated malignancy [2–6]. cART use alone has been suggested to prolong survival [7–9] and often leads to resolution of KS in HIV-positive patients [10]. These improved outcomes have been widely attributed to immune restoration and virologic suppression following cART use; however, the effects of specific cART classes on KS incidence have not been well described.
Previous reports have suggested that protease inhibitors (PIs), a class of antiretrovirals, may be effective in controlling KS independently of immune reconstitution and virologic suppression [11]. In vitro studies have demonstrated that PIs such as ritonavir, saquinavir, nelfinavir, and indinavir have direct effects on angiogenesis and tumor growth and on KS–herpesvirus 8 (HHV-8) replication [12]. However, clinical studies evaluating the effect of PIs on KS incidence have primarily been small case series or observational studies with poorly defined outcomes that have not indicated clear treatment benefit [13].
Furthermore, several recent studies have shown that KS incidence and progression may be affected by immune reconstitution inflammatory syndrome (IRIS), a paradoxical worsening of clinical status despite immune recovery [14–17]. IRIS results from an exaggerated activation of the immune system against persistent antigen or viable pathogens during immune restoration following cART initiation. IRIS clinically occurs with almost any opportunistic infection and some malignancies after cART initiation. Thus, evaluating the impact of specific antiretroviral therapies on KS incidence may be biased by the IRIS effect of cART initiation on KS development and progression. Our aim in this study was to better account for the influence of timing after cART initiation in order to more robustly evaluate the effect of specific cART medication classes on KS incidence among a large, national sample of HIV-infected male veterans.
METHODS
The institutional review board of the Baylor College of Medicine and the Research and Development Committee at the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, approved this study.
Study Population
The Department of Veterans Affairs (VA) HIV Clinical Case Registry (CCR) is a nationwide registry that contains health-related information (including demographic, laboratory, pharmacy, outpatient and hospitalization data, and vital status) on all known HIV-infected patients cared for by the VA since the registry's inception [18, 19]. Following identification of HIV-infected veterans, all past clinical data are electronically retrieved. The database is automatically updated electronically. On-site HIV coordinators provide maintenance and verification.
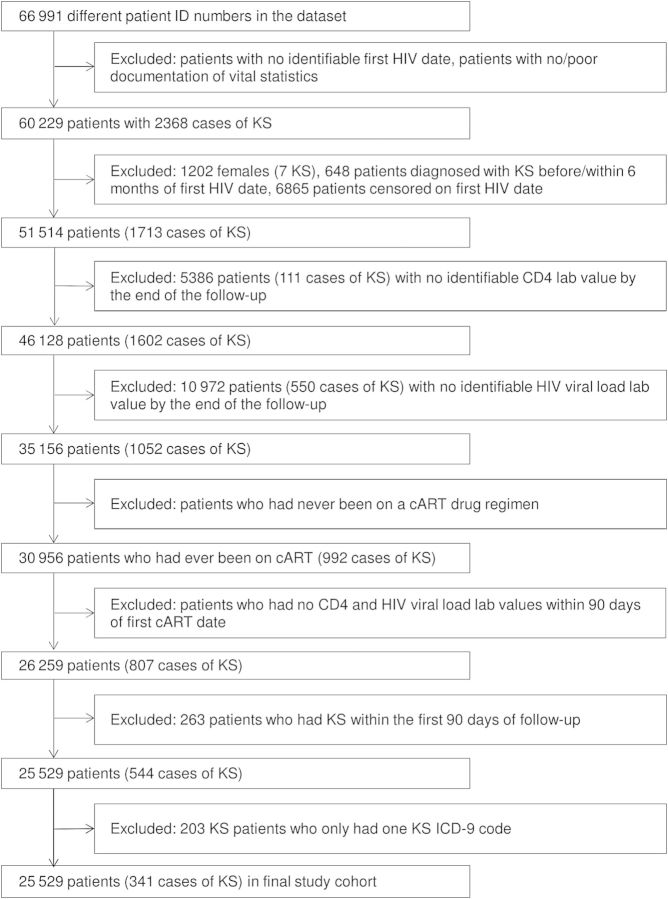
A total of 66 991 HIV-infected adult veterans were enrolled in the CCR between 1985 and 2010. Figure 1 describes criteria used to define the final study sample. The study population was restricted to HIV-infected veterans aged >18 years with documented CD4 and HIV measurement. Inclusion required a confirmed HIV diagnosis date based on (1) the presence of multiple International Classification of Diseases, Ninth Revision (ICD-9) codes for HIV (042 or V08) or (2) a combination of ICD-9 code for HIV, positive HIV-related test (eg, enzyme-linked immunosorbent assay, Western blot, quantifiable HIV RNA measurement) or prescription delivery of cART. To avoid inclusion of individuals erroneously added to the registry, individuals without adequate HIV diagnostics (ie, only a single ICD code for HIV and no laboratory or pharmacy records) or vital statistics were removed. HIV index date was defined as the earliest ICD-9 code, positive test, or prescription delivery. Due to the limited number of females in the population (<2%), only male veterans were included in our analyses. Additionally, we removed individuals whose death or censor date was the same as their HIV index date. Finally, we only included veterans who received cART with quantifiable CD4 or HIV RNA measurements within 90 days of cART initiation.
