Abstract
Objectives
Praziquantel is the only drug available for the treatment of schistosomiasis and the state of the exhausted drug discovery pipeline is alarming. We restarted investigations on the abandoned antischistosomal Ro 13-3978, an aryl hydantoin discovered in the early 1980s by Hoffmann La-Roche.
Methods
Newly transformed schistosomula and adult Schistosoma mansoni were studied in the presence of Ro 13-3978 in vitro. The metabolic stability of Ro 13-3978 was determined in vitro using human and mouse liver S9 fractions. Dose–response relationship, stage specificity, hepatic shift and scanning electron microscopy studies were carried out in S. mansoni-infected mice. In addition, efficacy experiments were conducted in rodents infected with Echinostoma caproni and Fasciola hepatica as well as in S. mansoni-infected immunocompromised nude (Foxn1nu) mice.
Results
Ro 13-3978 showed minor in vitro activity and no damage to the tegument was found. No cytotoxicity was detected for Ro 13-3978. Ro 13-3978 was metabolically stable. ED50 values of 138.9 and 14.6 mg/kg were calculated for the treatment of juvenile and adult S. mansoni infections, respectively, with a single oral dose of Ro 13-3978. SEM studies revealed severe damage to the worms 48 h post-treatment of infected mice. A single oral dose of Ro 13-3978 (100 mg/kg) administered to S. mansoni-infected (Foxn1nu) mice reduced the worm burden by 88%. Ro 13-3978 was not active against E. caproni and F. hepatica in vivo.
Conclusions
Ro 13-3978 has excellent antischistosomal properties in vivo. Structure–activity relationship studies with the aryl hydantoins have been launched in order to elucidate active pharmacophores, further investigate the mechanism of action and to identify a derivative with minimal antiandrogenic effects.
Keywords: schistosomiasis, Schistosoma mansoni, chemotherapy, drug discovery
Introduction
Schistosomiasis is a waterborne parasitic infection caused by six different trematode species, with Schistosoma mansoni, Schistosoma haematobium and Schistosoma japonicum being responsible for the largest public health burden.1,2 The disease gives rise to a persistent chronic disorder in endemic areas, resulting in common disabling complications such as anaemia, growth stunting, cognitive impairment and decreased aerobic capacity.2,3 Using the most recent disability-adjusted life year (DALY) metrics,4 an estimated 3.3 million DALYs have been attributed to schistosomiasis. To reduce the chronic health burden, millions of school-age children are treated each year in the framework of ‘preventive chemotherapy’ programmes with praziquantel.5 Praziquantel is a safe and effective drug against the chronic stage of the disease; however, it is the only commercially available drug for the treatment and control of schistosomiasis.5,6 Since the introduction of praziquantel several decades ago, drug discovery and development for this neglected tropical disease have been minimal and no backup drug is therefore available should praziquantel-resistant parasites evolve.6
In the early 1980s, Hoffmann La-Roche discovered the antischistosomal aryl hydantoin Ro 13-3978. The compound, a close structural analogue of the androgen receptor antagonist nilutamide, is a distant chemical cousin of the nitrothiazole, imidazolidinone niridazole7 (Figure 1), an obsolete schistosomicide, but is otherwise chemically unrelated to known antischistosomal drugs. We recently demonstrated that a single 400 mg/kg dose of nilutamide achieved a high worm burden reduction (WBR) in S. mansoni-infected mice.8 Furthermore, we showed that there is no correlation between antischistosomal activity and androgen receptor interaction for nilutamide, Ro 13-3978 and closely related aryl hydantoins.9 Drug discovery efforts by Hoffmann La-Roche revealed that Ro 13-3978 was equal to or more effective than praziquantel in different in vivo schistosome animal models; thus the compound was recommended for clinical testing.10 However, despite its remarkable and broad-spectrum activity, Ro 13-3978 was not further investigated.
Figure 1.
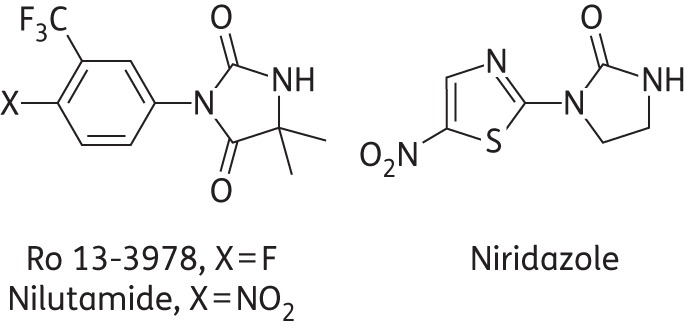
Structures of Ro 13-3978, nilutamide and niridazole.
The aim of the present study was to draw attention to the largely forgotten Ro 13-3978 by a thorough re-examination of its antischistosomal properties. In vitro studies with Ro 13-3978 were conducted against newly transformed schistosomula (NTS) and adult S. mansoni. Cytotoxicity studies were carried out using rat skeletal myoblast L6 cells. The dose–response relationship and stage specificity were evaluated in S. mansoni-infected mice. To begin to understand the pharmacokinetic/pharmacodynamic profile and mechanism of action of Ro 13-3978, metabolic stability, hepatic shift and scanning electron microscopy (SEM) studies and efficacy experiments in immunocompromised mice and mice treated with 1-aminobenzotriazole, a non-specific inhibitor of cytochrome P450, were conducted.
Materials and methods
Animals and parasites
In vivo experiments were carried out at the Swiss Tropical and Public Health Institute (Basel, Switzerland), in accordance with Swiss national and cantonal regulations on animal welfare (permission no. 2070). Female mice (NMRI strain, n = 86; weight ∼20–22 g) and female rats (n = 6; weight ∼80 g) were purchased from Charles River, Germany. Ten NMRI nude mice (weight ∼18–20 g) were purchased from Harlan, the Netherlands. Rodents were kept under environmentally controlled conditions (temperature ∼25°C; humidity ∼70%; 12 h light and 12 h dark cycle) and acclimatized for 1 week before infection. The animals had free access to water and rodent diet.
