Abstract
Hepatitis B virus (HBV) persistently infects approximately 350 million people, and approximately 600000 liver-related deaths are observed per year worldwide. HBV infection is also one of the major risk factors for hepatocellular carcinoma (HCC). The persistence of serum hepatitis B e antigen (HBeAg) and high level of serum HBV DNA are thought to reflect a high HBV replication status in hepatocytes, causing cirrhosis, HCC and liver-related deaths. It has been reported that antiviral therapy, such as peginterferon and nucleos(t)ide analogues (NUCs), could suppress liver-related death by inhibiting the HBV DNA levels and inducing seroconversion from HBeAg to antibody to HBe antigen. Currently, peginterferon is widely used, but there are also several disadvantages in the use of peginterferon, such as various adverse events, the administration route and duration. It is difficult to predict the effects of treatment and interferon is contraindicated for the patients with advanced fibrosis of the liver and cirrhosis. With respect to NUCs, entecavir and tenofovir disoproxil fumarate are current the first-choice drugs. NUCs can be administered orally, and their anti-viral effects are stronger than that of peginterferon. However, because cessation of NUC administration leads to high levels of viral replication and causes severe hepatitis, they must be administered for a long time. On the other hand, the use of both interferon and NUCs cannot eliminate covalently closed circular DNA of HBV. In this review, we evaluate the natural course of chronic HBV infection and then provide an outline of these representative drugs, such as peginterferon, entecavir and tenofovir disoproxil fumarate.
Keywords: Hepatocellular carcinoma, Peginterferon, Nucleotide analogue, Chronic hepatitis B
Core tip: Chronic hepatitis B virus (HBV) infection is one of the major causes of hepatocellular carcinoma, which is a cancer with poor prognosis. We reviewed the natural course of HBV infection and current standard therapies for chronic HBV infection. Peginterferon and nucleos(t)ide analogues, such as entecavir and tenofovir disoproxil fumarate, have several drug-specific advantages and disadvantages. It is difficult to eliminate covalently closed circular DNA of HBV with these current standard therapies. Further improvements of the therapeutic options for HBV infections should be needed.
INTRODUCTION
Approximately 350 million people are persistently infected with hepatitis B virus (HBV) and there are 600000 HBV-related deaths annually worldwide[1]. It has been reported that more than 90% of patients infected with HBV in their infancy or childhood become chronic HBV carriers[1]. Of them, approximately 15%-40% develop chronic hepatitis B. In the patients with chronic hepatitis B, approximately 90% could achieve seroconversion of hepatitis B e antigen (HBeAg) to antibody to HBe antigen (anti-HBe) and become inactive carriers. However, approximately 10% of patients with chronic hepatitis B have chronic active hepatitis and develop cirrhosis at a rate of approximately 2% per year, leading to liver failure and/or hepatocellular carcinoma (HCC)[2-5]. Globally, HBV infection is one of the major risk factors of HCC, and it accounts for up to 50% of all HCC patients. Positive serum HBeAg and a high level of serum HBV DNA are indicative of high HBV replication in the liver[6-8]. Therefore, it is important to suppress HBV replication to prevent hepatic failure and the development of cirrhosis and HCC. To prevent the disease progression, peginterferon and nucleos(t)ide analogues (NUCs) are now available as antivirals against HBV[9-12].
In general, the natural history of chronic HBV infection in birth or early childhood is divided into five phases as follows (Figure 1). In phase 1 (immune tolerance phase/asymptomatic carrier phase), HBV is actively replicating, but the host lacks an immune response. The serum alanine aminotransferase (ALT) level is within the normal limit and liver inflammation is almost absent. In phase 2 (immune clearance phase), in adulthood, the immune response to HBV becomes active, and an elevated serum ALT level and active hepatitis are observed. In phase 3 (inactive phase), as a result of an immune response, HBeAg is lost, anti-HBe emerges, the serum HBV DNA level is suppressed and liver inflammation is low[13]. Some patients cannot achieve seroconversion from HBeAg to anti-HBe and HBeAg persists as positive. For most of those with positive HBeAg, active hepatitis persists, and they often rapidly proceed to cirrhosis (HBeAg-positive hepatitis). In approximately 10%-20% of HBeAg-negative carriers, HBV DNA replication is reactivated and active hepatitis flares again (HBeAg-negative hepatitis) (phase 4)[14]. It should be noted that there are some developments in HCC even at low rates in this phase[15]. For approximately 4% to 20% of HBeAg-negative carriers, anti-HBe is lost and HBeAg reappears again (reverse seroconversion). In the natural course of HBeAg-negative carriers, HBs antigen (HBsAg) converts to negative and antibody to HBs antigen (anti-HBs) develops at a rate of 1% per year (phase 5, remission phase). In this phase, the both blood test and liver histology findings might be improved.