Figure 1.
Flow diagram for selection of study cohort. Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; ID, identification; KS, Kaposi sarcoma.
Kaposi Sarcoma Definition
The primary outcome was diagnosis of incident KS, identified from inpatient and outpatient ICD-9 codes (176.0–9). Cases were individuals with at least 1 KS ICD-9 code at an inpatient encounter or at least 2 codes at outpatient encounters on different dates. Follow-up for longitudinal analyses spanned from first cART to KS diagnosis, death, or 31 December 2010 (final date of current CCR iteration), whichever occurred first. To minimize inclusion of prevalent KS, individuals diagnosed with KS before or within 6 months after initial HIV diagnosis were excluded. For the same reason, KS cases diagnosed within 90 days after cART initiation were excluded. Chart review was conducted on all 149 study participants in the CCR with a KS ICD-9 code and a clinic visit at MEDVAMC. The positive predictive value for the ICD-9 code definition compared with chart review was 77.3%.
Calculating Combination Antiretroviral Therapy Use
cART use was abstracted from pharmacy records in the CCR, which included prescriptions dispensed at all VA facilities. cART use among HIV-infected individuals was defined as any combination of 2 classes of nucleoside reverse transcriptase inhibitors and 1 of either nonnucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) classes, integrase inhibitors, or CCR5 inhibitors and any combination of 2 classes.
Four classes of therapy regimens were defined: ritonavir-boosted PI (low-dose ritonavir administered concurrently with another PI), nonboosted PI (other than nelfinavir), nelfinavir, and NNRTI. PI classes were evaluated separately based on research demonstrating differential outcomes for patients receiving boosted vs nonboosted PIs and the prominent use of nelfinavir in previous KS research [12, 20, 21]. Utilization of each regimen was calculated as the aggregate number of therapy days delivered from the dispensed prescriptions. Treatment time excluded intervals of discontinued use or nonadherence (ie, treatment lapses based on prescription refill timing).
For each cART regimen type, 2 measures of drug exposure were examined as predictors of KS incidence in Poisson regression models. The first measure was the percentage of cART period, beginning at first cART, during which the drug regimen was followed. Preliminary examination of the data showed a nearly linear relation between this variable and KS incidence, justifying its use as a linear continuous independent variable. The second measure was total duration of drug regimen exposure. Preliminary examination showed marked nonlinearities in the relation between these durations and KS incidence. Accordingly, duration was categorized (<6, 6–11, 12–23, 24–35, ≥36 months).
Covariate Definitions
Potential confounders included age at HIV diagnosis, race/ethnicity, intravenous drug use, comorbid conditions captured using the Charlson comorbidity index (Deyo modification, calculated at start of follow-up and excluding points allotted for HIV infection) [22, 23], number of clinic visits per year, % time on cART, time since initiating cART, and era of HIV diagnosis (pre-cART, before 1996; early cART, 1996–2001; late cART, 2002–2010). Additional HIV disease factors were captured from the CCR laboratory database. Specifically, the lowest CD4 count before cART initiation was used to estimate pretreatment immune function. Time-updated CD4 count and HIV RNA measurements were also collected from cART initiation throughout follow-up to monitor fluctuations in immune status. CD4 variables were categorized (<200, 200–350, and >350 cells/µL). HIV RNA was captured as detectable or undetectable. For standardization of operational procedures at different contributing VA facilities over all study years, the value for undetectable HIV RNA was established as fewer than 500 copies/mL.
Statistical Analysis
Analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina). Distributions of sociodemographic and clinical characteristics among the study cohort were observed. Differences between KS cases and non-KS cases were evaluated using χ² tests for categorical variables and Wilcoxon tests for continuous variables.
