Abstract
Hepatocellular carcinoma (HCC) is the most rapidly increasing type of cancer in the United States. HCC is a highly malignant cancer, accounting for at least 14000 deaths in the United States annually, and it ranks third as a cause of cancer mortality in men. One major difficulty is that most patients with HCC are diagnosed when the disease is already at an advanced stage, and the cancer cannot be surgically removed. Furthermore, because almost all patients have cirrhosis, neither chemotherapy nor major resections are well tolerated. Clearly there is need of a multidisciplinary approach for the management of HCC. For example, there is a need for better understanding of the fundamental etiologic mechanisms that are involved in hepatocarcinogenesis, which could lead to the development of successful preventive and therapeutic modalities. It is also essential to define the cellular and molecular bases for malignant transformation of hepatocytes. Such knowledge would: (1) greatly facilitate the identification of patients at risk; (2) prompt efforts to decrease risk factors; and (3) improve surveillance and early diagnosis through diagnostic imaging modalities. Possible benefits extend also to the clinical management of this disease. Because there are many factors involved in pathogenesis of HCC, this paper reviews a multidisciplinary perspective of recent advances in basic and clinical understanding of HCC that include: molecular hepatocarcinogenesis, non-invasive diagnostics modalities, diagnostic pathology, surgical modality, transplantation, local therapy and oncological/target therapeutics.
Keywords: Genetic alterations, Epigenetic alterations, Diagnostic pathology, Diagnostic imaging, Surgical modality, Liver transplantation, Locoregional therapy, Sorafenib, Hepatocellular carcinoma, Liver resection
Core tip: Hepatocellular carcinoma (HCC) is one of the few tumors in which the incidence is on the rise worldwide, especially in the United States. The overall increase in the incidence warrants efforts to prevent and to more efficiently treat this disease. This necessitates the need for a multidisciplinary approach for the management of HCC, because there are many etiological factors involved in the pathogenesis and malignant transformation of the disease. For example, there is a need to improve surveillance and early diagnosis through diagnostic imaging modalities to facilitate identification of potential molecular targets for novel therapeutic strategies. In turn, this will facilitate the identification of patients at risk. This review summarizes current knowledge on the clinical management of the disease as well as etiologic mechanisms of malignant transformation for better diagnosis, prognosis, and treatment of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) constitutes the majority of primary liver cancers. It is the fifth most common malignancy in the world and is the third leading cause of cancer-related death worldwide[1,2]. More than half a million cases are newly diagnosed each year, with an almost equal annual mortality given its high fatality rates. The incidence of HCC continues to rise and is predicted to continue to be the third cause of cancer-related death by 2030[3]. Viral hepatitis and cirrhosis are known to be the most common risk factors for HCC, but the exact mechanisms of hepatocarcinogenesis remain unclear, particularly in patients without these risk factors. Fatty liver disease due to diabetes and obesity has recently been recognized as independent risk factor of HCC, but may also act synergistically with other risk factors such as viral hepatitis to contribute to the processes of hepatocarcinogenesis[4-6].
The treatment options for HCC include those of curative potential for early stage of the disease such as surgical resection, ablation, and liver transplantation. The only therapies that have been shown to prolong life for intermediate or advanced stage disease include liver-directed therapy with transarterial chemoembolization and systemic chemotherapy with sorafenib. As HCC is a complex type of cancer, optimal management requires a multidisciplinary-team approach including oncological surgeons, hepatologists, oncologists, radiologists, intervention radiologists, transplant surgeons, and pathologists, who routinely meet and discuss diagnosis and treatment options towards individualized management with the goal fulfilling precision medicine. This review aims to discuss the current understanding of the mechanisms and signaling pathways involved in hepatocarcinogenesis, pathological and radiological diagnosis, and management of HCC with a multidisciplinary approach.
GENETIC ALTERATIONS IN HCC
The genetic heterogeneity of HCC has complicated the search for driver mutations that initiate or promote HCC. Technological advancements in genomic research over the past decade, such as whole exome sequencing (WES), whole genome sequencing (WGS), and whole transcriptome analysis, have allowed more extensive genomic analyses of HCC. This section will focus on common genetic and epigenetic mutations in HCC, molecular classification of HCC, and the signaling pathways that may serve as therapeutic targets.
Multiple groups have performed whole exome as well as WGS of HCC in order to determine the most common genetic mutations involved in this disease[7-9]. Cleary et al[7] performed WES of 87 tumors and found that the most frequent mutations were TP53 (18%), CTNNB1 (10%) and MLL4 (7%) among others. Their work demonstrates the heterogeneity of HCC as they had a relatively even distribution of hepatitis C virus (HCV), HBV, and cirrhosis not otherwise specified in their cohort and no single mutation was present in > 20% of samples. Fujimoto et al[8] performed WGS on 27 tumors, all but two-harbored HCV or HBV. They also found TP53 to be the most frequent mutation (14/27 samples). Their work also found mutations in CTNNB1, MLL, as well as ARID1a/2. Kan et al[9] performed WGS on 88 tumors, 81 of which were positive for HBV. Their group also found TP53 as the most frequent mutation (35%) followed by CTNNB1 (16%) and JAK1 (9%). Pathway analysis was used to describe five major cellular pathways that are altered by the somatic mutations found by WGS: (1) P53/cell cycle; (2) Wnt/β-catenin (CTNNB1); (3) Chromatin remodeling (ARID); (4) PI3K/AKT/mammalian target of rapamycin (mTOR); and (5) Oxidative/ER stress. Overall, specific mutations were present in < 20% of all samples, further illustrating the genetic heterogeneity found in HCC (for further information about HCC genetic dysregulation in Table 1).
Table 1.
Genes frequently mutated in hepatocellular carcinoma
| Gene | Pathways/gene functions involved | Ref. |
| p53 | Genome integrity and cell cycle | Clearly et al[7] |
| CTNNB1 | Wnt/β-catenin signaling | Kan et al[9] |
| ARID1A | Chromatin remodeling | Fujimoto et al[8] |
| mTOR | PI3K/AKT/mTOR | Riechle et al[20] |
| NFE2L2 | Oxidative/ER stress | Guichard et al[16] |
| TERT promoter | Telomere stability | Nault et al[183] |
PI3K/AKT/mTOR: Phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin.
The great diversity of genetic alterations in HCC reflects the multiple etiologic factors that contribute to its pathogenesis. It is well known that HBV, HCV, alcoholic cirrhosis, aflatoxin-B, non-alcoholic steatohepatitis (NASH) and hemochromatosis all portend a higher risk of developing HCC. Multiple groups have used WES and WGS as well as genome wide association studies to define genetic “signatures” for different etiologies of HCC[10-12]. For example, those associated with alcoholic cirrhosis tend to have increased mutations in the chromatin-remodeling pathway, HCV-associated HCC were shown to have increased rates of CTNNB1 mutations (Wnt/β-catenin pathway) and ARID2 mutations (chromatin remodeling complex). In contrast, HBV-related tumors are commonly caused by integration of the viral HBx DNA into the host genome, which creates genetic instability and mutagenesis in cancer related genes such as TP53. Multiple groups have found common integration sites in the promoter sites or exons of TERT (MLL4), CCNE1, and ROCK1 genes that are significantly increased in HBV associated HCC. They also have a higher rate of differentially regulated TP53[13,14]. Aflatoxin B1 exposure results in a predictable mutation in codon 249 of TP53 which drives carcinogenesis[15]. Guichard et al[16] described a novel mutation in IRF2 that is present in HBV-related HCC which leads to TP53 inactivation. A recent study by Lau et al[17] found a common viral-human chimeric transcript resulting from HBV integration into a LINE1-element on chromosome 8p11.21, which functions as a long non-coding RNA to drive oncogenesis through its influence on Wnt/β-catenin pathway. Zain et al[18] have used genome wide analysis of copy number variation to identify rare or novel copy number variant that are associated with progression of NASH to cirrhosis and eventually HCC. There is, however, a paucity of data regarding the genetics of NASH and HCC, and given the worldwide rise in prevalence of NASH this presents a significant gap in our knowledge of the HCC cancer genetics.
Certain subtypes of HCC, however, have been associated with single driver mutations. Recently, WGS of fibrolamellar carcinoma has revealed a chimeric transcript of DNAJB1-PRKACA that is present in all tumor samples studied[19]. This rare variant of HCC occurring in young adults without cirrhosis also shows involvement of the mTORC1 and FGFR1 pathways[20].
There are also genetic signatures for clinical characteristics as well as risk assessment for certain HCCs. For example, Cleary et al[7] found that increased microvascular invasion was associated with MLL mutations, and those tumors with TP53 mutations were at higher risk for early recurrence. Multiple groups have also discovered various SNPs and their association with HCC risk[21]. Budhu et al[22] created 17-gene profile that was able to predict tumor metastasis and recurrence in an independent cohort. Huang et al[23] used WES of HBV related HCC with associated portal vein tumor thrombus (PVTT). They discovered novel mutations present only in the PVTT that suggest they may be involved in tumor progression[23].
The cumulative genetic and epigenetic alterations lead to changes in gene expression in HCC. Many groups have sub-classified HCC based on their transcriptome profile. Hoshida et al classified HCC into three groups, S1-S3. S1 tumors tend to have mutations in the Wnt/β-catenin pathway, have increased risk of early recurrence, and have increased vascular invasion as well as satellite lesions. S2 tumors are those with activating mutations in the MYC and PI3K/AKT pathways. They tend to be larger tumors that overexpress AFP. S3 tumors are well differentiated, have fewer inactivating mutations of p53, and tended to be smaller tumors[24]. Boyault’s group used whole transcriptome analysis of 103 HCC samples to classify six subgroups of HCC. G1-G3 groups were associated with increased genomic instability. G1-G 2 groups were both found to have AKT activation and were associated with HBV. For G1 groups, tumors had low copy numbers of HBV, while G2 tumors had higher copy numbers of HBV as well as TP53 and PIK3CA mutations. Tumors for G3 group were classified by having TP53 and cell cycle pathway mutations, whereas tumors for G4 group were heterogeneous, mostly with TLF-1 mutations. For G5-G6 groups, tumors were found to carry Wnt/β-catenin mutations such as CTNNB1. They also exhibited decreased expression of cell adhesion proteins and tended to have increased satellite lesions[25].
Epigenetic changes in HCC carcinogenesis and prognosis have also been investigated. There are a myriad of differentially regulated miRNA, alterations in DNA methylation, and dysregulations of histone complexes that occur in HCC. The complete list of miRNA is beyond the scope of this review. Seemingly, the most clinically relevant are the Let-7 miRNA, which are down regulated in HBV related HCC. Also, miRNA-196 seems to have a protective role in HCV related HCC. miRNA-26a and 195 are both down regulated inn HCC, which leads to decreased E2F expression and cell survival/proliferation[26-28].
DNA hypo-and hypermethylation can lead to differential regulation of tumor suppressors and oncogenes. In HCC, multiple groups have identified hypomethylation (activation) of oncogenes such as LINE-2, ALU, STAT2 as well as hypermethylation (suppression) of RB1, P16, APC, SOCS1, SOCS3, and RASSF1a. DNA methylation abnormalities occur early in the course of HCC, and seem to accumulate as the disease progresses[29]. Nagashio’s group has identified specific methylation signatures that differentiate malignant HCC from benign lesions with > 95% sensitivity and specificity. They have also shown that certain methylation sites such as RIZ1a and LINE-1 may have prognostic value[30].
The advent of WGS and WES has uncovered a myriad of novel mutations found in HCC. More studies are needed to define their role in hepatocarcinogenesis. This could lead to further targets for therapy, risk stratification, as well as development of biomarkers for early detection of HCC. As the technology improves we may be able to personalize targeted therapy for specific mutational profile found in each tumor.
