SUMMARY
Campylobacter jejuni infection is one of the most widespread infectious diseases of the last century. The incidence and prevalence of campylobacteriosis have increased in both developed and developing countries over the last 10 years. The dramatic increase in North America, Europe, and Australia is alarming, and data from parts of Africa, Asia, and the Middle East indicate that campylobacteriosis is endemic in these areas, especially in children. In addition to C. jejuni, there is increasing recognition of the clinical importance of emerging Campylobacter species, including Campylobacter concisus and Campylobacter ureolyticus. Poultry is a major reservoir and source of transmission of campylobacteriosis to humans. Other risk factors include consumption of animal products and water, contact with animals, and international travel. Strategic implementation of multifaceted biocontrol measures to reduce the transmission of this group of pathogens is paramount for public health. Overall, campylobacteriosis is still one of the most important infectious diseases that is likely to challenge global health in the years to come. This review provides a comprehensive overview of the global epidemiology, transmission, and clinical relevance of Campylobacter infection.
INTRODUCTION
Campylobacter species are Gram-negative spiral, rod-shaped, or curved bacteria with a single polar flagellum, bipolar flagella, or no flagellum, depending on the species (1). Campylobacter species are non-spore-forming, are approximately 0.2 to 0.8 by 0.5 to 5 μm, and are chemoorganotrophs which obtain their energy sources from amino acids or tricarboxylic acid cycle intermediates (2). Most Campylobacter species grow under microaerobic conditions and have a respiratory type of metabolism; however, several species (Campylobacter concisus, Campylobacter curvus, Campylobacter rectus, Campylobacter mucosalis, Campylobacter showae, Campylobacter gracilis, and, to a certain extent, Campylobacter hyointestinalis) require hydrogen or formate as an electron donor for microaerobic growth. In addition, certain species prefer anaerobic conditions for growth.
The Campylobacter genus was established in 1963 following the renaming of Vibrio fetus to Campylobacter fetus, forming the type species of this genus (3). The Campylobacter genus belongs to the family Campylobacteraceae, the order Campylobacterales, the class Epsilonproteobacteria, and the phylum Proteobacteria. Since its first description, the genus has grown to include several important human and animal pathogens that are primarily classified through phylogenetic means. The genus Campylobacter consists of 26 species, 2 provisional species, and 9 subspecies (as of December 2014).
Campylobacter jejuni is a major cause of gastroenteritis worldwide. Moreover, C. jejuni infection may lead to autoimmune conditions known as Guillain-Barré syndrome (GBS) and Miller Fisher syndrome. Many Campylobacter species are known pathogens in humans and animals (1). In humans, Campylobacter species have been associated with a range of gastrointestinal conditions, including inflammatory bowel diseases (IBD), Barrett's esophagus, and colorectal cancer (Fig. 1) (1). They have also been reported to be involved in extragastrointestinal manifestations, including bacteremia, lung infections, brain abscesses, meningitis, and reactive arthritis, in individual cases and small cohorts of patients (1). A full list of the clinical manifestations associated with Campylobacter infection is presented in Table 1. The precise role of Campylobacter species in the development of these clinical conditions is largely unknown. In this review, we describe the latest global epidemiological landscape of C. jejuni and other Campylobacter species in gastroenteritis and other diseases. We also discuss the modes of transmission and biocontrol methodologies to prevent transmission of campylobacteriosis.
FIG 1.
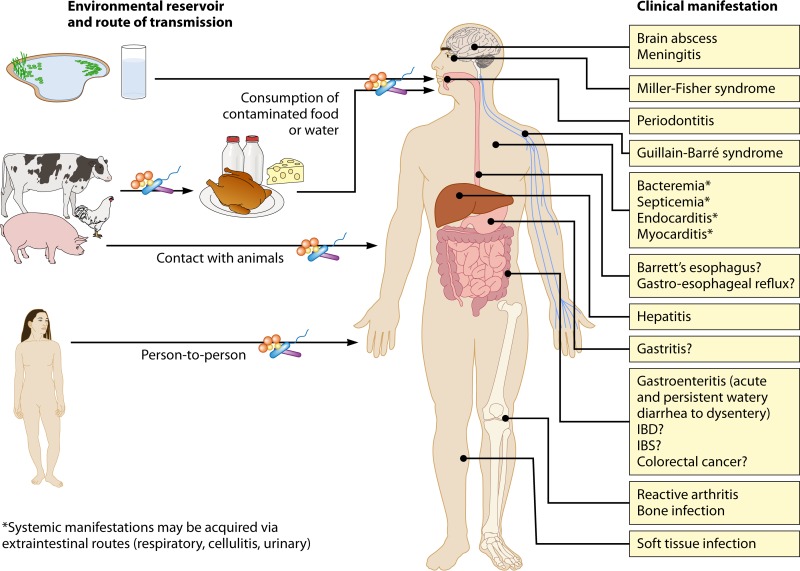
Environmental reservoirs, routes of transmission, and clinical manifestations associated with Campylobacter species. Campylobacter species can be transmitted to humans through consumption of undercooked or contaminated food or via contact with animals. Tap, bore, and pond waters are also sources of Campylobacter species. Person-to-person transmission (fecal-oral or via fomites) can occur. Ingestion of a sufficient dose of organisms via the oral-gastric route may lead to one or more gastrointestinal and/or extragastrointestinal manifestations; the outcome is dependent on the species or strains of Campylobacter involved in the infection. Abbreviations: IBD, inflammatory bowel diseases; IBS, irritable bowel syndrome. Question marks indicate conditions for which a role for Campylobacter is implicated but not certain.
TABLE 1.
Species within the genus Campylobacter and their clinical relevance to humans (as of December 2014)c
| Campylobacter speciesa | Clinical manifestations |
|---|---|
| C. coli | Established pathogen in gastroenteritis; also found in blood, meningitis, and acute cholecystitis |
| C. concisus | Emerging pathogen associated with gastroenteritis and IBD (Crohn's disease and ulcerative colitis); also found in Barrett's esophagitis, blood, and brain abscess |
| C. curvus | Found in gastroenteritis, ulcerative colitis, Barrett's esophagitis, blood, liver, and bronchial abscesses |
| C. fetusb | Associated with bacteremia; also found in gastroenteritis, brain abscesses, epidural abscess aspirate, cerebrospinal fluid, cellulitis, endocarditis, mycotic aneurysm of the abdominal aorta, and peritonitis |
| C. gracilis | Potential periodontal pathogen; also found in IBD, head and neck infection, and brain abscess |
| C. hominis | Found in blood and IBD (possibly a commensal in the intestine) |
| C. helveticus | Found in gastroenteritis |
| C. hyointestinalis | Found in gastroenteritis and blood |
| C. insulaenigrae | Found in gastroenteritis and blood |
| C. jejuni | Established pathogen in gastroenteritis and possible predisposing agent in IBD, postinfectious IBS, and celiac disease; infection may result in sequelae in the forms of Guillain-Barré syndrome, Miller Fisher syndrome, Bell's palsy (unilateral facial paralysis), and reactive arthritis; found in IBD, blood, myocarditis, meningitis, acute cholecystitis, urinary tract infection, and acute febrile illnesses associated with leukopenia or thrombocytopenia |
| C. lari | Associated with gastroenteritis; also found in blood |
| C. mucosalis | Found in gastroenteritis |
| C. rectus | Putative periodontal pathogen; also found in gastroenteritis, IBD, vertebral abscess, blood, necrotizing soft tissue infection, and pus |
| C. showae | Found in IBD, intraorbital abscess, and blood |
| C. sputorum | Found in gastroenteritis, axillary abscess, and blood |
| C. upsaliensis | Emerging pathogen in gastroenteritis; also found in breast abscess, blood, and placenta |
| C. ureolyticus | Associated with gastroenteritis and IBD; also found in oral, perianal, and soft tissue abscesses, soft tissue or bone infections, and ulcers or gangrenous lesions of the lower limb |
No disease association in humans has been reported for C. avium, C. canadensis, C. corcagiensis, C. cuniculorum, C. lanienae, C. lari subsp. concheus, C. peloridis, C. subantarcticus, C. troglodytis, C. volucris, “Campylobacter sp. Dolphin DP,” and “Campylobacter sp. Prairie Dog” (as of December 2014).
Includes C. fetus subsp. fetus, C. fetus subsp. venerealis, and C. fetus subsp. testudinum.
The table was updated from the work of Man (1).
GASTROENTERITIS
C. jejuni and C. coli are established causes of diarrhea in humans. A human experimental infection study revealed that the rate of colonization increased with increasing doses of C. jejuni, whereas the development of illness did not (4). Infection with a dose as low as 800 CFU resulted in diarrhea in some volunteers (4). However, it has been speculated further that the dose of C. jejuni required for the development of campylobacteriosis can be as low as 360 CFU (5). Mathematical modeling suggested that an intermediate dose of 9 × 104 CFU/ml has the highest ratio of illness to infection (6). In contrast, an association between dose and occurrence of disease was observed in humans experimentally infected with C. jejuni strain 81-176. In addition, exposure to C. jejuni strain 81-176 offered only short-term protection (7). This can be reconciled by the fact that the severity of disease, dose-response relationship, and illness/infection ratio are dependent, at least in part, on the strain used. These strain-specific differences were clearly observed when experimental infection of naive individuals with C. jejuni strain CG8421 failed to offer protection against a second bout of campylobacteriosis upon rechallenge with the same strain (8). Interestingly, an immunocompetent adult experimentally infected with C. jejuni experienced recrudescence of the infection at the conclusion of antibiotic therapy (9), suggesting that the incidence of recurrent infection may be underestimated.
Patients with C. jejuni or C. coli infection experience acute watery or bloody diarrhea, fever, weight loss, and cramps that last, on average, 6 days (1). Gastroenteritis induced by C. coli is clinically indistinguishable from that by C. jejuni. The onset of symptoms usually occurs 24 to 72 h following ingestion and may take longer to develop in those infected with a low dose. The peak of illness can last 24 to 48 h and may include abdominal pain that mimics appendicitis (10). Polymorphonuclear leukocytes and blood (gross or microscopic) can be observed in the stool, and diffuse inflammatory colitis is present in colonic biopsy specimens from infected patients (10). While infection with C. jejuni or C. coli can occur in patients of all ages, a recent study from Denmark showed that infection is more prevalent in toddlers (1 to 4 years) and young adults (15 to 24 years) than in other age groups (11). A recent comparison of the characteristics of patients infected with C. jejuni or C. coli indicated that slightly older patients (34.6 years versus 27.5 years) and those who traveled abroad were at a greater risk of being infected with C. coli than with C. jejuni (12). Studies have also shown that infections with C. jejuni and C. coli are more common during the summer months (11, 13). Although C. coli is less prevalent than C. jejuni in many geographic regions, C. coli infections can contribute as many as 25% of all gastroenteritis cases caused by Campylobacter species (14–18).
C. concisus, Campylobacter ureolyticus, Campylobacter upsaliensis, and Campylobacter lari are known as “emerging Campylobacter species,” a term used to describe their underappreciated roles in human and animal diseases. Emerging Campylobacter species are likely to contribute to the etiology of gastroenteritis, especially in cases which have no known association with other established pathogens (1, 19–21). Furthermore, many diagnostic laboratories fail to detect emerging Campylobacter species owing to a lack of the specialized cultivation techniques required to culture these organisms, including the use of microaerobic or anaerobic conditions enriched with hydrogen (1). Indeed, introduction of hydrogen as part of routine microaerobic culture for stool samples at a University Hospital in Bern, Germany, resulted in a significant increase in the rate of isolation of C. concisus (22). As a consequence, the incidence of C. concisus detection rose from 0.03 to 1.92%.
Patients infected with C. concisus and certain Campylobacter species other than C. jejuni and C. coli generally experience milder symptoms, with fewer individuals reporting fever, chills, weight loss, and mucus and blood in their stools than those infected with C. jejuni and C. coli (1, 23). In general, the milder severity of symptoms has been found to correlate with the low levels of fecal calprotectin in those infected with C. concisus (median, 53 mg/kg of feces; interquartile range, 20 to 169 mg/kg). For comparison, fecal calprotectin levels are higher (median, 631 mg/kg; interquartile range, 221 to 1,274 mg/kg) in those infected with C. jejuni or C. coli (24). Symptoms associated with C. concisus infection tend to be more persistent than those of C. jejuni or C. coli infection, with 80% of patients reporting diarrhea that lasted 14 days or more, whereas only 32% of those infected with C. jejuni or C. coli reported prolonged diarrhea (23). Similar to the case profile for C. concisus infection, infection with C. fetus is more common than infection with C. jejuni and C. coli in older patients (68.4 years versus 28.6 years) (12).
Epidemiology
There is evidence to suggest that there has been a rise in the global incidence of campylobacteriosis in the past decade. The numbers of cases of campylobacteriosis have increased in North America, Europe, and Australia. Although epidemiological data from Africa, Asia, and the Middle East are still incomplete, these data indicate that Campylobacter infection is endemic in these regions. Differences in the incidence and number of cases reported from different countries or regions within the same country may vary substantially (Fig. 2) (25, 26). It is likely that these variations arise, in part, from differences in the sensitivity of detection methodologies and the area, population, and scope of the case profile studied, as well as differences in the standard and stringency of biocontrol protocols, surveillance bias, food practices, and availability of natural reservoirs for Campylobacter species in these regions. Furthermore, the reported cases of C. jejuni and C. coli infections are likely to represent only the tip of the iceberg owing to underreporting (27).
FIG 2.
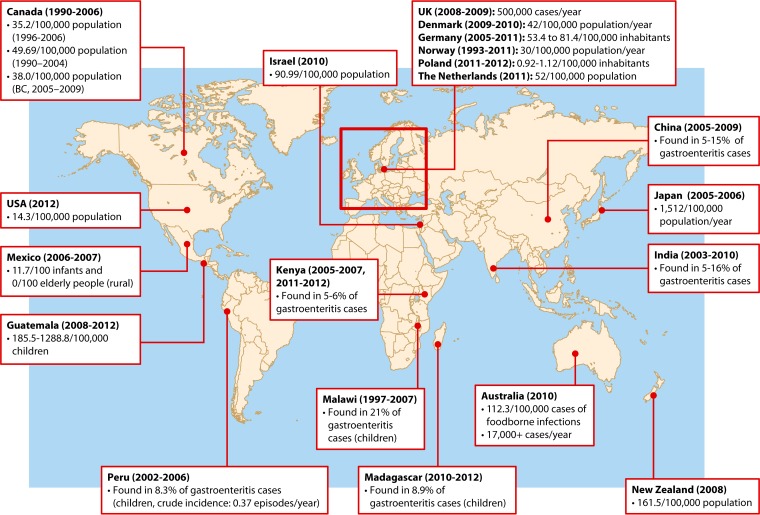
Incidence and prevalence of campylobacteriosis (C. jejuni/C. coli). The latest information on the global epidemiology of campylobacteriosis from the literature is shown, including data from the United Kingdom (47), Denmark (11), Germany (49), Norway (424), Poland (25, 50, 425), the Netherlands (51), Israel (67), China (60, 61), Japan (26), India (63–65), Australia (69), New Zealand (73), Madagascar (78), Malawi (77), Kenya (79, 426), Guatemala (41), Peru (427), Mexico (428), the United States (10 sites within The Food-Borne Diseases Active Surveillance Network) (34), and Canada (37–39). B.C., British Columbia. (Map adapted from an image from Wikimedia Commons [http://commons.wikimedia.org/wiki/File:A_large_blank_world_map_with_oceans_marked_in_blue.PNG].)
An additional factor that has been hypothesized to influence the prevalence of Campylobacter infections is population-level immunity (28). Population-level immunity refers to the host immune response against an infection within a population that can provide protection against transmission of an infection and/or disease for unprotected individuals. At the population level, this can have impacts on the epidemiology and risk assessment of campylobacteriosis (28). In developing countries where Campylobacter is endemic, infection is usually limited to children, with illness/infection ratios decreasing with age, suggesting that exposure in early life might lead to the development of protective immunity (13). This might reflect why asymptomatic Campylobacter infections are common in developing nations, which could also have an impact on the transmission of Campylobacter infections in these regions due to asymptomatic excretion (28). Asymptomatic excretion is also found in developed countries, with a number of studies showing that a majority of shedders are asymptomatic (29, 30).
Outbreaks caused by Campylobacter species are not uncommon. The Centers for Disease Control and Prevention defines a foodborne disease outbreak as the occurrence of more cases than expected in a particular area or among a specific group of people during a specific period, usually with a common cause. Since 2007, the numbers of individuals reported in published outbreaks of campylobacteriosis have ranged from 10 to more than 100 and have correlated with the type of event and environmental source of infection (Fig. 3). The most common reported sources of Campylobacter responsible for outbreaks are consumption of poultry products or water (Fig. 3). Between 1992 and 2009, 143 outbreaks were reported in England and Wales, United Kingdom. Of these, 114 were due to contaminated food or water, 2 to animal contact, and 22 to an unknown mode of transmission (31). According to records from the Centers for Disease Control and Prevention, there were 4,936 Campylobacter outbreaks in the United States between 1999 and 2008 (32). Between 2009 and 2010, 56 confirmed and 13 suspected outbreaks were reported to the U.S. National Outbreak Reporting System, among which 1,550 illnesses and 52 hospitalized cases were recorded (33). Unfortunately, outbreak data from developing nations are severely lacking. A full list of published campylobacteriosis outbreaks since 2007 is shown in Fig. 3. Below, we summarize the epidemiological data from different regions of the world.
FIG 3.
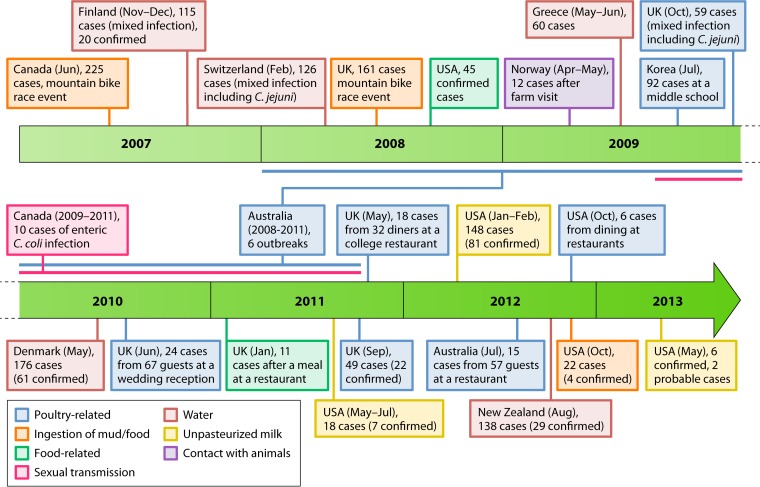
Timeline of published campylobacteriosis outbreaks since 2007 (381, 382, 429–447).
North and Central America.
In the United States, the annual number of campylobacteriosis cases, based on 10 years of outbreak data (1998 to 2008), was estimated to be 845,024 cases, resulting in 8,463 hospitalizations and 76 deaths (32). The U.S. Food-Borne Diseases Active Surveillance Network (1996 to 2012) reported an annual incidence of 14.3 per 100,000 population for Campylobacter infection (34). Batz and colleagues estimated the annual costs of campylobacteriosis to be $1.7 billion in the United States (35). Importantly, there was a 14% increase in the incidence of campylobacteriosis in 2012 compared to the 2006–2008 period, whereas the incidences of Cryptosporidium, Listeria, Salmonella, Shigella, Shiga-toxigenic Escherichia coli (STEC) O157, and Yersinia infections decreased over the same period (34). Analysis of seven states in the United States within the Food-Borne Diseases Active Surveillance Network revealed that of nine common foodborne pathogens, Campylobacter was the leading cause of travel-associated gastroenteritis from 2004 to 2009, accounting for 41.7% of cases (3,445 of 8,270 cases reported), followed by Salmonella (36.7%) and Shigella (13.0%) (36). The same study also found that Campylobacter species were the second most prevalent pathogens in non-travel-associated gastroenteritis, behind Salmonella species, accounting for 26.5% of the 14,782 cases reported between 2004 and 2009 (36).
In Quebec, Canada, 28,521 cases of campylobacteriosis were reported between 1996 and 2006, which yielded an estimated annual incidence of 35.2 cases per 100,000 persons (37). A higher incidence of campylobacteriosis (49.69 cases per 100,000 people) was reported from 1990 to 2004 in the Waterloo region of Ontario, Canada (38). In southwestern Alberta, Canada, 36.9% and 5.4% of the patients with diarrhea reported from 31 May to 31 October 2005 were positive for C. jejuni and C. coli, respectively (14). With a rate among the highest in the country, the province of British Columbia had an annual average of 38.0 cases per 100,000 people during 2005 and 2009 (39).
In Mexico, C. jejuni was the most common cause of acute gastroenteritis in infants and preschoolers in 2006 and 2007 (isolated in 15.7% of 5,459 cases) (40). Campylobacteriosis is also very common in children in Guatemala, with incidence rates of 185.5 to 1,288.8 per 100,000 children (41). One study estimated that the incidence of campylobacteriosis in Barbados in 2000 was 5.4 per 100,000 inhabitants—an incidence which had doubled in 2002 (42). However, there is no further report describing the incidence of human campylobacteriosis in this region. Overall, C. jejuni is a major pathogen in the United States and Canada, but its precise impact in regions of Central America is less clear.
South America.
In 2011, Fernández reviewed the available data on the prevalence of C. jejuni and C. coli in South America (43). The prevalences of C. jejuni ranged from 4.6 to 30.1% of diarrheic patients in Argentina (three studies), while those of C. coli were 0 to 1.4%, with C. coli being responsible for a third of the Campylobacter-related gastroenteritis cases in one of these studies. However, none of these studies included a control group. In Bolivia, the prevalences of C. jejuni ranged from 4.4 to 10.5%; however, of the two studies cited, one included a control group in which C. jejuni was detected in 9.6% of the controls. Similarly, two studies in Brazil found that the levels of detection of C. jejuni were similar between patients (5.8 to 9.6%) and controls (4.9 to 7.2%), while those of C. coli ranged from 2.2 to 6.0% for patients and from 1.2 to 2.0% for controls. Detection levels of C. jejuni and C. coli in gastroenteritis patients ranged from 0 to 14.1% in Chile, 0 to 14.4% in Colombia, 0 to 23.0% in Ecuador, 0.6 to 18.4% in Paraguay, 0 to 23.0% in Peru, 0 to 14.3% in Uruguay, and 0 to 13.0% in Venezuela (43).
In 2013, Collado and colleagues reported the detection of C. jejuni and emerging Campylobacter species in patients with gastroenteritis from southern Chile (44). In this study, fecal samples were collected from participants over the period from November 2010 to March 2012, and the presence of Campylobacter species was detected by PCR. Of the 140 patients, 11.4% tested positive for C. concisus DNA, compared to only 3.4% of the 116 healthy controls (P < 0.05) (44). Notably, the prevalence of C. concisus DNA in gastroenteritis patients was found to be similar to that of C. jejuni (10.7%). In Peru, a study that included 150 pediatric stool samples from the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort study detected C. jejuni/C. coli in 41.3% of the children with gastroenteritis and 18.7% of the controls (P = 0.007) (45). In contrast, the difference in the prevalences of other Campylobacter species, including C. hyointestinalis subsp. lawsonii, C. troglodytis, and C. upsaliensis, in children with gastroenteritis (33%) and in controls (24%) was not statistically significant (45). Taken together, the data from South America indicate that Campylobacter species contribute to the etiology of gastroenteritis, but the contribution of campylobacteriosis to this disease, relative to the contributions of other major pathogens, is unclear.
Europe.
The most current evaluation of the epidemiology of campylobacteriosis in 27 European Union (EU) state members indicated the incidences of Campylobacter infections to range from 29.9 to 13,500 per 100,000 population in 2009 (with the lowest incidences in Finland and Sweden and the highest in Bulgaria) (46). Overall, this equated to 9.2 million cases, compared to 6.2 million cases of salmonellosis, in 2009 (46). A United Kingdom-wide study conducted over the period from April 2008 to August 2009 identified Campylobacter species as the most common bacterial pathogens in cases of gastroenteritis (47). In this study, the reported rate of campylobacteriosis was 9.3 cases per 1,000 person-years in the community, with an estimated total of 500,000 cases and 80,000 general practitioner consultations across the United Kingdom annually (47). Investigation of the prevalence of pathogen-induced diarrhea in the United Kingdom in 2008 to 2009 revealed that the prevalence of Campylobacter species had not decreased compared to that observed 15 years prior (21). In contrast, the prevalences of enteroaggregative E. coli, Salmonella, and Yersinia enterocolitica had all decreased over the same 15-year period (21).
Similarly, in Germany, the prevalence of campylobacteriosis in 2011 was similar to the data from 2001; in contrast, over the same period, the prevalence of salmonellosis had decreased (48). In 2011, there were 70,560 reported cases of campylobacteriosis, a prevalence higher than that reported for Salmonella, Shigella, Yersinia, and Listeria infections (48). According to data from Hesse, Germany, the annual incidences of campylobacteriosis between 2005 and 2011 ranged from 53.4 to 81.4 cases per 100,000 persons (49). While in Poland the estimated incidence in 2012 was reported to be 1.12 cases per 100,000 inhabitants, it is likely that campylobacteriosis is underdiagnosed and underreported in this region (25, 50). Interestingly, a study from the Netherlands predicted that in 2060 the incidence of campylobacteriosis in this region will be similar to that in 2011, at 51 per 100,000 population (51). The same model estimated that salmonellosis would account for only 12 cases per 100,000 persons in 2060. However, it is important that these estimates were calculated using age-specific demographic forecasts for 10-year periods between 2020 and 2060, without having considered other factors, such as social clustering, health care systems, and food processing and preparation. Altogether, the estimated cost of illness in the Netherlands due to campylobacteriosis was €21 million per year (52). The high levels of campylobacteriosis across Europe may be reflected in the continuous increase in the number of C. jejuni-related GBS cases in Paris between 1996 and 2007 (mean annual increment, 7%; P = 0.007) (53).
Epidemiological data from Europe over the last 3 years have transformed our understanding of the clinical importance of emerging Campylobacter species in health and disease. Data collected from the Netherlands between March and April 2011 revealed that 71.4% of 493 gastroenteritis cases were PCR positive for Campylobacter DNA, among which 20 were C. jejuni-associated cases (4.1%). In addition, a further subset of samples was sequenced, which allowed the identification of other Campylobacter species, including C. concisus (4.1%), C. concisus or C. curvus (0.8%), C. ureolyticus (0.6%), C. gracilis (0.6%), C. showae or C. rectus (0.4%), C. upsaliensis (0.4%), C. hominis (0.2%), and C. sputorum (0.2%) (54). These results suggest that, in the Netherlands, the prevalence of C. concisus in gastroenteritis is similar to that of C. jejuni. A similar finding based on a culture-dependent approach has been reported for Denmark, where the prevalences of C. jejuni and C. concisus in adults and children were comparable, with the annual incidence of C. concisus infection reported to be 35 cases per 100,000 inhabitants in 2009 and 2010 (11, 55). This is in line with results from Portugal, where Campylobacter species were detected in 31.9% of diarrheic fecal samples, with C. jejuni and C. concisus being the most prevalent species (13.7% and 8.0%, respectively) (56). Furthermore, based on PCR analysis, the prevalences of C. ureolyticus in gastroenteritis cases in Ireland in the period from 2009 to 2012 ranged from 1.15 to 1.30% (57, 58). Furthermore, molecular screening for seven members of the Campylobacter genus by using PCR revealed the overall prevalence of Campylobacter species in patients with gastroenteritis from southern Ireland to be 4.7%, with C. jejuni being the predominant species, accounting for 66% of all Campylobacter species detected, followed by C. ureolyticus (22.3% of all Campylobacter species detected), C. coli (6.7%), C. fetus (2.1%), C. hyointestinalis (1.3%), C. upsaliensis (1.1%), and C. lari (0.5%) (59). Overall, there is compelling evidence from Europe to suggest that in addition to C. jejuni, emerging Campylobacter species contribute to the etiology of gastroenteritis in this region.
Asia and the Middle East.