Poisson regression models were constructed in order to evaluate the impact of the use of different cART regimens on KS incidence among users. Other covariates were included in the models to stratify or to control for confounding. Multivariable Poisson regression models were fit and adjusted for the clinically relevant covariates described previously (ie, age, race/ethnicity, Deyo comorbidity score, clinic visits/year, % time on cART, era of HIV diagnosis, lowest CD4 count before cART, CD4 count at event/censor, HIV viral load, and time from initiating cART). Effects of predictors and covariates were expressed as incidence rate ratios (IRRs) derived from Poisson regression model parameter estimates, with statistical significance of effects assessed by Wald test statistics derived from parameter estimates and their estimated covariances.
During the course of each participant's follow-up, the primary measures of exposure and some covariates (eg, CD4, HIV viral load) varied over time. Accordingly, the unit of analysis was an episode of drug regimen, with Poisson offset determined by episode length, nested within a participant's cART history. To adjust for within-participant correlation of KS incidence rates conditional on predictors among the drug regimen episodes, a general estimating equations approach was taken to the estimation of the Poisson model parameters and their covariances.
RESULTS
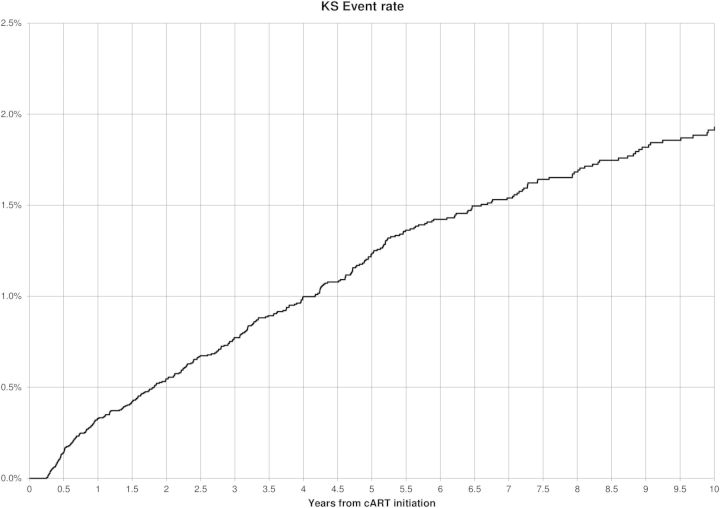
Table 1 displays characteristics of the 25 529 individuals in our cohort, consisting of male HIV-infected patients, 341 of whom were diagnosed with KS. Individuals contributed 169 106 person-years (incidence rate = 2.02/1000 person-years; Figure 2). KS cases were younger at HIV diagnosis (40 years vs 44.7 years; P < .0001), more likely to be white or Hispanic, and had shorter follow-up duration than noncases. Individuals with KS were less likely to be on a cART regimen 80%–100% of the time (30.8% vs 43.6%; P < .0001) and more likely to have ever had boosted PI and/or nelfinavir use (22.9% vs 13.3%; P = .02 and 41.9% vs 28.8%; P < .001, respectively). They were also less likely to have ever had nonboosted PI and/or NNRTI use (23.5% vs 29.5%; P < .0001 and 61.6% vs 73.0%; P < .0001, respectively). KS cases were more likely to be diagnosed with HIV in the pre-cART era (45.8% vs 26.9%; P < .0001) and have the lowest CD4 before cART initiation and CD4 at event/censor < 200 (54.6% vs 44.0% and 53.8% vs 23.4%, respectively; P < .0001) compared with noncases. KS cases at event/censor were also more likely to have detectable HIV viral loads 80%–100% of the time (71.2% vs 28.0%; P < .0001).
Table 1.