PATHOLOGY OF HCC
Pathology has played an important role in the diagnosis, staging and follow-up for the management of HCC. HCC is a morphologically heterogeneous tumor. Grossly HCC appears as circumscribed, yellow to greenish and soft tumors, often encapsulated with areas of hemorrhage and necrosis. Infiltrative borders can be seen but are not common. The background liver may or may not be cirrhotic (Figure 1A and B). Histologically HCC can show range of differentiation from well, moderate to poor, with a spectrum of architectural patterns including trabecular (greater than two cells in thickness yet still resembling hepatocytic cords), pseudoglandular or pseudoacinar to solid (Figure 1C). Normal structures, i.e., portal tracts are not present within the neoplastic tissue, where the blood is solely supplied by the artery; hence “unpaired arteries” are increased within HCC (Figure 1D), a phenomenon reflecting the neoangiogenic property during hepatocarcinogenesis and the hypervascularity observed by imaging. Sinusoidal capillarization is also a unique characteristic of HCC in which fenestrated hepatic sinusoids transform into continuous capillaries. In HCC, the neoplastic cells can appear similar to hepatocytes to markedly pleomorphic or small cell and undifferentiated. Features of hepatocytic differentiation may still retain in the neoplastic hepatocytes, such as bile, glycogen, steatosis, and Mallory-Denk bodies. Malignant features including enlarged and vesicular nuclei with prominent nucleoli are often seen. Mitotic figures are frequent and can appear bizarre in the poorly differentiated tumor. Although not always present, stromal invasion is a malignant feature of HCC that can be used to distinguish HCC from dysplastic nodule, where loss of ductular reaction by keratin 7 (CK7) or CK19 is observed by immunohistochemistry in HCC[31,32]. Reticulin stain has been traditionally useful to diagnose HCC, in which the thickened trabecula are highlighted by the loss of reticulin stain. In HCC, the sinusoidal endothelial cells stain positive for CD34[33,34], whereas they are negative in the non-neoplastic liver tissues. While glypican-3 is also a relatively sensitive and specific marker for HCC, the staining may be focal, and its sensitivity may decrease in well differentiated HCC, thus cautious interpretation is warranted[35,36]. Hepatocytic differentiation of HCC can be demonstrated by several markers such as Hep Par 1, polyclonal CEA, CD10, and the recently developed arginase (Arg-1), but they cannot distinguish HCC from benign hepatocytes. Hep Par 1 has a diffuse cytoplasmic granular staining pattern in normal and neoplastic hepatocytes[37-40]. In HCC, staining with polyclonal CEA and CD10 produces a canalicular staining pattern that has been attributed to cross reactivity with the biliary glycoprotein on the canalicular surface. The canalicular staining pattern is specific for HCC and is not seen in cholangiocarcinoma and metastatic adenocarcinoma, but its sensitivity has been variably reported, ranging from 50%-96%[41-45]. Arg-1 is a manganese metalloenzyme active in the urea cycle that is a recently developed immunohistochemical marker of hepatocellular neoplasms of high sensitivity and specificity when used alone or in combination with glypican-3 or Hep Par 1[46-48].
Figure 1.
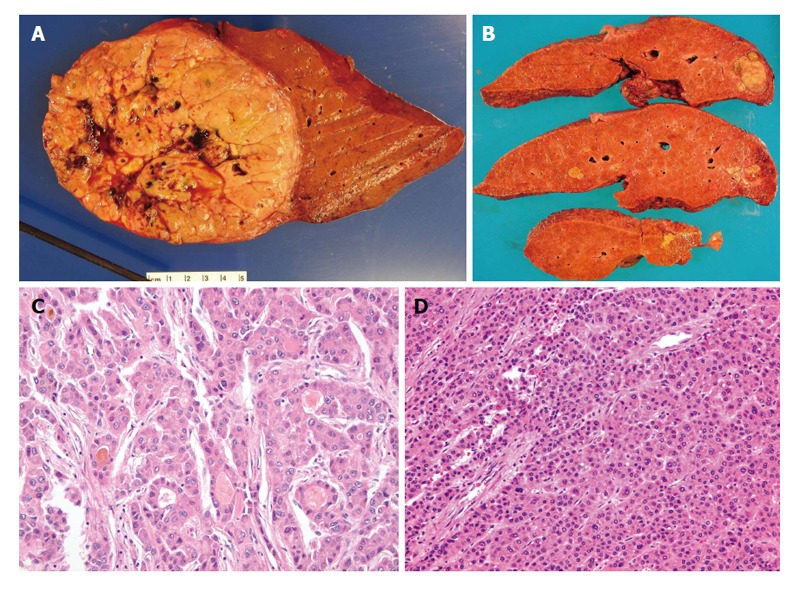
Pathology of classical hepatocellular carcinoma. A: Gross photo of a well circumscribed, soft, yellowish to tan, and lobulated hepatocellular carcinoma (HCC) in a background of non-cirrhotic liver; B: Gross photo of a yellow and greenish, soft and lobulated HCC in a background of cirrhotic liver; C: Microphotos of HCC showing the pseudoacinar and pseudoglandular patterns, some containg the yellowish bile within the pseudoglandular structure with increased nuclear sizes; D: Microphotos of HCC showing thickened trabeculi, with increased unpaired arteries. Notice there are no normal structures present, i.e., portal tracts.
The differential diagnosis of HCC from other hepatocytic lesions includes hepatocellular adenoma, focal nodular hyperplasia, and dysplastic and macroregenerative nodules, especially in well-differential HCC. Other malignant tumors that can cause diagnostic difficulties include cholangiocarcinoma and metastatic tumors including carcinoma from any sites and melanomas. These can be differentiated with clinical history, radiological findings, histomorphology, reticulin stain and immunohistochemical markers. Small samples of biopsy tissue material may cause diagnostic challenges.
Fibrolamellar variant of HCC (fibrolamellar carcinoma) consists approximately 0.5%-1% of all HCC. It has a unique clinical presentation, pathological feature, and biology than the typical HCC. It tends to occur in late teenage years and young adults years. Unlike the typical HCC that often arises in a background of chronic liver disease or cirrhosis, fibrolamellar carcinoma typically arises in liver without any underlying liver diseases. 60%-70% of fibrolamellar carcinomas have a central scar, which appear as thick lamellar bands of fibrosis under microscopy as one of the most characteristic low power feature (Figure 2A). The tumor cells are large and polygonal with abundant eosinophilic cytoplasm, large vesiculated nuclei, and large nucleoli (Figure 2B)[49].
Figure 2.
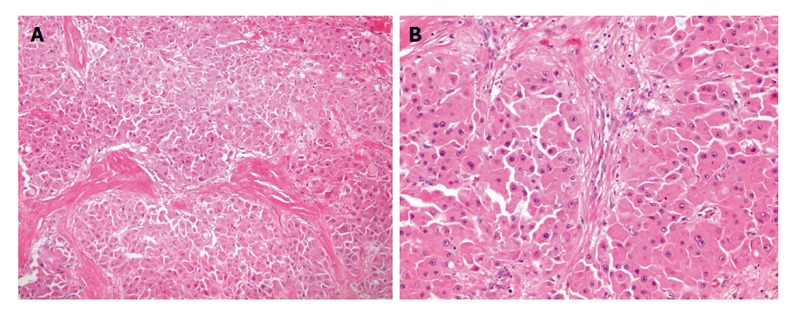
Pathology of fibrolamellar carcinoma. A: Microphotos of fibrolamellar carcinoma showing the thick lamellar bands of fibrosis under low power magnification; B: In higher power magnification, the tumor cells are large and polygonal with abundant eosinophilic and granular cytoplasm, large vesiculated nuclei, and prominent nucleoli.
For fear of needle tract seeding and risk of bleeding, HCC with the classic contrast enhanced imaging appearance, i.e., arterial enhancement with portal and delayed venous washout typically do not require preoperative tissue confirmation by core needle biopsy or fine needle aspiration biopsy, however, in equivocal cases, which are not uncommon, histopathology remains central in the diagnosis of HCC.
MEDICAL IMAGING OF HCC
Medical imaging has been an essential resource for the detection and management of HCC. The appropriate use of the different imaging modalities allows optimization of resources and more accurate results. Screening, characterization, staging, therapeutic interventions and response to treatment assessment are some of the most important uses of imaging studies.
Ultrasound
Ultrasound (US) imaging utilizes high frequency sound waves to generate images of the tissues. Most commonly it does not involve the use of intravenous (iv) contrast or radiation and therefore there are no contraindications for its use. It is also one of the least costly imaging modalities. These facts make it the exam of choice for screening of HCC in high-risk population[50,51] (Figure 3). Another application of US in HCC patients is to guide procedures including biopsies, radiofrequency ablation and ethanol injection of tumors.
Figure 3.
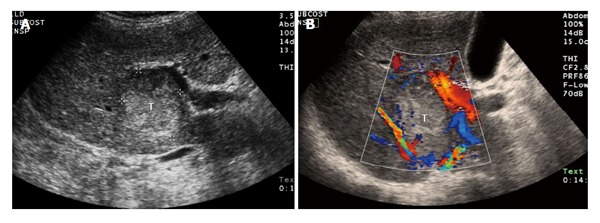
A 68-year-old male with cirrhosis and surgically proven hepatocellular carcinoma. A: Thirty-four seconds after intravenous injection of ultrasound contrast (microbubbles) there is tumor (T with dashed line) enhancement; B: One and half minutes after injection the tumor (T with dashed line) is washing out of contrast. The images on the right side are a conventional sonogram (non-contrasted) of the lesion. The image on the left is a pulse inversion harmonics ultrasound for better visualization of ultrasound contrast media.
The limitations of US are the low specificity for characterization of liver masses, thus it is frequently necessary to follow up the patients with contrasted computed tomography (CT) or magnetic resonance imaging (MRI) for confirmation of the diagnosis. Recent studies have demonstrated that the use of iv contrast for US increases the accuracy for tumor characterization of this modality, making it comparable to contrast enhanced CT or MRI[52]. US contrast is made of microscopic encapsulated gas bubbles. Although the Federal Drug Administration has not approved the use of US contrast for abdominal imaging in the United States it is routinely used for liver mass characterization in Europe, Canada and Asia. US contrasted studies show similar enhancing characteristics than other tomographic contrasted imaging modalities like CT or MRI (Figure 4).
Figure 4.
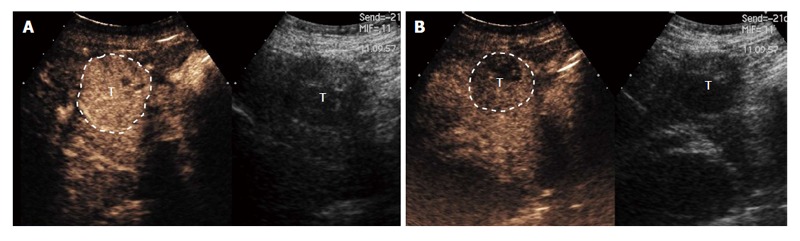
Ultrasound of hepatocellular carcinoma. A: Ultrasound of the liver demonstrates a heterogeneous tumor (T) in the right lobe of the liver that was later characterized as definite hepatocellular carcinoma by computed tomography; B: Same lesion (T) using color Doppler ultrasound images to demonstrate blood flow in the adjacent vessels.
New advances in US technology include the use of shear wave elastography[53,54]. This new technique uses estimations of the velocity of the sound in the tissue for quantitative assessment of fibrosis and prediction of the risk of HCC development. Shear wave elastography can also be used for characterization of liver tumors including HCC[55], and also for assessment of response to treatment[56].
CT
CT is the workhorse of medical imaging for diagnosis and staging of HCC. It utilizes measurements of the attenuation of X-rays to generate images. For accurate detection and diagnosis of HCC, the correct use of iodinated iv contrast, specifically with high injection rates and multiphase imaging with accurate timing for each phase (late arterial, portal venous and delayed) is extremely important. The almost exclusive arterial blood supply of HCC determines earlier arrival of injected iv contrast, compared to liver parenchyma mainly supplied by the portal vein. This early enhancement of HCC in contrasted studies is best captured in the set of images of arterial phase; later on, the HCC typically washes-out of contrast earlier than the liver parenchyma, best demonstrated in the 3-5 min delayed set of images. Also the tumor capsule shows characteristic enhancement in the delayed phase due to retention of contrast within the fibrous tissue of the capsule, as shown in Figure 5. Therefore a tumoral mass enhancing in the arterial phase, and washing out on the delayed phase in a high risk patient is a very specific finding for the diagnosis of HCC with a positive predictive value (PPV) of 98.8% for cirrhotic patients[57], and therefore allows the medical team to treat the patient without the need of a diagnostic biopsy.