Epidemiological data on campylobacteriosis in Asia and the Middle East are limited. Investigation of the etiology of gastroenteritis in three hospitals in Yangzhou, China, between July 2005 and December 2006 showed that 4.84% of 3,061 patients with diarrhea were PCR positive for C. jejuni, with the highest prevalence being detected in those younger than 7 years of age (60). Between 2005 and 2009, 14.9% (142/950 patients) of patients with gastroenteritis in a hospital in Beijing, China, were reported to be positive for Campylobacter species (127 with C. jejuni and 15 with C. coli) (61). Based on detection rates of Campylobacter in raw chicken and the consumption trend of chicken products in China from 2007 to 2010, Wang and colleagues predicted that 1.6% of the urban and 0.37% of the rural population are affected by campylobacteriosis every year (62). In the Miyagi Prefecture of Japan, Kubota and colleagues estimated that, from 2005 to 2006, the numbers of acute gastroenteritis episodes associated with Campylobacter, Salmonella, and Vibrio parahaemolyticus infections were 1,512, 209, and 100 per 100,000 population per year, respectively (26), suggesting that Campylobacter species are responsible for the majority of bacterial gastroenteritis cases in this region. The unusually high incidence of campylobacteriosis in this region may be attributed to a range of factors, such as unexpected outbreaks during the time frame examined and/or the methodologies used to estimate the incidence rate. Nevertheless, the high incidence of campylobacteriosis in certain regions of Asia highlights the need for further active surveillance of food safety.
In India, recent data from an infectious disease hospital in Kolkata reported that, in the period from January 2008 to December 2010, 7.0% (222/3,186 patients) of hospitalized patients with gastroenteritis were culture positive for Campylobacter species, with 70% of the isolates identified as C. jejuni (63). Based on real-time PCR analysis, Sinha and colleagues reported 16.2% (11/68 samples) of diarrheic stool samples from patients in the same region to be positive for Campylobacter species (64). Campylobacteriosis has also been reported to be most prevalent in children under the age of 5 years in this region (isolated in 10% of cases, compared to 3.7% for other age groups; P < 0.001) (63). In Vellore, South India, between January 2003 and May 2006, 4.5% of 349 children under the age of 5 years with diarrhea were positive by PCR for C. jejuni or C. coli (65). In addition, in a prospective case-control study conducted between 1 December 2007 and 3 March 2011 to identify the etiology of diarrhea in children aged 0 to 59 months, C. jejuni was reported to be significantly associated with moderate to severe diarrhea in children from Kolkata, India, Mirzapur, Bangladesh, and Karachi, Pakistan (66). However, a further study that examined 144 Bangladeshi children failed to identify a significant association between Campylobacter species, including C. jejuni/C. coli, C. troglodytis, C. hyointestinalis subsp. lawsonii, C. concisus, and C. upsaliensis, and diarrhea (45).
The most recent data from the Middle East show Campylobacter species to be a major and increasing cause of gastroenteritis in this region. For example, the annual incidence of campylobacteriosis in Israel increased from 31.04 cases per 100,000 population in 1999 to 90.99 cases per 100,000 population in 2010, with children under the age of 2 years having the highest incidence (356.12 cases per 100,000 population) (67). Consistently, a study conducted between 2007 and 2009 showed that of 99 hospitalized children with gastroenteritis reported in a hospital in Nahariya, Israel, 61% were positive for Campylobacter species, followed by Shigella (24%) and Salmonella (16%) (68). It is difficult to accurately assess the burden of Campylobacter infections in Asia owing to insufficient epidemiological data. There is nevertheless a trend indicating a possible rise in the incidence of campylobacteriosis in the Middle East.
Oceania.
Campylobacteriosis is the most commonly notified foodborne infection in Australia, with 16,968 notified cases (112.3 cases per 100,000 cases of notified foodborne infection) in 2010 (69–71). In 2010, the prevalence of Campylobacter infections increased 6% compared with the data from 2008 and 2009 (69–71). Similarly, an increase in salmonellosis notifications was observed, with 11,992 notifications (53.7 cases per 100,000 cases) in 2010, compared to 9,533 notifications (43.6 cases per 100,000 cases) in 2009 and 8,310 notifications (39 cases per 100,000 cases) in 2008 (69–71). In contrast, Gibney and colleagues reported Campylobacter to be the second leading cause of acute gastroenteritis in Australia in 2010, after norovirus, with the highest disability-adjusted life-year burden (72).
In New Zealand, over the period from 2002 to 2006, the incidence of campylobacteriosis was reported to be 353.8 cases per 100,000 population. However, in 2008, this high incidence dropped substantially, to 161.5 cases per 100,000 population, due to successful intervention strategies within the poultry sector in this region (discussed further below) (73). Epidemiological data available from 364 patients with campylobacteriosis from New Zealand, obtained through telephone and postal questionnaires, showed that 47% of the cases were due to consumption of contaminated food, 27.7% from direct contact with animals, 6.9% from overseas travel, 3.3% from consumption of contaminated water, and 11% from an unknown mode of transmission (74). A study from New Zealand suggested that emerging Campylobacter species are not associated with gastroenteritis cases (75). Among the fecal samples collected in that study, between 2007 and 2009, C. concisus (healthy controls, 53.1%; patients, 46.9%), C. ureolyticus (healthy controls, 24.5%; patients, 10.9%), C. hominis (healthy controls, 16.3%; patients, 8.6%), and C. gracilis (healthy controls, 6.1%; patients, 14.1%) were detected in samples from both patients and healthy controls, with similar frequencies (75). However, the authors concluded that given the level of genetic diversity within these species, in particular C. concisus, the possibility that they may play a role in disease cannot be ruled out.
Only one study has investigated the prevalence of Campylobacter species in the regions of Oceania other than Australia and New Zealand. Howard and colleagues reported the isolation of Campylobacter species from 143/1,167 (12%) gastroenteritis cases, compared to 20/660 (3%) controls, among children admitted to the Goroka Base Hospital, Papua New Guinea, between October 1985 and March 1990 (76). More data are required to elucidate the epidemiological landscape of campylobacteriosis in most Oceania regions.
Africa.
Data from a limited number of countries in Africa have indicated that Campylobacter infection is most prevalent in the pediatric population. A 10-year study (1997–2007) from Blantyre, Malawi, Africa, found that C. jejuni and C. coli were detected in 21% (415/1,941 children) of hospitalized children with diarrhea by real-time PCR, with C. jejuni accounting for 85% of all campylobacteriosis cases (77). Between 1997 and 1999, nondiarrheic children were also examined, and 14% were PCR positive for C. jejuni and C. coli (77). Although this prevalence was significantly lower than that for children with diarrhea within the same period (28%; P < 0.001) (77), these observations indicate that C. jejuni and C. coli are endemic in this pediatric population. These findings are supported by a study conducted in Moramanga, Madagascar, where the rate of Campylobacter isolation from diarrheic samples was reported to be 8.9% (41/459 samples), while that in nondiarrheic samples was 9.4% (278/2,965 samples) (78). In Kenya, samples collected from May 2011 to May 2012 at a hospital in Kisii for the detection of a range of enteric pathogens showed 5.8% (9/156 samples) of samples from patients with diarrhea to be culture positive for Campylobacter species, which was significantly higher than the incidence of 0.6% (1/156 samples) in the controls (P = 0.02) (79). A further study which described 138 Tanzanian children and the use of diverse detection techniques, including culture, enzyme immunoassay, and PCR, reported the detection of C. jejuni/C. coli in 34.8% of gastroenteritis cases and 30.4% of controls and other, non-jejuni/coli Campylobacter species in 47.8% of gastroenteritis cases and 42.0% of controls (45). These differences, however, did not reach statistical significance (45). Pioneering work conducted by Lastovica and colleagues in South Africa, who used culture methods optimal for the isolation of most Campylobacter species, unveiled a more complete and realistic epidemiological landscape of C. jejuni and emerging Campylobacter species. From 2005 to 2009, 5,443 strains of Campylobacter species were isolated from stools of children with diarrhea at the Red Cross Children's Hospital in Cape Town. Of these, 40% were C. jejuni (32.3% C. jejuni subsp. jejuni and 7.7% C. jejuni subsp. doylei), while the second most prevalent organism was C. concisus (24.6%) (80). From 1990 to 2009, Lastovica cultivated more than 2,000 clinical isolates of C. concisus (81), a valuable collection which could be characterized further by more extensive genomic sequence analyses. Overall, it is not unreasonable to conclude that C. jejuni and other Campylobacter species are endemic to children in most surveyed regions of Africa.
OTHER GASTROINTESTINAL MANIFESTATIONS
While gastroenteritis is a major clinical condition resulting from Campylobacter infection, these organisms have also been associated with a range of other serious conditions within the gastrointestinal tract, including IBD, esophageal diseases, periodontitis, functional gastrointestinal disorders, celiac disease, cholecystitis, and colon cancer. Here we review the epidemiology and impact of Campylobacter infection in these gastrointestinal diseases.
Inflammatory Bowel Diseases
IBD are chronic inflammatory conditions of the gastrointestinal tract which include Crohn's disease (CD) and ulcerative colitis (UC). The phenotype in patients with CD is characterized by transmural lesions that may occur in any site along the gastrointestinal tract, while patients with UC are affected by continuous submucosal inflammation restricted to the colon. Despite extensive research, the etiology of IBD has yet to be elucidated; however, the general hypothesis is that they are complex diseases in which a dysregulated immune response that leads to chronic inflammation arises as a result of a dysregulated gastrointestinal microbial ecology, host genetic factors, and a disruption of the gastrointestinal epithelium triggered by environmental factors (82).
The role of Campylobacter species in IBD has been investigated for the past 3 decades. C. jejuni was the initial focus of research (83–85), but it was not until 2009 that Gradel and colleagues provided evidence that indicated an association between C. jejuni infection and an increased risk of IBD (86). Furthermore, recent studies investigating the role of other emerging Campylobacter species in IBD have provided solid evidence that demonstrates an association between C. concisus and these gastrointestinal disorders (81, 87–94).
The association between emerging Campylobacter species (C. concisus, C. showae, C. hominis, C. gracilis, C. rectus, and C. ureolyticus) and CD was first described by the Mitchell group in 2009 (87). For a cohort of newly diagnosed pediatric CD patients, 82% of intestinal biopsy specimens were found to be positive for Campylobacter DNA by PCR, compared to 23% of control samples (87). Only the prevalence of C. concisus DNA was found to be significantly higher in patients with CD (51%) than in controls (2%) (P < 0.0001) (87). Consistent with this, a study by Tankovic and colleagues found that C. concisus was present in 21% (4/19 patients) of IBD patients but only 9% (1/11 controls) of controls (88). In 2010, Man and colleagues further reported that 65% of fecal samples from patients with CD were positive for C. concisus, compared to 33% of samples from healthy controls and 37% of samples from non-IBD controls, and the differences were statistically significant (P = 0.03 and P = 0.008, respectively) (89). Further analyses to investigate the fecal microbiota in a subset of these patients by using pyrosequencing techniques detected C. concisus in two CD samples but not in controls, which indicates that C. concisus DNA was present in sufficient quantity to be detected by less sensitive approaches (90). The increased prevalence of C. concisus DNA in CD patients compared to controls appears to be specific to the intestinal tract, because no difference in prevalence of C. concisus DNA was found for saliva samples from IBD patients (100%; 13 CD patients and 5 UC patients) and healthy controls (97%; 57/59 controls) (95). This raises the possibility that the oral cavity may be a natural reservoir for C. concisus.
In a study to investigate whether specific microorganisms were selectively transported to the lymph nodes of CD patients, O'Brien and colleagues used high-throughput sequencing and reported Campylobacteraceae DNA to be present in three CD patients (96). In line with this, a study by Kovach and colleagues identified 37 immunoreactive proteins of C. concisus, detected using sera collected from 10 C. concisus-positive children with CD (97). Of these proteins, flagellin B, the ATP synthase F1 α subunit, and outer membrane protein 18 were consistently recognized by all CD patients (97).
Similarly, the prevalence of C. concisus was reported to be higher in patients with UC (91–94). For example, Mahendran and colleagues found a higher prevalence of C. concisus, not only in colonic biopsy specimens from adult CD patients (53%; 8/15 patients) but also in those from UC patients (31%; 4/13 patients), than in controls (18%; 6/33 individuals) (P < 0.05) (91). Two further studies, conducted in Scotland, showed an increased prevalence of C. concisus DNA in both adults and children presenting with UC (92, 93). The first study isolated C. concisus from three children with IBD (two with CD and one with UC) but not from any of the controls; however, based on PCR, the prevalences of C. concisus were not significantly different between patients and controls (92). In contrast, the second study detected a significantly higher prevalence of C. concisus DNA (33.3%; 23/69 samples) in intestinal biopsy specimens from adult UC patients than in controls (10.8%; 7/65 individuals) (P = 0.0019) (93). More recently, Rajilic-Stojanovic and colleagues examined the fecal microbiota of 15 UC patients during remission and 15 controls, using a highly reproducible phylogenetic microarray assay that can detect and quantify more than 1,000 intestinal bacteria in a wide dynamic range. This showed the levels of Campylobacter and other pathogens (Fusobacterium, Peptostreptococcus, and Helicobacter) to be increased in the fecal samples from UC patients compared with those in controls (P = 0.0004) (94). The reason for the increased level of Campylobacter species during the remission stage of UC is unclear, and the identity of the Campylobacter species is unknown, as only the genus information was provided (94).
Despite solid evidence supporting an association between C. concisus and IBD, the observation that C. concisus is detected in the intestines of one-third of cohorts without IBD raises the possibility that C. concisus may simply be present as a result of dysbiosis and intestinal inflammation (1, 98). Although causality between C. concisus and IBD has not yet been established, recent studies have focused on identifying specific genetic variants of C. concisus or genomospecies that may be associated with disease. C. concisus is a genetically heterogeneous species which is defined by 2 to 4 genetically variable genomospecies (99–105). A recent study by our group addressed this issue by determining the levels of C. concisus exotoxin 9/DnaI, a putative virulence factor postulated to be associated with increased survival in the cell, in patients with CD (106–108). This showed exotoxin 9/DnaI levels to be significantly higher in fecal samples from CD patients [48.8 ± 20.7 pg (g feces)−1] than in controls [4.3 ± 1.1 pg (g feces)−1] (P = 0.037). Based on these findings, it is possible that IBD patients are colonized by strains harboring specific virulence factors (106). Furthermore, our group also identified the zonula occludens toxin (Zot) gene within the genomes of some C. concisus strains (109), and we showed that the levels of Zot gene DNA in patients with moderate to severe CD were increased compared to those for mild CD [for mild CD, 1.6 ± 0.7 pg (g feces)−1; and for moderate/severe CD, 4.4 ± 1.0 pg (g feces)−1] (P = 0.059) (110). Based on these and other findings on the pathogenicity and immunogenicity of C. concisus (108, 111, 112), we hypothesized that C. concisus strains can be subdivided into the following two pathotypes which differ from nonpathogenic strains: (i) adherent and invasive C. concisus (AICC), which possesses a superior ability to survive intracellularly within host cells (potentially involving exotoxin 9/DnaI and other virulence factors); and (ii) toxigenic C. concisus (AToCC), which produces Zot, with the potential to target tight junctions of host cells (98). Further characterization of the prevalence of these strains in IBD is required.
In addition to C. concisus, Mukhopadhya and colleagues found 21.7% (15/69 samples) of samples from UC patients and 3.1% (2/65 samples) of samples from controls (P = 0.0013) to be PCR positive for C. ureolyticus (93). Overall, further investigations are required to establish a causative role; nevertheless, these findings collectively indicate an important contribution of C. concisus to the pathogenesis of IBD.
Esophageal Diseases
Esophageal diseases include gastroesophageal reflux disease (GERD), Barrett's esophagus (BE), and esophageal adenocarcinoma. GERD is a chronic disorder in which mucosal damage to the esophagus occurs due to stomach acid or, occasionally, stomach content, which may contain bile, flowing back into the esophagus, which over time increases the risk of BE. In turn, BE is a preneoplastic condition defined by the replacement of normal squamous mucosa by metaplastic columnar mucosa in the distal esophagus. This event increases the predisposition to the development of esophageal adenocarcinoma.
Early studies have reported the bacterial composition to differ in individuals with a healthy esophagus, GERD, and BE. The bacterial communities detected in these sites are primarily characterized by members of four phyla: the Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (113–118). Recent studies have demonstrated that Campylobacter species, and C. concisus in particular, are among the dominant species present in patients with GERD and BE (113, 119). For example, in a study by Macfarlane and colleagues which examined the presence of aerobic, microaerobic, and anaerobic microorganisms in esophageal aspirates and mucosal samples from patients with BE, 57% of patients were reported to be colonized by Campylobacter species, the majority of which were C. concisus (113). In agreement with these findings, Blackett and colleagues reported Campylobacter species, almost exclusively C. concisus, to be increased in patients with GERD and BE, but not in esophageal adenocarcinoma patients, compared with healthy controls (119). This finding suggests a possible association between C. concisus colonization and reflux into the esophagus. Furthermore, the authors showed a strong correlation between C. concisus colonization and production of interleukin-18 (IL-18) (119), a cytokine that stimulates both innate and adaptive immune responses and has been widely associated with carcinogenesis (120).
Periodontal Diseases
C. rectus, C. gracilis, C. showae, and C. concisus have been identified as potential oral pathogens, while other Campylobacter species, including C. curvus, C. sputorum, and C. ureolyticus, have been isolated from the oral cavity; however, it remains unclear if they are linked to periodontal disease (1, 121–129). Gingivitis is a preventable and reversible clinical condition that includes erythema, edema, bleeding, sensitivity, tenderness, and enlargement. Periodontitis is a more severe condition characterized by a loss of clinical attachment level, reduction in bone level, and, ultimately, tooth loss. These oral inflammatory conditions are induced by biofilms that accumulate in the gingival margin and are reported to be initiated in periodontal tissue by a number of bacterial species, including C. rectus (121).
A number of studies have shown C. rectus to be associated with higher levels of clinical attachment loss, bleeding on probing of the sampled site, and probing depth (121, 130–133). Furthermore, the abundance of C. rectus has been reported to be elevated significantly in patients with chronic gingivitis and moderate periodontitis but not in severe periodontitis patients, suggesting that this organism is associated with the early stages of periodontitis (130, 134). C. gracilis has been isolated from the oral cavities of individuals presenting with dental caries (135–137), and its reported coaggregation with Actinomyces species has led to the suggestion that these Gram-negative obligate anaerobic rods contribute to the development of biofilms, dental plaque, and root caries (138).
Evidence that C. showae and C. concisus may also play a role in periodontal disease has been reported in a number of studies. For example, an increased prevalence and abundance of both species have been observed at active periodontal disease sites (121, 128, 129, 139–141). Furthermore, the findings that substantially increased levels of C. concisus are observed at periodontal sites with a more severe gingival bleeding index and that the presence of a systemic humoral immune response against C. concisus can be observed in patients with periodontal disease support the view that this species is an oral pathogen (142–144). In contrast, one study observed reduced levels of C. showae in plaque from white-spot or dentin lesions of patients with periodontal disease compared with the levels in healthy subjects (135), while another reported that C. concisus was associated with an increase in tooth attachment in a patient with periodontitis (145).
It has been speculated that oral colonization by Campylobacter species may lead to other pathological consequences in the body. For example, a study by Ercan and colleagues suggests that the presence of C. rectus in the oral cavity of pregnant women with periodontal disease may lead to adverse pregnancy outcomes, including preterm birth and low birth weight (146). Furthermore, this observation was reported to be more pronounced in those with generalized periodontitis and a high bleeding index. Whether this phenomenon relates to the ability of bacteria and their products to diffuse more readily when vascular permeability increases in gingival tissues during pregnancy remains to be determined. A small number of studies have investigated a possible etiological association between oral Campylobacter species and IBD. It is interesting that periodontal disease and IBD share some common clinical features and are both associated with an unusual microbiota. For example, levels of C. gracilis have been reported to be significantly higher at periodontitis sites of patients with CD than at those of patients with UC or than the levels in healthy controls (147). In addition, elevated levels of C. concisus can be found in periodontal lesions of IBD patients, and the oral cavity in these patients may be colonized by specific orally affiliated and enterically invasive C. concisus strains (148, 149).
Functional Gastrointestinal Disorders
C. jejuni and other Campylobacter species are associated with the development of foodborne gastroenteritis-associated sequelae, including postinfectious functional gastrointestinal disorders (PFGD). Two PFGD have received the most attention: irritable bowel syndrome (IBS) (150–156) and functional dyspepsia (FD) (156–161). IBS is defined by recurrent abdominal pain or discomfort during at least 3 days/month in the last 3 months, associated with an alteration of bowel habits (diarrhea, constipation, or both), in accordance with the Rome III classification system (162). FD is characterized by persistent or recurrent symptoms (pain or discomfort centered in the upper abdomen) in the last 3 months, in the absence of organic disease (including on upper endoscopy). The postinfectious forms of these disorders develop de novo despite clearance of the causative agent.
The mechanisms underlying postinfectious IBS are poorly understood but might include persistent changes in the gut microbiota as well as in mucosal immunocytes, enterochromaffin cells, mast cells, and enteric nerves (163). In addition, host factors, including female gender, depression, hypochondriasis, smoking, adverse life events in the preceding 3 months, and treatment with antibiotics, are risk factors for the development of postinfectious IBS (163).
The percentages of individuals presenting with gastroenteritis who develop postinfectious IBS range from 3.7% to 36% (163); however, studies exclusively investigating C. jejuni-associated postinfectious IBS showed percentages ranging from 9.0 to 13.8% (152, 164). Furthermore, long-term follow-up studies have revealed that C. jejuni-associated postinfectious IBS symptoms can persist for up to 10 years after the infectious event (165, 166). Current evidence suggests that both bacterial and host factors play a crucial role in the predisposition to C. jejuni-associated postinfectious IBS. These include increased cytotoxic virulence of the Campylobacter strain and increased transcellular bacterial translocation, a reduced absorptive capacity of the gut, and increased mucosal permeability in the host during acute gastroenteritis (167). Experimental evidence that Campylobacter toxins are important determinants for the development of chronic gastrointestinal symptoms following acute gastroenteritis comes from a study by Thornley and colleagues, who observed that Campylobacter strains associated with postinfectious IBS were more toxigenic to both HEp-2 and African green monkey kidney epithelial (Vero) cells (164). Further studies have shown that C. jejuni infection is the strongest risk factor for postinfectious IBS compared to Salmonella and Epstein-Barr virus infections (150, 151).
More recently, other Campylobacter species have been identified to play a role in postinfectious IBS. Nielsen and colleagues reported patients infected with C. jejuni, C. coli, and C. concisus to be more likely to develop IBS symptoms at 6 months postinfection (23). In a follow-up study, they assessed the risk of postinfectious IBS associated with C. concisus and found that patients with gastroenteritis associated with C. concisus carried a 25% risk of developing IBS (168).
Similar to the case for IBS, a number of studies have provided evidence for an association between Campylobacter infection and a risk of postinfectious FD. A meta-analysis of 19 studies found that following infections with several pathogens, including C. jejuni, Salmonella spp., Escherichia coli O157, Giardia lamblia, and norovirus, the prevalences of postinfectious FD were 9.6 and 30.5% in adults and children, respectively (157). Consistent with these findings, Ford and colleagues reported the odds ratio (OR) of postinfectious FD to be 2.30 (95% confidence interval [CI], 1.63 to 3.26) in a cohort study following a waterborne outbreak of infections with Campylobacter species and E. coli O157 (158). In addition, a recent study by Porter and colleagues reported the relative risk of Campylobacter-associated FD among active-duty U.S. military personnel with acute gastroenteritis from 1998 to 2009 to be 2.0 (95% CI, 1.3 to 3.0) (159). Of particular interest, Campylobacter species and E. coli O157 can be identified in blood tests and stool cultures from postinfectious FD patients (157). Overall, there is good evidence to suggest a link between Campylobacter infection and IBS or FD.
Colorectal Cancer
Increasing evidence indicates that dysbiosis of the gut microbiota contributes to the development of colorectal cancer. Currently, due to a lack of epidemiological studies, evidence supporting a role for Campylobacter species in colorectal cancer is very limited. However, a recent study by Warren and colleagues, investigating metatranscriptome data obtained from colorectal cancer and control tissues, demonstrated that Campylobacter species, predominantly C. showae, coaggregate with Fusobacterium and Leptotrichia species (169). This finding is of particular interest because previous studies have shown that Fusobacterium species are overrepresented in colorectal tumors compared to control specimens (170, 171). As part of their study, Warren and colleagues isolated a novel C. showae strain (CC57C) from colorectal cancer tissue, which they showed harbored a number of potential virulence genes, including a VirB10/D4 type IV secretion system. Furthermore, in vitro assays showed that this strain aggregates with another tumor strain of Fusobacterium nucleatum (CC53) (169). Based on these findings, Warren and colleagues raised the possibility that a Gram-negative anaerobic bacterial population comprising Campylobacter and Fusobacterium might be associated with colorectal cancer (169). Consistent with this, a study by Wu and colleagues (172) which used culture-independent pyrosequencing and reverse transcription-quantitative PCR (RT-qPCR) reported a specific microbial profile, characterized by significant increases in Bacteroides, Enterococcaceae, Fusobacterium, and Campylobacter species, to be associated with colorectal cancer. Although these studies provide an indication that Campylobacter species are present in patients with colorectal cancer, further studies will be required to determine if any relationship between Campylobacter and the development of colorectal cancer exists.
Celiac Disease
Celiac disease is a digestive disorder in which the immune system reacts abnormally to gluten, resulting in damage to the lining of the small intestine. Celiac disease is estimated to affect approximately 1% of people worldwide (173, 174), and the incidence of this disease has increased up to 5-fold in some countries, including the United States (175). In 2007, a case study described for the first time the development of celiac disease in a young healthy woman following infection with C. jejuni (176). More recently, Riddle and colleagues reviewed the U.S. Department of Defense medical encounter database to identify whether there is a risk of developing celiac disease following foodborne infection (177). They found that the rates of celiac disease were similar in those with prior diarrhea caused by bacteria (0.07 per 100,000 person-years) and matched controls (0.04 per 100,000 person-years). However, persons diagnosed with campylobacteriosis had a 3.5-fold higher rate of celiac disease (0.15 per 100,000 person-years) than unexposed individuals (177). Furthermore, no patients exposed to any of the other gastrointestinal pathogens, including Salmonella (nontyphoidal), Shigella, and Y. enterocolitica, developed celiac disease (177). To date, there are insufficient epidemiological data to conclude whether campylobacteriosis is associated with celiac disease.
Cholecystitis
Cholecystitis refers to inflammation of the gallbladder that usually arises when the cystic duct is blocked by gallstones, leading to the accumulation of bile within the gallbladder. C. jejuni has been implicated in the development of cholecystitis; however, this is considered rare given that only 15 cases have been described in the literature over the last 30 years (178). One possible reason for this is that the standard conditions used for culture of bacteria from bile samples do not generally favor the growth of Campylobacter species; thus, some cases of Campylobacter-associated cholecystitis may have been overlooked (178).
EXTRAGASTROINTESTINAL MANIFESTATIONS
In addition to gastrointestinal infection, Campylobacter species also cause a range of clinical manifestations in other parts of the body, as either a local isolated infection, a systemic manifestation after an episode of enteritis, or a postinfectious immune disorder. These manifestations include Guillain-Barré syndrome, Miller Fisher syndrome, brain abscesses and meningitis, bacteremia, sepsis, endocarditis and myocarditis, reactive arthritis, and clinical manifestations that result in complications in the reproductive tract. The clinical importance and epidemiology of these extragastrointestinal manifestations as a result of Campylobacter infection are discussed in the following sections.
Guillain-Barré Syndrome
GBS was first reported by Landry in 1859 (179, 180); however, it was not until 1916 that the French neurologists Guillain, Barré, and Strohl first described the clinical features of GBS (181). GBS is a neurologic condition characterized by a progressive symmetrical weakness in the limbs, with or without hyporeflexia, which can also affect respiratory and cranial nerve-innervated muscles (180). The two main subtypes of GBS are acute motor axonal neuropathy (AMAN) and acute inflammatory demyelinating polyneuropathy (AIDP), with each subtype displaying a distinct immunopathogenesis and response to treatment (182). AMAN is an axonal subtype that progresses more rapidly and is considered the major subtype (30 to 65% of patients) in Asia and Central and South America (182, 183), while AIDP is more prevalent in Europe and North America (182). The annual incidence of GBS is approximately 1.2 to 2.3 cases per 100,000 persons; however, the incidence increases with patient age and male gender (180).
