Abstract
Background. There are recognized needs to identify determinants of influenza vaccine effectiveness (VE), including the effect of repeated annual vaccination.
Methods. We recruited 321 households with 1426 members, including 833 children, and followed them during the 2012–2013 influenza season; specimens were collected from subjects with reported acute respiratory illnesses. We estimated the effectiveness of documented influenza vaccination in preventing laboratory-confirmed influenza, using adjusted Cox proportional hazards models. Antibody titers in a subset of subjects were determined by a hemagglutination inhibition assay to determine the subjects’ preseason susceptibility to influenza.
Results. Influenza was identified in 76 (24%) households and 111 (8%) individuals. VE point estimates indicated significant protection in adults (48%; 95% confidence interval [CI], 1%–72%), similar protection in children aged 9–17 years (49%; 95% CI, −16% to 78%), but no evidence of effectiveness in children aged <9 years (−4%; 95% CI, −110% to 49%). Lower VE was observed in those vaccinated in both the current and prior seasons, compared with those vaccinated in the current season only; susceptibility titers against type A but not type B were consistent with this observation. Residual protection from vaccination in the prior season was indicated by both VE and serologic results.
Conclusions. Prior vaccination appears to modify VE by both residual protection and reduced vaccine response.
Keywords: influenza, vaccine effectiveness, households with children, serologic susceptibility
(See the editorial commentary by Neuzil on pages 1517–8.)
Questions have been raised about the value of influenza vaccines, in part because of recognized variation in vaccine effectiveness (VE) related to the age of the vaccine recipients and the strains circulating but also because of methodological considerations [1–3]. As a result, assessments of VE are conducted annually in many countries [4–10]. Most evaluations are observational but use laboratory-confirmed outcomes; many estimate VE in preventing medically attended influenza, and use the test-negative design [4–9]. In the United States, multiple centers (ie, members of the US FLU VE Network) have collaborated annually since the 2008–2009 season to estimate VE in the healthcare setting with subject enrollment at the time of acute respiratory illness; similar studies have been conducted in Canada, Australia, and Europe [4–9]. Generally, these studies have indicated moderate VE in prevention of medically attended influenza, with lower effectiveness against influenza A(H3N2) viruses, compared with A(H1N1) and type B strains, and variation in effectiveness by age category [4–9].
Beginning with the 2010–2011 season, a complementary study using an alternative design was conducted among households with children in Michigan. In this study, the cohort of households was defined in advance and followed through the influenza season [10]. This approach allowed examination of symptomatic influenza illnesses of any severity and assessments of VE in preventing community and, separately, household-acquired influenza. Results from our 2010–2011 household study indicated lower VE than demonstrated in studies performed in healthcare settings in the same season [5, 9]. We also unexpectedly found lower effectiveness point estimates with repeated vaccination and no evidence that vaccination prevented household transmission once influenza was introduced. Here, we extend our evaluation of VE in households during the 2012–2013 season, with inclusion of serologic assessments of preseason susceptibility to assist in explaining observations. This influenza season was characterized by an early start and long duration, with circulation of primarily influenza A(H3N2) and influenza B/Yamagata lineage; these circulating viruses were considered vaccine like [11].
METHODS
Recruitment and Enrollment
Households were derived from persons who had selected a primary healthcare provider from the University of Michigan Health System in Ann Arbor [10]. Eligible households (shared residence) comprised at least 4 participating members, at least 2 of whom were children aged <18 years. At enrollment visits performed from June through September 2012, adult household members provided written informed consent for participation for themselves and their children, and children aged 7–17 years provided their oral assent. Demographic data were reported, and study access to health system medical records was granted. All study contacts with participants, including enrollment and illness visits, were performed at the research study site at the University of Michigan School of Public Health (UM-SPH). The study was approved by the institutional review board at the University of Michigan Medical School.
Influenza Surveillance and Laboratory Testing
Surveillance was performed from October 2012 through early May 2013. Households were instructed at enrollment and via weekly email reminders to report all acute respiratory illnesses in which ≥2 of the following symptoms were present: cough, fever or feverishness, nasal congestion, chills, headache, body aches, and/or sore throat. Subjects with eligible illnesses had a combined throat and nasal swab specimen (or, for children aged <3 years, a nasal swab specimen only) collected at an illness visit within 7 days of illness onset. Illnesses were followed for collection of data on whether the participant sought medical attention; healthcare contact for illness treatment was also documented, based on medical record review.
Respiratory tract specimens were tested for influenza virus by real-time reverse transcription polymerase chain reaction (RT-PCR) in the investigators' laboratory at the UM-SPH. The RT-PCR primers, probes, and testing protocol were developed and provided by the Influenza Division of the Centers for Disease Control and Prevention and designed for universal detection of influenza A and B viruses, subtype identification of influenza A viruses, and lineage determination of influenza B viruses.