Cercariae of S. mansoni and Echinostoma caproni were obtained from infected intermediate host snails (Biomphalaria glabrata) as described previously.11 Fasciola hepatica metacercariae were purchased from Baldwin Aquatics, USA.
Compounds
Ro 13-3978 was synthesized as previously described.10,12 For in vitro antischistosomal studies, compounds were dissolved in DMSO (Fluka, Buchs, Switzerland) to obtain 10 mg/mL stock solutions. For in vivo studies, compounds were suspended in 7% (v/v) Tween 80 (Fluka, Buchs, Switzerland) and 3% (v/v) ethanol before oral administration to rodents (10 mL/kg).
In vitro studies
NTS
S. mansoni cercariae were harvested from infected snails and mechanically transformed to NTS as described earlier.13 An NTS suspension at a concentration of 100 NTS per 50 μL was prepared using Medium 199 (Invitrogen, Carlsbad, CA, USA) supplemented with 5% inactivated FCS (iFCS; Connectorate AG, Dietikon, Switzerland) and 100 U/mL penicillin and 100 mg/mL streptomycin (Invitrogen). NTS suspensions were incubated (37°C, 5% CO2) for at least 24 h before experiments to ensure that conversion into schistosomula had been completed. NTS were incubated with 100 μg/mL Ro 13-3978 for 72 h at 37°C, 5% CO2. The experiment was conducted in triplicate and repeated once. The highest concentration of DMSO served as a control. NTS were evaluated by microscopic readout (Carl Zeiss, Germany, magnification ×80) using a viability scale scoring death, changes in motility, viability and morphological alterations.13
Adult S. mansoni
Adult schistosomes were removed by picking from the hepatic portal system and mesenteric veins of mice that had been infected with 100 S. mansoni cercariae 49 days earlier. The worms were washed and kept in RPMI 1640 culture medium (Invitrogen) supplemented with antibiotics and 5% iFCS at 37°C in an atmosphere of 5% CO2 until use. In one experiment, the medium was supplemented with a freshly prepared haemin solution (8%) or 2% (v/v) human red blood cells (RBCs: blood group AB, Rh positive) to test whether haemoglobin degradation is involved in the mechanism of action.14 Worms were incubated in the presence of 25–100 μg/mL Ro 13-3978 for up to 96 h. Phenotypes were monitored daily, scoring motility, viability and morphological alterations under an inverted microscope (Carl Zeiss, Germany, magnification ×80), using the viability scale mentioned above. In addition, membrane damage was checked by a Trypan Blue exclusion assay: Ro 13-3978-treated, live control and dead control worms (killed using a cell lysis buffer) were rinsed three times with non-enriched RPMI medium, then exposed to a solution of 50 μL of Trypan Blue (Invitrogen) in 500 μL of non-enriched RPMI for 10 min. Worms were then returned to the culture medium and checked for Trypan Blue staining. All experiments with adult S. mansoni were conducted in duplicate and repeated once.
Cytotoxicity studies on rat skeletal myoblast L6 cells
Cytotoxicity studies were conducted as described elsewhere.15 Briefly, rat skeletal myoblast L6 cells were seeded in 96-well plates (2 × 103 cells/well) (BD Falcon, USA) using RPMI 1640 medium supplemented with 10% iFCS and 1.7 μM l-glutamine (Sigma-Aldrich, Buchs, Switzerland). Following adhesion of the cells for 24 h at 37°C and 5% CO2, the IC50 of Ro 13-3978 was determined using concentrations of 0.12, 0.37, 1.11, 3.33, 10, 30 and 90 μM. Podophyllotoxin (Sigma-Aldrich) served as positive control. At 70 h post-incubation, 10 μL of resazurin dye (Sigma-Aldrich) was added and the plates incubated for another 2 h. The plates were then read using a SpectraMax M2 (Molecular Devices) plate reader with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The cytotoxicity experiment was conducted in duplicate and repeated three times.
Metabolic stability experiments
The in vitro metabolic stability of Ro 13-3978 was determined using human and mouse liver S9 fractions (XenoTech, LLC, Lenexa, KS, USA) following standard protocols.16,17 Briefly, Ro 13-3978 (2 mM in 0.1% methanol) was premixed with NADPH (1 mM), saccharolactone (5 mM), uridine 5′-diphospho-glucuronic acid (1 mM) and 3′-phosphoadenosin-5′-phosphosulphate (0.1 mM) in potassium phosphate buffer (100 mM, pH 7.4) at 37°C. Reactions were initiated by adding S9 fractions at 1 mg/mL protein concentration in final incubation volumes of 100 μL and were then quenched by adding 100 μL of ice-cold methanol at 0, 5, 10, 15, 30, 45, 60, 90 and 120 min, followed by centrifugation at 17 000 g for 10 min. Ten microlitres of the supernatants were then analysed by LC-MS/MS using an Acquity UPLC-BEH Shield RP18 column (2.1 × 100 mm, 1.7 mm; Waters, Milford, MA, USA) with a 2: 3 0.1% acetic acid:methanol isocratic mobile phase at a flow rate of 0.25 mL/min. MS/MS analyses were performed in negative electrospray ionization mode; specific detection of Ro 13-3978 was performed by monitoring the transition 288.9→177.9 m/z. For the detection of potential Ro 13-3978 metabolites, multiple reaction monitoring–information-dependent acquisition-enhanced product ion, neutral loss, precursor ion and enhanced MS scans were used. 7-Hydroxycoumarin and testosterone were used as control substrates for phase I and phase II metabolism.
In vivo studies
E. caproni
Mice were infected orally with 20–25 metacercarial cysts of E. caproni. Fourteen days post-infection, four mice were treated with a single oral 100 mg/kg Ro 13-3978 dose. Four untreated mice served as controls. One week post-treatment, mice were killed and dissected, and all worms present in the intestines counted.
F. hepatica
Six rats were infected with 20–25 F. hepatica metacercariae. Twelve weeks post-infection, three rats were treated with a single oral 100 mg/kg Ro 13-3978 dose while three rats remained untreated. One week post-treatment, livers and bile ducts were examined for flukes and worm burdens were calculated.