Figure 5.
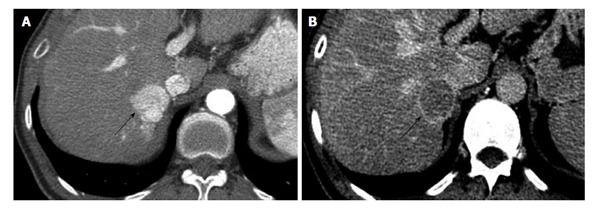
Computed tomography of hepatocellular carcinoma in 48-year-old male with hepatitis C. A: Arterial phase contrast enhanced CT of the liver shows a strongly enhancing mass (arrow) in the right lobe, adjacent to the IVC. B: The same lesion (arrow) washes-out of contrast on the delayed phase and shows a thin capsule, this is diagnostic for HCC and corresponds to LI-RADS category 5. HCC: Hepatocellular carcinoma; CT: Computed tomography; LI-RADS: Liver imaging reporting and data system; IVC: Inferior vena cava.
CT is commonly used for staging HCC, with excellent detection of vascular invasion and metastasis (Figure 6). CT can also demonstrate the presence of intratumoral calcifications, which sometimes can support the diagnosis of HCC.
Figure 6.
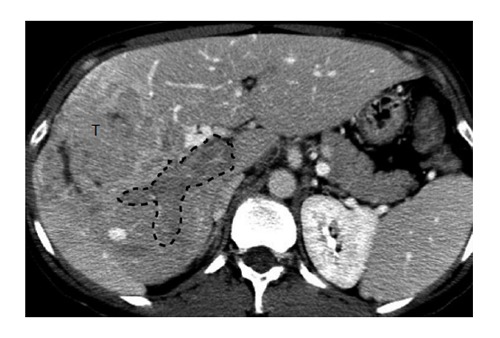
Portal vein invasion by hepatocellular carcinoma. Computed tomography in portal venous phase shows a right lobe mass (T) and lack of enhancement of the portal vein (outlined), consistent with tumor invasion.
Potential future advances in CT imaging of HCC include standard use of perfusion analysis and dual-energy imaging for assessment of response to therapy, with some studies showing changes in arterial perfusion (associated with improve in survival) earlier than changes in tumor diameter[58,59] Potential contraindications for CT include: anaphylaxis to iodinated contrast media, severe renal failure, and pregnancy.
MAGNETIC RESONANCE
MR generates medical images utilizing radiofrequency pulses and changes in magnetic gradients within a very strong magnetic field. Similar to CT, it is crucial to use iv contrast for detection and characterization of liver masses, as shown in Figure 7. In the case of MR, the contrast contains Gadolinium, a strong paramagnetic element that causes the surrounding molecules to release energy and show increased tissue intensity (enhancement). Injection rate and timing of the multiphase post-contrast images are also crucial for accuracy of the test. As with other modalities radiologists look for enhancement of the mass in arterial phase, and washout with capsular enhancement in delayed phase to make the diagnosis of HCC[60-63]. In addition, MR can demonstrate the presence of ancillary findings including tumoral fat, hemorrhage and increased signal in non-contrasted images. Contraindications include renal failure, first trimester pregnancy and pacemakers. Perfusion analysis and diffusion weighted imaging (DWI) to evaluate response to local therapy demonstrating enhancement changes earlier than size response. Whole body DWI for detection of metastatic disease have been used mostly in research setting but the standardization of protocols and other advances will allow the wide use of these techniques in the common clinical setting.
Figure 7.
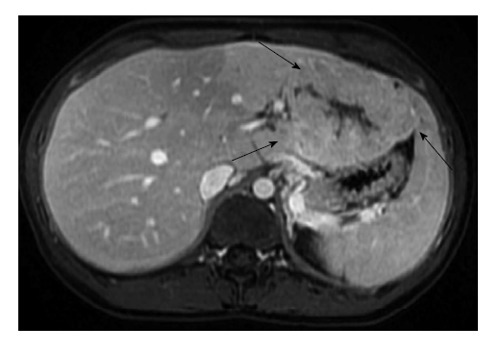
Magnetic resonance imaging of hepatocellular carcinoma in 19-year-old female. Post contrast liver magnetic resonance imaging in portal venous phase shows a large mass (arrows) arising from the left lobe of a liver without cirrhosis. This lesion that has some imaging similarities with focal nodular hyperplasia, corresponded to fibrolamellar carcinoma on pathologic analysis.
Angiography
Angiography is not used as a diagnostic tool anymore but has become a key therapeutic tool for HCC when used to deliver treatment in Trans Arterial Chemo-Embolization (TACE) and radioembolization with yttrium-90 (Y90) in patients with non-resectable tumors.
Positron emission tomography
Fluorodeoxyglucose (FDG) positron emission tomography (PET) and FDG CT-PET studies have low sensitivity for well-differentiated HCC and therefore are not commonly used to diagnose or stage the disease[64,65]. Nevertheless it could be of some value in cases of poorly differentiated tumors to identify metastasis. The development of new PET radiotracers for HCC could potentially increase the use for diagnosis, staging and assessment of response to therapy.
Accuracy of different imaging modalities for HCC diagnosis
Comparison between modalities like US, CT and MR is difficult due to differences in the methodology of the multiple published studies[60-63], but a systematic review by Colli et al[66] showed a sensitivity of 60% (95%CI: 44-76) and specificity of 97% (95%CI: 95-98) for US; for CT, the sensitivity was 68% (95%CI: 55-80) and specificity was 93% (95%CI: 89-96). The sensitivity for MR was 81% (95%CI: 70-91) and specificity was 85% (95%CI: 77-93). Accuracy of imaging tests correlates directly with tumor size with sensitivities and specificities around 30% for < 1 cm lesions and more than 90% for lesions > 2 cm[67].
Liver imaging reporting and data system
The American College of Radiology has directed an effort to standardize the reporting and data collection of CT and MRI for HCC in cirrhotic population, developing the liver imaging reporting and data system (LI-RADS) classification of lesions (http://www.acr.org/Quality-Safety/Resources/LIRADS). LI-RADS divide the lesions in 5 categories from benign (category1-2) to definitely HCC (category 5). The latest version of LI-RADS is now concordant with the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network classification, making it valuable for health care workers involved in the liver transplant teams[68]. The PPV for CT and MR in the category 5 lesions is so high that the patient does not require a biopsy to be treated. Table 2, shows the list of categories with the significance of each. Category 5 lesions can be treated without the need of histology confirmation. This classification should be applied only in cirrhotic patients.
Table 2.
Liver imaging reporting and data system categories
| LI-RADS category | Significance |
| 1 | Definitely benign |
| 2 | Probably benign |
| 3 | Indeterminate |
| 4 | Probably HCC |
| 5 | Definitely HCC |
LI-RADS: Liver imaging reporting and data system; HCC: Hepatocellular carcinoma.
SURGICAL RESECTION OF HCC
Liver resection (LR), also known as partial hepatectomy (PH) is a potentially curative surgical treatment option for patients with HCC, and is feasible in approximately 15% to 20% of all case presentations. The goal of LR is to remove the HCC with an adequate margin, while preserving as much functional liver parenchyma with minimal blood loss and no complications. The safety, result and outcomes of PH for HCC and cirrhotic patients have improved substantially over the last three decades. This has to be attributed to refined patient evaluation and selection, the ability to manipulate the future liver remnant volume, advances in surgical and anesthetic techniques, and the enhanced peri-operative management of these patients. The operative mortality for LR is less than 5% even in cirrhotic patients or those undergoing major PH, and the 5-year overall survival is over 50% for HCC[69-71].
Principles of LR for HCC
Patient selection: Patient selection for LR in HCC is unique in that in addition to the standard assessment of the patient’s ability to tolerate the procedure, anesthetic, potential complications, and the biology and stage of the cancer, the synthetic function of the liver parenchyma and the presence of portal hypertension must also be accounted for, as most patients will have some degree of fibrosis or cirrhosis, which will determine the liver’s capacity to regenerate and recover function following PH[72].
A LR patient must be medically fit for a major operation, have no significant medical co-morbidities, and should have a good Eastern Cooperative Oncology Group (ECOG) performance status and quality of life score. Although there is no strict cut-off in terms of chronological age, patients with advanced age (> 70) will have limited physiologic capacity for liver regeneration, which must be accounted for in the surgical planning[73].
Adequacy of hepatic reserve in the future liver remnant (FLR) is most commonly assessed using the Child-Turcotte-Pugh (CTP) score, where CTP A5 through B7 patients are considered reasonable candidates for LR. Pre-operative Model for End-stage Liver Disease (MELD) score of greater than 9 predicts increased operative mortality for major PHs and can supplement the CTP score[74].
The presence of portal hypertension is a relative contraindication to PH, where only select minor PHs are appropriate, and trans-jugular intra-hepatic porto-systemic gradient (PSG) measurements (significant portal hypertension when PSG measurements are greater than 10 mmHg) can help to elucidate equivocal cases[75]. Volumetric measurement using CT/MRI is important in planning major resections and in patients with cirrhosis. Although up to 80% of functional liver can be resected safely if two contiguous healthy liver segments are preserved, increased FLRs [FLR% = FLR/(total liver volume-tumor volume)] are necessary for fibrotic (> 30%) and cirrhotic (> 40%) livers. Preoperative portal vein embolization (PVE) is indicated in patients with small FLRs (≤ 20% in normal and ≤ 40% in fibrotic/cirrhotic liver), and a FLR volume increase > 5% with PVE predicts low risk of post-PH liver failure[76,77].
Biologic markers to predict HCC tumor biology are under development. Current surrogate prognostic factors, such as the stage and extent of the HCC used to formulate the operative plan is based on tumor size, number, and vascular invasion evaluated by multiphase liver protocol CT or MRI. For primary HCC tumors, Ho et al[78] reported that larger tumor sizes and AFP levels over 400 ng/mL were associated with postresection recurrence of HCC, exceeding the University of California at San Francisco (UCSF) criteria. Other markers such as retinoic acid-induced protein 3 as well as miRNA expression profiles could be used to predict poor prognosis to assess the risk of disease recurrence after liver transplantion[79,80].
Large tumor size has traditionally been a relative contraindication to LR given the elevated risk of vascular invasion. However, as many HCCs over 10 cm in size that do not invade the vasculature are amenable to PH with good results, identification of such tumors is important. Surgical techniques such as the anterior hanging maneuver can be employed to facilitate resection of such large HCCs[81-83].
Similarly, multifocal disease generally increases the risk of recurrence and is a relative contraindication to LR. However, select patients with multifocal HCC outside of the Milan criteria for orthotopic liver transplantation (OLT) can be offered PH in combination with ablative and catheter-directed therapies in the absence of vascular invasion or HCV as the etiology for cirrhosis. Routine use of TACE as a neo-adjuvant therapy has not been demonstrated beneficial. Y90 radio-embolization prior to LR may play a role in down-staging tumors and is being investigated[84,85].
LR for HCC invading the portal vein or hepatic veins remains controversial, as the outcomes have been disappointing. However, highly selected cases of HCCs with tumor thrombus not extending into the major vascular trunks, e.g., main portal or hepatic veins, can be resected with reasonable outcomes[86,87].
Ruptured HCC is a life-threatening condition occurring in approximately 4.5% to 14.5% of cases, and carries a grim prognosis. Control of bleeding is best accomplished using hepatic artery embolization. Surgical ligation of the hepatic artery with packing, plication or selective resection of the bleeding tumor can be considered in refractory cases. Interval PH can be considered in select cases where laparoscopy has ruled out peritoneal carcinomatosis, and can provide long-term survival in highly selected cases[88].