Blood Specimen Collection and Serologic Assay
Blood specimens were collected from participants aged ≥13 years who volunteered to have a single tube of blood collected at up to 3 time points annually; specimens were collected at summer enrollment visits and at scheduled visits before (during late fall) and after (during spring) the influenza season. Sera from all collected specimens were tested with the hemagglutination inhibition (HAI) assay [12, 13], using as antigens the virus strains present in the 2012–2013 North American influenza vaccine (H1N1: A/California/07/2009; H3N2: A/Texas/50/2012 [A/Victoria/361/2011-like]; B/Yamagata: B/Texas/20/2011 [B/Wisconsin/1/2010-like]), plus the alternative B lineage not contained in the vaccine (B Victoria: B/Brisbane/60/2008) [14]. Sera were also tested with the neuraminidase inhibition (NAI) assay [15, 16] against the A(H3N2) vaccine strain; this assay used a reassortant influenza virus with a mismatched hemagglutinin (HA) protein (H6 subtype), to avoid interference by HA-specific antibodies. Serologic testing was performed in the investigators' laboratory at the UM-SPH. Preseason susceptibility to influenza was estimated using antibody titers determined by the HAI and NAI assays in sera collected at the late fall preseason visits (or at summer enrollment, for those subjects without late fall preseason specimens and no evidence of influenza vaccine receipt) [12, 13, 15, 16]. For vaccinated subjects, these susceptibility measures represented postvaccination titers.
Statistical Analyses
Households were characterized by size and composition, and subjects were characterized by demographic characteristics, high-risk health status, and influenza vaccination status. Health system medical records were reviewed to document the presence of health conditions considered high risk for complications of influenza [17]. Documentation of influenza vaccine receipt (both current and prior season) was based on evidence in medical records or the Michigan Care Improvement immunization registry. Associations of subject characteristics with vaccination status and influenza outcomes were examined and compared by χ2 tests.
Cox proportional hazards models were used to estimate the effectiveness of receipt of at least 1 dose of influenza vaccine in preventing RT-PCR–confirmed influenza. Vaccination status was modeled as a time-varying covariate, with subjects considered vaccinated 14 days after vaccine receipt. VE was calculated as 100 × [1 − hazard ratio] and estimated in unadjusted and adjusted models; adjusted models included values for subject age and high-risk health status [10, 18]. We also estimated VE for each combination of current-season and prior-season vaccine exposure (ie, current only, both current and prior, and prior only), with subjects unvaccinated in both seasons as the reference group [6, 10, 19].
Analyses estimated the VE in preventing all influenza and, separately, community-acquired influenza (household index cases), household-acquired influenza (secondary cases resulting from exposure to household index cases), and medically attended influenza. A secondary (household-acquired) case was defined by transmission link to an index case if both cases were due to the same influenza virus type/subtype/lineage and if illness onset in the secondary case occurred 1–7 days after illness onset in the index case. Overall and community VE were estimated by comparing the hazard of influenza among vaccinated and unvaccinated subjects; cases that were household acquired were censored at the time of illness onset for community estimates. Household VE was estimated by comparing the hazard of influenza among vaccinated and unvaccinated subjects exposed to a household index case. The VE in preventing medically attended influenza was estimated by comparing the hazard of this outcome among vaccinated and unvaccinated subjects; cases that were not medically attended were censored at illness onset. Only the first case of influenza was considered for individuals with multiple influenza outcomes; similarly, only influenza outcomes resulting from the first introduction of influenza to a household were considered. Additional analyses estimated the influenza virus type–specific VE for all influenza cases.
HAI and NAI antibody titers, representing preseason assessments of susceptibility to each virus tested, were calculated for each subject as the reciprocal (eg, 160) of the highest dilution of sera (eg, 1:160) that inhibited hemagglutination or neuraminidase activity. Titers below the lower limit of detection (ie, <10) were considered as half the lower limit (ie, 5); titers greater than the upper limit of detection (ie, >5120) were considered twice the upper limit (ie, 10 240). Log transformation was applied to all HAI and NAI titers, and the mean and standard deviation (SD) of the transformed values were calculated and then exponentiated to obtain the geometric mean and SD. Geometric mean titers (GMTs) were estimated for each combination of current-season and prior-season vaccine exposure (ie, current only, both current and prior, prior only, and neither current nor prior) and by joint vaccination and case status. GMTs were compared across these categories, using Wilcoxon rank sum tests. All statistical analyses were performed using SAS software (release 9.2; SAS Institute). A P value of <.05 or a positive lower bound of a confidence interval (CI) were considered to indicate statistical significance.
RESULTS
Characteristics of Households and Participants
A total of 1426 subjects from 321 households were enrolled in the 2012–2013 study year; 162 households (50%) had participated in previous years. Household size ranged from 4 to 10 members (mean [±SD], 4.4 ± 0.8 members). All households had at least 2 participating children, and 228 households (71%) had ≥1 child aged <9 years.