Characteristics of 25 529 Men Ever Receiving Combination Antiretroviral Therapy Stratified by Kaposi Sarcoma Diagnosis
| Characteristic | Overall N = 25 529 |
KS N = 341 |
No KS N = 25 188 |
P Value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age at HIV diagnosis (y; mean [SD])a | 44.6 (10.4) | 40.0 (9.9) | 44.7 (10.4) | <.0001 |
| Race/ethnicity | .01 | |||
| White | 9549 (37.4) | 136 (39.9) | 9413 (37.4) | |
| African American | 12 911 (50.6) | 169 (49.6) | 12 742 (50.6) | |
| Hispanic | 1895 (7.4) | 30 (8.8) | 1865 (7.4) | |
| Unknown/Other | 1174 (4.6) | 6 (1.8) | 1168 (4.7) | |
| Use of intravenous drugs | .40 | |||
| No | 16 559 (64.9) | 214 (62.8) | 16 345 (65.0) | |
| Yes | 8940 (35.1) | 127 (37.2) | 8813 (35.0) | |
| Deyo comorbidity score | .23 | |||
| 0 | 13 345 (52.5) | 171 (50.2) | 13 174 (52.6) | |
| 1 | 7453 (29.3) | 96 (28.2) | 7357 (29.4) | |
| ≥2 | 4609 (18.2) | 74 (21.7) | 4535 (18.1) | |
| Follow-up duration (in years; mean [SD])a | 6.2 (4.3) | 3.4 (3.1) | 6.3 (4.3) | <.0001 |
| <1 y | 1845 (7.2) | 73 (21.4) | 1772 (7.0) | |
| 1–2 y | 2558 (10.0) | 47 (13.8) | 2511 (10.0) | |
| 2–3 y | 2169 (8.5) | 39 (11.4) | 2130 (8.5) | |
| ≥3 y | 18 957(74.3) | 182 (53.4) | 18 775 (74.5) | |
| Clinic visits per year | ||||
| <4 | 2509 (9.8) | 45 (13.2) | 2464 (9.8) | <.0001 |
| 4–5 | 4683 (18.3) | 52 (15.3) | 4631 (18.4) | |
| 6–9 | 9506 (37.2) | 91 (26.7) | 9415 (37.4) | |
| 10–19 | 7042 (27.6) | 107 (31.4) | 6935 (27.5) | |
| ≥20 | 1789 (7.0) | 46 (13.5) | 1743 (6.9) | |
| % time on cART | <.0001 | |||
| <80% | 14 432 (56.5) | 236 (69.2) | 14 196 (56.4) | |
| 80%–100% | 11 097 (43.5) | 105 (30.8) | 10 992 (43.6) | |
| cART use | ||||
| Any boosted PI | 3421 (13.4) | 78 (22.9) | 3343 (13.3) | .02 |
| Any nonboosted PI | 7505 (29.4) | 80 (23.5) | 7425 (29.5) | <.0001 |
| Any nelfinavir | 7391 (29.0) | 143 (41.9) | 7248 (28.8) | <.0001 |
| Any nonnucleoside reverse transcriptase inhibitor | 8601 (72.9) | 10 (61.6) | 18 391 (73.0) | <.0001 |
| Era of HIV diagnosis | <.0001 | |||
| Pre-cART (before 1996) | 6933 (27.2) | 156 (45.8) | 6777 (26.9) | |
| Early cART (1996–2002) | 10 416 (40.8) | 160 (46.9) | 10 256 (40.7) | |
| Late cART (2003–2010) | 8180 (32.4) | 25 (7.3) | 8155 (32.4) | |
| Lowest CD4 count before cART | <.0001 | |||
| CD4 <200 | 11 220 (44.2) | 186 (54.6) | 11 034 (44.0) | |
| CD4 200–350 | 7220 (28.4) | 94 (27.6) | 7126 (28.4) | |
| CD4 >350 | 6967 (27.4) | 61 (17.9) | 6906 (27.6) | |
| CD4 count at event/censor | <.0001 | |||
| CD4 <200 | 6070 (23.9) | 183 (53.8) | 5887 (23.4) | |
| CD4 200–350 | 4819 (18.9) | 67 (19.7) | 4752 (18.9) | |
| CD4 >350 | 14 571 (57.2) | 90 (26.5) | 14 481 (57.7) | |
| HIV viral load at event/censor | <.0001 | |||
| Detectable (≥500) | 6352 (25.0) | 242 (71.2) | 7051 (28.0) | |
| Undetectable (<500) | 18 229 (71.4) | 98 (28.8) | 18 131 (72.0) |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; KS, Kaposi sarcoma; PI, protease inhibitor; SD, standard deviation.
a Age at HIV diagnosis and follow-up duration are presented as mean values with SD in parentheses.
Figure 2.
Timing of Kaposi sarcoma (KS) events following combination antiretroviral therapy (cART) initiation.
Table 2 shows results from the multivariable Poisson model in which predictors of KS were evaluated. Individuals who were on cART 80%–100% time were less likely to have KS (IRR = 0.71; 95% confidence interval [CI], .55–.92) while adjusting for age, race/ethnicity, comorbidities, and other HIV virologic variables. Individuals aged 40–59 years had lower KS incidence than individuals aged <30 years (IRR = 0.52; 95% CI, .36–.76). African Americans had lower KS incidence than whites (IRR = 0.77; 95% CI, .60–.97). Individuals with moderate comorbidities (Deyo comorbidity score = 1) had lower KS incidence than individuals with minimal comorbidities (Deyo = 0; IRR = 0.73; 95% CI, .57–.94). KS incidence was lower in individuals diagnosed with HIV in more recent years (late cART IRR = 0.45; 95% CI, .28–.72). Individuals with the lowest CD4 count before cART initiation, ranging from 200 to 350, had higher KS incidence compared with those with a CD4 count of <200 (IRR = 1.33; 95% CI, 1.02–1.74). Individuals with current CD4 count at event/censor of 200–350 (IRR = 0.41; 95% CI, .31–.55) and >350 (IRR = 0.26; 95% CI, .19–.36) had lower KS incidence compared with <200. Individuals with undetectable HIV viral load had lower KS incidence (IRR = 0.43; 95% CI, .33–.56). Finally, individuals <1 year into their cART regimen were more likely to have KS incidence (IRR = 1.75; 95% CI, 1.27–2.40). Separate sensitivity analyses excluding individuals who died in the first year and individuals who died ever yielded similar results to the full model presented here (data not shown).