Technical considerations: What is considered an adequate width for the surgical resection margin has been a controversial topic of debate. The only randomized controlled trial evaluating the influence of the width of resection margin for HCC concluded that the recurrence rates decreased and 3- and 5-year survival rates increased when aiming for 2 cm margins compared with 1 cm margins. However, the meta-analysis of this and four non-randomized trials demonstrate no significant difference in recurrence rate, or 1-, 3-, and 5-year survival rates between resection margin < 1 cm and margin > 1 cm[89].
The liver consists of eight Couinaud segments with distinct vascular inflow/outflow and biliary drainage. Segment-based anatomical PH to remove all intra-segmental portal vein branches is not only less bloody given the ability to gain control of the inflow to the segment(s) and parenchymal division through relatively vessel-free regions, but has also shown to provide better 5-year overall and disease-free survival rates. This is presumably due to removal of microscopic tumor foci and is recommended when feasible. However, non-anatomical PH is oftentimes necessary in an effort to preserve as much FLR as possible in cirrhotic patients[90].
Hemorrhage is the most significant operative risk for LR especially for cirrhotic patients, and excessive bleeding is an independent risk factor for cancer recurrence and poor survival. Several surgical and anesthetic maneuvers have been developed to minimize intra-operative hemorrhage.
Low central venous pressure (CVP) anesthesia is preferred when feasible to minimize hemorrhage from the hepatic veins and inferior vena cava. Low CVP is maintained by iv fluid restriction and administration of diuretics and/or vasodilators. For open PHs, the patient is placed in Trendelenberg position to increase preload and cardiac output for better end-organ perfusion. For laparoscopic procedures, the patient is placed in reverse Trendelenberg position. Intermittent occlusion of the vascular inflow, or the Pringle maneuver with ischemic preconditioning, is selectively utilized for challenging parenchymal transections where potential massive hemorrhage is a concern[91].
Parenchymal division can be performed in a variety of ways, e.g., Clamp-crush technique, cavitron ultrasonic surgical aspirator, and Erbe Hydro-jet clear away the liver cells allowing for visualization of the vascular and biliary tributaries for ligation of these structures, whereas Harmonic scalpel, Sonocision, LigaSureand TissueLinkdissecting sealer are high-energy devices that can simultaneously seal blood vessels and transect liver tissue. No major difference in blood loss, morbidity or mortality has been demonstrated between these techniques, and choice is best left to the circumstances of the resection and surgeon preference and comfort level[92].
Comparison to other “Curative” modalities: Comparison of these two modalities is quite challenging given that PH and OLT have overlapping yet differing patient selection criteria. Meta-analyses have demonstrated that OLT increased late disease-free and overall survival rates when compared to PH. The benefit of OLT is offset by the higher short-term mortality, shortage of donor organ availability, and long transplant wait times associated with more patient deaths[93].
PH as bridge to salvage OLT, especially with the increasing number of patients with non-alcoholic fatty liver disease associated HCC where cirrhosis is not a mandatory step to development of HCC, and the advent of minimally invasive approaches to PH, is feasible and can be used to partially address the donor shortage issue. However, given current UNOS policies regarding retraction of tumor exception points when a solitary HCC is resected, a careful balance must be practiced with the patient’s interest in mind.
Multiple systematic reviews of randomized and non-randomized trials comparing PH to radio-frequency ablation (RFA) for patients with HCC meeting Milan criteria, i.e., small tumors, few in number, demonstrate that while PH afforded better long-term, i.e., 3- and 5-year disease-free and overall survival over RFA, it came at a cost of higher rates of complications and longer hospital stays. Cirrhotic patients with HCC tumor size less than 3 cm and three or less tumor numbers should be carefully evaluated and selected for either modality based on patient and tumor characteristics[94].
Laparoscopic surgical resection: The first laparoscopic PH for malignant disease was reported just over two decades ago. However, the last decade was met with an explosion in the number of reported cases totaling over 3000, and as more experience has accrued, this growth has been especially true in the treatment of HCC. It is well established that post-operative morbidity and longer-term complications such as incisional hernias are lower in laparoscopic PH compared to the open approach. Furthermore, liver-specific complications in cirrhotic patients are lower in the laparoscopic group, thought to be due in part to less severance of collateral vessels in the abdominal wall. Laparoscopic PH possesses advantages over the open approach in minimizing blood loss. With better visualization via 6- to 10-time, high-definition magnification, allowing for improved tissue handling and control of vessels, especially with the robotic approach which affords added dexterity, and a relative tamponade effect on the hepatic veins provided by the pneumoperitoneum, blood loss and transfusion requirements have been shown to be more favorable for the laparoscopic cohort. In an era of cost containment, the financial aspects are playing an increasingly important role. Studies directly comparing laparoscopic vs open PHs demonstrate that the total hospital costs for laparoscopic PHs are equivalent or less than those of open cases. The increased operating room costs are offset by the shorter length of stay following laparoscopic PH. Studies report equivalent to better margin status, recurrence rates, and overall survival figures for patients with HCC. Furthermore, significant decreases in operating room time, blood loss, transfusion and technical difficulty of salvage transplantations following laparoscopic PH for HCC have been reported. Also, there is emerging evidence that laparoscopic procedures may lessen the acute metabolic stress response accompanied by a transient state of post-operative immunosuppression, which may impact oncologic outcomes[95,96].
LIVER TRANSPLANTATION FOR HCC
Liver transplantation for HCC in the early years of transplantation was complicated by high rates of cancer recurrence and poor 5-year survival. In the late 1990s, there was emerging data suggesting that limiting transplant candidacy based on tumor characteristics could result in good outcomes, comparable to non-HCC patients. The hallmark study of Mazzaferro et al[96] in 1996 described 75% 4-year survival in a cohort of patients with HCC limited to a single tumor ≤ 5 cm or up to 3 tumors none greater than 3 cm[97]. These criteria, now known as the Milan Criteria, have become the standard for patient selection. In the United States, patients with HCC within Milan Criteria have been assigned priority with standardized exception points. When first created, this exception originally granted 29 MELD points, but was decreased to 24 points in 2003 and then 22 points in 2005, due to concerns that HCC patients were receiving excessive priority. There remains concern by some in the transplant community that HCC patients continue to have excess priority. With the increasing prevalence of HCC and the priority given to HCC for liver transplantation, the proportion of patients transplanted in the US with an HCC exception now exceeds 25% of total liver transplant volumes.
As patients with HCC await liver transplantation, there is a significant risk of tumor progression beyond transplant criteria, resulting in list drop out and exclusion from transplant. This risk can exceed 30% at one year for those with tumors > 3 cm[98]. Concern for tumor progression has resulted in the frequent use of locoregional tumor treatment as bridging therapy for those awaiting transplant. Many centers pursue locoregional therapy for patients who are likely to be on the waitlist for more than 6 mo prior to being transplanted. Modalities used to treat tumors prior to transplant include RFA, microwave ablation, TACE, transarterial radioembolization, percutaneous ethanol injection and irreversible electroporation. The impact of pre-transplant tumor treatment is not well understood, as studies have shown conflicting results. It appears that bridging therapy reduces the risk of list drop out, improving the likelihood that listed HCC patients will undergo transplant[99,100]. It is unclear if pre-transplant bridging therapy has any impact on post-transplant outcomes. Studies have been retrospective, and confounded by the fact that good response to locoregional therapy is likely a marker of favorable tumor biology. A study by Yao et al[100] showed an improvement in post-transplant survival in those who received bridging therapy; however, multiple additional studies show no impact on post-transplant survival[101-103].
Milan criteria remains the most commonly utilized inclusion criteria for liver transplantation, yet several other guidelines have been proposed and are being used by various centers around the globe. The rationale for more liberal tumor criteria is the concern that Milan Criteria may be too restrictive and exclude patients who could benefit from transplant with an acceptable risk of HCC recurrence. There are numerous criteria that have been proposed, including Up-To-Seven, UCSF, Toronto, Asan, CUN and Kyoto[104-108]. The best described of these include the Up-To-Seven criteria, in which the sum of the number of tumors and the diameter of the largest tumor (in cm) does not exceed 7. The UCSF criteria allow for a single tumor up to 6.5 cm, or up to three tumors none greater than 4.5 cm with a total tumor volume of less than 8 cm, with no extra hepatic disease or macrovascular invasion. The most recent published data report excellent 1- and 5-year survival of 90% and 75% for patients with HCC within UCSF criteria[105].
A similar concept is that of downstaging, in which patients with HCC beyond Milan Criteria undergo locoregional tumor treatment, and those with reduction in tumor burden within Milan Criteria are eligible for transplantation. This concept was first introduced in 1997 by Majno et al[108], noting improved post-transplant survival in those who responded to TACE with a reduction in tumor burden to meet Milan Criteria[109]. Multiple centers and UNOS Regions currently have proposed downstaging criteria, including the UCSF group, which has published their outcomes. UCSF downstaging criteria allows for initial tumor burden to include 1 lesion > 5 cm and ≤ 8 cm, 2 or 3 lesions each ≤ 5 cm with total tumor diameter ≤ 8 cm, 4 or 5 lesions none > 3 cm with total tumor diameter ≤ 8 cm, and no vascular invasion on imaging. Importantly, this algorithm requires 3 mo of imaging stability following downstaging to Milan prior to listing, to allow for observation of tumor biology. Five year patient survival in this cohort is excellent, at 80%[110].
In addition to tumor size and number, multiple additional factors have emerged as potential predictors of HCC recurrence following liver transplantation. The presence of vascular invasion on explant pathology is one of the strongest predictors of recurrence. Histologic grade of tumor differentiation has also been shown in multiple studies to be associated with the risk of tumor recurrence, with well-differentiated tumors having lower risk, and poorly-differentiated tumors being at high risk. The predictive value of pre-transplant alphafetoprotein (AFP) has been highlighted in multiple studies, with a strong association of AFP > 400 and AFP > 1000 with increased risk of post-transplant HCC recurrence[111]. As increased knowledge regarding molecular markers of HCC is gathered, certain microRNA sequences have been identified which can help predict tumor biology, including post-transplant recurrence[112]. It is possible that such biomarkers will help with selection of HCC patients for transplant in the future.
Once transplanted, the use of various immunosuppressive medications may impact the risk of cancer recurrence in HCC patients. While the data regarding the impact of steroids, calcineurin inhibitors and induction agents is highly variable, there is compelling data regarding the effects of the mTOR inhibitor sirolimus. Several studies, including a meta-analysis, have outlined a reduction in HCC recurrence and improved post-transplant survival for HCC patients receiving sirolimus[113,114]. If HCC does recur after transplant, surgical resection of isolated recurrences is often pursued. The use of sorafenib post-transplant has been reported in multiple studies, with mixed results regarding tolerability and efficacy[115,116].
LOCOREGIONAL THERAPY
When determining the most appropriate treatment for HCC, the patient’s underlying liver function and performance status play pivotal roles[117]. For patients who are not considered surgically resectable but otherwise may be treatment candidates, types of therapy include ablation and arterial embolization.
Ablation for HCC
Several ablation techniques have been used to treat HCC. RFA, microwave ablation, percutaneous ethanol injection, cryoablation, and irreversible electroporation are the most common modalities. For the purposes of this review, RFA will be discussed since that is the most common ablative technology used with the strongest evidence.
RFA is a thermal-based ablative technology, using energy to induce local coagulative necrosis. Via an alternating current, surrounding tissue heats from ion movement and friction. Tissue temperature in excess of 60 degrees Celsius induces local coagulative necrosis. RFA can be either done via a percutaneous route, laparascopic route, or via open surgery. It is often performed using real-time US guidance, in order to appropriately position the probe and to monitor the area of ablation (Figure 8). Numerous series have demonstrated consistently highly local tumor control rates, with relatively low rates of local tumor recurrence. Many have considered RFA to be near-equivalent to surgical resection for tumors < 3 cm, with similar 5-year survival rates[118,119]. While local tumor recurrence continues to be an issue with RFA, the recurrence rates are favorable compared to percutaneous ethanol injection[120], and have improved over time as new ablation devices and better imaging guidance have been utilized. Overall, there is a wide range of reported results both in terms of local tumor recurrence rates and overall survival. This is likely due to wide-ranging patient selection and varying levels of operator expertise. While several factors can affect local tumor recurrence rates, tumor size has been shown to be the most significant factor[121].