GBS is considered a postinfectious disease, and the major trigger of this disease is C. jejuni infection, with a direct correlation between annual rates of GBS and campylobacteriosis being reported. For example, following the implementation of stricter hygiene measures on poultry meat in New Zealand, decreased rates of GBS were observed, which correlated with a fall in the number of campylobacteriosis cases (184). Furthermore, outbreaks of GBS have been associated with outbreaks of C. jejuni infection (185). A recent systematic review reported the proportion of Campylobacter cases resulting in GBS to be 0.07% (95% CI, 0.03% to 0.15%) (186). Moreover, evidence exists to suggest that the number of C. jejuni-related GBS cases is increasing in some countries (53).
The underlying mechanism of the nerve damage associated with GBS is reported to be due to cross-reactivity between antibodies produced in response to C. jejuni lipooligosaccharide (LOS) and human gangliosides, such as the GM1 ganglioside (187). For example, C. jejuni strains expressing α2,3-sialylated GD1a/GM1a- and α2,8-sialylated GD1c-mimic LOS structures have been shown to interact with sialoadhesin and sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7), respectively (188, 189). T-cell responses are also important in GBS, with LOS that was α2,8-sialylated being shown to induce Th1 immune responses, while LOS containing α2,3-linked sialic acid induces Th2 responses (188). Evidence of this Th1/Th2 polarized immune response following C. jejuni infection comes from a study by Malik and colleagues, who showed that IL-10-deficient mice infected with colitogenic C. jejuni had upregulated Th1/17 but not Th2 responses, while GBS-associated C. jejuni enhanced Th2 responses but blunted Th1/17 responses (190). Thus, the GBS-associated C. jejuni strains may protect against colitis but instead promote autoimmunity. In addition to sialylated LOS structures in C. jejuni, two capsule biosynthesis genes (cj1421c and cj1428c) have been shown to have higher conservation rates among strains isolated from GBS patients than among strains from enteritis patients (191). Furthermore, a gene encoding a glucosyltransferase (cj1135) was reported to be more conserved in enteritis strains whose LOS did not mimic gangliosides, suggesting that this gene may function to silence the neuropathogenesis of C. jejuni LOS (191).
Miller Fisher Syndrome
Miller Fisher syndrome is a clinical variant of GBS that was discovered in 1956 by Charles Miller Fisher. This condition is defined by acute-onset ophthalmoparesis, areflexia, and ataxia, which arise from the development of anti-GQ1b antibodies following exposure to LOS from certain bacteria (192, 193). Several pathogens have been linked to the molecular mimicry that leads to the development of this condition. Of these, C. jejuni is the most frequently identified one (194). Siglec-7 has been shown to exclusively bind to C. jejuni strains that express terminal disialylated ganglioside mimics. C. jejuni binding to Siglec-7 is reported to correlate with the presence of anti-GQ1b antibodies and oculomotor weakness in patients, suggesting that this may be a potential trigger of Miller Fisher syndrome (195).
In some cases, patients are negative for antibodies against GQ1b (196, 197), indicating that antibodies against gangliosides other than GQ1b may be involved in the development of disease. For example, Oyazato and colleagues reported a recent atypical persistent case of Miller Fisher syndrome following Campylobacter enterocolitis where the patient had anti-GA1 antibody in his serum, but not anti-GQ1b and anti-GT1a (198). Further work is required to investigate the etiology and epidemiology of Campylobacter-associated Miller Fisher syndrome and the ability of Campylobacter species other than C. jejuni to trigger this disease.
Bacteremia and Septicemia
One of the most common extragastrointestinal manifestations of Campylobacter species is bacteremia, which is predominantly associated with C. jejuni, C. coli, and C. fetus infections (1). At least 10 different Campylobacter species have been documented in bacteremia cases, but bacteremia cases associated with C. lari, C. insulaenigrae, and C. upsaliensis infections are rare (1, 80, 199, 200). Campylobacter-associated bacteremia cases are often underreported (201). Most cases occur in elderly or immunocompromised patients with one or more concurrent pathologies, including liver cirrhosis or neoplasia; among these patients, 10 to 15% die within 30 days of disease diagnosis (202–204). A recent study conducted in a Danish population reported the estimated incidence of C. jejuni-, C. coli-, C. fetus-, and C. lari-associated bacteremia to be 2.9 cases per 1,000,000 person-years, with a peak incidence in patients older than 80 years (205). In contrast, a 10-year nationwide study in Finland concluded that C. jejuni and C. coli bacteremias affect predominately younger individuals without major underlying diseases (206). The disease generally results from a single gastroenteritis complication in children or as recurrent episodes in immunocompromised children without gastrointestinal symptoms (207). A number of Campylobacter species, including C. jejuni, C. coli, C. fetus, and C. upsaliensis, have also been associated with sepsis in both immunocompetent and immunocompromised children and adults (208–210). Furthermore, a number of cases of C. fetus-associated neonatal sepsis have been reported (discussed below) (211).
Cardiovascular Complications
Campylobacter species, mainly C. jejuni and C. fetus, have been detected in association with a wide spectrum of cardiovascular complications, including endocarditis, myocarditis, pericarditis, myopericarditis (pericarditis with concurrent myocardial involvement), atrial fibrillation, and aortitis with aortic dissection.
Myo(peri)carditis (refers to either myocarditis, pericarditis, or myopericarditis) associated with bacterial enteritis is a rare but serious condition in immunocompetent individuals. These conditions can lead to arrhythmia, dilated cardiomyopathy, congestive heart failure, and sudden cardiac death. Salmonella and Shigella are the main gastrointestinal pathogens linked to myo(peri)carditis, but the increasing incidence of campylobacteriosis worldwide over the last 10 years has drawn attention to Campylobacter-associated myo(peri)carditis. Interestingly, Becker and colleagues recently calculated the annual incidence rates of myocarditis, using data obtained from 6,204 individuals with stool cultures positive for Campylobacter and 62,040 matched controls, and they found the incidence rate to be 16.1 cases (95% CI, 2.3 to 114.4 cases) per 100,000 person-years in the population with Campylobacter-positive stool samples, compared to only 1.6 cases (95% CI, 0.2 to 11.4 cases) per 100,000 person-years in the control population (212). Notably, the same authors did not find a significant difference in the number of myopericarditis cases between the Campylobacter-infected and control populations (212). However, because of the rarity of these events (only two cases of myocarditis and two cases of pericarditis were found in the entire study sample), it is important to highlight the low statistical precision of this study.
A number of C. jejuni-associated myo(peri)carditis cases have been published since 1980 (213–230). These reports collectively indicate that patients usually present with symptoms such as thoracic pain, with concomitant electrocardiogram changes as well as increased levels of cardiac enzymes, 3 to 5 days after the onset of gastroenteritis. These studies also suggest that males are more susceptible, and microbiological stool cultures and/or serological analyses in these cases reveal C. jejuni as the only causative agent. In many cases, blood cultures remain sterile and the outcome is generally benign.
About 11 cases of C. fetus-associated myo(peri)carditis have been documented in the English literature. For these cases, blood cultures are generally positive and the outcome is more severe, sometimes even leading to death (225, 231). This might relate to the fact that, in contrast to C. jejuni, C. fetus tends to cause a more severe compromise of the pericardium, which might be due to colonization of the pericardium following bacteremia and septicemia and might explain the development of nonspecific symptoms, such as fever, malaise, and weight loss (232).
The mechanism by which Campylobacter species cause myo(peri)carditis remains unclear. It has been hypothesized that invasion of the cardiac tissue, bacterial exotoxins, circulating immune complexes, and cytotoxic T cells are involved (225). C. fetus-associated myo(peri)carditis may also require expression of bacterial surface layer proteins to confer evasion of the host immune system.
Campylobacter-associated endocarditis is an infrequent condition in which both native and prosthetic valves can be affected. Previous studies have shown that individuals with Campylobacter-associated endocarditis have either C. jejuni or C. fetus infection and that one-third of these patients suffer from concurrent chronic diseases, including hepatic cirrhosis, connective tissue disease, tuberculosis, or cancer (233–239). Furthermore, four cases of atrial fibrillation associated with C. jejuni infection have been reported in the literature (230, 240), and both C. jejuni and C. fetus infections have been associated with Campylobacter-associated aortitis (241–244). Overall, the limited current evidence suggesting an association between Campylobacter species and cardiovascular complications precludes causal inference.
Meningitis
Both C. jejuni and C. fetus subsp. fetus have been implicated in the development of meningitis in humans (245–252). Meningitis caused by C. fetus subsp. fetus has generally been reported for immunocompromised adults and is rare, with only eight cases reported from 1983 to 1998 (248). C. jejuni-associated meningitis is also rare and may affect both healthy and immunocompromised children and adults (246, 247, 251).
Extraoral Abscesses
Campylobacter species have been reported to be present in several types of abscesses outside the oral cavity. C. rectus has been associated with a chest wall infection (253), a breast abscess (254), and a vertebral abscess (255), while C. curvus has been associated with a liver abscess in a patient with complicated ovarian cancer and a bronchial abscess in a patient with lung cancer (254). C. gracilis and C. concisus have both been implicated in brain abscesses, while C. showae was detected in an intraorbital abscess (255). Most abscesses are polymicrobial in nature, making it difficult to assess the contribution of a specific Campylobacter species to the clinical outcome.
Reactive Arthritis
Reactive arthritis is a form of arthritis which most commonly occurs in patients in their 30s or 40s and develops following gastrointestinal or genitourinary infections. This condition can affect joints, such as knees and ankles, as well as the eyes and the genital, urinary, and gastrointestinal systems. Symptoms can begin approximately 1 month following infection and resolve within a year, although in some patients this condition may persist for up to 5 years (256). In 2007, a systematic review by Pope and colleagues reported the incidence of reactive arthritis associated with Campylobacter infection to be 1 to 5% (257). Other studies have estimated the risk of reactive arthritis associated with Campylobacter infection to be 3 to 13%, compared with 0 to 9% for E. coli O157:H7, 2 to 15% for Salmonella, 1 to 10% for Shigella, and 0 to 14% for Yersinia (256). Recently, Ajene and colleagues performed a comprehensive systematic review to identify the global incidence of reactive arthritis associated with infections by enteric pathogens, namely, Campylobacter, Salmonella, and Shigella (258). They found 25 articles, among which 14 cohort studies were chosen for calculation. Among a total of 63,206 patients infected by Campylobacter, 573 developed reactive arthritis, generating an incidence rate of 9 reactive arthritis cases per 1,000 cases of Campylobacter infection (258). Furthermore, the incidences of reactive arthritis resulting from Campylobacter infection ranged from 8 to 16% in adults and 0 to 6% in children. In comparison, the incidence rate for both Salmonella and Shigella infections was slightly higher, with 12 reactive arthritis cases per 1,000 infections (258). In a more recent meta-analysis assessing the proportion of Campylobacter cases that develop chronic sequelae, Keithlin and colleagues found that only 2.86% (95% CI, 1.40 to 5.61%) of patients infected with Campylobacter, mainly with C. jejuni and C. coli, developed reactive arthritis (186), suggesting that the incidence rate of Campylobacter-associated reactive arthritis is largely dependent on the geographic regions and cohorts used.
Complications of the Reproductive System
C. jejuni, C. coli, C. fetus subsp. fetus, and C. upsaliensis have been shown to cause septic abortion and neonatal sepsis in humans and animals (259, 260). These species are generally associated with abortion in pregnant women following an aggressive bowel infection that results in sepsis, with the infection eventually transmitted to the fetus (259). The clinical presentation and outcome of abortion caused by Campylobacter do not differ between species.
Other Campylobacter species, such as C. rectus and C. curvus, have been associated with premature birth and low birth weight in pregnant humans or mice (146, 261, 262). It has been shown that C. rectus translocates from the oral cavity to the reproductive tract, leading to inflammation that results in preterm birth (146). In mice, this inflammation appears to be mediated by the activation of Toll-like receptor 4 (TLR4) in placental tissues infected with C. rectus (262, 263). It remains to be seen if similar mechanisms control the inflammation that leads to preterm birth and intrauterine growth restriction following Campylobacter infection in humans.
CLINICAL MICROBIOLOGY
Isolation Methodologies in Clinical Settings
There is no gold standard or common method for the isolation of all Campylobacter species from clinical samples. Several selective agar media, using blood-based agar or blood-free agar, have been used for the isolation of Campylobacter species, particularly thermotolerant species (2). However, given the variability in antibiotic susceptibilities among Campylobacter species, these methods are effective for only a subset of species.
A more robust method—the Cape Town protocol—requires filtration of homogenized clinical samples through membrane filters with a pore size of 0.45 or 0.65 μm onto blood agar media (with or without vancomycin supplementation). The plates are then incubated at 37°C under microaerobic conditions (∼5% O2) enriched with CO2 and H2 (264). While not an absolute requirement, H2 enhances the growth of some Campylobacter species. The Cape Town protocol has been used successfully to isolate a range of Campylobacter species from fecal, intestinal biopsy, and saliva samples (264).
Enrichment procedures have been suggested to improve isolation rates from samples with a small starting number of Campylobacter cells (e.g., intestinal biopsy specimens). Enrichment of homogenized intestinal biopsy specimens in Ham's F-12 medium supplemented with vancomycin, followed by incubation at 37°C for 2 days and in combination with the Cape Town protocol, has been effective for the isolation of a number of Campylobacter species from patients with chronic gastroenteritis and IBD (108). Other enrichment broths that have been used to successfully isolate Campylobacter species include brucella-FBP (a combination of ferrous sulfate, sodium metabisulfite, and sodium pyruvate), Preston, Doyle and Roman, modified charcoal cefoperazone deoxycholate, Park and Sanders, Bolton, Hunt and Radle, and Hunt broths (265).
Laboratory Diagnosis
Laboratory diagnosis of Campylobacter infection requires the use of culture-dependent and/or culture-independent methodologies. In culture-dependent methodologies, single isolated colonies can be subjected to a range of conventional biochemical tests to identify phenotypic traits. Temperature, incubation time, and atmospheric conditions, for example, an H2-enriched atmosphere or growth at 42°C, can also be used to favor the growth of Campylobacter species during isolation procedures. The biochemical profile of an unknown organism is then matched to previously defined characteristics of the Campylobacter genus/species to enable identification of the organism to the genus or species level. In culture-independent methodologies, DNA or RNA can be isolated from clinical samples or from a pure culture. A genetic signature or marker of the organism can then be determined using sequencing techniques or genus- or species-specific PCR amplification of the gene of interest, for example, the 16S rRNA gene. Culture-independent tests are increasingly being used for the detection of Campylobacter species, and while in many cases they can enhance detection sensitivity, it has been proposed that this will have an impact on public health surveillance given that detailed analyses of isolates are required to monitor the distribution of different strains (266).
Biochemical identification.
Biochemical tests can be used to differentiate Campylobacter species from related genera and to identify organisms to the species level (2). The relevant biochemical tests used to differentiate between members of the Campylobacter genus have been reviewed by Lastovica (80). In that article, a biochemical flowchart to identify Campylobacter species is outlined, beginning with growth with or without supplementation of H2, followed by an indoxyl acetate test, a hippurate test, growth on MacConkey agar, an aryl sulfatase test, and production of H2S. Furthermore, detection of l-alanine aminopeptidase activity can be employed to differentiate between Campylobacter, Helicobacter, and Arcobacter species and other Gram-negative bacteria (80). However, the ability to differentiate between C. jejuni and C. coli would be the most relevant in the clinical setting. The only biochemical test that distinguishes between these two species is the hippurate hydrolysis test. C. jejuni isolates have the ability to hydrolyze hippurate, whereas C. coli isolates yield a negative test result.
Molecular identification.
The 16S rRNA gene has been used extensively for rapid detection and identification of many bacterial taxa, including Campylobacter species (267, 268). Owing to the sequence similarity among Campylobacter species, the 16S rRNA gene sequence cannot be used to differentiate between very closely related species, such as C. jejuni and C. coli (269). The larger 23S rRNA gene and the internal transcribed spacer (ITS) region, a region which lies between the 16S and 23S rRNA genes, have also been used to differentiate between Campylobacter species and strains (270–272). The 23S rRNA genes contain strain-specific intervening sequences, whereas the ITS region is highly variable in size and sequence composition depending on the species (273, 274). Indeed, a comprehensive analysis revealed the ITS region to be the most discriminatory region, compared with the 16S and 23S rRNA genes, for species and strain differentiation for the Campylobacter genus (270). When all three regions (16S rRNA-ITS-23S rRNA) were combined to create a phylogenetic tree, the resultant tree had the highest resolution in differentiating between members of the Campylobacter genus (270).
Several enzyme immunoassays are also available for the detection of C. jejuni and C. coli in clinical samples. A recent study by Granato and colleagues compared three commercially available kits with culture-based techniques and found that the three immunoassays had sensitivities that ranged from 98.5 to 99.3% and specificities that ranged from 98.0 to 98.2%, while standard culture had a sensitivity of 94.1% (275). Furthermore, a number of real-time assays are also available for the detection of Campylobacter species, some of which are capable of detecting more than one species at a time, including C. jejuni, C. coli, and C. lari (57). Interestingly, Javed and colleagues also described an assay to detect C. jejuni and C. coli based on the ability of recombinant receptor binding proteins from the C. jejuni bacteriophage NCTC12673 to agglutinate in the presence of these species (276).
More recently, due to the reduced costs of bacterial genome sequencing, the genomes of Campylobacter species and strains can now be sequenced fully, and many of these are completed or drafted. To date, the complete and draft genomes of over 100 Campylobacter species or strains—predominantly C. jejuni and C. coli strains—are available in the NCBI database. Genome sequencing and assembly have become commonplace in research labs for the characterization of isolates, and this will likely become routine practice in diagnostic facilities in the future.
Antibiotic Therapies
Most Campylobacter infections are self-limiting and require no therapeutic intervention other than supportive therapy, such as maintenance of hydration and electrolyte balance. However, antibiotics are employed in immunocompromised patients, patients whose symptoms are severe or persistent, and those with extraintestinal infections (277, 278).
Ciprofloxacin, a fluoroquinolone, is often used for the empirical treatment of gastroenteritis, particularly in travel-related cases. The major targets of quinolones in bacteria are DNA gyrase and topoisomerase IV, which are enzymes essential for DNA replication, transcription, recombination, and repair. However, in the case of campylobacteriosis, macrolides are the preferred choice of therapy (279). Macrolides bind to the 23S rRNA nucleotides 2,058 and 2,059 in the 50S ribosomal subunit, which results in blockage of the translocation step of protein synthesis, thereby preventing release of tRNA after peptide bond formation and resulting in the termination of peptide chain elongation. The reason for the increasing importance of macrolides for the treatment of Campylobacter infections is that the rates of ciprofloxacin resistance are relatively high for Campylobacter species due to the use of antibiotics in the poultry industry and animal husbandry operations (discussed further below) and, to a lesser extent, the indiscriminate use of ciprofloxacin for treatment of human diseases (280). This is particularly important in developing countries, where the use of fluoroquinolones is usually a suboptimal approach, for the above-mentioned reasons. For example, a recent randomized double-blind trial conducted in Thailand that compared macrolide regimens (single-dose and 3-day azithromycin treatments) with a fluoroquinolone regimen (3-day levofloxacin treatment) for the empirical management of traveler's diarrhea, mainly caused by C. jejuni/C. coli (64%), reported a cure rate of 96% with azithromycin, compared to 71% with levofloxacin (281). Similarly, the rate of microbiological eradication was superior with azithromycin-based regimens (96% to 100%) compared to the levofloxacin regimen (38%) (P = 0.001) (281). The high efficacy of azithromycin was supported by a further study comparing a single-dose azithromycin regimen to a 5-day erythromycin treatment in children with campylobacteriosis, which showed that azithromycin was significantly superior to erythromycin in eradicating the pathogen and accelerating the time to clinical cure (282).
A number of macrolides are also used in food animal production for both growth promotion and therapeutic reasons (283), and this has resulted in high levels of macrolide resistance being reported in some countries (284, 285). Consequently, ciprofloxacin has been recommended for the treatment of human infections caused by macrolide-resistant Campylobacter species.
Another problem related to antibiotic resistance in Campylobacter infections is the emergence of multidrug-resistant (MDR) strains (defined as strains with resistance to three or more antibiotics), which have been isolated in many countries throughout the world. The levels of MDR strains in humans are still relatively low overall (<25%) (286), but an increase in these strains in domesticated animals has raised concerns in relation to human disease (287). These concerns are well placed given that infections with Campylobacter species which are resistant to antibiotics have been associated with a longer duration of illness, an increased risk of invasive disease and death, and increased health care costs (288–290). In the case of severe Campylobacter infection in humans, treatment with aminoglycosides (e.g., gentamicin or kanamycin) is commonly employed (291). The mechanism of action of aminoglycosides involves binding to the 30S ribosomal subunit, where they interfere with the binding of formylmethionyl-tRNA to the ribosome, preventing the formation of the initiation complexes from which protein synthesis proceeds, and the rate of resistance to these antibiotics remains relatively low for Campylobacter species (3%) (286, 292).
RISK FACTORS, TRANSMISSION, AND ENVIRONMENTAL RESERVOIRS
A number of risk factors contribute to the susceptibility of humans to campylobacteriosis. A recent meta-analysis revealed that international travel was the most important risk factor for campylobacteriosis (OR, 4.9; 95% CI, 2.9 to 8.2), followed by consumption of undercooked chicken, environmental exposure, and direct contact with farm animals (293). In agreement with this, another study revealed that international travel was a risk factor for infection, with C. jejuni being one of six organisms that contributed to 70% of all gastrointestinal infections acquired during overseas travel between 1996 and 2005 (294). Moreover, campylobacteriosis was found to be the main cause of travel-related disease in Canada from 2005 to 2009, being responsible for 27.6% (123/446 cases) of cases (295). Similar levels have been reported in the United States, with 18% of Campylobacter infections being estimated to be associated with international travel (296). Domestic travel also plays a role in the transmission of human campylobacteriosis (297). In Europe, the proportion of diarrhea cases caused by Campylobacter species detected in travelers increased from 7% in 2008 to 12% in 2010 (298). In England, 17% of Campylobacter infections were classified as being associated with travel (299).
The level of risk for travel-related campylobacteriosis appears to be associated with the travel destination. A meta-analysis in 2009 showed that the locations with the highest levels of risk are Southeast Asia (32.4%; 162/500 cases), South Asia (7.8%; 39/499 cases), Africa (4.6%; 54/1,177 cases), and Latin America (2.5%; 51/2,031 cases) (300). More recently, Mughini-Gras and colleagues found that Dutch travelers visiting South Asia (OR, 28.9; 95% CI, 2.4 to 265.1), Southeast Asia and China (OR, 27.8; 95% CI, 4.5 to 170.9), sub-Saharan Africa (OR, 25.4; 95% CI, 2.7 to 310.7), Latin America and the Caribbean (OR, 20.8; 95% CI, 2.0 to 211.6), western Asia (OR, 10.6; 95% CI, 2.8 to 39.9), and northern Africa (OR, 10.6; 95% CI, 2.3 to 49.0) were at higher risk of developing campylobacteriosis than those traveling to western European countries. While travel does contribute to the overall burden of Campylobacter transmission, the transfer or spread of exotic or antibiotic-resistant strains to previously unexposed populations is a further concern. In addition, travel-related infections are often associated with consumption of contaminated meat or water (300), suggesting that a complex interrelationship exists between risk factors.
Consumption of contaminated food, particularly poultry products, unpasteurized milk, and water, is a risk factor for C. jejuni and C. coli infection (Fig. 1) (18, 301, 302). One approach to estimate the source attribution of campylobacteriosis cases is through the analysis of outbreak data. As expected, Campylobacter infections are mainly attributed to poultry products by using these techniques (303, 304). More recently, however, molecular techniques, such as multilocus sequence typing, porA/flaA typing, and gyrase subunit A typing, have played a more significant role in source attribution (305–310), allowing further insights into the contributions of the different reservoirs to the burden of campylobacteriosis in humans and the subsequent transfer of Campylobacter species to wildlife through environmental contamination.
Immunodeficiency is also a risk factor for campylobacteriosis (101, 311). For example, human immunodeficiency virus (HIV)-infected patients presenting with diarrhea are more frequently infected with Campylobacter than uninfected individuals with diarrhea (311). In addition, the incidence of Campylobacter-related illness among HIV-infected patients is higher than the incidence found in the general population (312). Below, we further examine the prevalence of Campylobacter species in the environment and discuss how these reservoirs facilitate transmission of Campylobacter to humans.
Poultry
Poultry is recognized as a primary source of food-related transmission of Campylobacter species to humans (313). A major contributing factor is the high carriage rate of Campylobacter within broiler chickens. Therefore, Campylobacter species are found in abundance on poultry farms and their surrounding environment, including the soil, water sources, dust, building surfaces, and the air (314). In addition to chickens, commercial turkeys and ducks can also serve as reservoirs of C. jejuni and C. coli (315–317). Furthermore, poultry is also an important reservoir of other Campylobacter species, such as C. lari, C. upsaliensis, and C. concisus (318, 319). Domesticated broiler chickens and imported chickens both contribute to the overall burden of Campylobacter infections (320).
It has been estimated that 71% of human campylobacteriosis cases in Switzerland between 2001 and 2012 were attributed to chickens (321, 322). A study investigating the prevalence of Campylobacter in crop (enlarged part of the digestive tract of birds that serves as a temporary storage space for food) and cecal samples from market-age broiler chickens found 62% of crop samples to be positive, compared to only 4% of cecal samples (P < 0.001), suggesting that the crop is an important niche for Campylobacter and may represent a major source of contamination during processing (323). Given that C. jejuni strains survive in chicken feces for up to 6 days after excretion, chicken feces may also be a potential source of transmission to the environment or humans when poultry manure is used as a fertilizer (324).
The UK Food Standards Agency reported preliminary findings showing that 72.9% of fresh whole retail chickens surveyed during 2014 to 2015 were infected with Campylobacter, with 18.9% of these harboring a level of >10,000 CFU/g, which is considered highly contaminated (325). Data from Canada also support the finding that broiler chickens are a major source of Campylobacter species. There are, however, indications that chicken-associated Campylobacter infections may be more common in urban dwellers than in rural dwellers (326, 327). One study reported the levels of thermotolerant Campylobacter species to be three times higher in organic broilers than in conventional broilers (54.2% versus 19.7%) (328), suggesting that the likelihood of purchasing Campylobacter-contaminated broiler meat is higher for organic sources than for conventional sources. Furthermore, the relative risk of becoming ill from Campylobacter on a per-serving basis is 1.7 times higher for consuming organic carcasses than for consuming conventional carcasses.
Domesticated Animals
Domesticated animals are another reservoir of Campylobacter species (313, 329). Fresh and frozen meats are frequently contaminated with Campylobacter, whereas commercial cooked products are less affected (1, 330). C. jejuni, C. coli, and C. lari accounted for 69, 30, and 1% of the contaminating bacteria in these products, respectively (330). In addition to consumption of contaminated meat from domesticated animals, contact with domesticated and companion animals poses a significant risk for the transmission of Campylobacter species (1, 301, 302, 321, 331, 332).
Recent studies from Denmark showed that cattle were the attributed source for 16 to 17% of the total cases of campylobacteriosis (320). Similarly, in Switzerland, cattle have been estimated to be responsible for 19.3% of Campylobacter infections, which is substantially higher than the contribution from pigs (1.2%) (321). The prevalence of Campylobacter in cattle varies significantly across studies, with rates ranging from 23% to close to 90% (333–335). The species detected in cattle include C. jejuni, C. coli, C. lari, and C. lanienae (335–337).
Campylobacter species are also prevalent in pigs and piglets. These bacteria are capable of colonizing piglets as early as 24 h after birth, as a result of exposure to contaminated feces (338). The carriage rates of Campylobacter range from 32.8 to 85.0%, depending on the age of the pig (339, 340). Similar to the trend in cattle, younger pigs tend to have higher levels of Campylobacter than older animals (333, 339). However, in contrast to cattle, pigs are more readily colonized by C. coli (340).
Sheep and goats have also been investigated for their rates of carriage of Campylobacter species. In Nigeria, 6.8% of intestinal samples from sheep were found to be positive for C. jejuni, C. coli, or C. lari (341). A higher prevalence rate (17.5%) was reported for cecal samples from sheep from Swiss abattoirs, with only C. jejuni and C. coli being detected (342). In both studies, C. jejuni was the major Campylobacter species detected (341, 342). Screening of fecal samples from 222 dairy goats on 12 farms in Spain failed to detect any Campylobacter species (343).