Table 2.
Multivariable Poisson Model Evaluating Predictors of Kaposi Sarcoma Including Time on Combination Antiretroviral Therapy in a Cohort of 25 529 Male Human Immunodeficiency Virus–Positive Veterans
| Parameter | Incidence Rate Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Age at HIV diagnosis (y) | ||
| <30 | 1.0 (Ref) | |
| 30–39 | 0.82 (.58–1.17) | .27 |
| 40–49 | 0.52 (.36–.76) | <.001 |
| 50–59 | 0.58 (.37–.90) | .02 |
| ≥60 | 0.70 (.38–1.28) | .25 |
| Race/Ethnicity | ||
| White | 1.0 (Ref) | |
| Black | 0.77 (.60–.97) | .03 |
| Hispanic | 1.01 (.68–1.51) | .95 |
| Unknown | 0.63 (.27–1.44) | .27 |
| Deyo comorbidity score | ||
| 0 | 1.0 (Ref) | |
| 1 | 0.73 (.57–.94) | .02 |
| ≥2 | 0.95 (.71–1.26) | .70 |
| Clinic visits per year | ||
| <4 | 1.0 (Ref) | |
| 4–5 | 1.01 (.66–1.52) | .98 |
| 6–9 | 0.94 (.65–1.36) | .73 |
| 10–19 | 1.21 (.85–1.73) | .28 |
| ≥20 | 1.46 (.96–2.22) | .08 |
| % of time on cART | ||
| <80% | 1.0 (Ref) | |
| 80%–100% | 0.71 (.55–.92) | <.001 |
| Era of HIV diagnosis | ||
| Pre-cART (before 1996) | 1.0 (Ref) | |
| Early cART (1996–2002) | 0.92 (.73–1.17) | .49 |
| Late cART (2003–2010) | 0.45 (.28–.72) | <.001 |
| Lowest CD4 count before cART | ||
| CD4 <200 | 1.0 (Ref) | |
| CD4 200–350 | 1.33 (1.02–1.74) | .03 |
| CD4 >350 | 1.11 (.77–1.59) | .58 |
| CD4 count at event/censor | ||
| CD4 <200 | 1.0 (Ref) | |
| CD4 200–350 | 0.41 (.31–.55) | <.0001 |
| CD4 >350 | 0.26 (.19–.36) | <.0001 |
| HIV viral load | ||
| Detectable (≥500) | 1.0 (Ref) | |
| Undetectable (<500) | 0.43 (.33–.56) | <.0001 |
| Time from initiating cART | ||
| <1 y | 1.75 (1.27–2.40) | <.001 |
| 1–2 y | 1.33 (.96–1.85) | .09 |
| 2–3 y | 1.22 (.86–1.72) | .26 |
| ≥3 y | 1.0 (Ref) | |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; Ref, reference.
Table 3 shows results from separate multivariable Poisson models in which the effect of percent time of different cART regimens on KS incidence was evaluated, stratified by number of years of cART drug use. The model examining ritonavir-boosted PIs demonstrated a protective effect on KS for every additional 10% time on drug, but only within year 3 after cART initiation (IRR = 0.79; 95% CI, .69–.90). The other drug regimens, stratified by number of years on cART, were not associated with KS.
Table 3.