Figure 8.
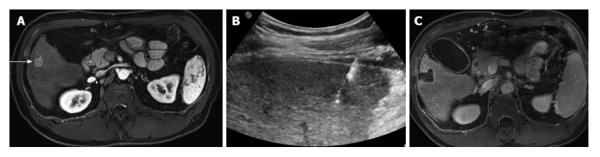
Radiofrequency ablation of a focal hepatocellular carcinoma. A: Contrast-enhanced MR of a 60-year-old male with cirrhosis demonstrates a single hepatocellular carcinoma in the right hepatic lobe (arrow); B: Ultrasound demonstrates a radiofrequency probe coursing through the hypoechoic tumor; C: Contrast-enhanced MR 1 mo after radio-frequency ablation demonstrates a large ablation defect, without any residual enhancement to suggest viable tumor. MR: Magnetic resonance.
While RFA has been used extensively in various types of tumors, several drawbacks remain. Thermal damage to adjacent non-target structures can result in significant complications. Additionally, ablation of tissue adjacent to flowing blood is affected by a “heat sink”, whereby sub-optimal temperatures are reached, resulting in incomplete ablation[122]. Due to these limitations, the appropriate use of RFA is often location dependent. Finally, several liver transplant centers consider RFA to be a relative contraindication for patients undergoing liver transplantation, due to the potential risk of “tract seeding”[123].
Embolization for HCC
Patients with HCC frequently present in later stages when curative treatments are no longer an option[124]. Therefore, the majority of patients with HCC eligible for treatment will undergo non-curative treatments, of which arterial chemoembolization is the most commonly performed procedure. As stated above in the section of surgical treatment, arterial embolization takes advantage of HCC’s reliance on the hepatic artery as its sole blood supply, as opposed to the portal vein. By utilizing hepatic arterial flow, therapeutics can be delivered in a selective manner to hypervascular tumors such as HCC. The mechanism of cell death may be from several causes depending on the embolic used. Tumor necrosis can result from tissue ischemia, a chemotherapeutic effect, or via internal radiation. By performing selective embolization (i.e., lobar or segmental), the degree of hepatic tissue exposed to the embolic agent can be potentially minimized and therefore complications and effectiveness can be optimized. Trans-arterial chemoembolization and Y90 radioembolization are the two most commonly used embolic procedures for HCC, and they will be discussed further.
Chemoembolization
Chemoembolization is defined as the infusion of a mixture of chemotherapeutic agents with or without ethiodized oil followed by embolization with particles such as polyvinyl alcohol, calibrated microspheres, or gelfoam[125]. This is performed by obtaining arterial access via the femoral artery. A microcatheter is then advanced into the hepatic artery, and typically advanced further into the vessel supplying the tumor.
Depending on the techniques employed, tumor death is caused by the cytotoxic effects through achieving high intra-tumoral concentration of chemotherapy, the ischemia induced by embolization, or both. In the case of oil-based chemoembolization, the mechanism of action relates to both the cytotoxic effects of the chemotherapy and ischemic effects induced by embolization. Embolization also prevents washout of the chemotherapeutic agent into the systemic circulation. Embolization with drug-eluting beads has gained popularity over traditional oil-based chemoembolization. Due to the prolonged binding properties of drug-eluting beads with doxorubicin, the drug is slowly released into the tumor reaching higher local concentration and decreased systemic concentration when compared to oil-based chemoembolization. This may allow for decreased side effects and improved tolerance in some patients[126].
Variation in patient selection and procedure technique among institutions has led to significant heterogeneity in response and survival. The publication of two randomized trials in 2002 established chemoembolization as standard of care for patients with unresectable HCC[127,128]. Llovet et al[127] reported survival probabilities at 1 year and 2 years, which were 75% and 50% for embolization, 82% and 63% for chemoembolization, and 63% and 27% for control (chemoembolization vs control, i.e., best supportive care, not tumor treatment, P = 0.009). The ensuing widespread use and proven results of chemoembolization have resulted in its incorporation into standard treatment guidelines for HCC[129,130].
Chemoembolization is generally considered the first line non-curative therapy for patients with early- and intermediate-stage HCC. Chemoembolization can also be used as a “bridge to transplant”. In these patients the procedure is performed to prevent disease progression beyond Milan criteria (single tumor ≤ 5 or three tumors ≤ 3 cm). Patients undergoing chemoembolization should have adequate hepatic function (Child-Pugh Class A or B) and acceptable functional performance status (ECOG 0-2). Chemoembolization can also be done in conjunction with an ablation procedure in intermediate-sized HCC (3-5 cm)[131].
Post-embolization syndrome is the most common side effect of chemoembolization. This is a constellation of symptoms including low-grade fever, abdominal pain, nausea, and ileus, often occurring 48-72 h following the procedure. Serious toxicities from chemoembolization include liver failure, biloma, and abscess formation.
Radioembolization
Y90 radioembolization is a relatively newer embolic procedure compared to chemoembolization. Many consider it to not be a true embolization procedure since the particle size is much smaller than those used in chemoembolization. Therefore, arterial stasis does not typically occur during radioembolization. Y90 is a beta emitter, with the radioactive element either embedded within or surface-coated on the particles. The average tissue penetration of beta radiation in this case is 2.5 mm, with a maximum distance of 11 mm. Y90 particles use internal radioactivity as its mechanism of action. Since HCC is hypervascular, preferential flow of microembolic particles to the tumor potentially increases dose to the tumor compared to the surround hepatic parenchyma. Using these principles, hypervascular tumors can receive lethal doses of radiation while the surrounding tissue is relatively spared (Figure 9).
Figure 9.
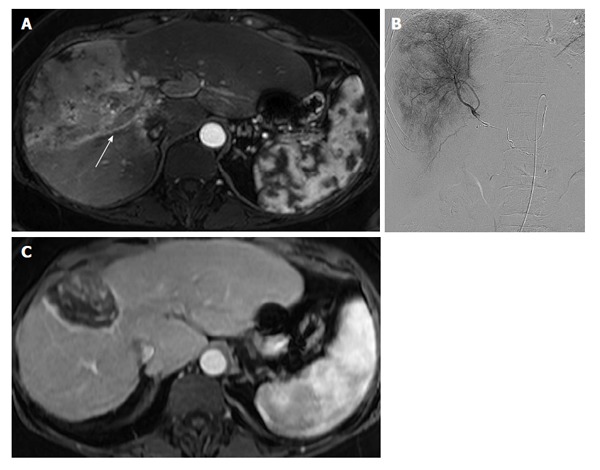
Yttrium-90 radioembolization of diffuse, infiltrative hepatocellular carcinoma with vascular invasion. A: Contrast-enhanced MR of a 55-year-old female with cirrhosis demonstrates an infiltrative hepatocellular carcinoma replacing the anterior right hepatic lobe. Tumor-associated portal venous thrombus is present in the right portal vein (arrow); B: Digital subtraction angiogram with a microcatheter in the right hepatic artery shows diffuse tumor hypervascularity. The patient underwent a right hepatic artery Y90 radioembolization; C: Contrast-enhanced MR 12 mo after radioembolization demonstrates complete necrosis of the entire tumor with marked reduction in size. MR: Magnetic resonance; Y90: Yttrium-90.
Radioembolization is a complex multi-step procedure[132]. The first is a mapping angiogram, where a diagnostic angiogram is performed in order to elucidate the hepatic arterial anatomy. During this initial step, extrahepatic vessels (i.e., gastroduodenal artery, right gastric artery) may be prophylactically coil embolized in order to prevent inadvertent non-target deposition of Y90 microspheres into the gut. At the conclusion of the mapping angiogram, a small dose of Tc99m-MAA is administered into the hepatic artery and a nuclear medicine scan is performed in order to determine the degree of shunting into the lungs, and to assess for the potential of extrahepatic uptake. Patients undergo a second angiogram 2-14 d later, and during this angiogram the Y90 infusion is performed.
Several studies have now reported tumor response rates, toxicity, and survival in patients undergoing Y90 radioembolization[133-135]. Salem et al[131] reported a prospective evaluation of 291 patients, where patients were stratified based on their tumor burden and degree of liver dysfunction. Patients with Child-Pugh A cirrhosis had significantly longer lengths of survival compared to those with advance liver dysfunction. Many series have also demonstrated the effectives of Y90 radioembolization in the setting of portal vein thrombosis[136]. These patients present a particular challenge given that blood supply to the liver depends primarily on the hepatic artery and portal flow is compromised by the obstructive tumor. If hepatic arterial vessels are embolized (as with chemoembolization) in order to treat advancing disease, blood flow to the liver is further compromised, increasing the risk of liver failure. Thanks to its minimally embolic properties, radioembolization can be used in these patients without compromising the hepatic arterial flow, preserving the functional liver reserve.
Fatigue is the most frequent observed toxicity. Abdominal pain and nausea can also occur, but typically at a lower incidence and severity compared to chemoembolization[137]. Serious complications include non-target embolization, which can result in gastrointestinal ulceration or cholecystitis. With increasing operator experience, the incidence of ulcers can be minimized. Finally, radiation induced liver disease occurs in 2%-20% of patients. In rare cases, this can be associated with progressive liver failure.
EXTERNAL BEAM RADIATION THERAPY IN HCC
Although HCC is considered a radioresponsive tumor, the role of external beam radiation therapy (EBRT) has historically been limited in the treatment of HCC due to the high radiosensitivity of normal liver tissue and the risk of radiation-induced liver disease (RILD) with whole liver RT[138]. RILD typically occur within 3-4 mo after EBRT and may progress to permanent liver failure and death with no established effective treatment other than supportive care. In general, the risk of RILD is considered to be primarily related to the volume of normal liver that is exposed to potentially hepatotoxic doses of EBRT. In HCC patients, the severity of cirrhosis has been found to be the predominant risk factor for the development of RILD[139-141]. In addition to RILD, other potential side effects of EBRT include fatigue, nausea, vomiting, and late gastrointestinal bleeding or ulceration if the target is located in close proximity to visceral organs.
Technological advances in the field of radiation oncology such as sophisticated radiation treatment planning and advanced imaging have allowed the ability to deliver high tumoricidal doses of radiation to a partial volume of the liver shaped closely to tumors (conformal EBRT). To date, published randomized data are lacking that have studied the efficacy of EBRT in comparison to alternative treatments or supportive care. However, the collective experience in treating HCC with EBRT is rapidly growing.
Phase II prospective and retrospective studies of conformal EBRT have used a range of moderate radiation doses (generally ≥ 45 Gy, 25-66 Gy) with various conventional or hypofractionated schemes (generally 1.8-2.0 Gy per fraction, 1.5-6 Gy) primarily inpatient with well-compensated liver function (Child-Pugh A cirrhosis 77%-100%) and high-risk tumors that were unsuitable for or refractory to other liver-directed therapies[142-145]. Despite these high-risk features, clinical outcomes have been encouraging with 1-year overall survival of 45%-65%, 1-year local control of 69%-81%, and RILD rates of approximately 15%. Caution in delivering EBRT to patients with more compromised liver function was underscored in a study from China that reported RILD in 60% of Child-Pugh B patients and an overall fatality of 85% in any patient that developed RILD[143].
Stereotactic body radiation therapy (SBRT) is a more recent technology that combines stereotaxy (accurate 3D target localization) and multiple finely collimated radiation beams to more precisely deliver ablative doses of radiation (24-54 Gy) over a small number (1-6) of fractions.SBRT is typically delivered to relatively small tumors 1-5 cm in size that are not in close proximity to visceral organs such as the stomach or bowel. The treatment planning and delivery of liver SBRT are complex. Robust assessment of tumor/organ motion and accurate image guidance are essential componentsin light ofthe high radiation doses that are delivered over steep dose gradients[146].