S. mansoni
To study the dose–response relationship of Ro 13-3978 in adult S. mansoni infections, groups of three to five NMRI mice were treated orally in a single experiment with single doses of Ro 13-3978 (12.5, 25, 50 and 100 mg/kg) at 49 days post-infection. To test whether metabolism contributes to the activity of Ro 13-3978, one group of mice (n = 5) infected with adult S. mansoni was orally treated with 50 mg/kg 1-aminobenzotriazole (Sigma-Aldrich) followed by 100 mg/kg Ro 13-3978, 2 h later. To assess whether a potential interaction of Ro 13-3978 with the immune response plays a role in its antischistosomal efficacy, immunocompromised NMRI nude mice (n = 5) were treated with a single 100 mg/kg oral dose of Ro 13-3978, 49 days post-infection.
Mice (four to five mice per group) harbouring juvenile infections (established 3 weeks post-infection) were treated orally in a single experiment with doses of Ro 13-3978 (25, 50, 100 and 200 mg/kg).
For the stage-specificity experiment, groups of four mice were treated with a single oral 100 mg/kg Ro 13-3978 dose, 2 and 1 day prior to infection with S. mansoni cercariae, on infection day and on days 7, 14, 22, 28, 35, 42 and 49 post-infection.
Untreated mice served as controls in all experiments. At day 21 post-treatment (days 21–49 for the stage-specificity experiment), animals were killed by the CO2 method and dissected. Worms were removed by picking, then sexed and counted as previously described.18
Finally, the hepatic shift was investigated as follows. Four mice infected with adult schistosomes were orally treated with 100 mg/kg Ro 13-3978 and after 4, 8, 28 and 48 h, one mouse was euthanized and dissected. All worms in the mesenteric veins, hepatic portal veins and pressed liver were counted.
SEM studies
SEM studies were conducted for both in vitro and in vivo Ro 13-3978-treated worms. In vitro worms were incubated as described above with 25–100 μg/mL Ro 13-3978 for 72 h. For the in vivo studies, two mice were orally treated with 100 mg/kg Ro 13-3978 and dissected at 24 and 48 h post-treatment. Worms were extracted from the mesenteric veins and liver as described above, rinsed twice in PBS, and fixed in 1 mL 2.5% glutaraldehyde for 3–24 h at room temperature. Subsequently, samples were dehydrated by incubating the worms for 30 min in ascending ethanol concentrations of 30, 50, 70, 90 and 100%. The worms were critically point-dried (Bomar SPC-900), mounted on aluminium stubs and sputter-coated with gold of 20 nm particle size (Baltec Med 020). Samples were visualized using a high-resolution SEM accelerating voltage of 5 kV (Philips XL30 ESEM). Control worms (untreated; Figure 4a–d) were prepared and visualized in the same manner.
Figure 4.
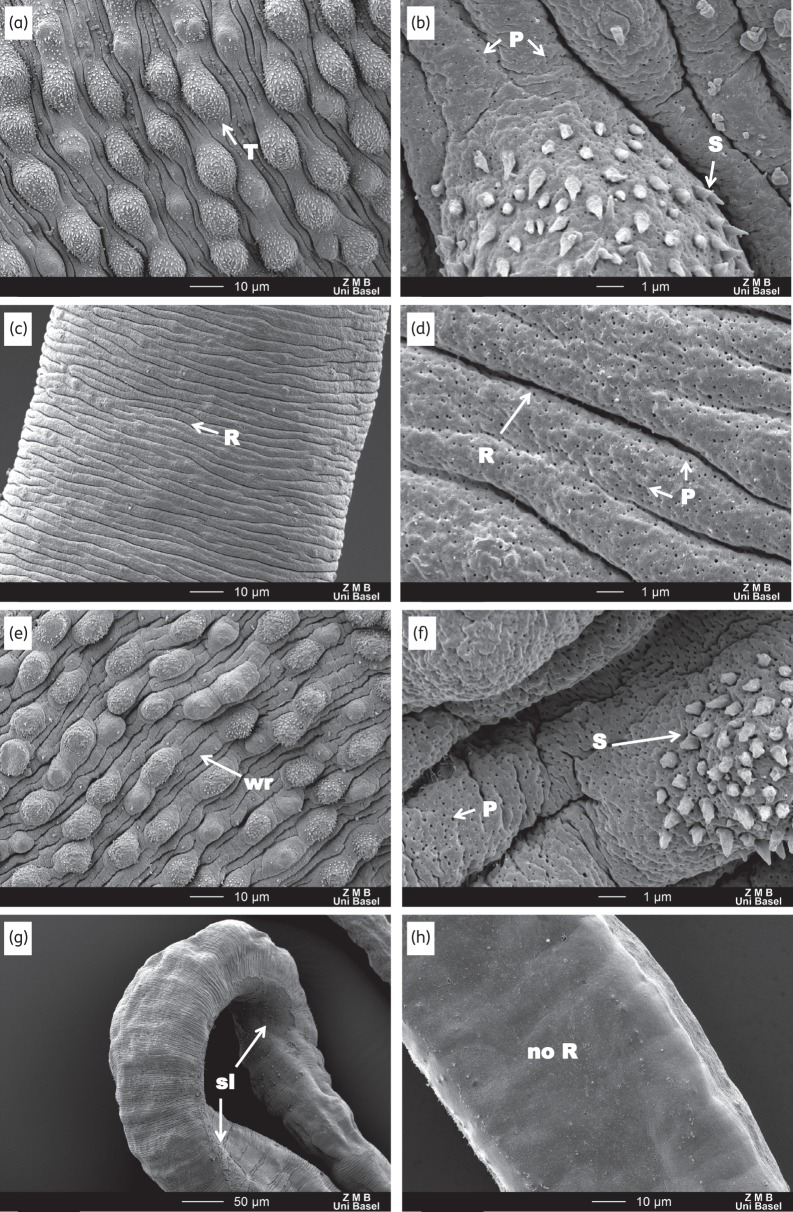
SEM observations of in vitro untreated and Ro 13-3978-treated (100 μg/mL for 72 h) worms. (a and b) Male control worms show a healthy tegument: tubercles (T) and spine (S) are intact and the tegument in-between is ridged and taut. Female control worms (c and d) are marked by a ridged (R) texture. Treated male worms (e and f) show a slight puffing and wrinkling (wr) of the between-tubercle tegument and some tubercles have a less rounded shape and fewer spikes. Female treated worms were mostly unaffected. However, two female worms (g and h) exhibit some areas of sloughing (sl) and a loss of ridged texture (no R). High magnification scans (×8000; b, d and f) reveal no differences between the control and treated worms, except that male treated worms appear to have slightly more pores (P; part f).