Dogs and cats are also carriers of Campylobacter species (344). Up to 58% of healthy dogs and 97% of diarrheic dogs have been determined to be positive for Campylobacter species (345). Interestingly, C. upsaliensis appears to be one of the major Campylobacter species colonizing dogs and cats (344); however, C. coli, C. concisus, C. fetus, C. gracilis, C. helveticus, C. jejuni, C. lari, C. mucosalis, C. showae, and C. sputorum have all been detected in these animals (345). Other domesticated animals that have been investigated as potential reservoirs of Campylobacter species include hamsters (346, 347), ferrets (348), pet reptiles (349), and rabbits (350). Based on the low rates of carriage of Campylobacter species in these animals, it is probable that they are not a major source of transmission of Campylobacter species to humans.
Wild Animals
Wild animals are potential reservoirs of Campylobacter species. Among all the host species studied, wild birds are most likely to carry Campylobacter species. Indeed, 35% of the cecal contents harvested from 445 wild ducks from Colorado tested positive for C. jejuni (351). A study in Norway examined 540 wild birds of 40 different species and found that 28.4% were positive for C. jejuni; crows, puffins, and gulls had the highest carriage rates, i.e., 89.8%, 51.3%, and 50.0%, respectively (352). A similar carriage rate was observed in wild birds in Italy (74/217 birds; 34.1%) (353).
Studies of other types of wild birds have reported lower Campylobacter carriage rates. For example, Llarena and colleagues examined fecal samples from 924 barnacle geese and reported the prevalences of C. jejuni to be 11.5% and 23.1% in 2011 and 2012, respectively (354). In the same study, the prevalence of C. coli in both 2011 and 2012 was only 0.2%. Furthermore, a study conducted in Norway failed to detect C. jejuni in geese. In contrast, 3.0% (6/200 birds) of pigeons and 20.0% (1/5 birds) of mallards carried C. jejuni (355). The prevalence of C. jejuni in waterfowl in northeastern Spain (318 adult waterfowl of nine fowl species) was 12.6% (356). A further study in Spain isolated C. jejuni from 1.0% (1/97 birds) of griffon vultures (357), while a study from the United States, conducted over the period from 2009 to 2010, isolated thermophilic Campylobacter species from 4.8% (9/188 birds) of captured wild birds (358). These results indicate that the prevalence of Campylobacter species varies greatly depending on the type of wild birds and their geographic regions.
Other wild animals tested for the presence of Campylobacter species include small rodents, deer, moose, reindeer, boars, mangabeys, porcupines, duikers, and turtles—all were found to have low levels or to be free of Campylobacter species (355, 358–362). Furthermore, a number of studies have suggested that the importance of wild animals as a reservoir of infection is limited, as strains isolated from wild animals are usually not clonally related to isolates from humans and chickens (317, 363, 364). This is in contrast to a number of studies which reported that Campylobacter strains isolated from wild bird feces around broiler houses can be recovered from the ceca of broilers in those houses (365–367). These studies provide evidence for the contribution of wild birds to Campylobacter contamination during poultry production and processing and suggest a potential role in human campylobacteriosis. Overall, current studies indicate that only some species of wild birds have high rates of carriage of Campylobacter species and that, currently, insufficient evidence exists to determine whether these isolates play an important role in human disease.
Water
Water is an effective vehicle of transmission of Campylobacter species to humans and animals, and contaminated water has been responsible for a number of outbreaks in different countries (368–371). Drinking undisinfected water is a leading risk factor for campylobacteriosis (372). Several studies have assessed the prevalence of Campylobacter species in different water sources. For example, Arvanitidou and colleagues isolated C. jejuni from 1.0% of drinking water samples (5/500 samples) in northern Greece (373). In Poland, Popowski and colleagues detected C. jejuni, C. coli, or C. lari in 70% of water samples from rivers or lakes in the Warsaw region (374), supporting a role for contaminated water in the direct transmission of Campylobacter species to humans. Individuals who utilize private wells rather than municipal surface water systems as a drinking water source are at a higher risk of developing campylobacteriosis than other reportable enteric diseases (375).
Farms that utilize private water supplies as a water source for their cattle are more likely to have cattle that test positive for Campylobacter, indicating that contaminated water can also serve as a means of transmission of Campylobacter to domesticated animals (334). Indeed, emptying and cleaning water troughs more regularly have been shown to reduce the risk of colonization of cattle by Campylobacter (334). Furthermore, it has been reported that cattle were more likely to test positive for Campylobacter following an outdoor grazing period in spring, when the water supply was lake water, than when they were confined indoors in winter, when their water supply was municipal chlorinated tap water (376). Other sources implicated in the contamination of outdoor water are wild bird feces and waste runoff from contaminated domesticated animals (377).
Given that contaminated water may be a common factor in transmission to different hosts, its role in the transmission cycle and as a source of Campylobacter contamination or infection may be underestimated. Indeed, Champion and colleagues showed that C. jejuni isolates from different sources (humans, chickens, bovines, ovines, and the environment) cluster into two clades: a “livestock clade” and a “nonlivestock clade.” Of these, 55.7% of human and 11.4% of livestock isolates were reported to group within the nonlivestock clade (378), suggesting that the environment, and particularly water sources, may be an underestimated reservoir for transmission of Campylobacter species.
Other Sources
Although it is not as common, another potential source of transmission is person-to-person transmission (fecal-oral or via fomites). The Health Protection Agency in the United Kingdom found that person-to-person transmission was the source of outbreaks in 3% (5/143 cases) of the campylobacteriosis cases from 1992 to 2009 (31). Furthermore, a study conducted in New Zealand that combined detailed epidemiological and genotyping data concluded that person-to-person transmission was responsible for 4% of campylobacteriosis cases in that country (74). Other studies, conducted in Australia and the Netherlands, provided similar figures for the contribution of person-to-person transmission to campylobacteriosis (300, 379).
Unpasteurized milk sourced from dairy cattle has also been implicated in a number of campylobacteriosis outbreaks (380–382). The number of outbreaks in the United States associated with unpasteurized milk increased from 30 in 2007 to 2009 to 51 in 2010 to 2012 (383). Unpasteurized milk may also serve as a source of several other Campylobacter species, including C. hyointestinalis subsp. hyointestinalis, C. fetus subsp. fetus, C. concisus, and C. ureolyticus (384, 385). Genomic analyses indicate that the presence of Campylobacter species in milk can be attributed to fecal contamination (386).
Several other transmission sources, including insects, have been investigated as reservoirs of Campylobacter species. For example, flies have been suggested to play a role in the transmission of Campylobacter species from contaminated sources to broiler chickens (387), and the lesser mealworm beetle, Alphitobius diaperinus, may act as a reservoir of C. jejuni (388). In one study, C. jejuni was reported to survive on the exterior of the beetle for 12 h and in the interior of larvae for 72 h, and it could be shed in the feces of larvae for 12 h after exposure. In this study, 90% of birds that consumed a single adult or larval beetle became C. jejuni positive, while 100% of those that consumed 10 adults or larvae became positive, a finding that suggests that beetles are capable of passing viable bacteria to chickens (388). In contrast, Skov and colleagues found that litter beetles, which are commonly present in chicken houses, do not play an important role as reservoirs of Campylobacter species (389). It is possible that only specific species of beetles are carriers of Campylobacter species.
Studies have also suggested that microbial eukaryotes may act as a nonvertebrate reservoir of Campylobacter species in the environment. For example, in a study by Axelsson-Olsson and colleagues, C. jejuni was found to survive longer when it was cocultured with the protozoan Acanthamoeba polyphaga than when it was cultured alone (390). Microscopically, C. jejuni was found to aggregate in vacuoles within amoebae, and it could be detected following rupture of the eukaryotic cells (390). Since a diverse range of microbial eukaryotic organisms (73.5% yeasts and fungi and 26.5% protozoa) are found in the poultry drinking water systems on farms (391), it is not surprising that eukaryotes infected with Campylobacter may play a role in the transmission of this organism to chickens.
IMPACT OF ANTIBIOTIC USAGE IN ANIMALS ON CAMPYLOBACTER RESISTANCE
The discovery in 1950 that the addition of antibiotics to animal feed at subtherapeutic levels could lead to increased growth rates in husbandry animals resulted in research into methods to improve or stabilize meat supplies to the consumer. Indeed, by the turn of the 20th century, the majority of antibiotic usage in the United States was for agricultural purposes. This approach has led to a dramatic increase in antibiotic resistance in several human pathogens that originate from domesticated animals, including Campylobacter species (392).
As early as 1999, Smith and colleagues reported an increase in quinolone-resistant C. jejuni in Minnesota residents, whose acquisition could be attributed to foreign travel and poultry products (393). Smith et al. proposed that the use of fluoroquinolones in the poultry industry, which started in the United States in 1995, was in part responsible for the increase in prevalence of quinolone-resistant C. jejuni (from 1.3% in 1992 to 10.2% in 1998; P < 0.001) (393). There is strong evidence to support the observation that fluoroquinolone use in food animals is associated with increased numbers of infections with resistant strains of Campylobacter in humans. Indeed, therapeutic fluoroquinolone administration to poultry flocks has been shown to select for ciprofloxacin-resistant C. jejuni in poultry (394). However, an increase in antibiotic resistance in Campylobacter isolates from poultry is not restricted to C. jejuni. In 2013, Wieczorek and colleagues found that C. coli had higher levels of resistance than C. jejuni, irrespective of the antimicrobial agents tested, including ciprofloxacin, nalidixic acid, tetracycline, and streptomycin (395).
This increase in antibiotic resistance has also been observed in Campylobacter isolates from cattle. For example, in a study by Di Labio and colleagues, investigation of antimicrobial resistance in 202 Campylobacter isolates from Swiss veal calves showed 67.8% of isolates to be resistant to at least one of the agents tested (396). Importantly, resistance mainly corresponded to antimicrobial agents that were utilized in the farming processes. In France, a 5-year survey of fecal samples from cattle between 2002 and 2006 recovered Campylobacter species from 16.5% of 2,255 samples (C. jejuni from 12.8% and C. coli from 3.7%). Investigation of the antibiotic susceptibility of these Campylobacter species showed an increase in the rates of resistance to quinolones and fluoroquinolones from 2002 to 2006, with the fluoroquinolone resistance rate rising from 29.7 to 70.4% (397). Interestingly, fluoroquinolones are not utilized in Australian livestock, and as a result, Campylobacter isolates from livestock in this region have negligible levels of resistance to fluoroquinolones, which in turn correspond to low resistance levels in human isolates (398). Low levels of resistance to quinolones are also observed in Finland and Sweden (284).
Similar to the case for cattle and poultry, the rate of macrolide resistance has been found to be high in C. coli isolates from pigs in Switzerland, a finding that reflects the use of these antimicrobials in Swiss livestock production (399). Indeed, the use of tylosin, erythromycin, and tilmicosin in pigs is considered a major driving factor of resistance to macrolides (400). While antibiotic use in agriculture is still commonplace in many countries, a number of countries have implemented bans on the nonmedicinal use of antimicrobials in livestock. It remains to be seen if such bans will result in a decrease in resistance rates in these countries.
CONTROLLING THE SPREAD OF CAMPYLOBACTERIOSIS
Current estimates suggest that transmission of 50 to 80% of all human cases of campylobacteriosis is related to chickens (401). The annual worldwide costs associated with Campylobacter infection are extremely high. For example, in the United States, the estimated annual cost of campylobacteriosis is reported to be $1.3 billion, while in the Netherlands, it is estimated to be €21 million (32, 52). Given the association between chickens and campylobacteriosis, as well as the high costs associated with this disease, many countries have invested in developing strategies to control the dissemination of Campylobacter species in the poultry industry (27). These strategies target a number of key areas, including reducing environmental exposure, reducing or removing Campylobacter from colonized chickens, and increasing the resistance of chickens to Campylobacter carriage. Based on an appraisal of all the literature published between 1980 and September 2008, Newell and colleagues have written an excellent review on Campylobacter and biosecurity on poultry farms, which outlines the different routes of transmission that occur on poultry farms and the degrees of success of strategies undertaken to prevent transmission of Campylobacter (402). We further highlight below the various methodologies that have been used to control Campylobacter transmission.
Reducing Campylobacter Transmission in Chickens
Horizontal transmission of Campylobacter in the farm environment represents the usual route of transmission. Once Campylobacter is introduced into a chicken flock, it spreads rapidly and results in colonization of the intestinal tracts of the majority of chickens within 1 week. The primary sites of colonization within the intestine are the crop, ceca, and small intestine (402, 403). In contrast, vertical transmission of Campylobacter from an infected bird to its offspring via internal contamination of eggs within the reproductive tract prior to shell formation is rare, although “pseudo-vertical transmission” of microorganisms from parent flocks to their chicks via fecal contamination of shells can occur (402). Once colonized, chickens remain colonized until they are slaughtered. Although they are colonized by large numbers of Campylobacter organisms (up to 109 bacteria/g), chickens usually exhibit no clinical or other adverse effects (402). An interesting study, however, recently showed that C. jejuni infection of some chicken breeds can lead to prolonged inflammation, damage of the gut, and diarrhea (404).
Human and vehicular traffic entering farms also appears to be an important route for Campylobacter species to enter poultry houses from the external environment. In particular, staff who handle neighboring livestock, especially poultry, are associated with an increased risk of Campylobacter-positive flocks, and the number of staff employed to look after the house and the frequency of visits per day are also associated with the level of risk (402). Given the abundant source of Campylobacter species on poultry farms and their environment, strict hygiene measures have been introduced on the majority of farms in an attempt to reduce Campylobacter colonization of chicks, although adherence to these measures differs considerably from farm to farm. Based on the current literature, the use of hygiene barriers at the entrance to poultry houses, the provision of hand-washing facilities, the performance of boot dips, and the use of house-specific boots and overshoes have all been shown to significantly decrease Campylobacter colonization of chicks (314, 402).
Although study results are conflicting, it appears that in some countries flies play an important role in transmitting Campylobacter species from fecal sources to poultry (402). For example, in a recent study conducted in Denmark, the prevalence of Campylobacter-positive flocks was reported to have reduced significantly, from 41.4% in 2003 to 2005, before fly screens were introduced, to 10.3% in 2006 to 2009, after fly screens were installed (P < 0.001; OR, 6.1; 95% CI, 3.1 to 12.4) (405). Furthermore, that study showed that over the summer period, no rise in the prevalence of Campylobacter species was observed in poultry houses with fly screens, while over the same period poultry houses without fly screens showed significant increases. Based on their findings, the authors estimated that if fly screens had been part of Danish biosecurity practices, the prevalence of Campylobacter-positive flocks during summer months could have been reduced by approximately 77% nationally (405).
Another important source of Campylobacter on chicken farms is fecal material from chickens already colonized with Campylobacter living on the same farm; the risk of colonization in a flock is associated with the presence of older birds (402). The number of chicken houses on the farm also appears to be an important factor, although the relationship between the number of houses and the level of increased risk appears to differ by geographic location. For example, in France, an increased infection risk was associated with three or more houses on the same site, while in the Netherlands, having five or more houses has been reported to give an elevated risk (402). Based on the finding that identical Campylobacter flaA types were present in isolates obtained from birds originating from different hatcheries, it has been suggested that flocks are colonized by C. jejuni following placement of chicks into broiler chicken houses and that C. jejuni strains within the farm are those carried over from the previous flock (406). Reductions in intestinal colonization levels of broiler chickens have been reported to lead to a considerable decline in the incidence of campylobacteriosis (407). Indeed, Rosenquist and colleagues reported that a 2-log10 reduction of Campylobacter levels on broiler carcasses resulted in a 30-fold decline in the incidence of human campylobacteriosis (328). Given this, a number of strategies aimed at reducing Campylobacter colonization in poultry, including the use of bacteriocins, bacteriophages, and probiotics, have been investigated.
Bacteriocins.
One approach to reduce Campylobacter colonization in chickens is the use of bacteriocins. Bacteriocins are antimicrobial peptides produced by a range of bacterial species. A number of effective anti-Campylobacter bacteriocins have been identified in commensal bacteria isolated from the intestines of chickens and are currently being investigated for the ability to control Campylobacter species at the farm level. For example, in a study by Svetoch and Stern, treatment of chickens with the bacteriocin L-1077, produced by Lactobacillus salivarius strain L-1077 isolated from broiler chickens, resulted in decreases in cecal C. jejuni counts of >4 log compared with those for untreated control birds (408). In addition, the effects of three other bacteriocins, OR-7 from Lactobacillus salivarius and E-760 and E50-52 from Enterococcus faecium, have been investigated (409). Administration of these three bacteriocins to chickens resulted in dramatic reductions of 5 to 8 log10 CFU in C. jejuni intestinal colonization (409). Investigation of the development of resistance of Campylobacter species to the E-760 bacteriocin in vitro indicated that low-level but not high-level bacteriocin resistance can be developed. Furthermore, in a chicken model of Campylobacter, the E-760 bacteriocin again selected only for low-level bacteriocin-resistant C. jejuni mutants. In the absence of bacteriocin selection pressure, this low level of bacteriocin resistance was shown not to be stable either in vitro or in vivo. Collectively, these findings indicate that bacteriocins may be promising agents for the control of C. jejuni in commercial broiler chickens.
Bacteriophages.
Bacteriophages have also been investigated for use in the biocontrol of Campylobacter species. The use of bacteriophages to reduce Campylobacter cecal colonization of chickens has shown encouraging results as far as reducing Campylobacter counts. A number of studies have reported that under experimental conditions, bacteriophage treatment of chickens which have already been colonized with Campylobacter results in reductions of 0.5 to 5.0 log10 CFU/g Campylobacter in the chicken gut (410–414). For example, in a field trial by Kittler and colleagues, a phage cocktail at levels of 5.8 to 7.5 log10 PFU/bird was added to the drinking water of chickens colonized with Campylobacter, and the results showed that, at slaughter, Campylobacter counts in the ceca of treated birds were significantly reduced (>3.2 log10 CFU/g cecal content) compared with those of the control group (P = 0.0011) (415).
While these results are encouraging, Campylobacter numbers generally return to pretreatment levels over time (416). One explanation for the lack of longevity in the reduction of Campylobacter numbers is the development of resistance to phage therapy (416). Given this, it has been suggested that bacteriophage treatment immediately prior to slaughter might be a better approach, as this has the potential to reduce the cecal Campylobacter load and, as a result, reduce infection in humans (416). Evidence exists to show that the rate of resistance to phage therapy can be reduced by administering multiple phages at the same time. For example, in a study by Fischer and colleagues, commercial broilers were inoculated with 104 CFU of a C. jejuni field strain (417), after which three groups of birds (n = 88 per group) were treated with either a single phage, a four-phage cocktail, or solvent only. The results showed that following treatment with the single phage and the phage cocktail, Campylobacter levels were permanently reduced, with a significant reduction being observed between 1 and 4 weeks posttreatment and a maximum reduction level of 2.8 log10 CFU/g cecal content. Investigation of bacteriophage resistance showed initial resistance rates of up to 43% in those treated with the single phage, whereas a resistance rate of 24% was observed in birds that had received the cocktail. These resistance levels, however, later stabilized at low levels (417). The feasibility of introducing phage therapy as part of poultry farming practices and the costs associated with this process on a large scale are not known.
Probiotics.
Probiotics have been shown in some studies to be valuable for inhibiting C. jejuni colonization. For example, a study by Ghareeb and colleagues demonstrated that Enterococcus faecium, L. salivarius, Lactobacillus reuteri, and Pediococcus acidilactici, originally isolated from the intestines of healthy chickens, could inhibit the growth of C. jejuni in vitro (418). In their study, they infected 1-day-old broiler chicks with 104 CFU of a field strain of C. jejuni orally. The chickens were then randomized into two groups, one of which received a multispecies probiotic (2 mg/chick/day) in their drinking water, while the other group received no probiotic treatment. At both 8 and 15 days postchallenge, cecal colonization by C. jejuni was shown to be reduced significantly in chickens treated with probiotics (mean, 3.0 log CFU/g and 2.5 log CFU/g, respectively; P < 0.001) compared with controls (mean, 6.8 log CFU/g and 8.0 log CFU/g, respectively; P < 0.001) (418). In a similar study by Nishiyama and colleagues, the ability of the probiotic strain Lactobacillus gasseri SBT2005 (LG2055) to reduce colonization in chicks was investigated (419). Approximately 24 h following hatching, chickens were infected with C. jejuni 81-176, and after 24 h, one group of chickens received LG2005 daily for 14 days, while a second group received no probiotic. At 14 days postinoculation, significantly increased levels of Lactobacillus gasseri were observed in chicks receiving C. jejuni plus LG2005 compared to those receiving C. jejuni alone (P < 0.001). Furthermore, a significant reduction in C. jejuni levels was observed in the ceca of chicks treated with LG2055 compared with the untreated controls (P < 0.001). Santini and colleagues also reported Bifidobacterium longum PCB 133 to have a marked anti-Campylobacter activity in vivo (420). In their study, they initially screened 55 lactic acid bacteria and bifidobacteria in vivo for probiotic properties against three strains of C. jejuni, with the ultimate goal of finding probiotics that could be used against Campylobacter in poultry. Based on their high activity against C. jejuni and their ability to survive in the gastrointestinal tract, Lactobacillus plantarum PCS 20 and B. longum PCB 133 were used in an in vivo trial in poultry. While L. plantarum PCS 20 showed no effect, B. longum PCB 133 gave encouraging results. Following 2 weeks of daily administration of B. longum to the chicks, the level of B. longum PCB 133 in chicken feces was found to have increased significantly, and it remained high even after a wash-out period of 6 days. Importantly, over the same time frame, the level of C. jejuni in chicken feces from chicks administered B. longum PCB 133 significantly decreased, showing a 1-log reduction (P < 0.05) compared to the level in chicks not receiving the probiotic (420).
Vaccination.
Clearly, if it were possible to successfully vaccinate chickens against Campylobacter, it would not only reduce Campylobacter levels in chickens but also reduce transmission to humans and eliminate postharvest procedures (421). While studies have investigated a range of vaccine candidates over the last 15 years, including the use of flagellar proteins, formalin-inactivated C. jejuni, C. jejuni inner membrane antigen, and killed C. jejuni, delivered intranasally, orally, orally using a Salmonella carrier, and intraperitoneally, respectively, none of these vaccines have completely prevented Campylobacter colonization in chickens (422). Interestingly, a recent study by Annamalai and colleagues which investigated the effect of nanoparticle-encapsulated outer membrane proteins (OMP) of C. jejuni as a vaccine in chickens has shown significant promise (422). In birds that received subcutaneous vaccination, C. jejuni colonization levels in cecal and cloacal contents at 7 days postchallenge were shown to be below the detection limit, whereas the other groups showed various degrees of colonization. Based on their study, Annamalai and colleagues considered the subcutaneous route of vaccination, using encapsulated OMP or OMP alone, to be efficacious in eliciting protective antibody responses and preventing colonization of C. jejuni in chickens (422).
Strategies aimed at the processing level.
Campylobacter in the intestinal tracts of chickens at slaughter has the potential to contaminate the slaughterhouse and the food processing environment as well as food products bought by consumers. At the processing level, treatment with organic acids, acidified sodium chlorite, trisodium phosphate, or UV light during primary processing, including the use of chemical dip tanks for carcasses, would help to reduce the number of Campylobacter organisms (423). Freezing or irradiating the entire poultry supply would further reduce bacterial numbers by directly killing the pathogen (423). While the postharvest measures outlined above have had some success in reducing the transmission of C. jejuni to humans, they do not completely eliminate transmission of Campylobacter-related gastroenteritis. In addition, postharvest strategies are expensive and have been reported to degrade the quality of meat (421). Nevertheless, Lake and colleagues have proposed that using multiple interventions at the level of primary processing is the most cost-effective approach to reducing poultry-related transmission, while the use of an irradiation method alone during primary processing is the least cost-effective (423).
Information describing safe food-handling procedures and risk factors associated with campylobacteriosis could be provided to consumers (423). This may help in limiting the level of cross-contamination in the kitchen that results from exposure to contaminated meat. A particular highlight is the success observed in New Zealand following regulatory interventions implemented by the New Zealand Food Safety Authority to reduce Campylobacter transmission in the poultry sector. Following the advent of these interventions, the incidence of campylobacteriosis in this country decreased by 50% from 2006 to 2008 (from 383 to 157 cases per 100,000 population) (73). As part of these interventions, poultry processers monitored and reported the prevalence of Campylobacter species in poultry flocks and levels of contamination in poultry carcass rinsates, after which mandatory performance targets were introduced. Key improvements were made in regard to hygiene practices in production and primary processing and modifications to the immersion-chiller conditions. In addition, a recent paper found that the prevalence of GBS in New Zealand also decreased as a result of the intervention strategy (184). The successes observed in New Zealand will hopefully encourage other countries which currently do not have regulated interventions in the poultry sector to take action.
CONCLUSIONS
Our evaluation of the epidemiology of campylobacteriosis has revealed an increasingly important role for Campylobacter infection in public health. While global efforts to control the transmission of enteric pathogens have been effective at reducing the incidence of a number of major foodborne pathogens, the prevalence of Campylobacter infection has nevertheless continued to increase across most developed nations. This rise may be a consequence of many factors, including but not limited to our improved ability to detect these species and our failure to effectively prevent transmission due to breakdowns in biosecurity. There are currently insufficient epidemiological data to provide an accurate assessment of the burdens of campylobacteriosis in Africa, Asia, and Central and South America. However, it is clear that in many of these regions, where data are available, Campylobacter species appear to be endemic in children.
The emergence of Campylobacter species other than C. jejuni and C. coli clearly warrants additional investigation. Additional research efforts focusing on their growth conditions, methods of detection, and elucidation of their mechanisms of pathogenesis could revolutionize our understanding of their global distribution and impact on infectious diseases.
It is now well established that poultry, particularly fresh and frozen chicken meat, is a major reservoir of Campylobacter species. Other domesticated animals, such as cattle and pigs, and environmental sources, such as contaminated water, also play a vital role in the direct transmission of these organisms to humans. Moreover, many cases of travel-associated Campylobacter infection can be attributed to consumption of contaminated meat products or water. Contaminated water contributes to transmission to domesticated birds and other animals, whereas the use of manure from domesticated animals may result in contamination of water sources, fulfilling a cycle of dissemination between natural reservoirs.
The lack of standardized biocontrol methodologies in the poultry sector, a principal source mediating Campylobacter transmission to humans, substantially contributes to the global increase in campylobacteriosis. This issue highlights the need for a worldwide campaign to encourage interventions within the poultry sector. The success achieved as a result of an intervention strategy introduced in New Zealand will hopefully inspire other government regulatory bodies to adopt similar methods to combat Campylobacter transmission. Without further action, Campylobacter-related infection will remain an important infectious disease that challenges global health in the years to come.
ACKNOWLEDGMENTS
N.O.K. is a recipient of an early career fellowship from the National Health and Medical Research Council, Australia. S.M.M. is a recipient of the R. G. Menzies Fellowship from the National Health and Medical Research Council, Australia.
We declare that we have no conflicts of interest.
Biographies

Nadeem O. Kaakoush, Ph.D., undertook his doctoral studies in pathology at the School of Medical Sciences at the University of New South Wales (UNSW), Sydney, Australia, where he investigated pathogenic Helicobacter and Campylobacter species. Following his doctoral studies, he took up a Research Associate position at the School of Biotechnology and Biomolecular Sciences at UNSW, examining the role of mucosa-associated bacteria in inflammatory bowel diseases. In 2011, Dr. Kaakoush was awarded a National Health and Medical Research Council early career fellowship to further his research in this field, and he has since expanded his research to investigate the overall clinical relevance of emerging Campylobacter species.

Natalia Castaño-Rodríguez holds an M.D. from Del Rosario University, Colombia, and an M.Phil. and Ph.D. from the University of New South Wales, Sydney, Australia. She is currently a Research Associate working in the Helicobacter and Campylobacter laboratory at the University of New South Wales. She has extensive experience conducting research in the fields of microbiology, immunology, and host immunogenetics, with a particular emphasis on the role of pattern recognition receptors in the host inflammatory response following Helicobacter and Campylobacter infections. More recently, her efforts have been focused on investigation of the role of host immunogenetics in Helicobacter pylori-related gastric cancer.

Hazel M. Mitchell was awarded a B.Sc. (with honors) from the University of Strathclyde, Glasgow, United Kingdom, a Dip.Ed. from the Jordanhill College of Education, Glasgow, and a Ph.D. from the University of New South Wales, Sydney, Australia. She previously held the positions of Senior Lecturer and Associate Professor in the School of Biotechnology and Biomolecular Sciences at the University of New South Wales and was promoted to Professor of Medical Microbiology in 2007. Her research studies focus on the role of mucosa-associated bacteria in gastrointestinal disease, in particular the role of emerging Campylobacter species and the intestinal microbiota in inflammatory bowel disease and the role of Helicobacter pylori and host genetic polymorphisms in gastric cancer. She is on the editorial board of Helicobacter and is an Associate Editor of Gut Pathogens. She has published 132 peer-reviewed journal articles and 16 book chapters and coedited a book entitled Helicobacter pylori in the 21st Century.