Phase I and II prospective trials, primarily in Child-Pugh A cirrhotic patients, have demonstrated comparable therapeutic efficacy compared to other liver-directed ablative therapies with 1-year overall survival and local control of 55%-75% and 75%-90%, respectively[147-149]. RILD reported as Child-Pugh deterioration of at least 2 points at 3 mo varies from 13%-29%. One study reported a decrease in RILD of 29% at 3 mo to 6% at 12 mo, suggesting the potential for hepatic recovery after RILD. Multiple modern retrospective studies confirm these prospective data: 1- to 2-year overall survival of 64%-79% and local control of 88%-100%[150-153]. When compared to a matched-pair cohort of patients managed with supportive care only, liver SBRT was found to improve overall survival from 42% to 73% at 2 years. In regards to treating Child-Pugh B patients, recent prospective data also highlight caution in treating Child-Pugh B patients with liver SBRT since Child-Pugh progression was noted in 63% of patients[154].
Charged particle radiation, such as protons and carbon ions, is a form of EBRT that has been employed to treat HCC due to its physical properties that allow the majority of the radiation energy to be deposited over a narrow range of tissue depth (Bragg peak) with little to no exit dose deposited beyond the Bragg peak. This unique dose deposition characteristic may allow higher doses of radiation to be delivered to HCC tumors and lower doses to surrounding normal tissues when compared with photon-based forms of EBRT; a theoretical advantage that is especially appealing in HCC patients where sparing as much remnant liver function as possible is critical.
Protons have been the most commonly studied charged particle in the treatment of HCC with the vast majority of experience from Japan. Multiple phase II prospective and retrospective studies using a hypofractionated approach delivering 63-77 GyE over 10-22 fractions have reported effective tumor control rates and low hepatotoxicity of < 10%[155-158]. Local control for small (< 5 cm) and large (5-10 cm) tumors has been excellent with protons, ranging from 81%-88% at 5 years in survivors[156,157]. Local control for high-risk bulky tumors (> 10 cm with portal venous thrombosis in 50%) has also been encouraging (87% at 2 years)[158].
EBRT has been explored in multiple other settings. Limited data with small numbers of patients investigating SBRT as bridging therapy for liver transplant when other bridging therapies were not suitable have shown that SBRT can be delivered safely without risk of intraoperative complications or long-term clinical compromise and effectively with at least some degree of pathologic response and pathologic complete response in 82%-100% and 20%-27% of tumors, respectively[159-161]. EBRT has been reported to treat HCC with portal venous thrombosis with the goals to restore portal flow for hepatic function maintenance and/or to eliminate arteriovenous shunting to allow successful future delivery of catheter-based therapies. The largest retrospective study to date with 412 patients treated with a combination of TACE and EBRT had a median survival of 11 mo, radiographic response rate of 40% and portal venous thrombus progression-free rate of 86%[162].
Thus, EBRT has the potential to be used in many different settings for the management of HCC: an alternative treatment for tumors that are unsuitable for other liver-directed treatments, salvage therapy for tumors refractory to other therapies, bridging therapy for liver transplant, and combination therapy to complement other treatment modalities. Additional prospective and randomized studies are needed to more clearly define its role in the routine management of HCC. The results of RTOG 1112, an active randomized phase III trial studying the role of SBRT (photons or protons) in addition to sorafenib in high-risk HCC patients, are eagerly awaited.
SORAFENIB IN THE TREATMENT OF ADVANCED HCC
The majority of patients diagnosed with HCC present with disease not amenable to surgical or potentially curative intervention[51]. Systemic therapy with sorafenib confers modest prolongation of overall survival and transient disease stability in appropriately selected patients with locally advanced or metastatic disease. Clinical investigations of new systemic agents suggesting efficacy and acceptable tolerance in patient with underlying cirrhosis have shown promise, and will hopefully fill the high unmet clinical need in the coming years.
Historically, cytotoxic chemotherapy including doxorubicin monotherapy was employed in the therapy of advanced HCC over several decades with negligible clinical benefit over supportive care without definitive survival advantage[163,164]. More recently, two pivotal phase III clinical trials (SHARP and Asia-Pacific) have established sorafenib as the current standard of care for first line systemic therapy of advanced HCC[165,166].
Sorafenib, an oral multikinase inhibitor affects multiple relevant cellular mechanisms based upon preclinical models including inhibition of neovascularization and cellular proliferation, in addition to induction of apoptosis. Key molecular targets thought to contribute to antitumor efficacy include VEGF, platelet derived growth factor recepter (PDGFR)-B, and RAF kinase inhibition[167]. The SHARP trial was a large randomized double-blind phase III trial comparing sorafenib 400 mg twice daily vs placebo and best supportive care[165]. This trial enrolled patients with advanced HCC naïve to systemic therapy, the vast majority of whom had Child-Pugh A or better hepatic function and ECOG performance status of 0-2. A statistically significant improvement in both overall survival (10.7 mo vs 7.9 mo) and time to disease progression (5.5 mo vs 2.8 mo) favored the active therapy arm, despite a low documented response rate of 2%. While the SHARP trial included mainly patients from European or North American sites with HCV and alcohol-related risk factors, significant improvements with sorafenib compared to placebo were also documented in a predominantly Asian, Hepatitis B infected population in the Asia-Pacific trial[166]. This second large phase III trial demonstrated a significant improvement in overall survival (6.5 mo vs 4.2 mo) favoring sorafenib.
Clinical observations based upon exploratory subset analyses suggest a relative advantage to patients with Hepatitis C related disease, while patients with Hepatitis B may achieve less benefit from sorafenib[167]. Notably, high quality data exists for patients with relatively preserved hepatic function, and the benefit of sorafenib in patients with Child-Pugh B cirrhosis remains unclear. The prospective, non-interventional phase IV GIDEON trial has documented a similar time to progression in Child-Pugh A vs Child-Pugh B populations, but higher rates of serious adverse events and dramatically lower overall survival rates in the Child-Pugh B populations (5.2 mo), bringing into question the relative benefit of sorafenib in the Child-Pugh B subgroup[168,169].
At this time, there is no data to suggest benefit of sorafenib in the adjuvant setting based upon preliminary results of the STORM trial. In this large phase 3 study, 1114 patients with HCC who had undergone surgical resection or RFA with curative intent were randomized to adjuvant sorafenib or placebo, with no differences detected in relapse free survival, time to recurrence or overall survival to date[170]. Additionally, the combination of sorafenib with catheter-based therapy remains of unclear clinical benefit and should only be considered in the context of clinical investigations at this time. Randomized phase 2 data from the SPACE trial assessing the potential impact of adding sorafenib to serial drug-eluting bead chemoembolization in intermediate-stage HCC patients, while meeting the predefined primary endpoint of improved time to progression (HR = 0.8, 95%CI: 0.59-1.08), showed no overall survival benefit and the expected toxicities predicted from combination therapy[171]. At this time, further investigation in phase 3 trials is necessary prior to adopting such a strategy, as the clinical benefit of such combinations remains unclear and benefits in delayed progression associated with early introduction of sorafenib may be outweighed by increased toxicity and impaired quality of life.
Thus, Sorafenib provides a transient period of disease stability despite low overall response rates, translating to a modest survival advantage in patients with advanced HCC (including macroscopic vascular invasion or metastatic disease) and Child-Pugh A or better hepatic function based upon these two trials. Side effects such as fatigue, nausea, diarrhea, anorexia, and weight loss have been documented and may prompt dose reductions. The common side effect of hand-foot skin reaction can be minimized by introduction of twice daily urea-based skin cream based upon randomized data, and anti-diarrheals and antiemetics play a key role in symptom management[172]. While this agent represents the current standard of care for systemic therapy in HCC, further clinical trials of new agents with enhanced efficacy and improved toxicity profile remains critical.
EXPERIMENTAL AGENTS FOR THE TREATMENT OF ADVANCED HCC
Enhanced understanding of the molecular pathogenesis of HCC and the parallel development of novel targeted therapeutics has led to a dramatic increase in interventional trials targeting advanced HCC over the past several years. Further investigation into the role of optimizing anti-angiogenic therapy, fine-tuning the spectrum of inhibition with various multi-kinase inhibitors, targeting of the hepatocyte growth factor/c-Met pathway, and early investigation into the role of immune checkpoint blockade agents provide some notable examples of current trends in clinical investigation and will be briefly summarized below.
Multiple signaling pathways contribute to angiogenesis in HCC, with subsequent attempts to target such mediators for therapeutic benefit representing a viable approach. VEGF is over-expressed in HCC and optimal inhibition of the ligand and its receptors remains an active area of investigation. Additionally, targeting of fibroblast growth factor (FGF), PDGF and angiopoietin 1/2 contribute to angiogenesis and represent rational targets for therapeutic intervention[173]. To date, trials attempting to enhance the anti-angiogenic effect of sorafenib, either through direct and potent targeting of the VEGF axis alone (i.e., VEGFR2 inhibitor Ramucirumab), through an altered spectrum of inhibitory targets (e.g., Brivanib inhibition of VEGFR/FGFR), or by modulation of the inhibitory profile of sorafenib (sunitinib, linifanib) have failed to demonstrate an advantage over Sorafenib in randomized phase 3 studies through lack of efficacy, increased toxicity, or both. In short, sorafenib has been surprisingly difficult to improve upon with such strategies, although currently lenvatinib, a multi-kinase inhibitor targeting VEGFR1-3, FGFR1, PDGFR a/b, remains under investigation in first line trials compared to sorafenib given promising phase 2 survival data and a reasonable toxicity profile[174].
Preclinical data suggests that Hepatocyte growth factor and its receptor c-Met play a key role in HCC angiogenesis, metastasis and cellular proliferation[175]. Targeted therapy of c-Met is currently under evaluation in several trials including two randomized phase 3 studies in advanced HCC after initial treatment with sorafenib. Previous clinical trial data suggests high tumoral c-Met expression is associated with poor overall prognosis compared to c-Met low HCC[176]. Additionally, data from a randomized, placebo-controlled phase 2 trial including patients with unresectable treatment-refractory HCC treatment with an oral c-Met inhibitor tivantinib was associated with significantly improved survival compared to placebo among the approximately 60% of patients with Met-high tumors (7.2 mo vs 3.8 mo, HR = 0.38). Trials of cabozantinib, a combination VEGFR2/c-Met inhibitor have shown promising median overall survival (15.1 mo) and time to progression data in a phase 2 randomized discontinuation study. Taken in combination, data supports continued investigation of c-Met inhibitors in advanced HCC populations and the potential for future biomarker driven selection of systemic therapy[177].
Immune checkpoint inhibition with agents targeting Programmed cell death 1 (PD1), it’s ligand programmed death ligand 1 (PD-L1), Cytotoxic T lymphocyte associated antigen 4 (CTLA-4) or other targets have shown dramatic results in multiple malignancies typically deemed refractory to more standard chemotherapy[178]. Immune checkpoint proteins elicit signals (often present in the tumor) limiting anti-tumor immune response, but such inhibitory signals can be negated with a variety of new agents allowing a more robust tumor specific T-cell repertoire. HCC is a rational target for such immune modulation based upon preclinical and observational data[179]. First, multiple case reports of spontaneous regression presumably due to immune response exist[180]. Second, there are observations of improved clinical outcomes after catheter-based therapy for HCC patients with higher titers of tumor-associated antigen specific T-cell subsets[181]. Third, higher expression of tumoral PD-L1 (and resultant inhibition of immune response) in resected HCC has been associated with worse overall survival and more rapid time to progression[182]. These observations and others have led to early investigation of immune checkpoint inhibitors in patients with HCC. Notably, a phase I trial of the CTLA-4 inhibitor tremelimumab in patients with hepatitis C and advanced HCC demonstrated an impressive 18% response rate with 45% of patients experiencing disease stability for over 6 mo with acceptable toxicity profiles. Currently, phase I trials of the PD1 inhibitor nivolumab are accruing in patients with HCC and Hepatitis B, C or no viral infection. The results of such studies are eagerly anticipated. Future combinations of immune checkpoint inhibitors in combination with local therapy or as adjuvant therapy should be strongly considered if initial trials demonstrate promising response rates and adequate safety profiles.