Statistics
Parasite viability values of treated and untreated NTS and adult schistosomes obtained from microscopic evaluation were averaged (means ± SD) using Microsoft Excel software. For in vivo studies, WBRs were determined by calculating the percentage reduction in mean worm burdens of the treatment groups relative to the untreated mice and the Kruskal–Wallis test was used to test for significance (P ≤ 0.5; StatsDirect statistical software, version 2.7.2.; StatsDirect Ltd, UK). The 50% effective dose values (ED50) were calculated using CompuSyn software (Version 3.0.1, 2007; ComboSyn Inc., USA).
Results
In vitro studies
Ro 13-3978 was tested against adult S. mansoni and NTS in vitro. Ro 13-3978 at 100 μg/mL moderately reduced the motility of both the schistosomula and adults at 24, 48 and 72 h post-incubation. Incubation with 25 or 50 μg/mL Ro 13-3978 produced the same result, although the slowed motility became pronounced only at ≥72 h. Occasional spasms were also observed in the adult worms. Both microscopic evaluation and the Trypan Blue exclusion test indicated no damage to the tegument. A similar behaviour of worms was observed in the presence of haemin. The addition of 2% RBC to the medium resulted in the death of worms at 100 μg/mL, but at lower concentrations (25 and 50 μg/mL) worms were still alive 72 h post-incubation with only slightly decreased motility. No cytotoxicity was detected for Ro 13-3978 (IC50 >90 μM).
Metabolic stability of Ro 13-3978
Ro 13-3978 was stable (>95%) in both human and mouse liver S9 fractions for up to 120 min, and no metabolites were detected using LC-MS/MS analysis. In contrast, under the same conditions, more than 65% of the positive controls 7-hydroxycoumarin (glucuronidation and sulphation) and testosterone (CYP hydroxylation) had been consumed within 30 min incubation (data not shown).
Dose–response relationship against juvenile and adult S. mansoni in vivo
In contrast to the marginal in vitro activity of Ro 13-3978, single oral doses of this aryl hydantoin showed high in vivo antischistosomal efficacies. Total and female WBRs following treatment of juvenile S. mansoni infections (22 days post-infection) with 50–200 mg/kg Ro 13-3978 and of adult S. mansoni infections (49 days post-infection) with 12.5–100 mg/kg Ro 13-3978 are summarized in Table 1. Adult S. mansoni infections were more susceptible to the compound than juvenile infections. Total and female WBRs of 88.2% and 85.4%, respectively, were observed with 200 mg/kg Ro 13-3978 against juvenile infections. In mice infected with adult S. mansoni, a single 100 mg/kg oral dose of Ro 13-3978 reduced total and female worm burden by 94.6% and 88.0%. From these data, ED50 values of 138.9 and 14.6 mg/kg were calculated for treatment with Ro 13-3978 of mice harbouring 22 and 49 day-old S. mansoni infections, respectively.
Table 1.
Dose–response relationship of Ro 13-3978 in mice harbouring juvenile and adult S. mansoni
| Stage of infection | Dosage (mg/kg) | No. of mice investigated | No. of mice cured | Mean number of worms (SD) |
Total WBR (%) | P value | Female WBR (%) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| liver | mesenteric veins | total | males | females | ||||||||
| control 1 | 8 | — | 1.0 (1.5) | 24.8 (8.0) | 25.9 (7.4) | 11.1 (4.2) | 14.8 (3.2) | — | — | — | — | |
| control 2 | 9 | — | 1.3 (1.9) | 24.0 (5.5) | 25.3 (5.3) | 13.0 (2.6) | 12.3 (3.4) | — | — | — | — | |
| control 3 | 9 | 0.9 (1.2) | 16.6 (5.2) | 17.4 (5.1) | 8.6 (3.3) | 8.9 (3.4) | — | — | — | — | ||
| control 4 | 10 | 1.9 (1.8) | 26.0 (13.8) | 27.9 (14.4) | 15.4 (8.1) | 12.5 (7.0) | — | — | — | — | ||
| Juvenile | 501 | 4 | 0 | 0 (0) | 27.3 (11.8) | 27.3 (11.8) | 10.3 (4.3) | 17.0 (7.8) | 0 | 0.005 | 0 | 0.01 |
| 1003a | 4 | 0 | 2.3 (2.1) | 4.0 (3.6) | 6.3 (4.3) | 2.5 (0.6) | 3.8 (3.9) | 63.8 | 57.3 | |||
| 2002a | 5 | 0 | 1.0 (1.0) | 2.0 (1.6) | 3.0 (1.9) | 1.2 (0.8) | 1.8 (1.3) | 88.1 | 85.4 | |||
| Adult | 12.51 | 4 | 0 | 0.5 (1.0) | 15.8 (11.6) | 16.3 (12.4) | 6.5 (4.1) | 9.8 (8.8) | 37.1 | <0.001 | 33.8 | <0.001 |
| 251 | 4 | 1 | 0.3 (0.5) | 4.3 (5.4) | 4.5 (5.3) | 2.3 (2.6) | 2.3 (2.6) | 82.6 | 84.5 | |||
| 503 | 4 | 1 | 0.3 (0.5) | 3.5 (2.6) | 3.8 (2.8) | 0 (0) | 3.8 (2.8) | 78.2 | 57.3 | |||
| 1004a | 4 | 2 | 0.3 (0.5) | 1.3 (1.5) | 1.5 (1.9) | 0 (0) | 1.5 (1.9) | 94.6 | 88.0 | |||
aData reproduced from Wang et al.,9 superscripts refer to the respective control group (1–4).