Participant characteristics, plus distributions of vaccination status and influenza outcomes, are presented in Table 1. Approximately 10% of subjects had high-risk health conditions, and 845 subjects (59%) had documented evidence of influenza vaccine receipt for the 2012–2013 season. Among vaccinated subjects, 728 (86%) received an inactivated vaccine, and 116 (14%) received the live attenuated vaccine (96% of live vaccine recipients were children aged <18 years). Vaccine coverage varied by age and race categories and was significantly higher in subjects with high-risk conditions. For the 2012–2013 influenza season, children aged <9 years were recommended to receive 1 dose of 2012–2013 vaccine if they had received at least 2 prior doses of vaccine since 1 July 2010; 2 doses of 2012–2013 vaccine were recommended otherwise [20]. These recommendations were used to classify study subjects <9 years of age as fully or partially vaccinated; 83% of vaccinated children <9 years of age were considered fully vaccinated.
Table 1.
Characteristics of Participating Household Members During the 2012–2013 Influenza Season, by Documented Influenza Vaccine Receipt and Influenza Case Status: Household Influenza Vaccine Effectiveness Study, Ann Arbor, Michigan
| Characteristic | All Subjects, No. (%) (n = 1426) | Vaccinated, Subjectsa Proportion (%) | Influenza Positive, Subjects, Proportion (%) |
|---|---|---|---|
| Age category | |||
| <9 y | 462 (32.4) | 316/462 (68.4)b,c | 45/462 (9.7) |
| 9–17 y | 371 (26.0) | 224/371 (60.4) | 26/371 (7.0) |
| 18–49 y | 536 (37.6) | 276/536 (51.5) | 35/536 (6.5) |
| ≥50 y | 57 (4.0) | 29/57 (50.9) | 5/57 (8.8) |
| Race | |||
| White | 1082 (75.9) | 668/1082 (61.7)c | 82/1082 (7.6) |
| Asian | 121 (8.5) | 74/121 (61.2) | 12/121 (9.9) |
| Black | 117 (8.2) | 61/117 (52.1) | 8/117 (6.8) |
| Other/unknown | 106 (7.4) | 42/106 (39.6) | 9/106 (8.5) |
| Sex | |||
| Female | 712 (49.9) | 434/712 (61.0) | 52/712 (7.3) |
| Male | 714 (50.1) | 411/714 (57.6) | 59/714 (8.3) |
| Documented high-risk health condition | |||
| Any | 136 (9.5) | 100/136 (73.5)c | 6/136 (4.4) |
| None | 1290 (90.5) | 745/1290 (57.8) | 105/1290 (8.1) |
| Documented influenza vaccinationa | |||
| Yes | 845 (59.3) | … | 51/845 (6.0)d |
| No | 581 (40.7) | … | 60/581 (10.3) |
| Overall | 1426 (100) | 845/1426 (59.3) | 111/1426e (7.8) |
a Defined as receipt of least 1 influenza vaccine dose anytime during the 2012–2013 vaccination period, as documented in the medical record or state immunization registry. Subjects with laboratory-confirmed influenza were considered vaccinated if vaccine was administered ≥14 days before illness onset.
b A total of 267 of 320 vaccinated children <9 years of age (83.4%) were considered fully vaccinated, as defined by Advisory Committee on Immunization Practices recommendations.
c P < .001, by χ2 analysis, for comparison of vaccinated subjects to unvaccinated subjects or subjects with laboratory-confirmed influenza to subjects without laboratory-confirmed influenza.
d P < .05, by χ2 analysis, for comparison of vaccinated subjects to unvaccinated subjects or subjects with laboratory-confirmed influenza to subjects without laboratory-confirmed influenza.
e A total of 116 influenza cases were identified in 111 individuals. The characteristics of those individuals are presented here.
Illness Surveillance and Influenza Outcomes
During surveillance, 695 subjects (49%) from 240 households (75%) reported 1227 acute respiratory illnesses, and 1133 specimens (92%) were collected. All illness specimens were tested for influenza virus by RT-PCR, and results for 116 (10%) were positive. Influenza virus types A and B circulated locally between mid-November 2012 and late April 2013, with type A predominating early and type B predominating late. Among the influenza cases, 65 (56%) were identified as influenza A(H3N2), 47 (41%) as influenza B, 3 (3%) as influenza A(H1N1)pdm09, and 1 (1%) as influenza A(H3N2)/influenza B coinfection; 37 (77%) of the influenza B cases were from the vaccine strain Yamagata lineage.
Influenza was identified in 76 households (24%) and 111 individuals (8%), including 5 individuals with 2 separate infections. Influenza virus infection risks were significantly lower in vaccinated subjects, compared with unvaccinated subjects (6.0% vs 10.3%; P = .003). Infection risks in children aged <9 years and considered fully vaccinated (10.6%) did not significantly differ from the risk for those considered only partially vaccinated (5.7%), who tended to be older. Influenza A(H3N2) infection risks were similar across age groups, but risks for both influenza B lineages were 2–3 times greater in children aged <9 years, compared with older children and adults (Table 2). Thirty-one influenza cases (27%) were considered household acquired, based on exposure to 85 index or coindex community-acquired infections. Thirty-five influenza illnesses (30%) were medically attended; the proportion of medically attended influenza illnesses was significantly higher for children, compared with adults (37% vs 17%; P = .02).
Table 2.