While cytotoxic chemotherapy alone has not demonstrated significant benefit, the combination of doxorubicin and sorafenib may show synergistic effects and is currently under evaluation in an ongoing randomized phase 3 trial. This trial builds on a completed randomized phase 2 data demonstrating significant benefit of the combination over doxorubicin alone, with improved overall survival (13.7 mo vs 6.5 mo) and time to progression (6.4 mo vs 2.8 mo)[183]. While initial data has suggested potential benefit when compared to historical controls of sorafenib monotherapy trials, a randomized comparison against sorafenib is required to establish superiority and tolerance of the combination.
These areas of investigation represent an overview of current trends in the effort to identify active systemic agents for the treatment of advanced HCC. Additional investigations in enzymatic therapy targeting impaired tumoral arginine metabolism, novel antibodies targeting HCC specific antigens, and modification of relevant pathways such as transforming growth factor-β and Wnt/β-catenin represent additional areas in development in this rapidly evolving field of clinical investigation.
Faced with an increasing incidence of HCC, the scope of treatment options has broadened significantly over the last decade to benefit a larger proportion of patients. The number and diversity of diagnostic modalities for HCC have also evolved over the past decade. Beyond the current guidelines outlined in the Barcelona Center for Liver Cancer staging system, a number of therapeutic modalities including radiation (either through radioembolization or external beam radiation), irreversible electroporation, and systemic drugs besides sorafenib are being incorporated into the treatment armamentarium for patients with HCC. High-volume centers such as the Liver Tumor Clinic at the University of Washington provide a multi-disciplinary approach that plays an important role in “designing” an appropriate treatment program based on the patient’s tumor, underlying liver disease and overall health. Many clinical trials are currently ongoing to explore new “druggable” targets and treatment combinations. In parallel, basic investigations into the molecular mechanisms of disease are shedding new lights into the pathogenesis of a highly heterogeneous set of tumors collectively classified as HCC. Findings from the “bench” science are expected to yield novel strategies towards disease classification, detection, treatment and prevention.
Footnotes
P- Reviewer: Chen Z, Gatselis NK S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: The authors have declared no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2014
First decision: December 17, 2014
Article in press: April 29, 2015
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 7.Cleary SP, Jeck WR, Zhao X, Chen K, Selitsky SR, Savich GL, Tan TX, Wu MC, Getz G, Lawrence MS, et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology. 2013;58:1693–1702. doi: 10.1002/hep.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 9.Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z, Gao H, Hao K, Willard MD, Xu J, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–1433. doi: 10.1101/gr.154492.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida N, Kudo M. Recent advancements in comprehensive genetic analyses for human hepatocellular carcinoma. Oncology. 2013;84 Suppl 1:93–97. doi: 10.1159/000345897. [DOI] [PubMed] [Google Scholar]
- 11.Hoshida Y. Molecular signatures and prognosis of hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2011;57:311–322. [PubMed] [Google Scholar]
- 12.Hoshida Y, Moeini A, Alsinet C, Kojima K, Villanueva A. Gene signatures in the management of hepatocellular carcinoma. Semin Oncol. 2012;39:473–485. doi: 10.1053/j.seminoncol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147–156. doi: 10.1055/s-0033-1345721. [DOI] [PubMed] [Google Scholar]
- 14.Qi LN, Li LQ, Chen YY, Chen ZH, Bai T, Xiang BD, Qin X, Xiao KY, Peng MH, Liu ZM, et al. Genome-wide and differential proteomic analysis of hepatitis B virus and aflatoxin B1 related hepatocellular carcinoma in Guangxi, China. PLoS One. 2013;8:e83465. doi: 10.1371/journal.pone.0083465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi LN, Bai T, Chen ZS, Wu FX, Chen YY, De Xiang B, Peng T, Han ZG, Li LQ. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China : role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int. 2015;35:999–1009. doi: 10.1111/liv.12460. [DOI] [PubMed] [Google Scholar]
- 16.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau CC, Sun T, Ching AK, He M, Li JW, Wong AM, Co NN, Chan AW, Li PS, Lung RW, et al. Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell. 2014;25:335–349. doi: 10.1016/j.ccr.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Zain SM, Mohamed R, Cooper DN, Razali R, Rampal S, Mahadeva S, Chan WK, Anwar A, Rosli NS, Mahfudz AS, et al. Genome-wide analysis of copy number variation identifies candidate gene loci associated with the progression of non-alcoholic fatty liver disease. PLoS One. 2014;9:e95604. doi: 10.1371/journal.pone.0095604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, Lim II, Gleason CE, Murphy JM, Rosenberg BR, Teegan L, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riehle KJ, Yeh MM, Yu JJ, Kenerson HL, Harris WP, Park JO, Yeung RS. mTORC1 and FGFR1 signaling in fibrolamellar hepatocellular carcinoma. Mod Pathol. 2015;28:103–110. doi: 10.1038/modpathol.2014.78. [DOI] [PubMed] [Google Scholar]
- 21.Kato N, Ji G, Wang Y, Baba M, Hoshida Y, Otsuka M, Taniguchi H, Moriyama M, Dharel N, Goto T, et al. Large-scale search of single nucleotide polymorphisms for hepatocellular carcinoma susceptibility genes in patients with hepatitis C. Hepatology. 2005;42:846–853. doi: 10.1002/hep.20860. [DOI] [PubMed] [Google Scholar]
- 22.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Deng Q, Wang Q, Li KY, Dai JH, Li N, Zhu ZD, Zhou B, Liu XY, Liu RF, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44:1117–1121. doi: 10.1038/ng.2391. [DOI] [PubMed] [Google Scholar]
- 24.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 26.Anwar SL, Lehmann U. DNA methylation, microRNAs, and their crosstalk as potential biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2014;20:7894–7913. doi: 10.3748/wjg.v20.i24.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeter S, Benator RM, Weinberg SM, Hartenberg MA. Sedation in pediatric CT: national survey of current practice. Radiology. 1990;175:745–752. doi: 10.1148/radiology.175.3.2343126. [DOI] [PubMed] [Google Scholar]
- 28.Khare S, Zhang Q, Ibdah JA. Epigenetics of hepatocellular carcinoma: role of microRNA. World J Gastroenterol. 2013;19:5439–5445. doi: 10.3748/wjg.v19.i33.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogribny IP, Rusyn I. Role of epigenetic aberrations in the development and progression of human hepatocellular carcinoma. Cancer Lett. 2014;342:223–230. doi: 10.1016/j.canlet.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagashio R, Arai E, Ojima H, Kosuge T, Kondo Y, Kanai Y. Carcinogenetic risk estimation based on quantification of DNA methylation levels in liver tissue at the precancerous stage. Int J Cancer. 2011;129:1170–1179. doi: 10.1002/ijc.26061. [DOI] [PubMed] [Google Scholar]
- 31.Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, Theise ND, Roncalli M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915–923. doi: 10.1002/cncr.22460. [DOI] [PubMed] [Google Scholar]
- 32.Lennerz JK, Chapman WC, Brunt EM. Keratin 19 epithelial patterns in cirrhotic stroma parallel hepatocarcinogenesis. Am J Pathol. 2011;179:1015–1029. doi: 10.1016/j.ajpath.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma. An immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med. 1995;119:173–178. [PubMed] [Google Scholar]
- 34.Cui S, Hano H, Sakata A, Harada T, Liu T, Takai S, Ushigome S. Enhanced CD34 expression of sinusoid-like vascular endothelial cells in hepatocellular carcinoma. Pathol Int. 1996;46:751–756. doi: 10.1111/j.1440-1827.1996.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723–1728. doi: 10.5858/132.11.1723. [DOI] [PubMed] [Google Scholar]
- 36.Anatelli F, Chuang ST, Yang XJ, Wang HL. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol. 2008;130:219–223. doi: 10.1309/WMB5PX57Y4P8QCTY. [DOI] [PubMed] [Google Scholar]
- 37.Fan Z, van de Rijn M, Montgomery K, Rouse RV. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol. 2003;16:137–144. doi: 10.1097/01.MP.0000052103.13730.20. [DOI] [PubMed] [Google Scholar]
- 38.Chu PG, Ishizawa S, Wu E, Weiss LM. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol. 2002;26:978–988. doi: 10.1097/00000478-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175–1181. doi: 10.1053/hupa.2002.130104. [DOI] [PubMed] [Google Scholar]
- 40.Morrison C, Marsh W, Frankel WL. A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Mod Pathol. 2002;15:1279–1287. doi: 10.1097/01.MP.0000037312.69565.24. [DOI] [PubMed] [Google Scholar]
- 41.Geramizadeh B, Boub R, Rahsaz M. Histologic differentiation of hepatocellular carcinoma from adenocarcinoma by a simple panel: evaluation of the pitfalls. Indian J Pathol Microbiol. 2007;50:507–510. [PubMed] [Google Scholar]
- 42.Stroescu C, Herlea V, Dragnea A, Popescu I. The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas. J Gastrointestin Liver Dis. 2006;15:9–14. [PubMed] [Google Scholar]
- 43.Ma CK, Zarbo RJ, Frierson HF, Lee MW. Comparative immunohistochemical study of primary and metastatic carcinomas of the liver. Am J Clin Pathol. 1993;99:551–557. doi: 10.1093/ajcp/99.5.551. [DOI] [PubMed] [Google Scholar]
- 44.Borscheri N, Roessner A, Röcken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. Am J Surg Pathol. 2001;25:1297–1303. doi: 10.1097/00000478-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Saad RS, Luckasevic TM, Noga CM, Johnson DR, Silverman JF, Liu YL. Diagnostic value of HepPar1, pCEA, CD10, and CD34 expression in separating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration cytology. Diagn Cytopathol. 2004;30:1–6. doi: 10.1002/dc.10345. [DOI] [PubMed] [Google Scholar]
- 46.Yan BC, Gong C, Song J, Krausz T, Tretiakova M, Hyjek E, Al-Ahmadie H, Alves V, Xiao SY, Anders RA, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010;34:1147–1154. doi: 10.1097/PAS.0b013e3181e5dffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krings G, Ramachandran R, Jain D, Wu TT, Yeh MM, Torbenson M, Kakar S. Immunohistochemical pitfalls and the importance of glypican 3 and arginase in the diagnosis of scirrhous hepatocellular carcinoma. Mod Pathol. 2013;26:782–791. doi: 10.1038/modpathol.2012.243. [DOI] [PubMed] [Google Scholar]
- 48.Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagn Pathol. 2012;7:149. doi: 10.1186/1746-1596-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torbenson M. Review of the clinicopathologic features of fibrolamellar carcinoma. Adv Anat Pathol. 2007;14:217–223. doi: 10.1097/PAP.0b013e3180504913. [DOI] [PubMed] [Google Scholar]
- 50.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 51.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J, et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1–243. doi: 10.3310/hta17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- 54.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]
- 55.Kapoor A, Kapoor A, Mahajan G, Sidhu BS, Lakhanpal VP. Real-time elastography in differentiating metastatic from nonmetastatic liver nodules. Ultrasound Med Biol. 2011;37:207–213. doi: 10.1016/j.ultrasmedbio.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Luo L, Chen J, Wang J, Zhou H, Li M, Jin Z, Chen N, Miao H, Lin M, et al. Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int. 2014;2014:901642. doi: 10.1155/2014/901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SE, Lee HC, Shim JH, Park HJ, Kim KM, Kim PN, Shin YM, Yu ES, Chung YH, Suh DJ. Noninvasive diagnostic criteria for hepatocellular carcinoma in hepatic masses > 2 cm in a hepatitis B virus-endemic area. Liver Int. 2011;31:1468–1476. doi: 10.1111/j.1478-3231.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 58.Reiner CS, Morsbach F, Sah BR, Puippe G, Schaefer N, Pfammatter T, Alkadhi H. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014;25:747–759. doi: 10.1016/j.jvir.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Ippolito D, Fior D, Bonaffini PA, Capraro C, Leni D, Corso R, Sironi S. Quantitative evaluation of CT-perfusion map as indicator of tumor response to transarterial chemoembolization and radiofrequency ablation in HCC patients. Eur J Radiol. 2014;83:1665–1671. doi: 10.1016/j.ejrad.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 60.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 61.Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–336. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Chon SB, Lee JM. Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:149–157. doi: 10.2214/ajr.04.1206. [DOI] [PubMed] [Google Scholar]
- 63.Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–454. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 64.Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–797. doi: 10.1016/s0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 65.Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–3319. doi: 10.1111/j.1572-0241.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 66.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 67.Addley HC, Griffin N, Shaw AS, Mannelli L, Parker RA, Aitken S, Wood H, Davies S, Alexander GJ, Lomas DJ. Accuracy of hepatocellular carcinoma detection on multidetector CT in a transplant liver population with explant liver correlation. Clin Radiol. 2011;66:349–356. doi: 10.1016/j.crad.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 69.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 70.Marín-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13–27. doi: 10.1016/s1040-8428(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 71.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 72.Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:289–299. doi: 10.1111/j.1477-2574.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faber W, Stockmann M, Schirmer C, Möllerarnd A, Denecke T, Bahra M, Klein F, Schott E, Neuhaus P, Seehofer D. Significant impact of patient age on outcome after liver resection for HCC in cirrhosis. Eur J Surg Oncol. 2014;40:208–213. doi: 10.1016/j.ejso.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215; discussion 1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron J, Deltenre P, Lebuffe G, Mathurin P, Pruvot FR. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg. 2012;99:855–863. doi: 10.1002/bjs.8753. [DOI] [PubMed] [Google Scholar]