Stage specificity of single oral 100 mg/kg Ro 13-3978 in mice harbouring S. mansoni
Given the lower activity of Ro 13-3978 against 22 day-old compared with 49 day-old S. mansoni, the entire stage specificity of Ro 13-3978 was tested in mice. Mice were treated with Ro 13-3978 1 and 2 days prior to infection, on infection day and on days 7, 14, 22, 28, 35, 42 and 49 post-infection (Figure 2). Seven and 14 day-old S. mansoni were not affected by the compound. Moderate WBRs (32.4%–66.8%) were observed in mice treated with 100 mg/kg Ro 13-3978 before infection. High activities (64.3%–94.6%) were documented in mice treated with Ro 13-3978 in weekly intervals from day 22 onwards.
Figure 2.
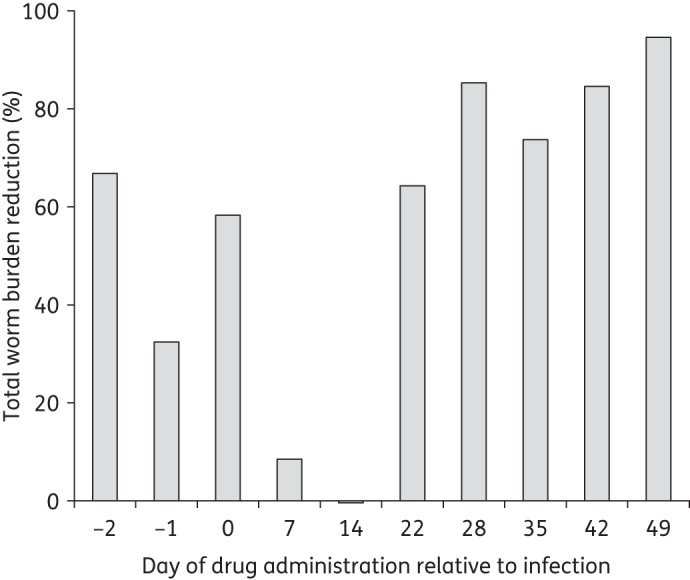
Stage specificity of single oral 100 mg/kg Ro 13-3978 in mice harbouring S. mansoni. Groups of four mice were treated 2 and 1 day prior to infection with S. mansoni cercariae, on infection day and on days 7, 14, 22, 28, 35, 42 and 49 post-infection. Worm burden reduction was evaluated 21–49 days post-treatment.
Hepatic shift
Figure 3 depicts the distribution of worms in the liver and the mesenteric veins. Four hours post-treatment, all worms were present in the mesenteric veins. Eight hours post-treatment, the first worms had shifted to the liver, but the majority of worms still resided in the mesenteric veins and all worms were still alive. Most of the worms had shifted to the liver 28 h post-treatment and all of these showed clearly reduced viability. On the next day (48 h post-treatment), all worms had died and the majority of worms had been expelled.
Figure 3.
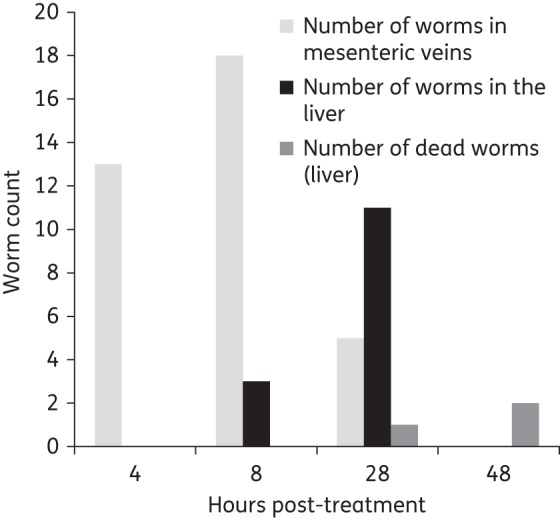
Hepatic shift observed in mice harbouring S. mansoni following 100 mg/kg Ro 13-3978. One mouse was dissected at 4, 8, 28 or 48 h post-treatment and worms were counted in the liver and mesenteric veins. Light grey bars, number of worms alive in the mesenteric veins; black bars, number of worms alive in the liver; and dark grey bars, number of dead worms found in the liver.