Separate Multivariable Poisson Models Examining Effect of Percent of Time on Different Drug Regimens Stratified by Number of Years on Combination Antiretroviral Therapy on the Incidence of Kaposi Sarcoma
| Drug Type | Year of Combination Antiretroviral Therapy Use | Incidence Rate Ratioa | P Value |
|---|---|---|---|
| Boosted PI | 1 | 1.01 (0.90–1.14) | .89 |
| (per additional 10% time) | 2 | 0.95 (0.79–1.13) | .54 |
| 3 | 0.79 (0.69–0.90) | <.001 | |
| Nonnucleoside reverse transcriptase inhibitor | 1 | 0.96 (0.89–1.03) | .22 |
| (per additional 10% time) | 2 | 1.09 (1.00–1.20) | .06 |
| 3 | 0.98 (0.92–1.04) | .53 | |
| Nelfinavir | 1 | 0.99 (0.91–1.07) | .78 |
| (per additional 10% time) | 2 | 1.08 (0.96–1.21) | .21 |
| 3 | 1.00 (0.93–1.08) | .98 | |
| Nonboosted PI | 1 | 1.09 (0.99–1.19) | .07 |
| (per additional 10% time) | 2 | 1.00 (0.83–1.22) | .97 |
| 3 | 1.02 (0.91–1.16) | .71 |
Abbreviation: PI, protease inhibitor.
a Adjusted for age, race, Deyo comorbidity score, clinic visits/year, % of time on combination antiretroviral therapy (cART), era of human immunodeficiency virus (HIV) diagnosis, lowest CD4 count before cART, CD4 count at event/censor, HIV viral load, and time from initiating cART.
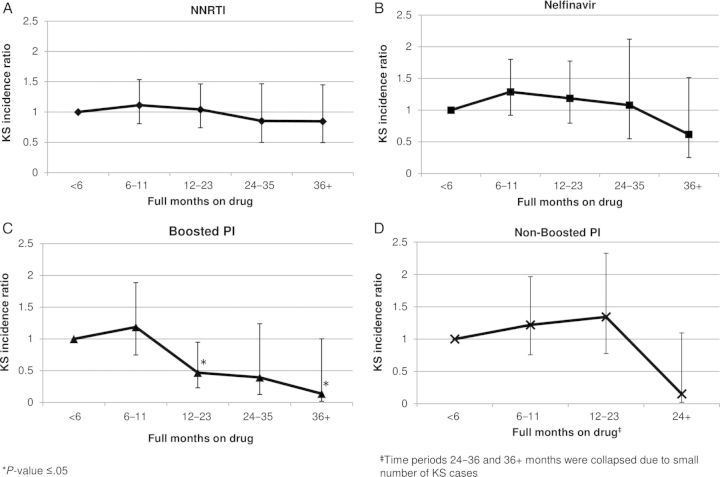
An examination of the effect of total number of months on each drug regimen on KS incidence is presented in Figure 3. There is no trend (P = .84) in the reduction of KS incidence with months on NNRTI (Figure 3A). Nelfinavir (Figure 3B) and nonboosted PIs (Figure 3D) demonstrated a relatively stable null effect on KS incidence until ≥36 and ≥24 months on drug, respectively, where there is not a statistically significant reduction in KS incidence. Ritonavir-boosted PIs demonstrate decreased KS incidence at 12–23 months on drug (IRR = 0.47; 95% CI, .23–.95) and again at ≥36 months on drug (IRR = 0.14; 95% CI, .02–1.00) compared with <6 months. There is also an overall trend toward lower KS incidence with longer duration on ritonavir-boosted PI (P = .02).
Figure 3.
Kaposi sarcoma (KS) incidence rate ratios by total duration of drug exposure for (A) nonnucleoside reverse transcriptase inhibitor (NNRTI), (B) nelfinavir, (C) boosted protease inhibitors (PIs), and (D) nonboosted PIs adjusted for age, race, comorbidities, total combination antiretroviral therapy (cART) exposure, human immunodeficiency virus (HIV) era, CD4 count, HIV viral load, time from initiating cART, and veterans health administration use.
DISCUSSION
To our knowledge, we are the first to investigate the impact of the type and duration of cART use on KS incidence, accounting specifically for timing of KS diagnosis after cART initiation, in a cohort of cART users. Our results suggest KS incidence is elevated in the first year after initiating cART; these results are similar to those from previous research that demonstrated an elevated KS incidence directly following cART initiation and secondary to KS-associated IRIS. Additionally, once adjustment was made for the effect associated with timing after cART initiation, as well as for other potential confounders including CD4 count and HIV viral load, we found that longer duration on boosted PIs provides a significant reduction in KS incidence. The observed protective effect was most pronounced after at least 1 year on cART. Longer duration on other cART classes was not associated with KS incidence.