Figure 1.
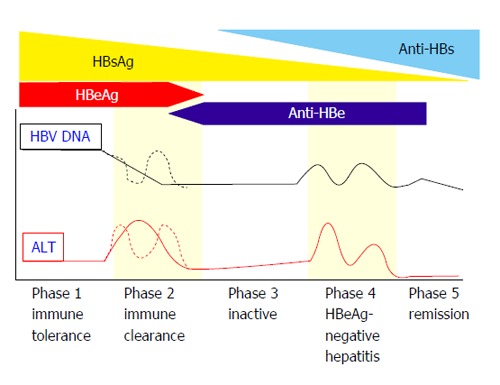
Natural course of hepatitis B virus infection[116]. HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; ALT: Alanine aminotransferase; HBsAg: Hepatitis B s antigen; Anti-HBe: Antibody to HBe antigen; Anti-HBs: Antibody to HBs antigen.
For acute-on-chronic liver failure and HCC, the risk factors are obvious and antiviral therapies could reduce the risk of developing acute-on-chronic liver failure and HCC[16]. In general, the indication and selection of antiviral therapies for persistent HBV infection is decided according to the age, disease phase, fibrosis stage and inflammatory activity of the liver, and risk of disease progression. In the immune tolerance phase, the rate of HBV clearance from the hepatocytes is very low because of the lack of a host immune response. In the low replication phase (inactive carriers), antiviral therapy may not be indicated if the liver histological findings are mild and the serum ALT level is within normal limits. In the remission phase (negative HBsAg), if HBV DNA is not detected, antiviral therapy may not be indicated because hepatitis calms down and the HCC development rates decrease[17] while NUC administration may be stopped. In young patients with HBeAg-positive chronic hepatitis and elevated serum ALT levels, there is a 7%-16% possibility of HBeAg-negative conversion. Then, strict observation without treatment may be chosen if there is no advanced fibrosis or possibility of fulminant hepatitis[16].
HBV-INFECTED PATIENTS WITH CIRRHOSIS
While cirrhotic patients are thought to be therapeutic indication even if HBeAg is negative, the serum ALT level is normal and serum HBV DNA level is suppressed at a low level. If advanced fibrosis is clinically suspected, the assessment of fibrosis should be performed by liver biopsy, abdominal ultrasonography, elastography or abdominal computed tomography[18-22]. If advanced fibrosis is observed, antiviral therapy should be started. HBV carriers who are not asymptomatic or inactive carriers are indicated to receive antiviral therapy, and inactive carriers with advanced fibrosis and a high serum HBV DNA level are also indicated to receive antiviral therapy.
At present, the complete elimination of covalently closed circular DNA (cccDNA) in nucleus of liver cells[23] seems difficult using peginterferon and NUCs. The best surrogate markers for antiviral treatment against HBV are HBsAg as a long-term marker as well as sustained normalization of the serum ALT level, negative serum HBV DNA level and negative HBeAg as short-term markers[16].
PEGINTERFERON THERAPY
Greenberg et al[24] reported the usefulness of interferon therapy for chronic hepatitis B in 1976. Interferon exerts antiviral activity, cell growth inhibition and immunomodulatory effects. It binds to the interferon receptors of hepatocytes, activates tyrosine type protein kinase Janus kinase 1 and induces phosphorylation and dimerization of signal transducer and activator of transcription 1 (STAT1). The STAT1 dimer translocates into the nucleus, induces interferon stimulated genes, and expresses various antiviral proteins that have antiviral effects[25]. The HBeAg-negative conversion rate of the interferon treated group was significantly higher than that of the untreated control group[26]. Interferon is non-antigen specific immunomodulator. Compared with NUCs, one of the advantages of interferon is that its treatment duration is limited and its effect is durable. The other benefit of interferon is that there is no risk of resistance mutants. However, interferon has no direct inhibitory effect on viral replication and its short-term effect, such as suppressing serum HBV DNA level, is inferior to NUCs. The other disadvantage of interferon is the difficulty in predicting the treatment effect and several adverse events, such as flu-like syndrome. Additionally, it is difficult to use interferon on patients with advanced liver fibrosis and cirrhosis.