Si Ming Man received his B.Med.Sc. (with honors) and M.S. (research) from the University of New South Wales, Sydney, Australia, for his work on Campylobacter infection in inflammatory bowel disease. He then obtained his Ph.D. from the University of Cambridge, United Kingdom, where he investigated the role of inflammasome activation in the host defense against Salmonella infection. He is a recipient of the National Health and Medical Research Council R. G. Menzies Fellowship and is currently based at St. Jude Children's Research Hospital, Memphis, TN. He has long-standing interests in understanding the molecular mechanisms regulating innate immunity in response to gastrointestinal pathogens. His current research program focuses on elucidating the molecular mechanisms involved in innate immune recognition of bacterial pathogens.
REFERENCES
- 1.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 2.Vandamme P, Dewhirst FE, Paster BJ, On SLW. 2005. Campylobacteraceae, p 1147–1160. In Garrity GM, Brenner DJ, Krieg NR, Staley JT (ed), Bergey's manual of systematic bacteriology, vol 2 Springer Science, New York, NY. [Google Scholar]
- 3.Sebald M, Veron M. 1963. Base DNA content and classification of vibrios. Ann Inst Pasteur (Paris) 105:897–910. [PubMed] [Google Scholar]
- 4.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 5.Hara-Kudo Y, Takatori K. 2011. Contamination level and ingestion dose of foodborne pathogens associated with infections. Epidemiol Infect 139:1505–1510. doi: 10.1017/S095026881000292X. [DOI] [PubMed] [Google Scholar]
- 6.Medema GJ, Teunis PF, Havelaar AH, Haas CN. 1996. Assessment of the dose-response relationship of Campylobacter jejuni. Int J Food Microbiol 30:101–111. doi: 10.1016/0168-1605(96)00994-4. [DOI] [PubMed] [Google Scholar]
- 7.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, Burg EF III, Moran AP, Applebee L, Bourgeois AL. 2010. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun 78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkpatrick BD, Lyon CE, Porter CK, Maue AC, Guerry P, Pierce KK, Carmolli MP, Riddle MS, Larsson CJ, Hawk D, Dill EA, Fingar A, Poly F, Fimlaid K, Hoq F, Tribble DR. 2013. Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model. Clin Infect Dis 57:1106–1113. doi: 10.1093/cid/cit454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baqar S, Tribble DR, Carmolli M, Sadigh K, Poly F, Porter C, Larsson CJ, Pierce KK, Guerry P, Darsley M, Kirkpatrick B. 2010. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. Clin Vaccine Immunol 17:80–86. doi: 10.1128/CVI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser MJ. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis 176(Suppl 2):S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen HL, Ejlertsen T, Engberg J, Nielsen H. 2013. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clin Microbiol Infect 19:445–450. doi: 10.1111/j.1469-0691.2012.03852.x. [DOI] [PubMed] [Google Scholar]
- 12.Bessede E, Lehours P, Labadi L, Bakiri S, Megraud F. 2014. Comparison of characteristics of patients infected by Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus. J Clin Microbiol 52:328–330. doi: 10.1128/JCM.03029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao MR, Naficy AB, Savarino SJ, Abu-Elyazeed R, Wierzba TF, Peruski LF, Abdel-Messih I, Frenck R, Clemens JD. 2001. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol 154:166–173. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 14.Inglis GD, Boras VF, Houde A. 2011. Enteric campylobacteria and RNA viruses associated with healthy and diarrheic humans in the Chinook Heath Region of Southwestern Alberta, Canada. J Clin Microbiol 49:209–219. doi: 10.1128/JCM.01220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmali MA, Penner JL, Fleming PC, Williams A, Hennessy JN. 1983. The serotype and biotype distribution of clinical isolates of Campylobacter jejuni and Campylobacter coli over a three-year period. J Infect Dis 147:243–246. doi: 10.1093/infdis/147.2.243. [DOI] [PubMed] [Google Scholar]
- 16.Gurtler M, Alter T, Kasimir S, Fehlhaber K. 2005. The importance of Campylobacter coli in human campylobacteriosis: prevalence and genetic characterization. Epidemiol Infect 133:1081–1087. doi: 10.1017/S0950268805004164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamidian M, Sanaei M, Bolfion M, Dabiri H, Zali MR, Walther-Rasmussen J. 2011. Prevalence of putative virulence markers in Campylobacter jejuni and Campylobacter coli isolated from hospitalized children, raw chicken, and raw beef in Tehran, Iran. Can J Microbiol 57:143–148. doi: 10.1139/W10-089. [DOI] [PubMed] [Google Scholar]
- 18.Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, Reddy S, Ahuja SD, Helfrick DL, Hardnett F, Carter M, Anderson B, Tauxe RV. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis 38(Suppl 3):S285–S296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 19.Adak GK, Meakins SM, Yip H, Lopman BA, O'Brien SJ. 2005. Disease risks from foods, England and Wales, 1996–2000. Emerg Infect Dis 11:365–372. doi: 10.3201/eid1103.040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, Millard G, Stafford R, Lalor K. 2005. Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis 11:1257–1264. doi: 10.3201/eid1108.041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam CC, O'Brien SJ, Tompkins DS, Bolton FJ, Berry L, Dodds J, Choudhury D, Halstead F, Iturriza-Gomara M, Mather K, Rait G, Ridge A, Rodrigues LC, Wain J, Wood B, Gray JJ. 2012. Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis 54:1275–1286. doi: 10.1093/cid/cis028. [DOI] [PubMed] [Google Scholar]
- 22.Casanova C, Schweiger A, von Steiger N, Droz S, Marschall J. 2015. Campylobacter concisus pseudo-outbreak caused by improved culture conditions. J Clin Microbiol 53:660–662. doi: 10.1128/JCM.02608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen HL, Engberg J, Ejlertsen T, Bucker R, Nielsen H. 2012. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect 18:E459–E465. doi: 10.1111/j.1469-0691.2012.03990.x. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. 2013. Evaluation of fecal calprotectin in Campylobacter concisus and Campylobacter jejuni/coli gastroenteritis. Scand J Gastroenterol 48:633–635. doi: 10.3109/00365521.2013.775329. [DOI] [PubMed] [Google Scholar]
- 25.Sadkowska-Todys M, Kucharczyk B. 2012. Campylobacteriosis in Poland in 2010. Przegl Epidemiol 66:255–258. [PubMed] [Google Scholar]
- 26.Kubota K, Kasuga F, Iwasaki E, Inagaki S, Sakurai Y, Komatsu M, Toyofuku H, Angulo FJ, Scallan E, Morikawa K. 2011. Estimating the burden of acute gastroenteritis and foodborne illness caused by Campylobacter, Salmonella, and Vibrio parahaemolyticus by using population-based telephone survey data, Miyagi Prefecture, Japan, 2005 to 2006. J Food Prot 74:1592–1598. doi: 10.4315/0362-028X.JFP-10-387. [DOI] [PubMed] [Google Scholar]
- 27.Wagenaar JA, French NP, Havelaar AH. 2013. Preventing Campylobacter at the source: why is it so difficult? Clin Infect Dis 57:1600–1606. doi: 10.1093/cid/cit555. [DOI] [PubMed] [Google Scholar]
- 28.Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, Gross U, Newell DG. 2009. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol 35:1–22. doi: 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- 29.Tompkins DS, Hudson MJ, Smith HR, Eglin RP, Wheeler JG, Brett MM, Owen RJ, Brazier JS, Cumberland P, King V, Cook PE. 1999. A study of infectious intestinal disease in England: microbiological findings in cases and controls. Commun Dis Public Health 2:108–113. [PubMed] [Google Scholar]
- 30.de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ, Vinje J, van Leusden F, Bartelds AI, van Duynhoven YT. 2001. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol 154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 31.Little CL, Gormley FJ, Rawal N, Richardson JF. 2010. A recipe for disaster: outbreaks of campylobacteriosis associated with poultry liver pate in England and Wales. Epidemiol Infect 138:1691–1694. doi: 10.1017/S0950268810001974. [DOI] [PubMed] [Google Scholar]
- 32.Batz MB, Hoffmann S, Morris JG Jr. 2012. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot 75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- 33.Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. 2013. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg Infect Dis 19:1305–1309. doi: 10.3201/eid1908.130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilliss D, Cronquist AB, Cartter M, Tobin-D'Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Lance S, Crim SM, Henao OL, Patrick M, Griffin PM, Tauxe RV. 2013. Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996–2012. MMWR Morb Mortal Wkly Rep 62:283–287. [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann S, Batz MB, Morris JG Jr. 2012. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 36.Kendall ME, Crim S, Fullerton K, Han PV, Cronquist AB, Shiferaw B, Ingram LA, Rounds J, Mintz ED, Mahon BE. 2012. Travel-associated enteric infections diagnosed after return to the United States, Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009. Clin Infect Dis 54(Suppl 5):S480–S487. doi: 10.1093/cid/cis052. [DOI] [PubMed] [Google Scholar]
- 37.Arsenault J, Berke O, Michel P, Ravel A, Gosselin P. 2012. Environmental and demographic risk factors for campylobacteriosis: do various geographical scales tell the same story? BMC Infect Dis 12:318. doi: 10.1186/1471-2334-12-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keegan VA, Majowicz SE, Pearl DL, Marshall BJ, Sittler N, Knowles L, Wilson JB. 2009. Epidemiology of enteric disease in C-EnterNet's pilot site—Waterloo region, Ontario, 1990 to 2004. Can J Infect Dis Med Microbiol 20:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BC Centre for Disease Control. 2011, posting date British Columbia annual summary of reportable diseases. BC Centre for Disease Control, Vancouver, British Columbia, Canada. [Google Scholar]
- 40.Larrosa-Haro A, Macias-Rosales R, Sanchez-Ramirez CA, Cortes-Lopez MC, Aguilar-Benavides S. 2010. Seasonal variation of enteropathogens in infants and preschoolers with acute diarrhea in western Mexico. J Pediatr Gastroenterol Nutr 51:534–536. doi: 10.1097/MPG.0b013e3181df5b66. [DOI] [PubMed] [Google Scholar]
- 41.Benoit SR, Lopez B, Arvelo W, Henao O, Parsons MB, Reyes L, Moir JC, Lindblade K. 2014. Burden of laboratory-confirmed Campylobacter infections in Guatemala 2008–2012: results from a facility-based surveillance system. J Epidemiol Glob Health 4:51–59. doi: 10.1016/j.jegh.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Workman SN, Sobers SJ, Mathison GE, Lavoie MC. 2006. Human Campylobacter-associated enteritis on the Caribbean island of Barbados. Am J Trop Med Hyg 74:623–627. [PubMed] [Google Scholar]
- 43.Fernández H. 2011. Campylobacter and campylobacteriosis: a view from South America. Rev Peru Med Exp Salud Publica 28:121–127. doi: 10.1590/S1726-46342011000100019. [DOI] [PubMed] [Google Scholar]
- 44.Collado L, Gutierrez M, Gonzalez M, Fernandez H. 2013. Assessment of the prevalence and diversity of emergent campylobacteria in human stool samples using a combination of traditional and molecular methods. Diagn Microbiol Infect Dis 75:434–436. doi: 10.1016/j.diagmicrobio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Platts-Mills JA, Liu J, Gratz J, Mduma E, Amour C, Swai N, Taniuchi M, Begum S, Penataro Yori P, Tilley DH, Lee G, Shen Z, Whary MT, Fox JG, McGrath M, Kosek M, Haque R, Houpt ER. 2014. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol 52:1074–1080. doi: 10.1128/JCM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havelaar AH, Ivarsson S, Lofdahl M, Nauta MJ. 2013. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol Infect 141:293–302. doi: 10.1017/S0950268812000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O'Brien SJ. 2012. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stingl K, Knüver MT, Vogt P, Buhler C, Krüger NJ, Alt K, Tenhagen BA, Hartung M, Schroeter A, Ellerbroek L, Appel B, Käsbohrer A. 2012. Quo vadis? Monitoring Campylobacter in Germany. Eur J Microbiol Immunol 2:88–96. doi: 10.1556/EuJMI.2.2012.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauri AM, Just M, McFarland S, Schweigmann A, Schlez K, Krahn J. 2013. Campylobacteriosis outbreaks in the state of Hesse, Germany, 2005–2011: raw milk yet again. Dtsch Med Wochenschr 138:357–361. doi: 10.1055/s-0032-1332884. [DOI] [PubMed] [Google Scholar]
- 50.Sadkowska-Todys M, Kucharczyk B. 2014. Campylobacteriosis in Poland in 2012. Przegl Epidemiol 68:239–241, 249–251. [PubMed] [Google Scholar]
- 51.Bouwknegt M, van Pelt W, Havelaar AH. 2013. Scoping the impact of changes in population age-structure on the future burden of foodborne disease in the Netherlands, 2020–2060. Int J Environ Res Public Health 10:2888–2896. doi: 10.3390/ijerph10072888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Havelaar AH, Nauta MJ, Mangen MJJ, de Koeijer AG, Bogaardt M-J, Evers EG, Jacobs-Reitsma WF, van Pelt W, Wagenaar JA, de Wit GA, van der Zee H. 29 July 2005, posting date Costs and benefits of controlling Campylobacter in the Netherlands—integrating risk analysis, epidemiology and economics. National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands. [Google Scholar]
- 53.Sivadon-Tardy V, Porcher R, Orlikowski D, Ronco E, Gault E, Roussi J, Durand MC, Sharshar T, Annane D, Raphael JC, Megraud F, Gaillard JL. 2014. Increased incidence of Campylobacter jejuni-associated Guillain-Barre syndromes in the Greater Paris area. Epidemiol Infect 142:1609–1613. doi: 10.1017/S095026881300263X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Boer RF, Ott A, Guren P, van Zanten E, van Belkum A, Kooistra-Smid AM. 2013. Detection of Campylobacter species and Arcobacter butzleri in stool samples by use of real-time multiplex PCR. J Clin Microbiol 51:253–259. doi: 10.1128/JCM.01716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. 2013. Clinical manifestations of Campylobacter concisus infection in children. Pediatr Infect Dis J 32:1194–1198. doi: 10.1097/INF.0b013e31829f0aff. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira S, Julio C, Queiroz JA, Domingues FC, Oleastro M. 2014. Molecular diagnosis of Arcobacter and Campylobacter in diarrhoeal samples among Portuguese patients. Diagn Microbiol Infect Dis 78:220–225. doi: 10.1016/j.diagmicrobio.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Koziel M, Kiely R, Blake L, O'Callaghan I, Corcoran GD, Lucey B, Sleator RD. 2013. Improved detection of bacterial pathogens in patients presenting with gastroenteritis by use of the EntericBio real-time Gastro Panel I assay. J Clin Microbiol 51:2679–2685. doi: 10.1128/JCM.00809-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bullman S, Corcoran D, O'Leary J, Lucey B, Byrne D, Sleator RD. 2011. Campylobacter ureolyticus: an emerging gastrointestinal pathogen? FEMS Immunol Med Microbiol 61:228–230. doi: 10.1111/j.1574-695X.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- 59.Bullman S, Corcoran D, O'Leary J, O'Hare D, Lucey B, Sleator RD. 2011. Emerging dynamics of human campylobacteriosis in southern Ireland. FEMS Immunol Med Microbiol 63:248–253. doi: 10.1111/j.1574-695X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 60.Huang JL, Xu HY, Bao GY, Zhou XH, Ji DJ, Zhang G, Liu PH, Jiang F, Pan ZM, Liu XF, Jiao XA. 2009. Epidemiological surveillance of Campylobacter jejuni in chicken, dairy cattle and diarrhoea patients. Epidemiol Infect 137:1111–1120. doi: 10.1017/S0950268809002039. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Sun XT, Zeng Z, Yu YY. 2011. Campylobacter enteritis in adult patients with acute diarrhea from 2005 to 2009 in Beijing, China. Chin Med J (Engl) 124:1508–1512. [PubMed] [Google Scholar]
- 62.Wang J, Guo YC, Li N. 2013. Prevalence and risk assessment of Campylobacter jejuni in chicken in China. Biomed Environ Sci 26:243–248. doi: 10.3967/0895-3988.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee P, Ramamurthy T, Bhattacharya MK, Rajendran K, Mukhopadhyay AK. 2013. Campylobacter jejuni in hospitalized patients with diarrhea, Kolkata, India. Emerg Infect Dis 19:1155–1156. doi: 10.3201/eid1907.121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinha A, SenGupta S, Guin S, Dutta S, Ghosh S, Mukherjee P, Mukhopadhyay AK, Ramamurthy T, Takeda Y, Kurakawa T, Nomoto K, Nair GB, Nandy RK. 2013. Culture-independent real-time PCR reveals extensive polymicrobial infections in hospitalized diarrhoea cases in Kolkata, India. Clin Microbiol Infect 19:173–180. doi: 10.1111/j.1469-0691.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 65.Rajendran P, Babji S, George AT, Rajan DP, Kang G, Ajjampur SS. 2012. Detection and species identification of Campylobacter in stool samples of children and animals from Vellore, South India. Indian J Med Microbiol 30:85–88. doi: 10.4103/0255-0857.93049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 67.Weinberger M, Lerner L, Valinsky L, Moran-Gilad J, Nissan I, Agmon V, Peretz C. 2013. Increased incidence of Campylobacter spp. infection and high rates among children, Israel. Emerg Infect Dis 19:1828–1831. doi: 10.3201/eid1911.120900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dayan N, Revivo D, Even L, Elkayam O, Glikman D. 2010. Campylobacter is the leading cause of bacterial gastroenteritis and dysentery in hospitalized children in the Western Galilee Region in Israel. Epidemiol Infect 138:1405–1406. doi: 10.1017/S0950268810000737. [DOI] [PubMed] [Google Scholar]
- 69.OzFoodNet Working Group. 2012. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2010. Commun Dis Intell Q Rep 36:E213–E241. [DOI] [PubMed] [Google Scholar]
- 70.OzFoodNet Working Group. 2010. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2009. Commun Dis Intell Q Rep 34:396–426. [DOI] [PubMed] [Google Scholar]
- 71.OzFoodNet Working Group. 2009. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2008. Commun Dis Intell Q Rep 33:389–413. [DOI] [PubMed] [Google Scholar]
- 72.Gibney KB, O'Toole J, Sinclair M, Leder K. 2014. Disease burden of selected gastrointestinal pathogens in Australia, 2010. Int J Infect Dis 28:176–185. doi: 10.1016/j.ijid.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Sears A, Baker MG, Wilson N, Marshall J, Muellner P, Campbell DM, Lake RJ, French NP. 2011. Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg Infect Dis 17:1007–1015. doi: 10.3201/eid/1706.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilpin BJ, Walsh G, On SL, Smith D, Marshall JC, French NP. 2013. Application of molecular epidemiology to understanding campylobacteriosis in the Canterbury region of New Zealand. Epidemiol Infect 141:1253–1266. doi: 10.1017/S0950268812001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cornelius AJ, Chambers S, Aitken J, Brandt SM, Horn B, On SL. 2012. Epsilonproteobacteria in humans, New Zealand. Emerg Infect Dis 18:510–512. doi: 10.3201/eid1803.110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howard P, Alexander ND, Atkinson A, Clegg AO, Gerega G, Javati A, Kajoi M, Lupiwa S, Lupiwa T, Mens M, Saleu G, Sanders RC, West B, Alpers MP. 2000. Bacterial, viral and parasitic aetiology of paediatric diarrhoea in the highlands of Papua New Guinea. J Trop Pediatr 46:10–14. doi: 10.1093/tropej/46.1.10. [DOI] [PubMed] [Google Scholar]
- 77.Mason J, Iturriza-Gomara M, O'Brien SJ, Ngwira BM, Dove W, Maiden MC, Cunliffe NA. 2013. Campylobacter infection in children in Malawi is common and is frequently associated with enteric virus co-infections. PLoS One 8:e59663. doi: 10.1371/journal.pone.0059663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Randremanana RV, Randrianirina F, Sabatier P, Rakotonirina HC, Randriamanantena A, Razanajatovo IM, Ratovoson R, Richard V. 2014. Campylobacter infection in a cohort of rural children in Moramanga, Madagascar. BMC Infect Dis 14:372. doi: 10.1186/1471-2334-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swierczewski BE, Odundo EA, Koech MC, Ndonye JN, Kirera RK, Odhiambo CP, Cheruiyot EK, Shaffer DN, Ombogo AN, Oaks EV. 2013. Enteric pathogen surveillance in a case-control study of acute diarrhea in Kisii Town, Kenya. J Med Microbiol 62:1774–1776. doi: 10.1099/jmm.0.059139-0. [DOI] [PubMed] [Google Scholar]
- 80.Lastovica A. 2006. Emerging Campylobacter spp.: the tip of the iceberg. Clin Microbiol Newsl 28:49–56. doi: 10.1016/j.clinmicnews.2006.03.004. [DOI] [Google Scholar]
- 81.Lastovica AJ. 2009. Clinical relevance of Campylobacter concisus isolated from pediatric patients. J Clin Microbiol 47:2360. doi: 10.1128/JCM.00568-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Man SM, Kaakoush NO, Mitchell HM. 2011. The role of bacteria and pattern-recognition receptors in Crohn's disease. Nat Rev Gastroenterol Hepatol 8:152–168. doi: 10.1038/nrgastro.2011.3. [DOI] [PubMed] [Google Scholar]
- 83.Blaser MJ, Hoverson D, Ely IG, Duncan DJ, Wang WL, Brown WR. 1984. Studies of Campylobacter jejuni in patients with inflammatory bowel disease. Gastroenterology 86:33–38. [PubMed] [Google Scholar]
- 84.Weber P, Koch M, Heizmann WR, Scheurlen M, Jenss H, Hartmann F. 1992. Microbic superinfection in relapse of inflammatory bowel disease. J Clin Gastroenterol 14:302–308. doi: 10.1097/00004836-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Boyanova L, Gergova G, Spassova Z, Koumanova R, Yaneva P, Mitov I, Derejian S, Krastev Z. 2004. Campylobacter infection in 682 Bulgarian patients with acute enterocolitis, inflammatory bowel disease, and other chronic intestinal diseases. Diagn Microbiol Infect Dis 49:71–74. doi: 10.1016/j.diagmicrobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. 2009. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, Man SM, Day AS, Leach ST, Lemberg DA, Dutt S, Stormon M, Otley A, O'Loughlin EV, Magoffin A, Ng PH, Mitchell H. 2009. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn's disease. J Clin Microbiol 47:453–455. doi: 10.1128/JCM.01949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tankovic J, Burghoffer B, Petit JC. 2009. Frequent detection by real-time PCR of bacteria from the Helicobacter and Campylobacter genera in stool samples from inflammatory bowel disease patients, abstr R2084. Abstr 19th Eur Congr Clin Microbiol Infect Dis, Helsinki, Finland. [Google Scholar]
- 89.Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, Mitchell H. 2010. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn's disease. Inflamm Bowel Dis 16:1008–1016. doi: 10.1002/ibd.21157. [DOI] [PubMed] [Google Scholar]
- 90.Kaakoush NO, Day AS, Huinao KD, Leach ST, Lemberg DA, Dowd SE, Mitchell HM. 2012. Microbial dysbiosis in pediatric patients with Crohn's disease. J Clin Microbiol 50:3258–3266. doi: 10.1128/JCM.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahendran V, Riordan SM, Grimm MC, Tran TAT, Major J, Kaakoush NO, Mitchell H, Zhang L. 2011. Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS One 6:e25417. doi: 10.1371/journal.pone.0025417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen R, Berry SH, Mukhopadhya I, Thomson JM, Saunders KA, Nicholl CE, Bisset WM, Loganathan S, Mahdi G, Kastner-Cole D, Barclay AR, Bishop J, Flynn DM, McGrogan P, Russell RK, El-Omar EM, Hold GL. 2013. The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: The BISCUIT Study. PLoS One 8:e58825. doi: 10.1371/journal.pone.0058825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM, Hold GL. 2011. Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS One 6:e21490. doi: 10.1371/journal.pone.0021490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajilic-Stojanovic M, Shanahan F, Guarner F, de Vos WM. 2013. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis 19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 95.Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, Riordan SM, Grimm M, Leach ST, Ismail Y. 2010. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol 48:2965–2967. doi: 10.1128/JCM.02391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Brien CL, Pavli P, Gordon DM, Allison GE. 2014. Detection of bacterial DNA in lymph nodes of Crohn's disease patients using high throughput sequencing. Gut 63:1596–1606. doi: 10.1136/gutjnl-2013-305320. [DOI] [PubMed] [Google Scholar]
- 97.Kovach Z, Kaakoush NO, Lamb S, Zhang L, Raftery MJ, Mitchell H. 2011. Immunoreactive proteins of Campylobacter concisus, an emergent intestinal pathogen. FEMS Immunol Med Microbiol 63:387–396. doi: 10.1111/j.1574-695X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 98.Kaakoush NO, Mitchell HM, Man SM. 2014. Role of emerging Campylobacter species in inflammatory bowel diseases. Inflamm Bowel Dis 20:2189–2197. doi: 10.1097/MIB.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 99.Matsheka MI, Elisha BG, Lastovica AL, On SL. 2002. Genetic heterogeneity of Campylobacter concisus determined by pulsed field gel electrophoresis-based macrorestriction profiling. FEMS Microbiol Lett 211:17–22. doi: 10.1111/j.1574-6968.2002.tb11197.x. [DOI] [PubMed] [Google Scholar]
- 100.Matsheka MI, Lastovica AJ, Zappe H, Elisha BG. 2006. The use of (GTG)5 oligonucleotide as an RAPD primer to type Campylobacter concisus. Lett Appl Microbiol 42:600–605. doi: 10.1111/j.1472-765X.2006.01900.x. [DOI] [PubMed] [Google Scholar]
- 101.Aabenhus R, Permin H, On SL, Andersen LP. 2002. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis 34:248–252. doi: 10.1080/00365540110080566. [DOI] [PubMed] [Google Scholar]
- 102.Aabenhus R, On SL, Siemer BL, Permin H, Andersen LP. 2005. Delineation of Campylobacter concisus genomospecies by amplified fragment length polymorphism analysis and correlation of results with clinical data. J Clin Microbiol 43:5091–5096. doi: 10.1128/JCM.43.10.5091-5096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aabenhus R, Permin H, Andersen LP. 2005. Characterization and subgrouping of Campylobacter concisus strains using protein profiles, conventional biochemical testing and antibiotic susceptibility. Eur J Gastroenterol Hepatol 17:1019–1024. doi: 10.1097/00042737-200510000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Engberg J, Bang DD, Aabenhus R, Aarestrup FM, Fussing V, Gerner-Smidt P. 2005. Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteristics. Clin Microbiol Infect 11:288–295. doi: 10.1111/j.1469-0691.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 105.On SL, Harrington CS. 2000. Identification of taxonomic and epidemiological relationships among Campylobacter species by numerical analysis of AFLP profiles. FEMS Microbiol Lett 193:161–169. doi: 10.1111/j.1574-6968.2000.tb09419.x. [DOI] [PubMed] [Google Scholar]
- 106.Kaakoush NO, Castano-Rodriguez N, Day AS, Lemberg DA, Leach ST, Mitchell HM. 2014. Campylobacter concisus and exotoxin 9 levels in paediatric patients with Crohn's disease and their association with the intestinal microbiota. J Med Microbiol 63:99–105. doi: 10.1099/jmm.0.067231-0. [DOI] [PubMed] [Google Scholar]
- 107.Deshpande NP, Kaakoush NO, Wilkins MR, Mitchell HM. 2013. Comparative genomics of Campylobacter concisus isolates reveals genetic diversity and provides insights into disease association. BMC Genomics 14:585. doi: 10.1186/1471-2164-14-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaakoush NO, Deshpande NP, Wilkins MR, Tan CG, Burgos-Portugal JA, Raftery MJ, Day AS, Lemberg DA, Mitchell H. 2011. The pathogenic potential of Campylobacter concisus strains associated with chronic intestinal diseases. PLoS One 6:e29045. doi: 10.1371/journal.pone.0029045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaakoush NO, Man SM, Lamb S, Raftery MJ, Wilkins MR, Kovach Z, Mitchell H. 2010. The secretome of Campylobacter concisus. FEBS J 277:1606–1617. doi: 10.1111/j.1742-4658.2010.07587.x. [DOI] [PubMed] [Google Scholar]