- 76.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 77.Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159–167. doi: 10.1159/000343829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho CM, Wu CY, Lee PH, Lai HS, Ho MC, Wu YM, Hu RH. Analysis of the risk factors of untransplantable recurrence after primary curative resection for patients with hepatocellular carcinoma. Ann Surg Oncol. 2013;20:2526–2533. doi: 10.1245/s10434-013-2940-7. [DOI] [PubMed] [Google Scholar]
- 79.Zheng J, Guo X, Gao X, Liu H, Tu Y, Zhang Y. Overexpression of retinoic acid-induced protein 3 predicts poor prognosis for hepatocellular carcinoma. Clin Transl Oncol. 2014;16:57–63. doi: 10.1007/s12094-013-1040-2. [DOI] [PubMed] [Google Scholar]
- 80.Barry CT, D’Souza M, McCall M, Safadjou S, Ryan C, Kashyap R, Marroquin C, Orloff M, Almudevar A, Godfrey TE. Micro RNA expression profiles as adjunctive data to assess the risk of hepatocellular carcinoma recurrence after liver transplantation. Am J Transplant. 2012;12:428–437. doi: 10.1111/j.1600-6143.2011.03788.x. [DOI] [PubMed] [Google Scholar]
- 81.Truant S, Boleslawski E, Duhamel A, Bouras AF, Louvet A, Febvay C, Leteurtre E, Huet G, Zerbib P, Dharancy S, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. 2012;38:1189–1196. doi: 10.1016/j.ejso.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 82.Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1874–1878. doi: 10.1007/s00268-010-0546-9. [DOI] [PubMed] [Google Scholar]
- 83.Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Y, Zhang X, Wu L, Ye F, Su X, Shi L, Li B. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. doi: 10.1186/1471-230X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, Yamaoka Y. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65–75, ix. doi: 10.1016/s1055-3207(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 86.Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, Kianmanesh R, Ng IO, Curley SA, Yamaoka Y, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 88.Tang YH, Wen TF, Chen X. Resection margin in hepatectomy for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2012;59:1393–1397. doi: 10.5754/hge10600. [DOI] [PubMed] [Google Scholar]
- 89.Tang YH, Wen TF, Chen X. Anatomic versus non-anatomic liver resection for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2013;60:2019–2025. [PubMed] [Google Scholar]
- 90.Wong TC, Lo CM. Resection strategies for hepatocellular carcinoma. Semin Liver Dis. 2013;33:273–281. doi: 10.1055/s-0033-1351782. [DOI] [PubMed] [Google Scholar]
- 91.Lu WP, Dong JH. Hepatectomy for hepatocellular carcinoma in the era of liver transplantation. World J Gastroenterol. 2014;20:9237–9244. doi: 10.3748/wjg.v20.i28.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11:190. doi: 10.1186/1477-7819-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamiyama T, Tahara M, Nakanishi K, Yokoo H, Kamachi H, Kakisaka T, Tsuruga Y, Matsushita M, Todo S. Long-term outcome of laparoscopic hepatectomy in patients with hepatocellular carcinoma. Hepatogastroenterology. 2014;61:405–409. [PubMed] [Google Scholar]
- 94.Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol. 2014;20:4892–4899. doi: 10.3748/wjg.v20.i17.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu XB, Hu WM. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657–6668. doi: 10.3748/wjg.v18.i45.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 97.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 98.Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]
- 99.Alba E, Valls C, Dominguez J, Martinez L, Escalante E, Lladó L, Serrano T. Transcatheter arterial chemoembolization in patients with hepatocellular carcinoma on the waiting list for orthotopic liver transplantation. AJR Am J Roentgenol. 2008;190:1341–1348. doi: 10.2214/AJR.07.2972. [DOI] [PubMed] [Google Scholar]
- 100.Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R, Kerlan R, Venook A, Ascher NL, Emond JC, Roberts JP. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 101.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 102.DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, Ghanekar A, Guba M, Cattral MS, McGilvray ID, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24–32. doi: 10.1111/j.1477-2574.2010.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 104.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 105.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 106.Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272–278. doi: 10.1002/lt.21368. [DOI] [PubMed] [Google Scholar]
- 107.Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637–1644. doi: 10.1002/lt.21281. [DOI] [PubMed] [Google Scholar]
- 108.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-703. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yao FY, Hirose R, LaBerge JM, Davern TJ, Bass NM, Kerlan RK, Merriman R, Feng S, Freise CE, Ascher NL, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 110.Kashkoush S, El Moghazy W, Kawahara T, Gala-Lopez B, Toso C, Kneteman NM. Three-dimensional tumor volume and serum alpha-fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria. Clin Transplant. 2014;28:728–736. doi: 10.1111/ctr.12373. [DOI] [PubMed] [Google Scholar]
- 111.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14:633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 113.Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, Guo Z, He X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 114.Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, Estfan B, Romero-Marrero C, Aucejo F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555–561. doi: 10.1111/ctr.12150. [DOI] [PubMed] [Google Scholar]
- 115.Castelli G, Burra P, Giacomin A, Vitale A, Senzolo M, Cillo U, Farinati F. Sorafenib use in the transplant setting. Liver Transpl. 2014;20:1021–1028. doi: 10.1002/lt.23911. [DOI] [PubMed] [Google Scholar]
- 116.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 117.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 119.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 120.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 122.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 123.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 124.Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, Rilling WS, Geschwind JF, Salem R, Vedantham S, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S425–S434. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 125.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 127.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 128.Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D’Angelica MI, Davila R, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 130.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 131.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 132.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 133.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 134.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 135.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 136.Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, Leung T, Han KH, Poon RT. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311–318. doi: 10.1159/000348325. [DOI] [PubMed] [Google Scholar]
- 137.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, Cheng SH, Tsai SY, Jian JJ, Lin YM, Cheng TI, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–162. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 140.Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, Yang YL, Chen L, Wang AY, Fu XL, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 141.Xu ZY, Liang SX, Zhu J, Zhu XD, Zhao JD, Lu HJ, Yang YL, Chen L, Wang AY, Fu XL, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:189–195. doi: 10.1016/j.ijrobp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 142.Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 143.Liang SX, Zhu XD, Lu HJ, Pan CY, Li FX, Huang QF, Wang AY, Chen L, Fu XL, Jiang GL. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–2188. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 144.Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, Merle P. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 145.Seong J, Lee IJ, Shim SJ, Lim do H, Kim TH, Kim JH, Jang HS, Kim MS, Chie EK, Kim JH, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int. 2009;29:147–152. doi: 10.1111/j.1478-3231.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 146.Brock KK. Imaging and image-guided radiation therapy in liver cancer. Semin Radiat Oncol. 2011;21:247–255. doi: 10.1016/j.semradonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 149.Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 150.Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, Lartigau E, Mirabel X. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 152.Kimura T, Togami T, Nishiyama Y, Ohkawa M, Takashima H. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non-small-cell lung cancer treated with involved-field radiation therapy: does incidental irradiation contribute to the low incidence of elective nodal failure? Int J Radiat Oncol Biol Phys. 2010;77:337–343. doi: 10.1016/j.ijrobp.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 153.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, Iwabuchi S, Kunieda E. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 154.Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412–417. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 156.Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K, Tokuuye K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 157.Mizumoto M, Okumura T, Hashimoto T, Fukuda K, Oshiro Y, Fukumitsu N, Abei M, Kawaguchi A, Hayashi Y, Ookawa A, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81:1039–1045. doi: 10.1016/j.ijrobp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 158.Sugahara S, Oshiro Y, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tokita M, et al. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:460–466. doi: 10.1016/j.ijrobp.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 159.Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895–900. doi: 10.1016/j.ijrobp.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 160.O’Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- 161.Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, Cattral MS, McGilvray ID, Levy GA, Renner E, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23:299–306. doi: 10.1111/j.1432-2277.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 162.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 163.Harding JJ, Abou-Alfa GK. Treating advanced hepatocellular carcinoma: How to get out of first gear. Cancer. 2014;120:3122–3130. doi: 10.1002/cncr.28850. [DOI] [PubMed] [Google Scholar]
- 164.Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479–483. doi: 10.1002/1097-0142(19880801)62:3<479::aid-cncr2820620306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 165.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 166.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 167.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 168.Bolondi L, Caspary W, Bennouna J, Thompson B, Van Steenbergen WF. Clinical benefit of sorafenib in hepatitis C patients with hepatocellular carcinoma (HCC): subgroup analysis of the SHARP trial (abstract). Data presented at the 2008 ASCO Gastrointestinal Cancers Symposium. United States: Orlando,Florida; 2008. abstract 129 pp. [Google Scholar]
- 169.Marrero JALR, Ye SL, Kudo M, Bronowicki JP, Van Steenbergen WF. Final Analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib[Sor]) in >3000 Sor-treated patients [pts]: Clinical findings in pts with liver dysfunction. 2013 ASCO Annual Meeting. United States: ASC University; 2013. abstract 4126 pp. [Google Scholar]
- 170.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RTP, Han KH, Tak WY, et al. STORM: A Phase III randomized, double-blind, placebo-controlled trial of adjuvant sorafenib after resection or ablation to prevent recurrence of hepatocellular carcinoma. J Clin Oncol. 2014;32 Suppl:abstract 4006. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 171.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma. J Clin Oncol. 2012;30 Supp 4:abstract LBA154. [Google Scholar]
- 172.Ren ZK, Kang H. A randomized controlled study of the prophylactic effect of urea-based cream on the hand foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol. 2012;30 Suppl:abstract 4008. doi: 10.1200/JCO.2013.52.9651. [DOI] [PubMed] [Google Scholar]
- 173.Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther Adv Med Oncol. 2013;5:41–50. doi: 10.1177/1758834012458480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mitsunaga S, Ikeda M, Ueno H, Nakachi K, Morizane C, Kondo S, Shimizu S, Kojima Y, Suzuki T, Tamai T, et al. Phase I/II study of lenvatinib (E7080), a multitargeted tyrosine kinase inhibitor, in patients with advanced hepatocellular carcinoma: Phase I results. J Clin Oncol. 2013:31 Suppl 4; abstract 231. [Google Scholar]
- 175.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–2318. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 177.Xiang Q, Chen W, Ren M, Wang J, Zhang H, Deng DY, Zhang L, Shang C, Chen Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–2970. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 178.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 179.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 181.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 182.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 183.Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M, Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983–1992. doi: 10.1002/hep.27372. [DOI] [PubMed] [Google Scholar]