Compared to standard interferon, peginterferon-alpha has a long half-life and gains its long-acting effect through the addition of polyethylene glycol high molecular proteins to interferon. Its administration is performed only once per week. There have been several reports about peginterferon for HBeAg-positive or HBeAg-negative patients (Tables 1-3)[27-35]. In the comparison trial with standard interferon-alpha and peginterferon-alpha in Asia, the combined responses, defined as HBeAg loss, HBV DNA suppression (< 500000 copies/mL) and ALT normalization, were 28% vs 12%, respectively (P = 0.036), and the superiority of peginterferon-alpha to standard interferon has been demonstrated[27]. A report comparing three groups of 48 wk of peginterferon-alpha-2a alone, 48 wk of peginterferon-alpha2a plus lamivudine, and 48 wk of lamivudine alone in 814 HBe positive patients reported that the HBeAg seroconversion rates 24 wk after the end of administration were 32%, 27% and 19%, respectively, and the peginterferon-alpha-2a alone group had a significantly higher effect than lamivudine alone group[28]. The HBsAg-negative conversion rate was 3%[28]. In a NEPTUNE trial exploring the appropriate dose and duration of interferon treatment, the HBeAg seroconversion rate of 180 μg peginterferon-alpha-2a for 48 wk was significantly higher than that of 24 wk or 90 μg. Therefore, 180 μg peginterferon-alpha-2a administrations for 48 wk were considered the standard treatment[30]. The durable effect after stopping (peg)interferon administration is one specific advantage of this therapy. In another report[31], 81% of HBeAg-positive patients who achieved HBeAg-negative conversion by peginterferon-alpha-2b sustained their effect at years 3 after stopping interferon administration, and 27% of patients who could not achieve HBeAg-negative conversion at week 26 achieved HBeAg-negative conversion at years 3 (Table 2)[31]. In that report[31], 11% of all patients and 30% of patients who achieved HBeAg-negative conversion at month 6 achieved HBsAg-negative conversion even though 31% of all patients in this trial were genotype A and 47% were given additional NUCs. In a multicenter, randomized control trial conducted in Europe on peginterferon-alpha-2a for HBeAg-negative patients comparing three groups treated with 48 wk of peginterferon-alpha-2a alone, 48 wk of peginterferon-alpha-2a plus lamivudine and 48 wk of lamivudine alone, the serum ALT normalization rates 24 wk after end of administration were 59%, 60% and 44% and the serum HBV DNA negative rates were 43%, 44% and 29%, respectively (Table 3)[33]. HBsAg converted to negative in 7 patients in the peginterferon-alpha-2a alone group and 5 patients in the peginterferon-alpha-2a plus lamivudine group. A meta-analysis comparing peginterferon with NUCs has already been published and it was reported that peginterferon-alpha achieved a higher serum HBsAg-negative conversion rate compared to lamivudine monotherapy[36]. In a European multicenter trial conducted over 3 years, 8.7% of all patients and 44% of HBV DNA-negative patients treated with peginterferon-alpha-2a alone had HBsAg-negative conversion[35]. For a longer duration of peginterferon administration in HBeAg-negative patients, 180 μg of peginterferon-alpha-2a administered for 48 wk or 96 wk (49 wk or later, the peginterferon dose was down to 135 μg) were compared and the serum HBV DNA suppression rates (< 2000 IU/mL) were 29% vs 12% and serum HBsAg-negative conversion rates were 0% vs 6%, respectively. Also, the 96 wk administration was superior to 48 wk administration[37]. Patients in this study were infected with HBV genotype D. The HBeAg-negative patients treated by peginterferon-alpha-2a had worse results than HBeAg-positive patients treated by the same regimen.
Table 1.
Treatment efficacy at 24 wk after the end of peginterferon treatment in hepatitis B e antigen-positive chronic hepatitis B
| Ref. | No. of patients | Formula of therapy | Seroconversion from HBeAg to anti-HBe (%) | Suppression of HBV DNA (%) | Normalization of ALT (%) | HBsAg loss (n) |
| Cooksley et al[27] | 51 | IFNα-2a 4.5 MIU three times weekly for 24 wk | 25 | 25a | 26 | 0 |
| 49 | Peg-IFNα-2a 90 μg weekly for 24 wk | 37 | 43a | 43 | 0 | |
| 46 | Peg-IFNα-2a 180 μg weekly for 24 wk | 35 | 39a | 35 | 0 | |
| 48 | Peg-IFNα-2a 270 μg weekly for 24 wk | 27 | 27a | 31 | 0 | |
| Lau et al[28] | 214 | Peg-IFNα-2a 180 μg weekly plus placebo for 48 wk | 32 | 32b | 41 | 8 |
| 271 | Peg-IFNα-2a 180 μg weekly plus LAM 100 mg daily for 48 wk | 27 | 34b | 39 | 8 | |
| 272 | LAM 100 mg/d for 48 wk | 19 | 22b | 28 | 0 | |
| Chan et al[29] | 50 | Peg-IFNα-2b 1.5 μg/kg weekly for 32 wk plus LAM 100 mg daily for 52 wk | 36 | 36a | 50 | 1 |
| 50 | LAM 100 mg daily for 52 wk | 14 | 14a | 30 | 0 | |
| Liaw et al[30] | 140 | Peg-IFNα-2a 90 μg weekly for 24 wk | 14 | 21c | 30 | 1 |
| 136 | Peg-IFN α-2a 180 μg weekly for 24 wk | 22 | 21c | 30 | 0 | |
| 136 | Peg-IFNα-2a 90 μg weekly for 48 wk | 25 | 32c | 43 | 3 | |
| 136 | Peg-IFNα-2a 180 μg weekly for 48 wk | 36 | 42c | 52 | 3 |
a < 500000 copies/mL; b < 100000 copies/mL; c < 20000 copies/mL. LAM: Lamivudine; Peg-IFN: Peginterferon; HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; Anti-HBe: Antibody to HBe antigen; ALT: Alanine aminotransferase.