SEM studies
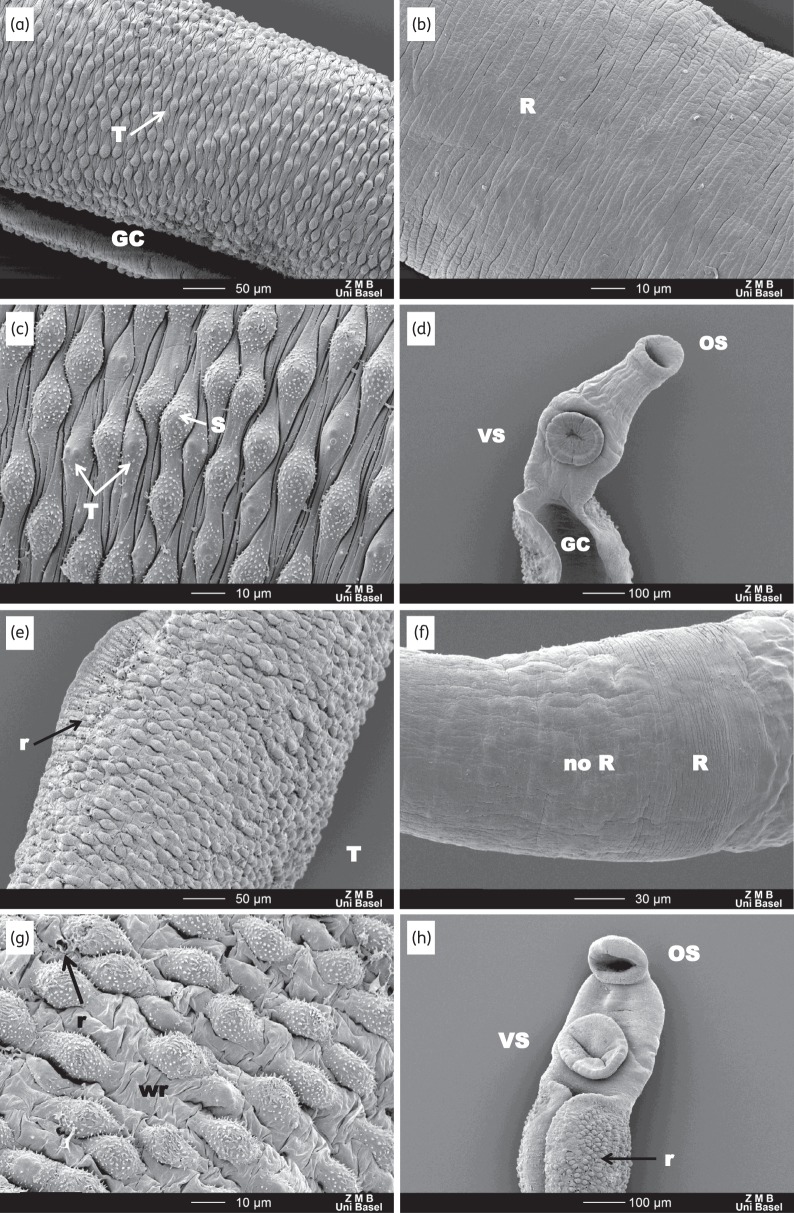
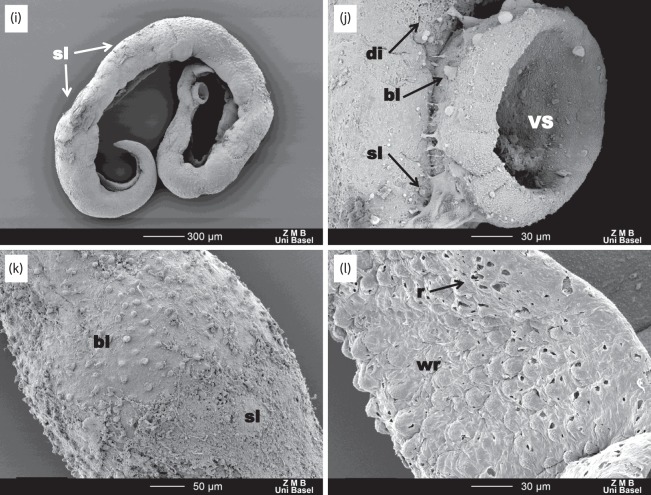
As reported above, in vitro-treated worms showed moderately slowed activity at 72 h without an apparent change to the tegument. SEM examinations of the male worms revealed no obvious changes in tegument, except that it appeared less taut (Figure 4e and f). Most female worms appeared unaffected; however, two female worms from the 100 and 25 μg/mL treatment arms were marked by a few patches of mild to moderate sloughing and a few patches where a loss of ridged texture had occurred (Figure 4g and h). In S. mansoni-infected mice treated with a 100 mg/kg oral dose of Ro 13-3978, a progressive effect was observed (Figure 5). At 24 h post-treatment (Figure 5e–h), the worms were slightly more contorted. The tegument was still mostly intact; however, the areas between the tubercles were very wrinkled and small ruptures could be seen on the external tegument at the edges of the gynecophoral canal as well as the edges of the oral sucker. Moreover, the female tegument had started to lose its ridged texture (parallel ridges disappeared). At 48 h post-treatment, severe damage to the worms could be observed throughout all regions. The tegument showed blebbing, sloughing and rupturing all along the body, as well as the head and suckers (Figure 5i–l), and the edges of the gynecophoral canal were deeply furrowed.
Figure 5.
SEM observations of untreated worms and worms extracted from mice treated with 100 mg/kg Ro 13-3978, 24 and 48 h post-treatment. (a–d) Tegument of control worms with intact tubercles (T) and spines (S). OS, VS and GC denote oral sucker, ventral sucker and gynecophoral canal, respectively. (e–h) At 24 h there is mild damage to the male and female worms. For male worms, the tegument between the tubercles exhibits extensive wrinkling (wr) and some tubercles exhibit rupturing (r). The female worms lose features—the ridges (R) found in the control worms start to disappear. (i–l) At 48 h, extensive damage to the tegument is observed: along with wrinkling and rupturing (l), massive blebbing (bl), sloughing (sl) and general disintegration (di) are apparent along the entire length of the worm.
Activity of Ro 13-3978 in immunocompromised mice and mice pretreated with 1-aminobenzotriazole
To further elucidate whether active metabolites or immunological mechanisms might help to account for the striking difference between the in vitro and in vivo activity of Ro 13-3978, further in vivo studies were conducted (Table 2). We found that very similar percentage WBR values were observed when single 100 mg/kg oral doses of Ro 13-3978 were administered to S. mansoni-infected mice with (100% WBR) and without (95% WBR) pretreatment with 1-aminobenzotriazole as a pan-CYP450 inhibitor, an unsurprising result given that this aryl hydantoin appears to be metabolically inert (see above). The same dose of Ro 13-3978 administered to S. mansoni-infected immunocompromised nude (Foxn1nu) mice reduced the worm burden by 88%.
Table 2.
Activity of Ro 13-3978 administered at 100 mg/kg in immunocompromised mice and in mice pretreated with 1-aminobenzotriazole
| Experiment | Dosage (mg/kg) | No. of mice investigated | No. of mice cured | Mean number of worms (SD) |
Total WBR (%) | P value | Female WBR (%) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| liver | mesenteric veins | total | males | females | ||||||||
| Nude mice | control | 5 | 0 | 0.8 (1.1) | 18.2 (7.5) | 19.4 (7.3) | 9.8 (4.1) | 9.6 (3.2) | — | 0.014 | — | 0.014 |
| 100 | 4 | 0 | 0 | 2.3 (1.5) | 2.3 (1.5) | 0 | 2.3 (1.5) | 88.2 | 76.1 | |||
| Pretreatment with 1-aminobenzotriazole | control | 8 | 0 | 0.3 (0.5) | 35.1 (14.0) | 35.4 (13.8) | 18.8 (6.3) | 16.6 (7.6) | — | 0.01 | — | 0.01 |
| 100 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | |||
Activity of Ro 13-3978 against E. caproni and F. hepatica
We tested whether Ro 13-3978 exhibits blood fluke-specific in vivo activity or whether the range of activity would also include the liver fluke F. hepatica and the intestinal fluke E. caproni. No effect on the worm burden was observed when rodents infected with these two trematode species were treated with 100 mg/kg oral doses of Ro 13-3978 (Table 3).