Influenza Virus Infection Risks During the 2012–2013 Influenza Season, by Subject Age Category and Influenza A Subtype and B Lineage: Household Influenza Vaccine Effectiveness Study, Ann Arbor, Michigan
| Age | All Subjects | Influenza A(H3N2)a | Influenza A(H1N1) | Influenza B/Yamagataa,b | Influenza B/Victoriab |
|---|---|---|---|---|---|
| Overall | 1426 (100) | 66/1426 (4.6) | 3/1426 (0.2) | 37/1426 (2.6) | 10/1426 (0.7) |
| <9 y | 462 (32.4) | 22/462 (4.8) | 1/462 (0.2) | 20/462 (4.3) | 7/462 (1.5) |
| 9–17 y | 371 (26.0) | 15/371 (4.0) | 0/371 (0.0) | 8/371 (2.2) | 2/371 (0.5) |
| ≥18 y | 593 (41.6) | 29/593 (4.9) | 2/593 (0.3) | 9/593 (1.5) | 1/593 (0.2) |
Data are influenza positive, subjects, and proportion (%).
a Six individuals with both influenza A(H3N2) and influenza B/Yamagata (1 with coinfection and 5 with separate infections) are counted in both columns.
b One individual with influenza B, for which the lineage could not be determined, is excluded.
Determinants of Influenza VE
Influenza virus infection risks for vaccinated and unvaccinated subjects and results from unadjusted and adjusted VE models are presented in Table 3. Risks for overall, community-acquired, household-acquired, and medically attended illnesses were 7.8%, 5.8%, 10.2%, and 2.2% (based on first illnesses and first household introductions only), respectively. Infection risks were highest in children aged <9 years and, with the exception of influenza B infection in young children, lower in vaccinated subjects.
Table 3.
Estimates of Vaccine Effectiveness in Preventing All Influenza Outcomes During the 2012–2013 Influenza Season, by Age and Influenza Virus Type, and Community-Acquired, Household-Acquired, and Medically Attended Influenza: Household Influenza Vaccine Effectiveness Study, Ann Arbor, Michigan
| Analysis Set | Influenza Positive, Subjects, Proportion (%) |
VE,a % (95% CI) |
||
|---|---|---|---|---|
| Vaccinated | Unvaccinated | Unadjusted | Adjustedb | |
| Agec | ||||
| Overall | 51/845 (6.0) | 60/581 (10.3) | 30 (−9 to 55) | 32 (−6 to 56) |
| <9 y | 27/316 (8.5) | 18/146 (12.3) | 3 (−97 to 52) | −4 (−110 to 49) |
| 9–17 y | 11/224 (4.9) | 15/147 (10.2) | 46 (−24 to 76) | 49 (−16 to 78) |
| ≥18 y | 13/305 (4.3) | 27/288 (9.4) | 49 (5–73) | 48 (1–72) |
| Influenza A, by agec | ||||
| Overall | 28/845 (3.3) | 41/581 (7.1) | 43 (2–67) | 40 (−4 to 65) |
| <9 y | 12/316 (3.8) | 11/146 (7.5) | 31 (−73 to 73) | 21 (−98 to 68) |
| 9–17 y | 6/224 (2.7) | 9/147 (6.1) | 47 (−62 to 83) | 48 (−62 to 84) |
| ≥18 y | 10/305 (3.3) | 21/288 (7.3) | 48 (−3 to 74) | 45 (−12 to 73) |
| Influenza B, by agec | ||||
| Overall | 28/845 (3.3) | 20/581 (3.4) | −11 (−131 to 47) | 7 (−94 to 55) |
| <9 y | 19/316 (6.0) | 8/146 (5.5) | −52 (−325 to 46) | −53 (−326 to 45) |
| 9–17 y | 5/224 (2.2) | 6/147 (4.1) | 44 (−96 to 84) | 51 (−53 to 85) |
| ≥18 y | 4/305 (1.3) | 6/288 (2.1) | 35 (−133 to 82) | 45 (−92 to 84) |
| Community-acquired influenzad | 39/845 (4.6) | 43/581 (7.4) | 27 (−13 to 54) | 30 (−9 to 55) |
| Household-acquired influenzae | 11/139 (7.9) | 16/126 (12.7) | 31 (−73 to 73) | 37 (−73 to 77) |
| Medically attended influenzaf | 13/845 (1.5) | 18/581 (3.1) | 39 (−26 to 71) | 43 (−18 to 72) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Defined as the effectiveness of at least 1 dose of influenza vaccine in preventing laboratory-confirmed influenza and calculated as follows: 100 × [1 − hazard ratio]. Vaccination status was modeled as time-varying covariate, with subjects considered vaccinated 14 days after vaccine receipt. To adjust for correlation of exposures and outcomes among subjects in the same household, robust variances for model parameter estimates were computed using sandwich estimators [17].
b Models adjusted for age in months (natural cubic spline) and documentation (present or absent) of high-risk health status in the medical record.
c Five subjects had separate influenza A and influenza B virus infections, and 1 subject had influenza A and B virus coinfection. Outcomes for these 6 individuals were considered in analyses of VE against both influenza A and influenza B virus infection, but only the first outcome was considered in analysis of VE against all influenza virus infections.
d Eighty-five cases of influenza were defined as community acquired, but 3 cases are excluded here because they occurred in a subject with a prior case of community-acquired influenza.
e Thirty-one cases of influenza were defined as household acquired, but 4 cases are excluded here because they occurred as a result of a second introduction of influenza (different type/subtype/lineage and/or >7 days from prior case) to a household.
f Thirty-five influenza illnesses were medically attended, based on self-report or medical record documentation of healthcare contact for illness treatment, but 4 are excluded here because they occurred in a subject with a prior case of influenza.