- 110.Kaakoush NO, Castaño-Rodríguez N, Day AS, Lemberg DA, Leach ST, Mitchell HM. 2015. Faecal levels of zonula occludens toxin in paediatric patients with Crohn's disease and their association with the intestinal microbiota. J Med Microbiol 64:303–306. doi: 10.1099/jmm.0.000005. [DOI] [PubMed] [Google Scholar]
- 111.Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK, Norman J, Day AS, Zhang L, Mitchell HM. 2010. Host attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J Infect Dis 202:1855–1865. doi: 10.1086/657316. [DOI] [PubMed] [Google Scholar]
- 112.Kaakoush NO, Deshpande NP, Man SM, Burgos-Portugal JA, Khattak FA, Raftery MJ, Wilkins MR, Mitchell HM. 2015. Transcriptomic and proteomic analyses reveal key innate immune signatures in the host response to the gastrointestinal pathogen Campylobacter concisus. Infect Immun 83:832–845. doi: 10.1128/IAI.03012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macfarlane S, Furrie E, Macfarlane GT, Dillon JF. 2007. Microbial colonization of the upper gastrointestinal tract in patients with Barrett's esophagus. Clin Infect Dis 45:29–38. doi: 10.1086/518578. [DOI] [PubMed] [Google Scholar]
- 114.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. 2004. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A 101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pei Z, Yang L, Peek RM Jr, Levine SM, Pride DT, Blaser MJ. 2005. Bacterial biota in reflux esophagitis and Barrett's esophagus. World J Gastroenterol 11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. 2009. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu N, Ando T, Ishiguro K, Maeda O, Watanabe O, Funasaka K, Nakamura M, Miyahara R, Ohmiya N, Goto H. 2013. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett's esophagus. BMC Infect Dis 13:130. doi: 10.1186/1471-2334-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osias GL, Bromer MQ, Thomas RM, Friedel D, Miller LS, Suh B, Lorber B, Parkman HP, Fisher RS. 2004. Esophageal bacteria and Barrett's esophagus: a preliminary report. Dig Dis Sci 49:228–236. doi: 10.1023/B:DDAS.0000017443.44802.4b. [DOI] [PubMed] [Google Scholar]
- 119.Blackett KL, Siddhi SS, Cleary S, Steed H, Miller MH, Macfarlane S, Macfarlane GT, Dillon JF. 2013. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett's and oesophageal carcinoma: association or causality? Aliment Pharmacol Ther 37:1084–1092. doi: 10.1111/apt.12317. [DOI] [PubMed] [Google Scholar]
- 120.Palma G, Barbieri A, Bimonte S, Palla M, Zappavigna S, Caraglia M, Ascierto PA, Ciliberto G, Arra C. 2013. Interleukin 18: friend or foe in cancer. Biochim Biophys Acta 1836:296–303. doi: 10.1016/j.bbcan.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Macuch PJ, Tanner AC. 2000. Campylobacter species in health, gingivitis, and periodontitis. J Dent Res 79:785–792. doi: 10.1177/00220345000790021301. [DOI] [PubMed] [Google Scholar]
- 122.Kaakoush NO, Mitchell HM. 2012. Campylobacter concisus—a new player in intestinal disease. Front Cell Infect Microbiol 2:4. doi: 10.3389/fcimb.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Etoh Y, Dewhirst FE, Paster BJ, Yamamoto A, Goto N. 1993. Campylobacter showae sp. nov., isolated from the human oral cavity. Int J Syst Bacteriol 43:631–639. doi: 10.1099/00207713-43-4-631. [DOI] [PubMed] [Google Scholar]
- 124.Loesche WJ, Gibbons RJ, Socransky SS. 1965. Biochemical characteristics of Vibrio sputorum and relationship to Vibrio bubulus and Vibrio fetus. J Bacteriol 89:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Palenstein Helderman WH. 1975. Total viable count and differential count of Vibrio (Campylobacter) sputorum, Fusobacterium nucleatum, Selenomonas sputigena, Bacteroides ochraceus and Veillonella in the inflamed and noninflamed human gingival crevice. J Periodont Res 10:294–305. doi: 10.1111/j.1600-0765.1975.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 126.Duerden BI, Goodwin L, O'Neil TC. 1987. Identification of Bacteroides species from adult periodontal disease. J Med Microbiol 24:133–137. doi: 10.1099/00222615-24-2-133. [DOI] [PubMed] [Google Scholar]
- 127.Tanner AC, Dzink JL, Ebersole JL, Socransky SS. 1987. Wolinella recta, Campylobacter concisus, Bacteroides gracilis, and Eikenella corrodens from periodontal lesions. J Periodont Res 22:327–330. doi: 10.1111/j.1600-0765.1987.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 128.Tanner ACR, Badger S, Lai CH, Listarten MA, Visconti RA, Socransky SS. 1981. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int J Syst Bacteriol 31:432–445. doi: 10.1099/00207713-31-4-432. [DOI] [Google Scholar]
- 129.Henne K, Fuchs F, Kruth S, Horz HP, Conrads G. 2014. Shifts in Campylobacter species abundance may reflect general microbial community shifts in periodontitis progression. J Oral Microbiol 6:25874. doi: 10.3402/jom.v6.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HJ, Kim JK, Cho JY, Lee JM, Hong SH. 2012. Quantification of subgingival bacterial pathogens at different stages of periodontal diseases. Curr Microbiol 65:22–27. doi: 10.1007/s00284-012-0121-8. [DOI] [PubMed] [Google Scholar]
- 131.Lopez R, Dahlen G, Retamales C, Baelum V. 2011. Clustering of subgingival microbial species in adolescents with periodontitis. Eur J Oral Sci 119:141–150. doi: 10.1111/j.1600-0722.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 132.von Troil-Linden B, Torkko H, Alaluusua S, Jousimies-Somer H, Asikainen S. 1995. Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. J Dent Res 74:1789–1795. doi: 10.1177/00220345950740111201. [DOI] [PubMed] [Google Scholar]
- 133.Ihara H, Miura T, Kato T, Ishihara K, Nakagawa T, Yamada S, Okuda K. 2003. Detection of Campylobacter rectus in periodontitis sites by monoclonal antibodies. J Periodont Res 38:64–72. doi: 10.1034/j.1600-0765.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- 134.Tanner A, Maiden MF, Macuch PJ, Murray LL, Kent RL Jr. 1998. Microbiota of health, gingivitis, and initial periodontitis. J Clin Periodontol 25:85–98. doi: 10.1111/j.1600-051X.1998.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 135.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnson CC, Reinhardt JF, Edelstein MA, Mulligan ME, George WL, Finegold SM. 1985. Bacteroides gracilis, an important anaerobic bacterial pathogen. J Clin Microbiol 22:799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rawlinson A, Eleys K, Bennett KW, Goodwin L. 1994. Bacteroides gracilis in periodontal health and disease. Microb Ecol Health Dis 7:201–205. doi: 10.3109/08910609409141355. [DOI] [Google Scholar]
- 138.Shen S, Samaranayake LP, Yip HK. 2005. Coaggregation profiles of the microflora from root surface caries lesions. Arch Oral Biol 50:23–32. doi: 10.1016/j.archoralbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 139.Haririan H, Andrukhov O, Bertl K, Lettner S, Kierstein S, Moritz A, Rausch-Fan X. 2014. Microbial analysis of subgingival plaque samples compared to that of whole saliva in patients with periodontitis. J Periodontol 85:819–828. doi: 10.1902/jop.2013.130306. [DOI] [PubMed] [Google Scholar]
- 140.Moore WE, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, Palcanis KG, Ranney RR. 1985. Comparative bacteriology of juvenile periodontitis. Infect Immun 48:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moore LV, Moore WE, Cato EP, Smibert RM, Burmeister JA, Best AM, Ranney RR. 1987. Bacteriology of human gingivitis. J Dent Res 66:989–995. doi: 10.1177/00220345870660052401. [DOI] [PubMed] [Google Scholar]
- 142.Kamma JJ, Nakou M, Manti FA. 1994. Microbiota of rapidly progressive periodontitis lesions in association with clinical parameters. J Periodontol 65:1073–1078. doi: 10.1902/jop.1994.65.11.1073. [DOI] [PubMed] [Google Scholar]
- 143.Ebersole JL, Taubman MA, Smith DJ, Haffajee AD. 1985. Effect of subgingival scaling on systemic antibody responses to oral microorganisms. Infect Immun 48:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taubman MA, Haffajee AD, Socransky SS, Smith DJ, Ebersole JL. 1992. Longitudinal monitoring of humoral antibody in subjects with destructive periodontal diseases. J Periodont Res 27:511–521. doi: 10.1111/j.1600-0765.1992.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 145.Haffajee AD, Socransky SS, Ebersole JL, Smith DJ. 1984. Clinical, microbiological and immunological features associated with the treatment of active periodontosis lesions. J Clin Periodontol 11:600–618. doi: 10.1111/j.1600-051X.1984.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 146.Ercan E, Eratalay K, Deren O, Gur D, Ozyuncu O, Altun B, Kanli C, Ozdemir P, Akincibay H. 2013. Evaluation of periodontal pathogens in amniotic fluid and the role of periodontal disease in pre-term birth and low birth weight. Acta Odontol Scand 71:553–559. doi: 10.3109/00016357.2012.697576. [DOI] [PubMed] [Google Scholar]
- 147.Brito F, Zaltman C, Carvalho AT, Fischer RG, Persson R, Gustafsson A, Figueredo CM. 2013. Subgingival microflora in inflammatory bowel disease patients with untreated periodontitis. Eur J Gastroenterol Hepatol 25:239–245. doi: 10.1097/MEG.0b013e32835a2b70. [DOI] [PubMed] [Google Scholar]
- 148.Ismail Y, Mahendran V, Octavia S, Day AS, Riordan SM, Grimm MC, Lan R, Lemberg D, Tran TA, Zhang L. 2012. Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS One 7:e38217. doi: 10.1371/journal.pone.0038217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Dyke TE, Dowell VR Jr, Offenbacher S, Snyder W, Hersh T. 1986. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun 53:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moss-Morris R, Spence M. 2006. To “lump” or to “split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom Med 68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- 151.Neal KR, Hebden J, Spiller R. 1997. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dunlop SP, Jenkins D, Neal KR, Spiller RC. 2003. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 153.Stermer E, Lubezky A, Potasman I, Paster E, Lavy A. 2006. Is traveler's diarrhea a significant risk factor for the development of irritable bowel syndrome? A prospective study. Clin Infect Dis 43:898–901. doi: 10.1086/507540. [DOI] [PubMed] [Google Scholar]
- 154.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. 2006. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 131:445–450. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 155.Okhuysen PC, Jiang ZD, Carlin L, Forbes C, DuPont HL. 2004. Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in North American travelers to Mexico. Am J Gastroenterol 99:1774–1778. doi: 10.1111/j.1572-0241.2004.30435.x. [DOI] [PubMed] [Google Scholar]
- 156.Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, Di Lorenzo C. 2008. Post-infectious functional gastrointestinal disorders in children. J Pediatr 152:812–816, 816.e1. doi: 10.1016/j.jpeds.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 157.Futagami S, Itoh T, Sakamoto C. 2015. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther 41:177–188. doi: 10.1111/apt.13006. [DOI] [PubMed] [Google Scholar]
- 158.Ford AC, Thabane M, Collins SM, Moayyedi P, Garg AX, Clark WF, Marshall JK. 2010. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: a cohort study. Gastroenterology 138:1727–1736. doi: 10.1053/j.gastro.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 159.Porter CK, Choi D, Cash B, Pimentel M, Murray J, May L, Riddle MS. 2013. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol 13:46. doi: 10.1186/1471-230X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parry SD, Stansfield R, Jelley D, Gregory W, Phillips E, Barton JR, Welfare MR. 2003. Does bacterial gastroenteritis predispose people to functional gastrointestinal disorders? A prospective, community-based, case-control study. Am J Gastroenterol 98:1970–1975. doi: 10.1111/j.1572-0241.2003.07664.x. [DOI] [PubMed] [Google Scholar]
- 161.Futagami S, Shindo T, Kawagoe T, Horie A, Shimpuku M, Gudis K, Iwakiri K, Itoh T, Sakamoto C. 2010. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol 105:1835–1842. doi: 10.1038/ajg.2010.151. [DOI] [PubMed] [Google Scholar]
- 162.Drossman DA. 2006. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 163.Spiller R, Garsed K. 2009. Postinfectious irritable bowel syndrome. Gastroenterology 136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 164.Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. 2001. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis 184:606–609. doi: 10.1086/322845. [DOI] [PubMed] [Google Scholar]
- 165.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM. 2010. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 166.Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster AV, Klosterhalfen S, Autenrieth IB, Zipfel S, Frick JS. 2011. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil 23:e479–e488. doi: 10.1111/j.1365-2982.2011.01779.x. [DOI] [PubMed] [Google Scholar]
- 167.Grover M, Camilleri M, Smith K, Linden DR, Farrugia G. 2014. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterol Motil 26:156–167. doi: 10.1111/nmo.12304. [DOI] [PubMed] [Google Scholar]
- 168.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. 2014. Psychometric scores and persistence of irritable bowel after Campylobacter concisus infection. Scand J Gastroenterol 49:545–551. doi: 10.3109/00365521.2014.886718. [DOI] [PubMed] [Google Scholar]
- 169.Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. 2013. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. 2012. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. 2012. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y, Chen Y, Yang F, Lu N, Wang Z, Luan C, Liu Y, Wang B, Xiang C, Wang Y, Zhao F, Gao GF, Wang S, Li L, Zhang H, Zhu B. 2013. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol 66:462–470. doi: 10.1007/s00248-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 173.Riddle MS, Gutierrez RL, Verdu EF, Porter CK. 2012. The chronic gastrointestinal consequences associated with Campylobacter. Curr Gastroenterol Rep 14:395–405. doi: 10.1007/s11894-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 174.Altobelli E, Paduano R, Petrocelli R, Di Orio F. 2014. Burden of celiac disease in Europe: a review of its childhood and adulthood prevalence and incidence as of September 2014. Ann Ig 26:485–498. doi: 10.7416/ai.2014.2007. [DOI] [PubMed] [Google Scholar]
- 175.Riddle MS, Murray JA, Porter CK. 2012. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol 107:1248–1255. doi: 10.1038/ajg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Verdu EF, Mauro M, Bourgeois J, Armstrong D. 2007. Clinical onset of celiac disease after an episode of Campylobacter jejuni enteritis. Can J Gastroenterol 21:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Riddle MS, Murray JA, Cash BD, Pimentel M, Porter CK. 2013. Pathogen-specific risk of celiac disease following bacterial causes of foodborne illness: a retrospective cohort study. Dig Dis Sci 58:3242–3245. doi: 10.1007/s10620-013-2733-7. [DOI] [PubMed] [Google Scholar]
- 178.Vaughan-Shaw PG, Rees JR, White D, Burgess P. 2010. Campylobacter jejuni cholecystitis: a rare but significant clinical entity. BMJ Case Rep 2010:bcr1020092365. doi: 10.1136/bcr.10.2009.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Landry O. 1859. Note sur la paralysie ascendante aigue. Gaz Hebd Med Chir 6:472–474, 486–488. [Google Scholar]
- 180.van Doorn PA, Ruts L, Jacobs BC. 2008. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol 7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 181.Guillain G, Barré J, Strohl A. 1916. Sur un syndrome de radiculo-nevrite avec hyperalbuminose du liquide cephalorachidien sans reaction cellulaire. Remarques sur les caracteres cliniques et graphiques des reflexes tendineux. Bull Soc Med Hop Paris 28:1462–1470. [Google Scholar]
- 182.Kuwabara S, Yuki N. 2013. Axonal Guillain-Barre syndrome: concepts and controversies. Lancet Neurol 12:1180–1188. doi: 10.1016/S1474-4422(13)70215-1. [DOI] [PubMed] [Google Scholar]
- 183.Bae JS, Yuki N, Kuwabara S, Kim JK, Vucic S, Lin CS, Kiernan MC. 2014. Guillain-Barre syndrome in Asia. J Neurol Neurosurg Psychiatry 85:907–913. doi: 10.1136/jnnp-2013-306212. [DOI] [PubMed] [Google Scholar]
- 184.Baker MG, Kvalsvig A, Zhang J, Lake R, Sears A, Wilson N. 2012. Declining Guillain-Barre syndrome after campylobacteriosis control, New Zealand, 1988–2010. Emerg Infect Dis 18:226–233. doi: 10.3201/eid1802.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jackson BR, Zegarra JA, Lopez-Gatell H, Sejvar J, Arzate F, Waterman S, Nunez AS, Lopez B, Weiss J, Cruz RQ, Murrieta DY, Luna-Gierke R, Heiman K, Vieira AR, Fitzgerald C, Kwan P, Zarate-Bermudez M, Talkington D, Hill VR, Mahon B. 2014. Binational outbreak of Guillain-Barre syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol Infect 142:1089–1099. doi: 10.1017/S0950268813001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keithlin J, Sargeant J, Thomas MK, Fazil A. 2014. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health 14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Freddo L, Yu RK, Latov N, Donofrio PD, Hays AP, Greenberg HS, Albers JW, Allessi AG, Keren D. 1986. Gangliosides GM1 and GD1b are antigens for IgM M-protein in a patient with motor neuron disease. Neurology 36:454–458. doi: 10.1212/WNL.36.4.454. [DOI] [PubMed] [Google Scholar]
- 188.Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, Garcia-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. 2011. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun 79:2681–2689. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heikema AP, Koning RI, Duarte Dos Santos Rico S, Rempel H, Jacobs BC, Endtz HP, van Wamel WJ, Samsom JN. 2013. Enhanced, sialoadhesin-dependent uptake of Guillain-Barre syndrome-associated Campylobacter jejuni strains by human macrophages. Infect Immun 81:2095–2103. doi: 10.1128/IAI.01437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS. 2014. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol 7:802–817. doi: 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Taboada EN, van Belkum A, Yuki N, Acedillo RR, Godschalk PC, Koga M, Endtz HP, Gilbert M, Nash JH. 2007. Comparative genomic analysis of Campylobacter jejuni associated with Guillain-Barre and Miller Fisher syndromes: neuropathogenic and enteritis-associated isolates can share high levels of genomic similarity. BMC Genomics 8:359. doi: 10.1186/1471-2164-8-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fisher M. 1956. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia). N Engl J Med 255:57–65. doi: 10.1056/NEJM195607122550201. [DOI] [PubMed] [Google Scholar]
- 193.Chiba A, Kusunoki S, Shimizu T, Kanazawa I. 1992. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol 31:677–679. doi: 10.1002/ana.410310619. [DOI] [PubMed] [Google Scholar]
- 194.Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, Hirata K, Yuki N. 2005. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology 64:1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- 195.Heikema AP, Jacobs BC, Horst-Kreft D, Huizinga R, Kuijf ML, Endtz HP, Samsom JN, van Wamel WJ. 2013. Siglec-7 specifically recognizes Campylobacter jejuni strains associated with oculomotor weakness in Guillain-Barre syndrome and Miller Fisher syndrome. Clin Microbiol Infect 19:E106–E112. doi: 10.1111/1469-0691.12073. [DOI] [PubMed] [Google Scholar]
- 196.Lee KY. 2012. Anti-GQ1b-negative Miller Fisher syndrome after Campylobacter jejuni enteritis. Pediatr Neurol 47:213–215. doi: 10.1016/j.pediatrneurol.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 197.Koga M, Gilbert M, Takahashi M, Li J, Hirata K, Kanda T, Yuki N. 2012. GQ1b-seronegative Fisher syndrome: clinical features and new serological markers. J Neurol 259:1366–1374. doi: 10.1007/s00415-011-6360-y. [DOI] [PubMed] [Google Scholar]
- 198.Oyazato Y, Shiihara T, Kusunoki S, Adachi M, Ohnishi N, Taniguchi H, Nishiyama A, Watanabe A, Kobayashi M, Kamioka I. 2012. A case of anti-GA1 antibody-positive Fisher syndrome with elevated tau protein in cerebrospinal fluid. Brain Dev 34:329–332. doi: 10.1016/j.braindev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 199.Tauxe RV, Patton CM, Edmonds P, Barrett TJ, Brenner DJ, Blake PA. 1985. Illness associated with Campylobacter laridis, a newly recognized Campylobacter species. J Clin Microbiol 21:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chua K, Gurtler V, Montgomery J, Fraenkel M, Mayall BC, Grayson ML. 2007. Campylobacter insulaenigrae causing septicaemia and enteritis. J Med Microbiol 56:1565–1567. doi: 10.1099/jmm.0.47366-0. [DOI] [PubMed] [Google Scholar]
- 201.Louwen R, Baarlen P, Vliet AH, Belkum A, Hays JP, Endtz HP. 2012. Campylobacter bacteremia: a rare and under-reported event? Eur J Microbiol Immunol 2:76–87. doi: 10.1556/EuJMI.2.2012.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pigrau C, Bartolome R, Almirante B, Planes AM, Gavalda J, Pahissa A. 1997. Bacteremia due to Campylobacter species: clinical findings and antimicrobial susceptibility patterns. Clin Infect Dis 25:1414–1420. doi: 10.1086/516127. [DOI] [PubMed] [Google Scholar]
- 203.Pacanowski J, Lalande V, Lacombe K, Boudraa C, Lesprit P, Legrand P, Trystram D, Kassis N, Arlet G, Mainardi JL, Doucet-Populaire F, Girard PM, Meynard JL. 2008. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis 47:790–796. doi: 10.1086/591530. [DOI] [PubMed] [Google Scholar]
- 204.Liao CH, Chuang CY, Huang YT, Lee PI, Hsueh PR. 2012. Bacteremia caused by antimicrobial resistant Campylobacter species at a medical center in Taiwan, 1998–2008. J Infect 65:392–399. doi: 10.1016/j.jinf.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 205.Nielsen H, Hansen KK, Gradel KO, Kristensen B, Ejlertsen T, Ostergaard C, Schonheyder HC. 2010. Bacteraemia as a result of Campylobacter species: a population-based study of epidemiology and clinical risk factors. Clin Microbiol Infect 16:57–61. doi: 10.1111/j.1469-0691.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 206.Feodoroff B, Lauhio A, Ellstrom P, Rautelin H. 2011. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-year period, 1998–2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin Infect Dis 53:e99–e106. doi: 10.1093/cid/cir509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ben-Shimol S, Carmi A, Greenberg D. 2013. Demographic and clinical characteristics of Campylobacter bacteremia in children with and without predisposing factors. Pediatr Infect Dis J 32:e414–e418. doi: 10.1097/INF.0b013e31829baae0. [DOI] [PubMed] [Google Scholar]
- 208.Blaser MJ, Perez GP, Smith PF, Patton C, Tenover FC, Lastovica AJ, Wang WI. 1986. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J Infect Dis 153:552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- 209.Nagy MT, Hla SM. 2013. Campylobacter fetus sepsis in an immunocompetent patient with haematological complication. BMJ Case Rep 2013:bcr2013008610. doi: 10.1136/bcr-2013-008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chusid MJ, Wortmann DW, Dunne WM. 1990. “Campylobacter upsaliensis” sepsis in a boy with acquired hypogammaglobulinemia. Diagn Microbiol Infect Dis 13:367–369. doi: 10.1016/0732-8893(90)90003-E. [DOI] [PubMed] [Google Scholar]
- 211.Fujihara N, Takakura S, Saito T, Iinuma Y, Ichiyama S. 2006. A case of perinatal sepsis by Campylobacter fetus subsp. fetus infection successfully treated with carbapenem—case report and literature review. J Infect 53:e199–e202. doi: 10.1016/j.jinf.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 212.Becker S, Ejlertsen T, Kristensen B, Norgaard M, Nielsen H. 2007. Is the incidence of perimyocarditis increased following Campylobacter jejuni infection? Eur J Clin Microbiol Infect Dis 26:927–929. doi: 10.1007/s10096-007-0393-2. [DOI] [PubMed] [Google Scholar]
- 213.Florkowski CM, Ikram RB, Crozier IM, Ikram H, Berry ME. 1984. Campylobacter jejuni myocarditis. Clin Cardiol 7:558–559. doi: 10.1002/clc.4960071008. [DOI] [PubMed] [Google Scholar]
- 214.Cox ID, Fluck DS, Joy MD. 2001. Campylobacter myocarditis; loose bowels and a baggy heart. Eur J Heart Fail 3:105–107. doi: 10.1016/S1388-9842(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 215.Westling K, Evengard B. 2001. Myocarditis associated with Campylobacter infection. Scand J Infect Dis 33:877–878. doi: 10.1080/003655401753186286. [DOI] [PubMed] [Google Scholar]
- 216.Cunningham C, Lee CH. 2003. Myocarditis related to Campylobacter jejuni infection: a case report. BMC Infect Dis 3:16. doi: 10.1186/1471-2334-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Reda E, Mansell C. 2005. Myocarditis in a patient with Campylobacter infection. N Z Med J 118:U1634. [PubMed] [Google Scholar]
- 218.Mera V, Lopez T, Serralta J. 2007. Take traveller's diarrhoea to heart. Travel Med Infect Dis 5:202–203. doi: 10.1016/j.tmaid.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 219.Pena LA, Fishbein MC. 2007. Fatal myocarditis related to Campylobacter jejuni infection: a case report. Cardiovasc Pathol 16:119–121. doi: 10.1016/j.carpath.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 220.Braun KP, Theissig F, Ernst H, May M, Krulls-Munch J. 2008. Campylobacter jejuni-associated hepatitis and myocardial injury. Med Klin (Munich) 103:346–348. doi: 10.1007/s00063-008-1042-y. [DOI] [PubMed] [Google Scholar]
- 221.Turley AJ, Crilley JG, Hall JA. 2008. Acute myocarditis secondary to Campylobacter jejuni enterocolitis. Resuscitation 79:165–167. doi: 10.1016/j.resuscitation.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 222.Kratzer C, Wolf F, Graninger W, Weissel M. 2010. Acute cardiac disease in a young patient with Campylobacter jejuni infection: a case report. Wien Klin Wochenschr 122:315–319. doi: 10.1007/s00508-010-1381-6. [DOI] [PubMed] [Google Scholar]
- 223.Ponka A, Pitkanen T, Pettersson T, Aittoniemi S, Kosunen TU. 1980. Carditis and arthritis associated with Campylobacter jejuni infection. Acta Med Scand 208:495–496. [DOI] [PubMed] [Google Scholar]
- 224.Hull SR, Varma MP. 2011. Myopericarditis following Campylobacter infection. Ir J Med Sci 180:753–755. doi: 10.1007/s11845-009-0314-8. [DOI] [PubMed] [Google Scholar]
- 225.Alzand BS, Ilhan M, Heesen WF, Meeder JG. 2010. Campylobacter jejuni: enterocolitis and myopericarditis. Int J Cardiol 144:e14–e16. doi: 10.1016/j.ijcard.2008.12.101. [DOI] [PubMed] [Google Scholar]
- 226.Panikkath R, Costilla V, Hoang P, Wood J, Gruden JF, Dietrich B, Gotway MB, Appleton C. 2014. Chest pain and diarrhea: a case of Campylobacter jejuni-associated myocarditis. J Emerg Med 46:180–183. doi: 10.1016/j.jemermed.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 227.Murphy D, Jolly C, MacDonald S, Troughton R. 2013. Myocarditis associated with Campylobacter jejuni. N Z Med J 126:95–98. [PubMed] [Google Scholar]
- 228.De Cock D, Hiltrop N, Timmermans P, Dymarkowski S, Van Cleemput J. 2012. Myocarditis associated with Campylobacter enteritis: report of three cases. Circ Heart Fail 5:e19–e21. doi: 10.1161/CIRCHEARTFAILURE.111.964882. [DOI] [PubMed] [Google Scholar]