As with most AIDS-defining opportunistic diseases, incidence of KS and other AIDS-associated cancers has declined significantly following cART introduction. These positive changes in HIV epidemiology have largely been attributed to the restoration of immune function. However, several recent in vitro and in vivo studies suggest HIV-specific PIs, originally designed to block the development and maturation of the HIV virion by targeting the viral protease, exhibit pleiotropic cellular effects including anticancer activity [11]. Gills et al [24] assessed anticancer properties of 6 PIs and found that nelfinavir, ritonavir, and saquinavir inhibited proliferation of every cell line in the NCI60 cell line panel. They also described potential mechanisms of action, including inhibition of Akt signaling, matrix metalloproteases (MMP) activity, nuclear factor κB, and the proteasome and induction of endoplasmic reticulum stress, autophagy, and apoptosis.
Studies have also shown that PIs directly affect KS development independent of HIV protease inhibition, primarily by targeting the proteasome and MMPs [25]. Ritonavir and saquinavir have been reported to inhibit proliferation of KS cell lines through induction of apoptosis of tumor cells by modulating proteasomal proteolysis without affecting proliferation or survival of noncancerous cells [26, 27]. Additionally, in nude mice injected with KS cells from human lesions, indinavir and saquinavir were shown to prevent development of angioproliferative KS-type lesions by blocking MMP2 activation, thus, inhibiting angiogenesis and cell invasion [12]. Ritonavir and saquinavir have also been shown to block production of inflammatory cytokines and chemokines (eg, tumor necrosis factor-α, interleukin [IL]-6, IL-8), which are critical to KS development and proliferation [27–29]. Furthermore, experimental models have shown that HIV and HHV-8 replication are reciprocally enhanced through HIV Tat protein activity. In mouse models, Tat viral proteins have been shown to stimulate the growth of KS lesions by promoting migration and proliferation of cytokine-activated endothelial cells and by deregulating angiogenic factors [30]. Ritonavir has been shown to inhibit nuclear factor-κB transcriptional activity induced by HIV Tat viral proteins, thereby interfering with HHV-8 replication and KS development [27, 31, 32].
While evidence from experimental models supports a direct inhibitory role of PIs on KS development and progression, results from previous clinical studies have not shown reduced KS risk associated with PI use compared with other cART regimens. Portsmouth et al [6] analyzed 1204 KS cases in a cohort of 8640 HIV-infected individuals and determined that PI- and NNRTI-based cARTs were equally effective in protecting against KS. Additionally, Stebbing et al [33] identified 198 KS cases from 4480 HIV-infected individuals and found that ritonavir-based therapy did not reduce KS incidence compared with other regimens. These and other similar clinical findings attribute the reduction in KS incidence to improved overall immune function rather than differential effects of specific therapy.
However, these prior clinical studies have not directly measured certain clinical effects (including timing after cART initiation and amount of time on specific cART regimens) that may impact the interpretation of these previous studies. Specifically, KS has been shown to arise from an IRIS-associated pathogenesis secondary to cART initiation [17]. IRIS may manifest clinically as new or worsening KS, which may skew the association between cART use and KS incidence. By adjusting for possible IRIS effects and amount of drug exposure, our study is a more detailed investigation of the effect of different drug classes than previous clinical studies. Our results suggest that timing around cART initiation may play an important role in interpreting the effects of different cART regimens on KS incidence. Under these assumptions, our findings indicate that use of boosted PI-based regimens do provide an advantage in reducing long-term KS incidence and reinforce what is known about anticancer and anti-KS activity of PIs from experimental models described previously. Additional studies in other cohorts are warranted to confirm our findings. Further evaluations of KS incidence should carefully incorporate similar attributions for timing of cART initiation.
Findings from the current study should be viewed within the context of the study design. Data for the present retrospective cohort study were extracted from a national, system-wide VA registry of HIV-infected veterans. Certain limitations are inherent in large registry-based observational analyses. First, use of ICD-9 codes for identification of KS diagnoses presents the potential for misclassification, and findings should be interpreted under this assumption. Additionally, our statistical models were limited by available data, which did not include certain KS risk factors (eg, mode of HIV transmission [except intravenous drug use]). Also, our data ends at 2010; thus, there was limited exposure to cART medications introduced more recently (eg, atazanavir, darunavir). There is also potential for variable follow-up visit frequency to affect time-updating laboratory values (eg, CD4, HIV viral load). We attempted to reduce potential information bias by segmenting follow-up into 1-week intervals and adjusting for number of clinic visits annually in our analysis. Finally, this study was conducted exclusively on male veterans, which may limit generalizability to other populations. Despite these limitations, the large number of HIV-infected individuals and KS cases contributing data on cART exposure is a considerable strength, enabling execution of the complex analyses described.