Adjusted VE against all influenza outcomes was 32% (95% CI, −6% to 56%). VE point estimates indicated significant protection in adults (48%; 95% CI, 1%–72%), similar but nonsignificant protection in children 9–17 years (49%; CI, −16% to 78%), but no evidence of protection in children <9 years (−4%; 95% CI, −110% to 49%). In children aged <9 years and 9–17 years, VE estimates for inactivated and live attenuated vaccines were similar to overall estimates by age group and not statistically different (data not shown).
VE against influenza A outcomes (96% were influenza A[H3N2]) was 40% (95% CI, −4% to 65%); VE against influenza B outcomes was 7% (95% CI, −94% to 55%), with no evidence of VE against influenza B in children <9 years. Vaccine was 30% (95% CI, −9% to 55%) effective in preventing community-acquired influenza, 37% (95% CI, −73% to 77%) in preventing household-acquired influenza, and 43% (95% CI, −18% to 72%) in preventing medically attended influenza.
We also estimated overall VE for each combination of current-season and prior-season vaccine exposure, using subjects who were unvaccinated in both seasons as the reference group [6, 10, 19]. Children aged <9 years were excluded here because definitions of fully vaccinated in the current season depended on prior vaccination in this age group. Results indicated the lowest infection risk and highest VE point estimate for the relatively few subjects vaccinated in the current year only (88%; 95% CI, 7%–98%), with higher infection risks and lower but still significant VE for the larger number of subjects vaccinated in both the current and prior year (47%; 95% CI, 11%–69%; Table 4).
Table 4.
Estimates of Vaccine Effectiveness (VE) in Preventing Laboratory-Confirmed Influenza During the 2012–2013 Influenza Season, by 2-Year Vaccination Status Among Subjects ≥9 Years of Age: Household Influenza VE Study, Ann Arbor, Michigan
| Vaccination Status | Influenza Positive, Subjects, Proportion (%) | VE,a % (95% CI) |
|
|---|---|---|---|
| Unadjusted | Adjustedb | ||
| 2012–2013 only | 1/88 (1.1) | 87 (2–98) | 88 (7–98) |
| Both 2011–2012 and 2012–2013 | 23/441 (5.2) | 48 (10–69) | 47 (11–69) |
| 2011–2012 only | 6/91 (6.6) | 38 (−67 to 77) | 43 (−66 to 80) |
| Neither year | 36/344 (10.5) | Reference | Reference |
Abbreviation: CI, confidence interval.
a Calculated as 100 × [1 − hazard ratio].
b Models adjusted for age in months (natural cubic spline) and documentation (present or absent) of high-risk health status in the medical record.
Serologic Assessments
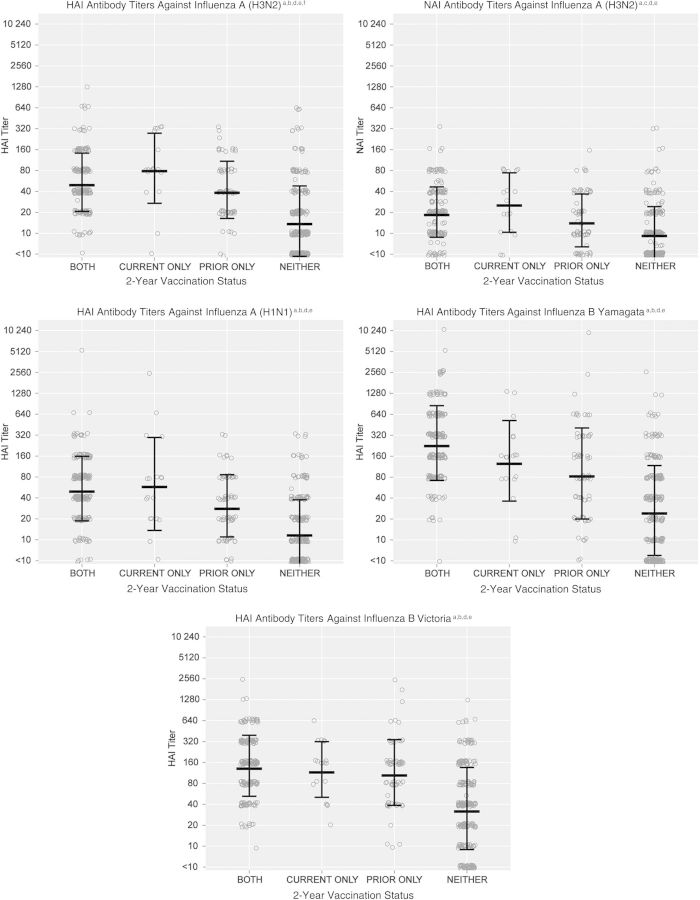
HAI antibody titers measured in sera collected from 504 subjects (66% of all subjects aged ≥13 years) were used to estimate preseason susceptibility to the 3 vaccine strains plus the alternate influenza B lineage; NAI antibody titers for the same subjects targeted the influenza A(H3N2) vaccine strain only. Figure 1 presents the distributions of titers against each influenza virus strain, based on each combination of current-season and prior-season vaccine exposure, with GMTs and SDs denoted by linked lines. Results indicated