Table 3.
Activity of Ro 13-3978 administered at 100 mg/kg against F. hepatica and E. caproni
| Experiment | Dosage (mg/kg) | No. of rodents investigated | No. of rodents cured | Mean number of worms (SD) | Total WBR (%) | P value |
|---|---|---|---|---|---|---|
| F. hepatica | control | 3 | 0 | 5.3 (2.3) | — | >0.05 |
| 100 | 3 | 0 | 5.6 (1.5) | 0 | ||
| E. caproni | control | 4 | 0 | 12.5 (6.5) | — | >0.05 |
| 100 | 4 | 0 | 14.3 (2.8) | 0 |
Discussion
At least 230 million people worldwide are infected with Schistosoma spp.,2 yet we only have the lone drug praziquantel for treatment and control of this neglected tropical disease. A recent systematic review, which assessed the state of the research and development pipeline of drugs and vaccines for neglected diseases, highlighted a clear deficiency in this field.19 No drug is currently undergoing clinical testing for schistosomiasis;19 hence a backup drug will not be available in the next decade.
We restarted investigations on Ro 13-3978, an aryl hydantoin with excellent antischistosomal properties, discovered in the early 1980s by Hoffmann La-Roche.10 Our experiments confirmed that Ro 13-3978 has high activity against S. mansoni in the mouse model, with mature S. mansoni being more susceptible to the drug than juvenile flukes. We found that Ro 13-3978 had a single oral ED50 of 15 mg/kg in S. mansoni-infected mice (adult worms), a value close to the previously reported ED50 of 38 mg/kg.10 In this same schistosome mouse model, praziquantel is considerably less effective against adult S. mansoni, with reported ED50 values ranging from 172 to 202 mg/kg,10,20 and it has no significant activity against juvenile stages of the parasite. In contrast, in mice infected with juvenile S. mansoni, Ro 13-3978 had a single oral ED50 of 140 mg/kg. Interestingly, 7 and 14 day-old worms were not affected by Ro 13-3978, which might be explained by the location of the developing worm in the host (worms migrate via the heart and lungs to the liver, where they establish after ∼2.5 weeks).21
Considerable unpublished preclinical data on Ro 13-3978 have been generated by Hoffmann La-Roche, ranging from parasitological to toxicological studies. Most importantly, Ro 13-3978 is active against all three major schistosome species—S. mansoni, S. haematobium and S. japonicum.10 When Ro 13-3978 was administered to three different monkey species (Cebus monkeys, baboons and Erythrocebus monkeys) infected with S. mansoni, high activity was observed.10 Our studies further document that, in contrast to praziquantel, the activity of Ro 13-3978 is schistosome-specific with no activity observed on the intestinal fluke E. caproni and the liver fluke F. hepatica. A range of toxicology studies were conducted at Hoffmann La-Roche, which confirmed that the drug is safe when administered at a single dose (unpublished data). For this class of aryl hydantoins, antiandrogenic effects were observed in multiple-dose studies using male castrated rats,12 which is likely to be of less concern with single-dose treatment regimens. A recent study9 demonstrated that, in contrast to nilutamide, Ro 13-3978 had no measurable interaction with the androgen receptor at concentrations up to 27 μM (7.8 μg/mL), although it did block dihydrotestosterone-induced cell proliferation in an androgen-dependent cell line.
The mechanism of action of Ro 13-3978 is not known; particularly striking is the difference between its in vitro and in vivo efficacy. In in vitro assays, worms were slightly affected by Ro 13-3978; only moderate impairment to motility was seen and only at very high concentrations. Interestingly, supplementation of the medium with 2% RBC resulted in the death of worms, although only at a high concentration of 100 μg/mL. Further, SEM studies showed no membrane damage for most worms—results that, based on in vitro data alone, would have dismissed Ro 13-3978 from any further consideration. Yet in vivo studies have repeatedly confirmed the high activity of this compound. From the data that we have generated to date, the lack of significant in vitro activity of Ro 13-3978 is not explained by active metabolites or by effects on host immunology. In fact, experiments using human and mouse S9 fractions and 1-aminobenzotriazole-treated S. mansoni-infected mice indicate that Ro 13-3978 is not metabolized. Our data with the largely T cell-deficient Foxn1nu mice suggests that cytotoxic T cell activity is not required for Ro 13-3978 to effectively clear adult S. mansoni from the host. On the other hand, the antischistosomal drugs oxamniquine and praziquantel were found to kill fewer adult S. mansoni worms in T cell-deprived mice than in immunologically intact controls.22 However, T cell-independent worm antigens may be released or unveiled following Ro 13-3978 treatment, allowing for a humoral response to either be generated or effectively coat the adult worm in vivo. Further studies are in progress to identify the most likely mechanisms that might account for the high in vivo efficacy of Ro 13-3978.
The hepatic shift, which characterizes the forced migration of schistosomes to the liver, indicates that the onset of action of Ro 13-3978 starts rather slowly—at around 1 day post-treatment. For comparison, in parallel experiments with praziquantel, by 30 min post-treatment, the majority of worms shift to the liver.23 Mefloquine, another recently identified antischistosomal agent, showed a hepatic shift 72 h post-treatment.24
SEM studies corroborate the slow onset of action of Ro 13-3978. At 24 h post-treatment, all of the worms were still found in the mesenteric veins and the corresponding images show that the worms are still mostly intact, although some damage (occasional ruptures in the tegument) can be seen. At 48 h post-treatment, the damage is clearly visible throughout the worm: the tegument is completely disintegrated, characterized by blebbing, rupturing and sloughing. At this time point, the vast majority of the worms were shifted to the liver and the females expelled. Damage to the tegument is likely not caused by the hepatic shift, as previous non-effective treatments, e.g. the inactive enantiomer of praziquantel (S-praziquantel),23 have also caused a hepatic shift, followed by a return to the mesenteric veins, with the worms completely intact and viable.