- 229.Kotilainen P, Lehtopolku M, Hakanen AJ. 2006. Myopericarditis in a patient with Campylobacter enteritis: a case report and literature review. Scand J Infect Dis 38:549–552. doi: 10.1080/00365540500372903. [DOI] [PubMed] [Google Scholar]
- 230.Hannu T, Mattila L, Rautelin H, Siitonen A, Leirisalo-Repo M. 2005. Three cases of cardiac complications associated with Campylobacter jejuni infection and review of the literature. Eur J Clin Microbiol Infect Dis 24:619–622. doi: 10.1007/s10096-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 231.Abbass K, Emig M, Bernstein JM. 2011. Sepsis and pericarditis caused by Campylobacter fetus: case report and literature review. J Ark Med Soc 108:88–89. [PubMed] [Google Scholar]
- 232.Uzoigwe C. 2005. Campylobacter infections of the pericardium and myocardium. Clin Microbiol Infect 11:253–255. doi: 10.1111/j.1469-0691.2004.01028.x. [DOI] [PubMed] [Google Scholar]
- 233.Farrugia DC, Eykyn SJ, Smyth EG. 1994. Campylobacter fetus endocarditis: two case reports and review. Clin Infect Dis 18:443–446. doi: 10.1093/clinids/18.3.443. [DOI] [PubMed] [Google Scholar]
- 234.Dinant S, Schurink CA, Deckers JW, Severin JA. 2011. Aortic homograft endocarditis caused by Campylobacter jejuni. J Clin Microbiol 49:4016–4017. doi: 10.1128/JCM.00935-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 235.Haruyama A, Toyoda S, Kikuchi M, Arikawa T, Inami S, Otani N, Amano H, Matsuda R, Inoue T. 2011. Campylobacter fetus as cause of prosthetic valve endocarditis. Tex Heart Inst J 38:584–587. [PMC free article] [PubMed] [Google Scholar]
- 236.Caramelli B, Mansur AJ, Grinberg M, Mendes CM, Pileggi F. 1988. Campylobacter fetus endocarditis on a prosthetic heart valve. South Med J 81:802–803. doi: 10.1097/00007611-198806000-00028. [DOI] [PubMed] [Google Scholar]
- 237.Peetermans WE, De Man F, Moerman P, van de Werf F. 2000. Fatal prosthetic valve endocarditis due to Campylobacter fetus. J Infect 41:180–182. doi: 10.1053/jinf.2000.0699. [DOI] [PubMed] [Google Scholar]
- 238.Morrison VA, Lloyd BK, Chia JK, Tuazon CU. 1990. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev Infect Dis 12:387–392. doi: 10.1093/clinids/12.3.387. [DOI] [PubMed] [Google Scholar]
- 239.Prendki V, Marmor S, Zeller V, Lhotellier L, Megraud F, Desplaces N. 2013. Campylobacter infection after prosthetic joint surgery. Scand J Infect Dis 45:706–710. doi: 10.3109/00365548.2013.800225. [DOI] [PubMed] [Google Scholar]
- 240.Kell RJA, Ellis ME. 1985. Transient atrial fibrillation in Campylobacter jejuni infection. BMJ 291:1542. doi: 10.1136/bmj.291.6508.1542.20742561 [DOI] [Google Scholar]
- 241.Bucknell SJ, Le T, Amerena J, Hill DG, McDonald M. 2000. Aortic dissection associated with Campylobacter aortitis. Heart Lung Circ 9:88–91. doi: 10.1046/j.1444-2892.2000.00027.x. [DOI] [PubMed] [Google Scholar]
- 242.Abassade P, Cremieux O, Korach JM, Templier F, Morette C, Wolff M, Baudouy PY, Farge C. 1994. Campylobacter fetus subspecies fetus endoaortitis on a Bentall tube prosthesis. Apropos of a case. Arch Mal Coeur Vaiss 87:1483–1487. [PubMed] [Google Scholar]
- 243.Blabey RG Jr, Parry MF, Bull SM, Weed CB. 1983. Mycotic aneurysm of the abdominal aorta: successful management of Campylobacter fetus aortitis. Conn Med 47:129–130. [PubMed] [Google Scholar]
- 244.Gazaigne L, Legrand P, Renaud B, Bourra B, Taillandier E, Brun-Buisson C, Lesprit P. 2008. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis 27:185–189. doi: 10.1007/s10096-007-0415-0. [DOI] [PubMed] [Google Scholar]
- 245.Thomas K, Chan KN, Ribeiro CD. 1980. Campylobacter jejuni/coli meningitis in a neonate. Br Med J 280:1301–1302. doi: 10.1136/bmj.280.6227.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Goossens H, Henocque G, Kremp L, Rocque J, Boury R, Alanio G, Vlaes L, Hemelhof W, Van den Borre C, Macart M, et al. 1986. Nosocomial outbreak of Campylobacter jejuni meningitis in newborn infants. Lancet ii:146–149. [DOI] [PubMed] [Google Scholar]
- 247.Ruef C, Fah L, Caduff F. 1988. Campylobacter jejuni: sepsis and meningitis in an adult without risk factors. Schweiz Med Wochenschr 118:302–304. [PubMed] [Google Scholar]
- 248.Dronda F, Garcia-Arata I, Navas E, de Rafael L. 1998. Meningitis in adults due to Campylobacter fetus subspecies fetus. Clin Infect Dis 27:906–907. doi: 10.1086/517168. [DOI] [PubMed] [Google Scholar]
- 249.Burch KL, Saeed K, Sails AD, Wright PA. 1999. Successful treatment by meropenem of Campylobacter jejuni meningitis in a chronic alcoholic following neurosurgery. J Infect 39:241–243. doi: 10.1016/S0163-4453(99)90059-2. [DOI] [PubMed] [Google Scholar]
- 250.Umehara Y, Kudo M, Kawasaki M. 2009. Campylobacter fetus meningitis in a patient with Crohn's disease. Inflamm Bowel Dis 15:645–646. doi: 10.1002/ibd.20686. [DOI] [PubMed] [Google Scholar]
- 251.Kogawa S, Furukawa K. 2010. Campylobacter jejuni meningitis in an immunocompetent adult male. Rinsho Shinkeigaku 50:262–264. doi: 10.5692/clinicalneurol.50.262. [DOI] [PubMed] [Google Scholar]
- 252.Tsoni K, Papadopoulou E, Michailidou E, Kavaliotis I. 2013. Campylobacter jejuni meningitis in a neonate: a rare case report. J Neonatal Perinatal Med 6:183–185. doi: 10.3233/NPM-1366612. [DOI] [PubMed] [Google Scholar]
- 253.Spiegel CA, Telford G. 1984. Isolation of Wolinella recta and Actinomyces viscosus from an actinomycotic chest wall mass. J Clin Microbiol 20:1187–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Han XY, Tarrand JJ, Rice DC. 2005. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J Clin Microbiol 43:2513–2515. doi: 10.1128/JCM.43.5.2513-2515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.de Vries JJ, Arents NL, Manson WL. 2008. Campylobacter species isolated from extra-oro-intestinal abscesses: a report of four cases and literature review. Eur J Clin Microbiol Infect Dis 27:1119–1123. doi: 10.1007/s10096-008-0550-2. [DOI] [PubMed] [Google Scholar]
- 256.Batz MB, Henke E, Kowalcyk B. 2013. Long-term consequences of foodborne infections. Infect Dis Clin North Am 27:599–616. doi: 10.1016/j.idc.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 257.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. 2007. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum 37:48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ajene AN, Fischer Walker CL, Black RE. 2013. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr 31:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Simor AE, Karmali MA, Jadavji T, Roscoe M. 1986. Abortion and perinatal sepsis associated with Campylobacter infection. Rev Infect Dis 8:397–402. doi: 10.1093/clinids/8.3.397. [DOI] [PubMed] [Google Scholar]
- 260.Gurgan T, Diker KS. 1994. Abortion associated with Campylobacter upsaliensis. J Clin Microbiol 32:3093–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mendz GL, Petersen R, Quinlivan JA, Kaakoush NO. 2014. Potential involvement of Campylobacter curvus and Haemophilus parainfluenzae in preterm birth. BMJ Case Rep 2014:bcr2014205282. doi: 10.1136/bcr-2014-205282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Arce RM, Barros SP, Wacker B, Peters B, Moss K, Offenbacher S. 2009. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta 30:156–162. doi: 10.1016/j.placenta.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Arce RM, Caron KM, Barros SP, Offenbacher S. 2012. Toll-like receptor 4 mediates intrauterine growth restriction after systemic Campylobacter rectus infection in mice. Mol Oral Microbiol 27:373–381. doi: 10.1111/j.2041-1014.2012.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lastovica AJ, le Roux E. 2000. Efficient isolation of campylobacteria from stools. J Clin Microbiol 38:2798–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kim SA, Lee YM, Hwang IG, Kang DH, Woo GJ, Rhee MS. 2009. Eight enrichment broths for the isolation of Campylobacter jejuni from inoculated suspensions and ground pork. Lett Appl Microbiol 49:620–626. doi: 10.1111/j.1472-765X.2009.02714.x. [DOI] [PubMed] [Google Scholar]
- 266.Iwamoto M, Huang JY, Cronquist AB, Medus C, Hurd S, Zansky S, Dunn J, Woron AM, Oosmanally N, Griffin PM, Besser J, Henao OL. 2015. Bacterial enteric infections detected by culture-independent diagnostic tests—FoodNet, United States, 2012–2014. MMWR Morb Mortal Wkly Rep 64:252–257. [PMC free article] [PubMed] [Google Scholar]
- 267.Kulkarni SP, Lever S, Logan JM, Lawson AJ, Stanley J, Shafi MS. 2002. Detection of Campylobacter species: a comparison of culture and polymerase chain reaction based methods. J Clin Pathol 55:749–753. doi: 10.1136/jcp.55.10.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Maher M, Finnegan C, Collins E, Ward B, Carroll C, Cormican M. 2003. Evaluation of culture methods and a DNA probe-based PCR assay for detection of Campylobacter species in clinical specimens of feces. J Clin Microbiol 41:2980–2986. doi: 10.1128/JCM.41.7.2980-2986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.On SL. 2001. Taxonomy of Campylobacter, Arcobacter, Helicobacter and related bacteria: current status, future prospects and immediate concerns. Symp Ser Soc Appl Microbiol 2001:1S–15S. doi: 10.1046/j.1365-2672.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- 270.Man SM, Kaakoush NO, Octavia S, Mitchell H. 2010. The internal transcribed spacer region, a new tool for use in species differentiation and delineation of systematic relationships within the Campylobacter genus. Appl Environ Microbiol 76:3071–3081. doi: 10.1128/AEM.02551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Eyers M, Chapelle S, Van Camp G, Goossens H, De Wachter R. 1993. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J Clin Microbiol 31:3340–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fermer C, Engvall EO. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters, Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J Clin Microbiol 37:3370–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Christensen H, Jorgensen K, Olsen JE. 1999. Differentiation of Campylobacter coli and C. jejuni by length and DNA sequence of the 16S-23S rRNA internal spacer region. Microbiology 145:99–105. doi: 10.1099/13500872-145-1-99. [DOI] [PubMed] [Google Scholar]
- 274.Hurtado A, Owen RJ. 1997. A molecular scheme based on 23S rRNA gene polymorphisms for rapid identification of Campylobacter and Arcobacter species. J Clin Microbiol 35:2401–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Granato PA, Chen L, Holiday I, Rawling RA, Novak-Weekley SM, Quinlan T, Musser KA. 2010. Comparison of premier CAMPY enzyme immunoassay (EIA), ProSpecT Campylobacter EIA, and ImmunoCard STAT! CAMPY tests with culture for laboratory diagnosis of Campylobacter enteric infections. J Clin Microbiol 48:4022–4027. doi: 10.1128/JCM.00486-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Javed MA, Poshtiban S, Arutyunov D, Evoy S, Szymanski CM. 2013. Bacteriophage receptor binding protein based assays for the simultaneous detection of Campylobacter jejuni and Campylobacter coli. PLoS One 8:e69770. doi: 10.1371/journal.pone.0069770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis 32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 278.Smith JL, Fratamico PM. 2010. Fluoroquinolone resistance in Campylobacter. J Food Prot 73:1141–1152. [DOI] [PubMed] [Google Scholar]
- 279.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol 4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Graham JP, Boland JJ, Silbergeld E. 2007. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep 122:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, Armstrong A, Hshieh P, Fox A, Maley EA, Lebron C, Faix DJ, Lawler JV, Nayak G, Lewis M, Bodhidatta L, Scott DA. 2007. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 44:338–346. doi: 10.1086/510589. [DOI] [PubMed] [Google Scholar]
- 282.Vukelic D, Trkulja V, Salkovic-Petrisic M. 2010. Single oral dose of azithromycin versus 5 days of oral erythromycin or no antibiotic in treatment of Campylobacter enterocolitis in children: a prospective randomized assessor-blind study. J Pediatr Gastroenterol Nutr 50:404–410. doi: 10.1097/MPG.0b013e3181a87104. [DOI] [PubMed] [Google Scholar]
- 283.McEwen SA, Fedorka-Cray PJ. 2002. Antimicrobial use and resistance in animals. Clin Infect Dis 34(Suppl 3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 284.Wieczorek K, Osek J. 2013. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res Int 2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Garin B, Gouali M, Wouafo M, Perchec AM, Pham MT, Ravaonindrina N, Urbes F, Gay M, Diawara A, Leclercq A, Rocourt J, Pouillot R. 2012. Prevalence, quantification and antimicrobial resistance of Campylobacter spp. on chicken neck-skins at points of slaughter in 5 major cities located on 4 continents. Int J Food Microbiol 157:102–107. doi: 10.1016/j.ijfoodmicro.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 286.Hakanen AJ, Lehtopolku M, Siitonen A, Huovinen P, Kotilainen P. 2003. Multidrug resistance in Campylobacter jejuni strains collected from Finnish patients during 1995–2000. J Antimicrob Chemother 52:1035–1039. doi: 10.1093/jac/dkg489. [DOI] [PubMed] [Google Scholar]
- 287.Qin SS, Wu CM, Wang Y, Jeon B, Shen ZQ, Zhang Q, Shen JZ. 2011. Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int J Food Microbiol 146:94–98. doi: 10.1016/j.ijfoodmicro.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 288.Nelson JM, Smith KE, Vugia DJ, Rabatsky-Ehr T, Segler SD, Kassenborg HD, Zansky SM, Joyce K, Marano N, Hoekstra RM, Angulo FJ. 2004. Prolonged diarrhea due to ciprofloxacin-resistant Campylobacter infection. J Infect Dis 190:1150–1157. doi: 10.1086/423282. [DOI] [PubMed] [Google Scholar]
- 289.Helms M, Simonsen J, Olsen KE, Molbak K. 2005. Adverse health events associated with antimicrobial drug resistance in Campylobacter species: a registry-based cohort study. J Infect Dis 191:1050–1055. doi: 10.1086/428453. [DOI] [PubMed] [Google Scholar]
- 290.Quinn T, Bolla JM, Pages JM, Fanning S. 2007. Antibiotic-resistant Campylobacter: could efflux pump inhibitors control infection? J Antimicrob Chemother 59:1230–1236. doi: 10.1093/jac/dkl470. [DOI] [PubMed] [Google Scholar]
- 291.Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C, Shen J. 2012. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob Agents Chemother 56:5332–5339. doi: 10.1128/AAC.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Moore JE, Barton MD, Blair IS, Corcoran D, Dooley JS, Fanning S, Kempf I, Lastovica AJ, Lowery CJ, Matsuda M, McDowell DA, McMahon A, Millar BC, Rao JR, Rooney PJ, Seal BS, Snelling WJ, Tolba O. 2006. The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect 8:1955–1966. doi: 10.1016/j.micinf.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 293.Domingues AR, Pires SM, Halasa T, Hald T. 2012. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect 140:970–981. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- 294.Swaminathan A, Torresi J, Schlagenhauf P, Thursky K, Wilder-Smith A, Connor BA, Schwartz E, Vonsonnenberg F, Keystone J, O'Brien DP. 2009. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect 59:19–27. doi: 10.1016/j.jinf.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 295.Ravel A, Nesbitt A, Marshall B, Sittler N, Pollari F. 2011. Description and burden of travel-related cases caused by enteropathogens reported in a Canadian community. J Travel Med 18:8–19. doi: 10.1111/j.1708-8305.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 296.Ricotta EE, Palmer A, Wymore K, Clogher P, Oosmanally N, Robinson T, Lathrop S, Karr J, Hatch J, Dunn J, Ryan P, Blythe D. 2014. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am J Public Health 104:e108–e114. doi: 10.2105/AJPH.2013.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zinszer K, Michel P, Hardardottir H, Kristinsson KG, Sigmundsdottir G, St-Onge L, Reiersen J, Charland K, Lowman R. 2010. The impact of domestic travel on estimating regional rates of human campylobacteriosis. Epidemiol Infect 138:1735–1743. doi: 10.1017/S0950268810001081. [DOI] [PubMed] [Google Scholar]
- 298.Gautret P, Cramer JP, Field V, Caumes E, Jensenius M, Gkrania-Klotsas E, de Vries PJ, Grobusch MP, Lopez-Velez R, Castelli F, Schlagenhauf P, Hervius Askling H, von Sonnenburg F, Lalloo DG, Loutan L, Rapp C, Basto F, Santos O'Connor F, Weld L, Parola P. 2012. Infectious diseases among travellers and migrants in Europe, EuroTravNet 2010. Euro Surveill 17:20205 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20205. [PubMed] [Google Scholar]
- 299.Zenner D, Gillespie I. 2011. Travel-associated Salmonella and Campylobacter gastroenteritis in England: estimation of under-ascertainment through national laboratory surveillance. J Travel Med 18:414–417. doi: 10.1111/j.1708-8305.2011.00553.x. [DOI] [PubMed] [Google Scholar]
- 300.Mughini-Gras L, Smid JH, Wagenaar JA, De Boer A, Havelaar AH, Friesema IH, French NP, Graziani C, Busani L, Van Pelt W. 2014. Campylobacteriosis in returning travellers and potential secondary transmission of exotic strains. Epidemiol Infect 142:1277–1288. doi: 10.1017/S0950268813002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kapperud G, Skjerve E, Bean NH, Ostroff SM, Lassen J. 1992. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J Clin Microbiol 30:3117–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Neimann J, Engberg J, Molbak K, Wegener HC. 2003. A case-control study of risk factors for sporadic Campylobacter infections in Denmark. Epidemiol Infect 130:353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Greig JD, Ravel A. 2009. Analysis of foodborne outbreak data reported internationally for source attribution. Int J Food Microbiol 130:77–87. doi: 10.1016/j.ijfoodmicro.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 304.Pires SM, Vigre H, Makela P, Hald T. 2010. Using outbreak data for source attribution of human salmonellosis and campylobacteriosis in Europe. Foodborne Pathog Dis 7:1351–1361. doi: 10.1089/fpd.2010.0564. [DOI] [PubMed] [Google Scholar]
- 305.Strachan NJ, Gormley FJ, Rotariu O, Ogden ID, Miller G, Dunn GM, Sheppard SK, Dallas JF, Reid TM, Howie H, Maiden MC, Forbes KJ. 2009. Attribution of Campylobacter infections in northeast Scotland to specific sources by use of multilocus sequence typing. J Infect Dis 199:1205–1208. doi: 10.1086/597417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sheppard SK, Colles F, Richardson J, Cody AJ, Elson R, Lawson A, Brick G, Meldrum R, Little CL, Owen RJ, Maiden MC, McCarthy ND. 2010. Host association of Campylobacter genotypes transcends geographic variation. Appl Environ Microbiol 76:5269–5277. doi: 10.1128/AEM.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Strachan NJ, MacRae M, Thomson A, Rotariu O, Ogden ID, Forbes KJ. 2012. Source attribution, prevalence and enumeration of Campylobacter spp. from retail liver. Int J Food Microbiol 153:234–236. doi: 10.1016/j.ijfoodmicro.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 308.Mughini Gras L, Smid JH, Wagenaar JA, de Boer AG, Havelaar AH, Friesema IH, French NP, Busani L, van Pelt W. 2012. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: a combined case-control and source attribution analysis. PLoS One 7:e42599. doi: 10.1371/journal.pone.0042599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ragimbeau C, Colin S, Devaux A, Decruyenaere F, Cauchie HM, Losch S, Penny C, Mossong J. 2014. Investigating the host specificity of Campylobacter jejuni and Campylobacter coli by sequencing gyrase subunit A. BMC Microbiol 14:205. doi: 10.1186/s12866-014-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Baily JL, Meric G, Bayliss S, Foster G, Moss SE, Watson E, Pascoe B, Mikhail J, Pizzi R, Goldstone RJ, Smith DG, Willoughby K, Hall AJ, Sheppard SK, Dagleish MP. 2015. Evidence of land-sea transfer of the zoonotic pathogen Campylobacter to a wildlife marine sentinel species. Mol Ecol 24:208–221. doi: 10.1111/mec.13001. [DOI] [PubMed] [Google Scholar]
- 311.Kownhar H, Shankar EM, Rajan R, Vengatesan A, Rao UA. 2007. Prevalence of Campylobacter jejuni and enteric bacterial pathogens among hospitalized HIV infected versus non-HIV infected patients with diarrhoea in southern India. Scand J Infect Dis 39:862–866. doi: 10.1080/00365540701393096. [DOI] [PubMed] [Google Scholar]
- 312.Larsen IK, Gradel KO, Helms M, Hornstrup MK, Jurgens G, Mens H, Rosager CL, Clausen TH, Kronborg G, Nielsen H. 2011. Non-typhoidal Salmonella and Campylobacter infections among HIV-positive patients in Denmark. Scand J Infect Dis 43:3–7. doi: 10.3109/00365548.2010.517780. [DOI] [PubMed] [Google Scholar]
- 313.Skirrow MB. 1977. Campylobacter enteritis: a “new” disease. Br Med J 2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ellis-Iversen J, Ridley A, Morris V, Sowa A, Harris J, Atterbury R, Sparks N, Allen V. 2012. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol Infect 140:916–924. doi: 10.1017/S095026881100118X. [DOI] [PubMed] [Google Scholar]
- 315.Gu W, Siletzky RM, Wright S, Islam M, Kathariou S. 2009. Antimicrobial susceptibility profiles and strain type diversity of Campylobacter jejuni isolates from turkeys in eastern North Carolina. Appl Environ Microbiol 75:474–482. doi: 10.1128/AEM.02012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Giacomelli M, Andrighetto C, Lombardi A, Martini M, Piccirillo A. 2012. A longitudinal study on thermophilic Campylobacter spp. in commercial turkey flocks in northern Italy: occurrence and genetic diversity. Avian Dis 56:693–700. doi: 10.1637/10141-032312-Reg.1. [DOI] [PubMed] [Google Scholar]
- 317.Colles FM, Ali JS, Sheppard SK, McCarthy ND, Maiden MC. 2011. Campylobacter populations in wild and domesticated mallard ducks (Anas platyrhynchos). Environ Microbiol Rep 3:574–580. doi: 10.1111/j.1758-2229.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tresierra-Ayala A, Bendayan ME, Bernuy A, Pereyra G, Fernandez H. 1994. Chicken as potential contamination source of Campylobacter lari in Iquitos, Peru. Rev Inst Med Trop Sao Paulo 36:497–499. [DOI] [PubMed] [Google Scholar]
- 319.Kaakoush NO, Sodhi N, Chenu JW, Cox JM, Riordan SM, Mitchell HM. 2014. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog 6:18. doi: 10.1186/1757-4749-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Boysen L, Rosenquist H, Larsson JT, Nielsen EM, Sorensen G, Nordentoft S, Hald T. 2014. Source attribution of human campylobacteriosis in Denmark. Epidemiol Infect 142:1599–1608. doi: 10.1017/S0950268813002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kittl S, Heckel G, Korczak BM, Kuhnert P. 2013. Source attribution of human Campylobacter isolates by MLST and fla-typing and association of genotypes with quinolone resistance. PLoS One 8:e81796. doi: 10.1371/journal.pone.0081796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wei W, Schupbach G, Held L. 2014. Time-series analysis of Campylobacter incidence in Switzerland. Epidemiol Infect 2014:1–8. doi: 10.1017/S0950268814002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Byrd JA, Corrier DE, Hume ME, Bailey RH, Stanker LH, Hargis BM. 1998. Incidence of Campylobacter in crops of preharvest market-age broiler chickens. Poult Sci 77:1303–1305. doi: 10.1093/ps/77.9.1303. [DOI] [PubMed] [Google Scholar]
- 324.Ahmed MF, Schulz J, Hartung J. 2013. Survival of Campylobacter jejuni in naturally and artificially contaminated laying hen feces. Poult Sci 92:364–369. doi: 10.3382/ps.2012-02496. [DOI] [PubMed] [Google Scholar]
- 325.Food Standards Agency. 2014, posting date A microbiological survey of Campylobacter contamination in fresh whole UK-produced chilled chickens at retail sale—interim report to cover quarters 1–3. Food Standards Agency, London, United Kingdom. [Google Scholar]
- 326.Deckert AE, Taboada E, Mutschall S, Poljak Z, Reid-Smith RJ, Tamblyn S, Morrell L, Seliske P, Jamieson FB, Irwin R, Dewey CE, Boerlin P, McEwen SA. 2014. Molecular epidemiology of Campylobacter jejuni human and chicken isolates from two health units. Foodborne Pathog Dis 11:150–155. doi: 10.1089/fpd.2013.1610. [DOI] [PubMed] [Google Scholar]
- 327.Levesque S, Fournier E, Carrier N, Frost E, Arbeit RD, Michaud S. 2013. Campylobacteriosis in urban versus rural areas: a case-case study integrated with molecular typing to validate risk factors and to attribute sources of infection. PLoS One 8:e83731. doi: 10.1371/journal.pone.0083731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rosenquist H, Boysen L, Krogh AL, Jensen AN, Nauta M. 2013. Campylobacter contamination and the relative risk of illness from organic broiler meat in comparison with conventional broiler meat. Int J Food Microbiol 162:226–230. doi: 10.1016/j.ijfoodmicro.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 329.Blaser MJ, LaForce FM, Wilson NA, Wang WL. 1980. Reservoirs for human campylobacteriosis. J Infect Dis 141:665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- 330.Moore JE, Wilson TS, Wareing DR, Humphrey TJ, Murphy PG. 2002. Prevalence of thermophilic Campylobacter spp. in ready-to-eat foods and raw poultry in northern Ireland. J Food Prot 65:1326–1328. [DOI] [PubMed] [Google Scholar]
- 331.Levallois P, Chevalier P, Gingras S, Dery P, Payment P, Michel P, Rodriguez M. 2014. Risk of infectious gastroenteritis in young children living in Quebec rural areas with intensive animal farming: results of a case-control study (2004–2007). Zoonoses Public Health 61:28–38. doi: 10.1111/zph.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.de Perio MA, Niemeier RT, Levine SJ, Gruszynski K, Gibbins JD. 2013. Campylobacter infection in poultry-processing workers, Virginia, USA, 2008–2011. Emerg Infect Dis 19:286–288. doi: 10.3201/eid1902.121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Nielsen EM. 2002. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Lett Appl Microbiol 35:85–89. doi: 10.1046/j.1472-765X.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- 334.Ellis-Iversen J, Cook AJ, Smith RP, Pritchard GC, Nielen M. 2009. Temporal patterns and risk factors for Escherichia coli O157 and Campylobacter spp. in young cattle. J Food Prot 72:490–496. [DOI] [PubMed] [Google Scholar]
- 335.Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J Appl Microbiol 85:472–480. doi: 10.1046/j.1365-2672.1998.853511.x. [DOI] [PubMed] [Google Scholar]
- 336.Leatherbarrow AJ, Griffiths R, Hart CA, Kemp R, Williams NJ, Diggle PJ, Wright EJ, Sutherst J, Houghton P, French NP. 2007. Campylobacter lari: genotype and antibiotic resistance of isolates from cattle, wildlife and water in an area of mixed dairy farmland in the United Kingdom. Environ Microbiol 9:1772–1779. doi: 10.1111/j.1462-2920.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 337.Guevremont E, Normand V, Lamoureux L, Cote C. 2008. Genetic detection of Campylobacter lanienae in fecal matter and stored manure from swine and dairy cattle. Foodborne Pathog Dis 5:361–364. doi: 10.1089/fpd.2007.0054. [DOI] [PubMed] [Google Scholar]
- 338.Harvey RB, Young CR, Anderson RC, Droleskey RE, Genovese KJ, Egan LF, Nisbet DJ. 2000. Diminution of Campylobacter colonization in neonatal pigs reared off-sow. J Food Prot 63:1430–1432. [DOI] [PubMed] [Google Scholar]
- 339.Weijtens MJ, Bijker PG, Van der Plas J, Urlings HA, Biesheuvel MH. 1993. Prevalence of Campylobacter in pigs during fattening; an epidemiological study. Vet Q 15:138–143. doi: 10.1080/01652176.1993.9694392. [DOI] [PubMed] [Google Scholar]