significantly higher HAI GMTs for subjects with each vaccine exposure, compared with GMTs for subjects unvaccinated in both years against each influenza virus strain. HAI GMTs to both the vaccine and alternative-lineage influenza B strains were similarly high (>80) and not statistically different for each vaccine exposure; the highest GMTs were estimated for those subjects vaccinated both years. In contrast, HAI GMTs against the influenza A(H3N2) and influenza A(H1N1)pdm09 strains were lower overall (≤80), with slightly higher GMTs for subjects vaccinated in the current season only, compared with subjects vaccinated both years, and significantly higher GMTs for influenza A(H3N2) (P = .03). NAI GMTs against the influenza A(H3N2) target were lower (<40) than the corresponding HAI GMTs but also suggested a slightly higher GMT for individuals vaccinated in the current season only, compared with individuals vaccinated both years (P = .12).
Figure 1.
Preseason susceptibility, based on antibody titers measured by hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) assays, in subjects, by combinations of influenza vaccination status in the 2011–2012 and 2012–2013 seasons. aAntibody titers measured by HAI and NAI assays in sera collected from a subset of subjects aged ≥13 years at preseason visits (or at enrollment, for subjects without preseason specimens and no evidence of influenza vaccine receipt) were used to estimate preseason susceptibility to influenza; bSera were tested with the HAI assay, using as antigens the virus strains present in the 2012–2013 North American influenza vaccine (A[H1N1]: A/California/07/2009; A[H3N2]: A/Texas/50/2012 [A/Victoria/361/2011-like]; B/Yamagata: B/Texas/20/2011 [B/Wisconsin/1/2010-like]) plus the alternative influenza B lineage not contained in the vaccine (B Victoria: B/Brisbane/60/2008); cSera were tested with the NAI assay, using the influenza A(H3N2) strain present in the 2012–2013 North American influenza vaccine (A/Texas/50/2012 [A/Victoria/361/2011-like]) as the antigen; dEach circle indicates the titer of an individual observation. Linked lines indicate the geometric mean titer (GMT) ± the geometric standard deviation; eAll vaccination groups (both years, current only, and prior only) had significantly higher GMTs (P < .001) than those unvaccinated both years for all antigens; fGMTs were significantly higher (P = .03) among those vaccinated in the current year only, compared with those vaccinated both years for the H3 antigen but not for the other antigens.
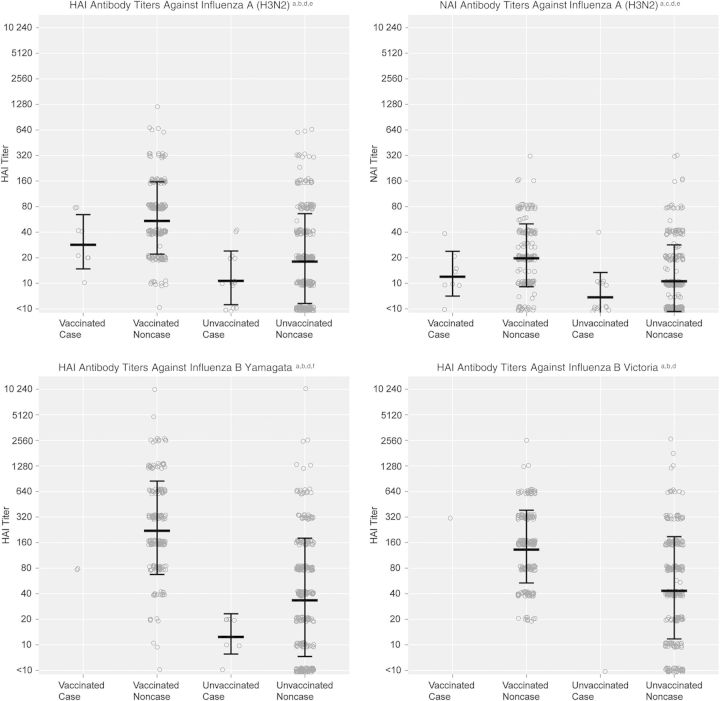
Figure 2 presents HAI and NAI susceptibility titers for vaccinated and unvaccinated subjects who ultimately were influenza cases or noncases, based on virus identification by RT-PCR. Thirty-four influenza cases (31% of 111 persons with influenza) had susceptibility assessments. Influenza A(H3N2) and influenza B/Yamagata cases had significantly lower HAI and NAI GMTs than noncases overall, indicating greater susceptibility. This pattern was consistent, although not statistically significant, when stratified by vaccination status.