In conclusion, we have confirmed that Ro 13-3978 has excellent antischistosomal properties against juvenile and adult S. mansoni infections in vivo. The aryl hydantoins should be considered candidates for the antischistosomal drug discovery and development pipeline. Structure–activity relationship studies have been launched to identify a derivative with minimal antiandrogenic effects to elucidate active pharmacophores and to further investigate the mechanism of action of this compound class.
Funding
This work was supported by grants from the European Research Council (ERC 614739-A_HERO) and the US National Institutes of Health (R21 AI097802-02). The sponsors of the study had no role in: the design and the conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the manuscript.
Transparency declarations
None to declare.
Acknowledgements
We acknowledge helpful discussions with Yazen Alnouti of the University of Nebraska Medical Center and Paul H. Davis of the University of Nebraska—Omaha. We also are indebted to Eva Bieler of the University of Basel Centre for Microscopy for her assistance and expertise in SEM techniques.
References
- 1.Gryseels B. Schistosomiasis. Infect Dis Clin N Am 2012; 26: 383–97. [DOI] [PubMed] [Google Scholar]
- 2.Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. Lancet 2014; 383: 2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terer CC, Bustinduy AL, Magtanong RV, et al. Evaluation of the health-related quality of life of children in Schistosoma haematobium-endemic communities in Kenya: a cross-sectional study. PLoS Negl Trop Dis 2013; 7: e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–223. [DOI] [PubMed] [Google Scholar]
- 5.Knopp S, Becker SL, Ingram KJ, et al. Diagnosis and treatment of schistosomiasis in children in the era of intensified control. Expert Rev Anti Infect Ther 2013; 11: 1237–58. [DOI] [PubMed] [Google Scholar]
- 6.Keiser J, Utzinger J. Antimalarials in the treatment of schistosomiasis. Curr Pharm Des 2012; 18: 3531–8. [PubMed] [Google Scholar]
- 7.da Rocha Pitta MG, da Rocha Pitta MG, de Melo Rego MJ, et al. The evolution of drugs on schistosoma treatment: looking to the past to improve the future. Mini Rev Med Chem 2013; 13: 493–508. [DOI] [PubMed] [Google Scholar]
- 8.Keiser J, Vargas M, Vennerstrom JL. Activity of antiandrogens against juvenile and adult Schistosoma mansoni in mice. J Antimicrob Chemother 2010; 65: 1991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Zhao Q, Min J, et al. Antischistosomal versus antiandrogenic properties of aryl hydantoin Ro 13–3978. Am J Trop Med Hyg 2014; 90: 1156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link H, Stohler HR. 3-Arylhydantoine, eine substanzklasse mit schistosomizider wirkung. Eur J Med Chem 1984; 19: 261–5. [Google Scholar]
- 11.Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 2010; 137: 589–603. [DOI] [PubMed] [Google Scholar]
- 12.Bernauer K, Link H, Stohler HR. Antiandrogenic and schistosomicidal imidazolidine derivatives. 1980 Hoffmann La-Roche patent 4,234,736.
- 13.Manneck T, Haggenmüller Y, Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 2010; 137: 85–98. [DOI] [PubMed] [Google Scholar]
- 14.Ingram K, Ellis W, Keiser J. Antischistosomal activities of mefloquine-related arylmethanols. Antimicrobial Agents Chemother 2012; 56: 3207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperandeo NR, Brun R. Synthesis and biological evaluation of pyrazolylnaphthoquinones as new potential antiprotozoal and cytotoxic agents. Chembiochem 2003; 4: 69–72. [DOI] [PubMed] [Google Scholar]
- 16.Gautam N, Bathena SP, Chen Q, et al. Pharmacokinetics, protein binding and metabolism of a quinoxaline urea analog as an NF-κB inhibitor in mice and rats by LC-MS/MS. Biomed Chromatogr 2013; 27: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Bathena SP, Alnouti Y. Metabolite profiling of praziquantel and its analogs during the analysis of in vitro metabolic stability using information-dependent acquisition on a hybrid triple quadrupole linear ion trap mass spectrometer. Drug Metab Pharmacokinet 2010; 25: 487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao SH, Keiser J, Chollet J, et al. The in vitro and in vivo activities of synthetic trioxolanes on major human schistosome species. Antimicrobial Agents Chemother 2007; 51: 1440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedrique B, Strub-Wourgaft N, Some C, et al. The drug and vaccine landscape for neglected diseases (2000–11): a systematic assessment. Lancet Global Health 2013; 1: 371–e9. [DOI] [PubMed] [Google Scholar]
- 20.Keiser J, Manneck T, Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J Antimicrob Chemother 2011; 66: 1791–7. [DOI] [PubMed] [Google Scholar]
- 21.Pearce EJ, James SL. Post lung-stage schistosomula of Schistosoma mansoni exhibit transient susceptibility to macrophage-mediated cytotoxicity in vitro that may relate to late phase killing in vivo. Parasite Immunol 1986; 8: 513–27. [DOI] [PubMed] [Google Scholar]
- 22.Sabah AA, Fletcher C, Webbe G, et al. Schistosoma mansoni: reduced efficacy of chemotherapy in infected T-cell-deprived mice. Exp Parasitol 1985; 60: 348–54. [DOI] [PubMed] [Google Scholar]
- 23.Meister I, Ingram-Sieber K, Cowan N, et al. Activity of praziquantel enantiomers and main metabolites against Schistosoma mansoni. Antimicrob Agents Chemother 2014; 58: 5466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram K, Duthaler U, Vargas M, et al. Disposition of mefloquine and enpiroline is highly influenced by a chronic Schistosoma mansoni infection. Antimicrob Agents Chemother 2013; 57: 4506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]