CONCLUSIONS
In summary, after accounting for potential IRIS effects, we found that longer duration on boosted PIs reduces KS incidence after at least 1 year on treatment. Our results provide preliminary clinical evidence that supports previous experimental findings of anti-KS activity of some PIs. Additional research is needed to measure newer cART regimens and evaluate the long-term clinical benefit of PI- vs NNRTI-based cART on KS incidence in other cohorts and prospective studies.
Notes
Financial support. This work was supported by a pilot grant from the AIDS Malignancy Consortium at Baylor College of Medicine, Dan L. Duncan Cancer Center (P30CA125123-04S1). This work was also supported in part by resources and the use of facilities at the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey Veterans Affairs Medical Center, and the Baylor-University of Texas–Houston Center for AIDS Research, a National Institutes of Health–funded program (AI036211). E. Y. C. (R01CA163103) also received support from the National Cancer Institute. The funders had no role in study design, data collection and analysis, or preparation of this report.
Disclaimer. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30(suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1999; 21(suppl 1):S34–41. [PubMed] [Google Scholar]
- 5.Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 2000; 92:1823–30. [DOI] [PubMed] [Google Scholar]
- 6.Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS 2003; 17:F17–22. [DOI] [PubMed] [Google Scholar]
- 7.Holkova B, Takeshita K, Cheng DM, et al. Effect of highly active antiretroviral therapy on survival in patients with AIDS-associated pulmonary Kaposi's sarcoma treated with chemotherapy. J Clin Oncol 2001; 19:3848–51. [DOI] [PubMed] [Google Scholar]
- 8.Stebbing J, Sanitt A, Nelson M, et al. A prognostic index for AIDS-associated Kaposi's sarcoma in the era of highly active antiretroviral therapy. Lancet 2006; 367:1495–502. [DOI] [PubMed] [Google Scholar]
- 9.Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer 2002; 98:916–22. [DOI] [PubMed] [Google Scholar]
- 10.Lebbe C, Blum L, Pellet C, et al. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 1998; 12:F45–9. [DOI] [PubMed] [Google Scholar]
- 11.Gantt S, Casper C, Ambinder RF. Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr Opin Oncol 2013; 25:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sgadari C, Barillari G, Toschi E, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med 2002; 8:225–32. [DOI] [PubMed] [Google Scholar]
- 13.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS 2009; 23:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002; 81:213–27. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch HH, Kaufmann G, Sendi P, et al. Immune reconstitution in HIV-infected patients. Clin Infect Dis 2004; 38:1159–66. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5:361–73. [DOI] [PubMed] [Google Scholar]
- 17.Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J Clin Oncol 2005; 23:5224–8. [DOI] [PubMed] [Google Scholar]
- 18.Backus L, Mole L, Chang S, et al. The Immunology Case Registry. J Clin Epidemiol 2001; 54(suppl 1):S12–5. [DOI] [PubMed] [Google Scholar]
- 19.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc 2009; 16:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffito M, Maitland D, Samarasinghe Y, et al. The pharmacokinetics of HIV protease inhibitor combinations. Curr Opin Infect Dis 2005; 18:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society–USA panel. JAMA 2008; 300:555–70. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 24.Gills JJ, Lopiccolo J, Tsurutani J, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res 2007; 13:5183–94. [DOI] [PubMed] [Google Scholar]
- 25.Monini P, Sgadari C, Toschi E, et al. Antitumour effects of antiretroviral therapy. Nat Rev Cancer 2004; 4:861–75. [DOI] [PubMed] [Google Scholar]
- 26.Gaedicke S, Firat-Geier E, Constantiniu O, et al. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res 2002; 62:6901–8. [PubMed] [Google Scholar]
- 27.Pati S, Pelser CB, Dufraine J, et al. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood 2002; 99:3771–9. [DOI] [PubMed] [Google Scholar]
- 28.Weichold FF, Bryant JL, Pati S, et al. HIV-1 protease inhibitor ritonavir modulates susceptibility to apoptosis of uninfected T cells. J Hum Virol 1999; 2:261–9. [PubMed] [Google Scholar]
- 29.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001; 1:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ensoli B, Gendelman R, Markham P, et al. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 1994; 371:674–80. [DOI] [PubMed] [Google Scholar]
- 31.Ensoli B, Sturzl M, Monini P. Reactivation and role of HHV-8 in Kaposi's sarcoma initiation. Adv Cancer Res 2001; 81:161–200. [DOI] [PubMed] [Google Scholar]
- 32.Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis 2011; 24:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stebbing J, Portsmouth S, Nelson M, et al. The efficacy of ritonavir in the prevention of AIDS-related Kaposi's sarcoma. Int J Cancer 2004; 108:631–3. [DOI] [PubMed] [Google Scholar]