Figure 2.
Preseason susceptibility, based on antibody titers measured by hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) assays, in subjects, by influenza vaccination and infection status. aAntibody titers measured by HAI and NAI assays in sera collected from a subset of subjects aged ≥13 years at preseason visits (or at enrollment, for subjects without preseason specimens and no evidence of influenza vaccine receipt) were used to estimate preseason susceptibility to influenza; bSera were tested with the HAI assay, using as antigens the virus strains present in the 2012–2013 North American influenza vaccine (A[H1N1]: A/California/07/2009; A[H3N2]: A/Texas/50/2012 [A/Victoria/361/2011-like]; B/Yamagata: B/Texas/20/2011 [B/Wisconsin/1/2010-like]) plus the alternative influenza B lineage not contained in the vaccine (B Victoria: B/Brisbane/60/2008); cSera were tested with the NAI assay, using the influenza A(H3N2) strain present in the 2012–2013 North American influenza vaccine (A/Texas/50/2012 [A/Victoria/361/2011-like]) as the antigen; dEach circle indicates the titer of an individual observation. Linked lines indicate the geometric mean titer ± the geometric standard deviation; eInfluenza A(H3N2) cases had significantly lower geometric mean HAI and NAI titers than noncases overall (P = .02 and P = .02, respectively); this pattern was consistent for both vaccinated subjects (P = .06 and P = .09, respectively) and unvaccinated subjects (P = .17 and P = .07, respectively); fInfluenza B/Yamagata cases had significantly lower geometric mean HAI titers than noncases overall (P = .01); this pattern was consistent for both vaccinated subjects (P = .13) and unvaccinated subjects (P = .11).
DISCUSSION
During the 2012–2013 season, we observed substantial circulation of influenza A(H3N2) and influenza B viruses in a highly vaccinated population of households with children. VE point estimates indicated significant protection, near 50%, in adults (90% were <50 years of age), similar but nonsignificant protection in children aged 9–17 years, but no evidence of effectiveness in children aged <9 years; VE was especially poor in young children (mean age, 5.0 years; median age, 5.3 years) against influenza B. Findings from this season extend those previously reported for the 2010–2011 season [10]. In that season, we observed lower VE than demonstrated in studies performed in healthcare settings in the same season, an apparent negative effect of prior vaccination on current-season VE estimates, and no evidence that vaccination prevented household transmission once influenza was introduced [10].
The observed effect of lower VE point estimates with repeated vaccination was demonstrated subsequently in the US Flu VE Network in the 2011–2012 season, in a similar study performed in Australia, and now in the current household study (during the 2012–2013 season) [6, 19]. To explain this observation, serologic specimens were collected from a subset of older children and adults to estimate preseason susceptibility to influenza. Preseason HAI and NAI antibody titers against influenza A(H3N2) were consistent with the observed patterns of VE for each combination of current-season and prior-season vaccination status, with significantly higher GMTs observed for those vaccinated in the current year only. The situation was different for influenza B, where observed GMTs were slightly but not significantly higher for subjects vaccinated in both years, compared with those vaccinated in the current year only. Residual protection for those vaccinated in the prior season only was indicated in both serologic and VE results.
These observations are consistent with 2 mechanisms by which past vaccination may reduce current-season VE. The first supports the hypothesis that a small or no change in strains included in the vaccine from year to year may reduce antibody response and, possibly, VE [21–24]. The influenza B vaccine strain in 2012–2013 had switched lineages from B Victoria to B Yamagata, while the A(H3N2) vaccine strain was updated, owing to evidence of antigenic drift in circulating viruses [14]. The second is that inclusion of subjects in the unvaccinated comparison group who were vaccinated in the prior season reduces overall estimates of current-season VE, owing to residual immunity. Confirmatory studies of the effects of longer-term vaccination and infection histories and their methodological implications are warranted. Serologic studies were not performed on young children and, as such, offer no explanation for the poor vaccine performance demonstrated in that age group. Low VE and poor serologic responses with nonadjuvanted vaccine were demonstrated in a recent clinical trial of children aged <6 years [25]. Our results support the recommendation for annual vaccination, given current vaccine options, because those who were unvaccinated in the current year appear to be at greater risk, when considering both VE and serologic evidence.
In the 2012–2013 season, we again observed lower overall VE point estimates, compared with VE estimates, in preventing medically attended influenza from the US Flu VE Network (32% vs 56%) [26]; our estimates for prevention of medically attended influenza were slightly higher (43%) than those for prevention of all influenza. Data from a recent clinical trial suggested that the influenza vaccine is more efficacious in preventing severe than mild illness [27]. Given that prior vaccination appears to modify VE both in terms of residual protection and reduced vaccine response, differing patterns of vaccination coverage and history in the 2 study populations may also have contributed to differences in VE. Instability of VE estimates due to the relatively small sample size of our cohort may also explain some of the differences; based on our sample size, vaccine coverage, and influenza virus infection risk, we had 80% power to estimate significant overall VE as low as 42% and less power for stratified estimates.