- 340.Alter T, Gaull F, Kasimir S, Gurtler M, Mielke H, Linnebur M, Fehlhaber K. 2005. Prevalences and transmission routes of Campylobacter spp. strains within multiple pig farms. Vet Microbiol 108:251–261. doi: 10.1016/j.vetmic.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 341.Raji MA, Adekeye JO, Kwaga JK, Bale JO. 2000. Bioserogroups of Campylobacter species isolated from sheep in Kaduna State, Nigeria. Small Rumin Res 37:215–221. doi: 10.1016/S0921-4488(00)00125-5. [DOI] [PubMed] [Google Scholar]
- 342.Zweifel C, Zychowska MA, Stephan R. 2004. Prevalence and characteristics of Shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. isolated from slaughtered sheep in Switzerland. Int J Food Microbiol 92:45–53. doi: 10.1016/j.ijfoodmicro.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 343.Cortes C, de la Fuente R, Contreras A, Sanchez A, Corrales JC, Martinez S, Orden JA. 2006. A survey of Salmonella spp and Campylobacter spp in dairy goat faeces and bulk tank milk in the Murcia region of Spain. Ir Vet J 59:391–393. doi: 10.1186/2046-0481-59-7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Baker J, Barton MD, Lanser J. 1999. Campylobacter species in cats and dogs in South Australia. Aust Vet J 77:662–666. doi: 10.1111/j.1751-0813.1999.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 345.Chaban B, Ngeleka M, Hill JE. 2010. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol 10:73. doi: 10.1186/1471-2180-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Fox JG, Zanotti S, Jordan HV. 1981. The hamster as a reservoir of Campylobacter fetus subspecies jejuni. J Infect Dis 143:856. doi: 10.1093/infdis/143.6.856. [DOI] [PubMed] [Google Scholar]
- 347.Fox JG, Hering AM, Ackerman JI, Taylor NS. 1983. The pet hamster as a potential reservoir of human campylobacteriosis. J Infect Dis 147:784. doi: 10.1093/infdis/147.4.784. [DOI] [PubMed] [Google Scholar]
- 348.Fox JG, Ackerman JI, Newcomer CE. 1983. Ferret as a potential reservoir for human campylobacteriosis. Am J Vet Res 44:1049–1052. [PubMed] [Google Scholar]
- 349.Giacomelli M, Piccirillo A. 2014. Pet reptiles as potential reservoir of Campylobacter species with zoonotic potential. Vet Rec 174:479. doi: 10.1136/vr.102243. [DOI] [PubMed] [Google Scholar]
- 350.Kohler R, Krause G, Beutin L, Stephan R, Zweifel C. 2008. Shedding of food-borne pathogens and microbiological carcass contamination in rabbits at slaughter. Vet Microbiol 132:149–157. doi: 10.1016/j.vetmic.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 351.Luechtefeld NA, Blaser MJ, Reller LB, Wang WL. 1980. Isolation of Campylobacter fetus subsp. jejuni from migratory waterfowl. J Clin Microbiol 12:406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kapperud G, Rosef O. 1983. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl Environ Microbiol 45:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Levre E, Valentini P, Brunetti M, Sacchelli F. 1989. Stationary and migratory avifauna as reservoirs of Salmonella, Yersinia and Campylobacter. Ann Ig 1:729–740. [PubMed] [Google Scholar]
- 354.Llarena AK, Skarp-de Haan CP, Rossi M, Hanninen ML. 2015. Characterization of the Campylobacter jejuni population in the barnacle geese reservoir. Zoonoses Public Health 62:209–221. doi: 10.1111/zph.12141. [DOI] [PubMed] [Google Scholar]
- 355.Lillehaug A, Monceyron Jonassen C, Bergsjo B, Hofshagen M, Tharaldsen J, Nesse LL, Handeland K. 2005. Screening of feral pigeon (Colomba livia), mallard (Anas platyrhynchos) and graylag goose (Anser anser) populations for Campylobacter spp., Salmonella spp., avian influenza virus and avian paramyxovirus. Acta Vet Scand 46:193–202. doi: 10.1186/1751-0147-46-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Antilles N, Sanglas A, Cerda-Cuellar M. 20 September 2013. Free-living waterfowl as a source of zoonotic bacteria in a dense wild bird population area in northeastern Spain. Transbound Emerg Dis doi: 10.1111/tbed.12169. [DOI] [PubMed] [Google Scholar]
- 357.Marin C, Palomeque MD, Marco-Jimenez F, Vega S. 2014. Wild griffon vultures (Gyps fulvus) as a source of Salmonella and Campylobacter in eastern Spain. PLoS One 9:e94191. doi: 10.1371/journal.pone.0094191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sippy R, Sandoval-Green CM, Sahin O, Plummer P, Fairbanks WS, Zhang Q, Blanchong JA. 2012. Occurrence and molecular analysis of Campylobacter in wildlife on livestock farms. Vet Microbiol 157:369–375. doi: 10.1016/j.vetmic.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 359.Pacha RE, Clark GW, Williams EA, Carter AM, Scheffelmaier JJ, Debusschere P. 1987. Small rodents and other mammals associated with mountain meadows as reservoirs of Giardia spp. and Campylobacter spp. Appl Environ Microbiol 53:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wacheck S, Fredriksson-Ahomaa M, Konig M, Stolle A, Stephan R. 2010. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog Dis 7:307–312. doi: 10.1089/fpd.2009.0367. [DOI] [PubMed] [Google Scholar]
- 361.Bachand N, Ravel A, Onanga R, Arsenault J, Gonzalez JP. 2012. Public health significance of zoonotic bacterial pathogens from bushmeat sold in urban markets of Gabon, Central Africa. J Wildl Dis 48:785–789. doi: 10.7589/0090-3558-48.3.785. [DOI] [PubMed] [Google Scholar]
- 362.Marin C, Ingresa-Capaccioni S, Gonzalez-Bodi S, Marco-Jimenez F, Vega S. 2013. Free-living turtles are a reservoir for Salmonella but not for Campylobacter. PLoS One 8:e72350. doi: 10.1371/journal.pone.0072350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Petersen L, Nielsen EM, Engberg J, On SL, Dietz HH. 2001. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl Environ Microbiol 67:3115–3121. doi: 10.1128/AEM.67.7.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Broman T, Palmgren H, Bergstrom S, Sellin M, Waldenstrom J, Danielsson-Tham ML, Olsen B. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J Clin Microbiol 40:4594–4602. doi: 10.1128/JCM.40.12.4594-4602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hiett KL, Stern NJ, Fedorka-Cray P, Cox NA, Musgrove MT, Ladely S. 2002. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl Environ Microbiol 68:6220–6236. doi: 10.1128/AEM.68.12.6220-6236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Craven SE, Stern NJ, Line E, Bailey JS, Cox NA, Fedorka-Cray P. 2000. Determination of the incidence of Salmonella spp., Campylobacter jejuni, and Clostridium perfringens in wild birds near broiler chicken houses by sampling intestinal droppings. Avian Dis 44:715–720. doi: 10.2307/1593118. [DOI] [PubMed] [Google Scholar]
- 367.Stern NJ, Myszewski MA, Barnhart HM, Dreesen DW. 1997. Flagellin A gene restriction fragment length polymorphism patterns of Campylobacter spp. isolates from broiler production sources. Avian Dis 41:899–905. doi: 10.2307/1592344. [DOI] [PubMed] [Google Scholar]
- 368.Taylor DN, Brown M, McDermott KT. 1982. Waterborne transmission of Campylobacter enteritis. Microb Ecol 8:347–354. doi: 10.1007/BF02010674. [DOI] [PubMed] [Google Scholar]
- 369.Rogol M, Sechter I, Falk H, Shtark Y, Alfi S, Greenberg Z, Mizrachi R. 1983. Waterborne outbreak of Campylobacter enteritis. Eur J Clin Microbiol 2:588–590. doi: 10.1007/BF02016571. [DOI] [PubMed] [Google Scholar]
- 370.Hanninen ML, Haajanen H, Pummi T, Wermundsen K, Katila ML, Sarkkinen H, Miettinen I, Rautelin H. 2003. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl Environ Microbiol 69:1391–1396. doi: 10.1128/AEM.69.3.1391-1396.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Richardson G, Thomas DR, Smith RM, Nehaul L, Ribeiro CD, Brown AG, Salmon RL. 2007. A community outbreak of Campylobacter jejuni infection from a chlorinated public water supply. Epidemiol Infect 135:1151–1158. doi: 10.1017/S0950268807007960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kapperud G, Espeland G, Wahl E, Walde A, Herikstad H, Gustavsen S, Tveit I, Natas O, Bevanger L, Digranes A. 2003. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am J Epidemiol 158:234–242. doi: 10.1093/aje/kwg139. [DOI] [PubMed] [Google Scholar]
- 373.Arvanitidou M, Stathopoulos GA, Katsouyannopoulos VC. 1994. Isolation of Campylobacter and Yersinia spp. from drinking waters. J Travel Med 1:156–159. doi: 10.1111/j.1708-8305.1994.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 374.Popowski J, Lekowska-Kochaniak A, Korsak D. 1997. The incidence of heat tolerant Campylobacter in rivers and lakes of the Warsaw region. Rocz Panstw Zakl Hig 48:253–262. [PubMed] [Google Scholar]
- 375.Galanis E, Mak S, Otterstatter M, Taylor M, Zubel M, Takaro TK, Kuo M, Michel P. 2014. The association between campylobacteriosis, agriculture and drinking water: a case-case study in a region of British Columbia, Canada, 2005–2009. Epidemiol Infect 142:2075–2084. doi: 10.1017/S095026881400123X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Hanninen ML, Niskanen M, Korhonen L. 1998. Water as a reservoir for Campylobacter jejuni infection in cows studied by serotyping and pulsed-field gel electrophoresis (PFGE). Zentralbl Veterinarmed B 45:37–42. [DOI] [PubMed] [Google Scholar]
- 377.Carter PE, McTavish SM, Brooks HJ, Campbell D, Collins-Emerson JM, Midwinter AC, French NP. 2009. Novel clonal complexes with an unknown animal reservoir dominate Campylobacter jejuni isolates from river water in New Zealand. Appl Environ Microbiol 75:6038–6046. doi: 10.1128/AEM.01039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Champion OL, Gaunt MW, Gundogdu O, Elmi A, Witney AA, Hinds J, Dorrell N, Wren BW. 2005. Comparative phylogenomics of the food-borne pathogen Campylobacter jejuni reveals genetic markers predictive of infection source. Proc Natl Acad Sci U S A 102:16043–16048. doi: 10.1073/pnas.0503252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Vally H, Glass K, Ford L, Hall G, Kirk MD, Shadbolt C, Veitch M, Fullerton KE, Musto J, Becker N. 2014. Proportion of illness acquired by foodborne transmission for nine enteric pathogens in Australia: an expert elicitation. Foodborne Pathog Dis 11:727–733. doi: 10.1089/fpd.2014.1746. [DOI] [PubMed] [Google Scholar]
- 380.Centers for Disease Control and Prevention. 2013. Recurrent outbreak of Campylobacter jejuni infections associated with a raw milk dairy—Pennsylvania, April-May 2013. MMWR Morb Mortal Wkly Rep 62:702. [PMC free article] [PubMed] [Google Scholar]
- 381.Castrodale LJ, Gerlach RF, Xavier CM, Smith BJ, Cooper MP, McLaughlin JB. 2013. Sharing milk but not messages: campylobacteriosis associated with consumption of raw milk from a cow-share program in Alaska, 2011. J Food Prot 76:744–747. doi: 10.4315/0362-028X.JFP-12-329. [DOI] [PubMed] [Google Scholar]
- 382.Longenberger AH, Palumbo AJ, Chu AK, Moll ME, Weltman A, Ostroff SM. 2013. Campylobacter jejuni infections associated with unpasteurized milk—multiple states, 2012. Clin Infect Dis 57:263–266. doi: 10.1093/cid/cit231. [DOI] [PubMed] [Google Scholar]
- 383.Mungai EA, Behravesh CB, Gould LH. 2015. Increased outbreaks associated with nonpasteurized milk, United States, 2007–2012. Emerg Infect Dis 21:119–122. doi: 10.3201/eid2101.140447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Serraino A, Florio D, Giacometti F, Piva S, Mion D, Zanoni RG. 2013. Presence of Campylobacter and Arcobacter species in in-line milk filters of farms authorized to produce and sell raw milk and of a water buffalo dairy farm in Italy. J Dairy Sci 96:2801–2807. doi: 10.3168/jds.2012-6249. [DOI] [PubMed] [Google Scholar]
- 385.Koziel M, Lucey B, Bullman S, Corcoran GD, Sleator RD. 2012. Molecular-based detection of the gastrointestinal pathogen Campylobacter ureolyticus in unpasteurized milk samples from two cattle farms in Ireland. Gut Pathog 4:14. doi: 10.1186/1757-4749-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Revez J, Zhang J, Schott T, Kivisto R, Rossi M, Hanninen ML. 2014. Genomic variation between Campylobacter jejuni isolates associated with milk-borne-disease outbreaks. J Clin Microbiol 52:2782–2786. doi: 10.1128/JCM.00931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Jonsson ME, Chriel M, Norstrom M, Hofshagen M. 2012. Effect of climate and farm environment on Campylobacter spp. colonisation in Norwegian broiler flocks. Prev Vet Med 107:95–104. doi: 10.1016/j.prevetmed.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 388.Strother KO, Steelman CD, Gbur EE. 2005. Reservoir competence of lesser mealworm (Coleoptera: Tenebrionidae) for Campylobacter jejuni (Campylobacterales: Campylobacteraceae). J Med Entomol 42:42–47. doi: 10.1603/0022-2585(2005)042[0042:RCOLMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 389.Skov MN, Spencer AG, Hald B, Petersen L, Nauerby B, Carstensen B, Madsen M. 2004. The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis 48:9–18. doi: 10.1637/5698. [DOI] [PubMed] [Google Scholar]
- 390.Axelsson-Olsson D, Waldenstrom J, Broman T, Olsen B, Holmberg M. 2005. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl Environ Microbiol 71:987–992. doi: 10.1128/AEM.71.2.987-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Snelling WJ, McKenna JP, Hack CJ, Moore JE, Dooley JS. 2006. An examination of the diversity of a novel Campylobacter reservoir. Arch Microbiol 186:31–40. doi: 10.1007/s00203-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 392.Barton MD. 2014. Impact of antibiotic use in the swine industry. Curr Opin Microbiol 19:9–15. doi: 10.1016/j.mib.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 393.Smith KE, Besser JM, Hedberg CW, Leano FT, Bender JB, Wicklund JH, Johnson BP, Moore KA, Osterholm MT. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. Invest Team N Engl J Med 340:1525–1532. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 394.Gupta A, Nelson JM, Barrett TJ, Tauxe RV, Rossiter SP, Friedman CR, Joyce KW, Smith KE, Jones TF, Hawkins MA, Shiferaw B, Beebe JL, Vugia DJ, Rabatsky-Ehr T, Benson JA, Root TP, Angulo FJ. 2004. Antimicrobial resistance among Campylobacter strains, United States, 1997–2001. Emerg Infect Dis 10:1102–1109. doi: 10.3201/eid1006.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wieczorek K, Kania I, Osek J. 2013. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from poultry carcasses in Poland. J Food Prot 76:1451–1455. doi: 10.4315/0362-028X.JFP-13-035. [DOI] [PubMed] [Google Scholar]
- 396.Di Labio E, Regula G, Steiner A, Miserez R, Thomann A, Ledergerber U. 2007. Antimicrobial resistance in bacteria from Swiss veal calves at slaughter. Zoonoses Public Health 54:344–352. doi: 10.1111/j.1863-2378.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 397.Chatre P, Haenni M, Meunier D, Botrel MA, Calavas D, Madec JY. 2010. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from cattle between 2002 and 2006 in France. J Food Prot 73:825–831. [DOI] [PubMed] [Google Scholar]
- 398.Cheng AC, Turnidge J, Collignon P, Looke D, Barton M, Gottlieb T. 2012. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg Infect Dis 18:1453–1460. doi: 10.3201/eid1809.111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Egger R, Korczak BM, Niederer L, Overesch G, Kuhnert P. 2012. Genotypes and antibiotic resistance of Campylobacter coli in fattening pigs. Vet Microbiol 155:272–278. doi: 10.1016/j.vetmic.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 400.Juntunen P, Olkkola S, Hanninen ML. 2011. Longitudinal on-farm study of the development of antimicrobial resistance in Campylobacter coli from pigs before and after danofloxacin and tylosin treatments. Vet Microbiol 150:322–330. doi: 10.1016/j.vetmic.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 401.EFSA. 2011. Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J 9:2105. doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 402.Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G, Allen VM. 2011. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl Environ Microbiol 77:8605–8614. doi: 10.1128/AEM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.van Gerwe T, Bouma A, Wagenaar JA, Jacobs-Reitsma WF, Stegeman A. 2010. Comparison of Campylobacter levels in crops and ceca of broilers at slaughter. Avian Dis 54:1072–1074. doi: 10.1637/9113-101809-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 404.Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. 2014. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 5:e01364-14. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Bahrndorff S, Rangstrup-Christensen L, Nordentoft S, Hald B. 2013. Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerg Infect Dis 19:425–430. doi: 10.3201/eid1903.111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Yano S, Kira T, Morishita Y, Ishihara K, Asai T, Iwata T, Akiba M, Murase T. 2013. Colonization of chicken flocks by Campylobacter jejuni in multiple farms in Japan. Poult Sci 92:375–381. doi: 10.3382/ps.2012-02710. [DOI] [PubMed] [Google Scholar]
- 407.Lammerding AM, Fazil A. 2000. Hazard identification and exposure assessment for microbial food safety risk assessment. Int J Food Microbiol 58:147–157. doi: 10.1016/S0168-1605(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 408.Svetoch EA, Stern NJ. 2010. Bacteriocins to control Campylobacter spp. in poultry—a review. Poult Sci 89:1763–1768. doi: 10.3382/ps.2010-00659. [DOI] [PubMed] [Google Scholar]
- 409.Hoang KV, Stern NJ, Lin J. 2011. Development and stability of bacteriocin resistance in Campylobacter spp. J Appl Microbiol 111:1544–1550. doi: 10.1111/j.1365-2672.2011.05163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wagenaar JA, Van Bergen MA, Mueller MA, Wassenaar TM, Carlton RM. 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet Microbiol 109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 411.Atterbury RJ, Dillon E, Swift C, Connerton PL, Frost JA, Dodd CE, Rees CE, Connerton IF. 2005. Correlation of Campylobacter bacteriophage with reduced presence of hosts in broiler chicken ceca. Appl Environ Microbiol 71:4885–4887. doi: 10.1128/AEM.71.8.4885-4887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Loc Carrillo C, Atterbury RJ, el-Shibiny A, Connerton PL, Dillon E, Scott A, Connerton IF. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl Environ Microbiol 71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.El-Shibiny A, Scott A, Timms A, Metawea Y, Connerton P, Connerton I. 2009. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J Food Prot 72:733–740. [DOI] [PubMed] [Google Scholar]
- 414.Carvalho CM, Gannon BW, Halfhide DE, Santos SB, Hayes CM, Roe JM, Azeredo J. 2010. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol 10:232. doi: 10.1186/1471-2180-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Kittler S, Fischer S, Abdulmawjood A, Glunder G, Klein G. 2013. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl Environ Microbiol 79:7525–7533. doi: 10.1128/AEM.02703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, Rasschaert G, Heyndrickx M, Pasmans F. 2011. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet Microbiol 152:219–228. doi: 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 417.Fischer S, Kittler S, Klein G, Glunder G. 2013. Impact of a single phage and a phage cocktail application in broilers on reduction of Campylobacter jejuni and development of resistance. PLoS One 8:e78543. doi: 10.1371/journal.pone.0078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnes M, Bohm J, Schatzmayr G. 2012. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult Sci 91:1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- 419.Nishiyama K, Seto Y, Yoshioka K, Kakuda T, Takai S, Yamamoto Y, Mukai T. 2014. Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS One 9:e108827. doi: 10.1371/journal.pone.0108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Santini C, Baffoni L, Gaggia F, Granata M, Gasbarri R, Di Gioia D, Biavati B. 2010. Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int J Food Microbiol 141(Suppl 1):S98–S108. doi: 10.1016/j.ijfoodmicro.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 421.Saxena M, John B, Mu M, Van TTH, Taki A, Coloe PJ, Smooker PM. 2013. Strategies to reduce Campylobacter colonisation in chickens. Procedia Vaccinol 7:40–43. doi: 10.1016/j.provac.2013.06.008. [DOI] [Google Scholar]
- 422.Annamalai T, Pina-Mimbela R, Kumar A, Binjawadagi B, Liu Z, Renukaradhya GJ, Rajashekara G. 2013. Evaluation of nanoparticle-encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poult Sci 92:2201–2211. doi: 10.3382/ps.2012-03004. [DOI] [PubMed] [Google Scholar]
- 423.Lake RJ, Horn BJ, Dunn AH, Parris R, Green FT, McNickle DC. 2013. Cost-effectiveness of interventions to control Campylobacter in the New Zealand poultry meat food supply. J Food Prot 76:1161–1167. doi: 10.4315/0362-028X.JFP-12-481. [DOI] [PubMed] [Google Scholar]
- 424.Steens A, Eriksen HM, Blystad H. 2014. What are the most important infectious diseases among those ≥65 years: a comprehensive analysis on notifiable diseases, Norway, 1993–2011. BMC Infect Dis 14:57. doi: 10.1186/1471-2334-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Sadkowska-Todys M, Kucharczyk B. 2013. Campylobacteriosis in Poland in 2011. Przegl Epidemiol 67:227–229, 341–342. [PubMed] [Google Scholar]
- 426.O'Reilly CE, Jaron P, Ochieng B, Nyaguara A, Tate JE, Parsons MB, Bopp CA, Williams KA, Vinje J, Blanton E, Wannemuehler KA, Vulule J, Laserson KF, Breiman RF, Feikin DR, Widdowson MA, Mintz E. 2012. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med 9:e1001256. doi: 10.1371/journal.pmed.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Lee G, Pan W, Penataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. 2013. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Zaidi MB, Campos FD, Estrada-Garcia T, Gutierrez F, Leon M, Chim R, Calva JJ. 2012. Burden and transmission of zoonotic foodborne disease in a rural community in Mexico. Clin Infect Dis 55:51–60. doi: 10.1093/cid/cis300. [DOI] [PubMed] [Google Scholar]
- 429.Yu JH, Kim NY, Cho NG, Kim JH, Kang YA, Lee HG. 2010. Epidemiology of Campylobacter jejuni outbreak in a middle school in Incheon, Korea. J Korean Med Sci 25:1595–1600. doi: 10.3346/jkms.2010.25.11.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Parry A, Fearnley E, Denehy E. 2012. ‘Surprise’: outbreak of Campylobacter infection associated with chicken liver pate at a surprise birthday party, Adelaide, Australia, 2012. Western Pac Surveill Response J 3:16–19. doi: 10.5365/wpsar.2012.3.4.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Merritt T, Combs B, Pingault N. 2011. Campylobacter outbreaks associated with poultry liver dishes. Commun Dis Intell Q Rep 35:299–300. [DOI] [PubMed] [Google Scholar]
- 432.Wensley A, Coole L. 2013. Cohort study of a dual-pathogen point source outbreak associated with the consumption of chicken liver pate, UK, October 2009. J Public Health (Oxf) 35:585–589. doi: 10.1093/pubmed/fdt020. [DOI] [PubMed] [Google Scholar]
- 433.Inns T, Foster K, Gorton R. 2010. Cohort study of a campylobacteriosis outbreak associated with chicken liver parfait, United Kingdom, June 2010. Euro Surveill 15:19704 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19704. [DOI] [PubMed] [Google Scholar]
- 434.Griffiths SL, Salmon RL, Mason BW, Elliott C, Thomas DR, Davies C. 2010. Using the Internet for rapid investigation of an outbreak of diarrhoeal illness in mountain bikers. Epidemiol Infect 138:1704–1711. doi: 10.1017/S0950268810001561. [DOI] [PubMed] [Google Scholar]
- 435.Farmer S, Keenan A, Vivancos R. 2012. Food-borne Campylobacter outbreak in Liverpool associated with cross-contamination from chicken liver parfait: implications for investigation of similar outbreaks. Public Health 126:657–659. doi: 10.1016/j.puhe.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 436.Abid M, Wimalarathna H, Mills J, Saldana L, Pang W, Richardson JF, Maiden MCJ, McCarthy ND. 2013. Duck liver-associated outbreak of campylobacteriosis among humans, United Kingdom, 2011. Emerg Infect Dis 9:1310–1313. doi: 10.3201/eid1908.121535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Rasanen S, Lappalainen S, Kaikkonen S, Hamalainen M, Salminen M, Vesikari T. 2010. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol Infect 138:1227–1234. doi: 10.1017/S0950268809991671. [DOI] [PubMed] [Google Scholar]
- 438.Breitenmoser A, Fretz R, Schmid J, Besl A, Etter R. 2011. Outbreak of acute gastroenteritis due to a washwater-contaminated water supply, Switzerland, 2008. J Water Health 9:569–576. doi: 10.2166/wh.2011.158. [DOI] [PubMed] [Google Scholar]
- 439.Gubbels SM, Kuhn KG, Larsson JT, Adelhardt M, Engberg J, Ingildsen P, Hollesen LW, Muchitsch S, Molbak K, Ethelberg S. 2012. A waterborne outbreak with a single clone of Campylobacter jejuni in the Danish town of Koge in May 2010. Scand J Infect Dis 44:586–594. doi: 10.3109/00365548.2012.655773. [DOI] [PubMed] [Google Scholar]
- 440.Moller-Stray J, Eriksen HM, Bruheim T, Kapperud G, Lindstedt BA, Skeie A, Sunde M, Urdahl AM, Oygard B, Vold L. 2012. Two outbreaks of diarrhoea in nurseries in Norway after farm visits, April to May 2009. Euro Surveill 17:20321 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20321. [DOI] [PubMed] [Google Scholar]
- 441.Karagiannis I, Sideroglou T, Gkolfinopoulou K, Tsouri A, Lampousaki D, Velonakis EN, Scoulica EV, Mellou K, Panagiotopoulos T, Bonovas S. 2010. A waterborne Campylobacter jejuni outbreak on a Greek island. Epidemiol Infect 138:1726–1734. doi: 10.1017/S0950268810002116. [DOI] [PubMed] [Google Scholar]
- 442.Gardner TJ, Fitzgerald C, Xavier C, Klein R, Pruckler J, Stroika S, McLaughlin JB. 2011. Outbreak of campylobacteriosis associated with consumption of raw peas. Clin Infect Dis 53:26–32. doi: 10.1093/cid/cir249. [DOI] [PubMed] [Google Scholar]
- 443.Stuart TL, Sandhu J, Stirling R, Corder J, Ellis A, Misa P, Goh S, Wong B, Martiquet P, Hoang L, Galanis E. 2010. Campylobacteriosis outbreak associated with ingestion of mud during a mountain bike race. Epidemiol Infect 138:1695–1703. doi: 10.1017/S095026881000049X. [DOI] [PubMed] [Google Scholar]
- 444.Gaudreau C, Helferty M, Sylvestre JL, Allard R, Pilon PA, Poisson M, Bekal S. 2013. Campylobacter coli outbreak in men who have sex with men, Quebec, Canada, 2010–2011. Emerg Infect Dis 19:764–767. doi: 10.3201/eid1905.121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zeigler M, Claar C, Rice D, Davis J, Frazier T, Turner A, Kelley C, Capps J, Kent A, Hubbard V, Ritenour C, Tuscano C, Qiu-Shultz Z, Leaumont CF. 2014. Outbreak of campylobacteriosis associated with a long-distance obstacle adventure race—Nevada, October 2012. MMWR Morb Mortal Wkly Rep 63:375–378. [PMC free article] [PubMed] [Google Scholar]
- 446.Bartholomew N, Brunton C, Mitchell P, Williamson J, Gilpin B. 2014. A waterborne outbreak of campylobacteriosis in the South Island of New Zealand due to a failure to implement a multi-barrier approach. J Water Health 12:555–563. doi: 10.2166/wh.2014.155. [DOI] [PubMed] [Google Scholar]
- 447.Edwards DS, Milne LM, Morrow K, Sheridan P, Verlander NQ, Mulla R, Richardson JF, Pender A, Lilley M, Reacher M. 2014. Campylobacteriosis outbreak associated with consumption of undercooked chicken liver pate in the East of England, September 2011: identification of a dose-response risk. Epidemiol Infect 142:352–357. doi: 10.1017/S0950268813001222. [DOI] [PMC free article] [PubMed] [Google Scholar]