As demonstrated previously in the 2010–2011 household study [10], infection risks in the current season were higher in the household than in the community, but in contrast to the 2010–2011 season, VE estimates for prevention of household-acquired and community-acquired influenza were similar. In the 2010–2011 season, adults were at particular risk of household-acquired influenza despite vaccination, and we speculated that this excess risk may have been a consequence of providing care for individuals with illness [10]. Those results were not confirmed in the current season; while household secondary infection risks remained high in adults, poor vaccine performance was limited to children aged <9 years.
A particular strength of the longitudinal design of the household study is the ability to allow vaccination status in models estimating VE to vary with time. In seasons when subjects continue to be vaccinated after the start of influenza virus circulation, effectiveness estimates can be biased upward; an extreme example of this was seen during the 2009 pandemic, when vaccine became available in most of the United States after the outbreak [4]. In the current study, nearly one third of those vaccinated received vaccine after the first case of influenza was identified. As a result, comparison of the infection risks for vaccinated and unvaccinated subjects would suggest higher VE than that estimated by the models (Table 3). This adjustment is more difficult in test-negative design studies, in which the relationship between calendar time, vaccination, and infection must be explicitly defined and modeled [2, 3].
Household cohort studies can make a significant contribution to annual VE evaluations. They are less susceptible to selection bias, because of enrollment before infection; they can evaluate VE in preventing influenza illnesses of any severity; and they can make use of longitudinal data on vaccination and infection histories if households are followed for >1 season. However, they are logistically challenging to organize and sustain, and sample size can be an issue. This problem can be at least partially addressed by recruiting a larger cohort; this strategy is easily justified by the added information provided.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC (U01 IP000474) and the National Institute of Allergy and Infectious Diseases (R01 AI097150).
Potential conflicts of interest. S. E. O. has received grant support from Sanofi Pasteur. A. S. M. has received grant support from Sanofi Pasteur and consultancy fees from Sanofi, GlaxoSmithKline, and Novavax. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Osterholm MT, Kelley NS, Sommer A, Belongia A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 2.Foppa IM, Haber M, Ferdinands JM, et al. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104–9. [DOI] [PubMed] [Google Scholar]
- 3.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 19:2165–8. [DOI] [PubMed] [Google Scholar]
- 4.Griffin MR, Monto AS, Belongia EA, et al. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One 2011; 6:e23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treanor J, Talbot HK, Ohmit SE, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multi-centre case control study. Euro Surveill 2013; 18:pii:20390. [DOI] [PubMed] [Google Scholar]
- 8.Fielding JE, Grant KA, Tran T, Kelly HA. Moderate influenza vaccine effectiveness in Victoria, Australia, 2011. Euro Surveill 2012; 17:11. [PubMed] [Google Scholar]
- 9.Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010–2011. Clin Infect Dis 2012; 55:333–42. [DOI] [PubMed] [Google Scholar]
- 10.Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC FluView. 2012–2013 influenza season surveillance summary. http://www.cdc.gov/flu/weekly/pdf/12–13%20Season%20Summary.pdf. Accessed 4 December 2014.
- 12.de Jong JC, Palache AM, Beyer WEP, Rimmelzwaan GF, Boon ACM, Osterhaus ADME. Haemagglutination-inhibiting antibody to influenza virus. In: Brown F, Haaheim LR, Schild GC, eds. Laboratory correlates of immunity to influenza—a reassessment. Basel, Switzerland: Karger, 2003:63–73. [Google Scholar]
- 13.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879–85. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. Weekly Epidemiologic Report 2012; 87:83–96. [PubMed] [Google Scholar]
- 15.Lambre CR, Terzidis H, Greffard A, Webster RG. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods 1990; 135:49–57. [DOI] [PubMed] [Google Scholar]
- 16.Cate TR, Rayford Y, Nino D, et al. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 2010; 28:2076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;55(RR-08):1–62. [PubMed] [Google Scholar]
- 18.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Statistical Assoc 1989; 84:1065–73. [Google Scholar]
- 19.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required [correspondence]. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP) – United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2012; 61:613–8. [PubMed] [Google Scholar]
- 21.Beyer WE, de Bruijn IA, Palache AM, Westerdorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination; a met-analysis of serologic and field studies. Arch Intern Med 1999; 159:182–8. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008; 3:e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DJ, Lapedes AS, Forrest S, et al. Modeling the effects of updating the influenza vaccine on the efficacy of repeated vaccination. In: Osterhaus ADME, Cox N, Hampson A, eds. Options for the control of influenza virus IV. International Congress Series 1219 Amsterdam: Excerpta Medica, 2001:655–60. [Google Scholar]
- 25.Vesikari T, Knuf M, Wutzler P, et al. Oil-in-Water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–16. [DOI] [PubMed] [Google Scholar]
- 26.Jackson L, Jackson ML, Phillips CH, et al. Interim adjusted estimates of seasonal influenza vaccine effectiveness - United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481–91. [DOI] [PubMed] [Google Scholar]