Table 3.
Treatment efficacy of peginterferon treatment in hepatitis B e antigen-negative chronic hepatitis B
| Ref. | No. of patients | Therapy regimen | HBV DNA suppression (%) | ALT normalization (%) | HBsAg loss (n) |
| cMarcellin et al[33] | 177 | Peg-IFNα-2a 180 μg weekly plus placebo for 48 wk | 43a | 59 | 7 |
| 179 | Peg-IFNα-2a 180 μg weekly plus LAM 100 mg daily for 48 wk | 44a | 60 | 5 | |
| 181 | LAM 100 mg daily for 48 wk | 29a | 44 | 0 | |
| cPapadopoulos et al[34] | 88 | Peg-IFNα-2b 1.5 μg/kg weekly plus LAM 100 mg daily for 48 wk | 59 (60 IU/mL below) | 27 | NA |
| 35 | Peg-IFNα-2b 1.5 μg/kg weekly for 48 wk | 42 | 40 | NA | |
| dMarcellin et al[35] | 116 | Peg-IFNα-2a 180 μg weekly plus placebo for 48 wk | 28b | 31 | 9 (8%) |
| 114 | Peg-IFNα-2a 180 μg daily plus LAM 100 mg daily for 48 wk | 25b | 31 | 9 (8%) | |
| 85 | LAM 100 mg daily for 48 wk | 15b | 18 | 0 (0%) |
a < 20000 copies/mL; b < 10000 copies/mL. The treatment efficacies were assessed at 24-wk follow-upc or approximately 3-year follow-upd. LAM: Lamivudine; Peg-IFN: Peginterferon; ALT: Alanine aminotransferase; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; NA: Not available.
Table 2.
Long-term treatment efficacy of peginterferon treatment in hepatitis B e antigen-positive chronic hepatitis B
| Ref. | No. of patients | Formula of therapy | Seroconversion from HBeAg to anti-HBe (%) | Suppression of HBV DNA (%) | Normalization of ALT (%) | HBsAg loss (n) |
| cBuster et al[31] | 91 | Peg-IFNα-2b for 52 wk | 35 | 25a | 30 | 7 (8%) |
| 81 | Peg-IFNα-2b plus LAM for 52 wk | 25 | 31a | 30 | 12 (15%) | |
| dWong et al[32] | 85 | Peg-IFNα-2b for 32 wk plus LAM for 52 or 104 wk | 60 | 13b | 57 | 2 (2.4%) |
a < 10000 copies/mL; b < 100 copies/mL. The treatment efficacies were assessed at approximately 3-year follow-upc or approximately 5-year follow-upd. LAM: Lamivudine; Peg-IFN: Peginterferon; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; ALT: Alanine aminotransferase.
While the prediction of the treatment effect by pre-treatment factors is difficult for (peg)interferon therapy, some reports have showed that measuring the serum HBsAg level at weeks 12, 24 and 48 after starting interferon administration contributed to predicting the therapeutic response (HBeAg seroconversion, HBV DNA-negative conversion and HBsAg-negative conversion) for both HBeAg-negative and HBeAg-positive patients[38,39].
With respect to the factors affecting the outcome of interferon therapy, the HBV genotype[40-42], age[43] and fibrosis of the liver[44] were reported to affect the therapeutic outcome of standard interferon. On the other hand, peginterferon is highly effective and the age and HBV genotypes are no longer related to the treatment effect of peginterferon except for HBV genotype A[45,46]. For the other HBV genotypes, the therapeutic effects of genotypes C and B for HBeAg-positive and HBeAg-negative patients have been reported to be equivalent[28,35,47-49]. The pretreatment level of HBsAg could not predict the treatment effect, but its reduction rate and level during treatment can predict the therapeutic effect for both HBeAg-positive[39,50] and HBeAg-negative patients[48,51], and it is thought to be useful marker for predicting the therapeutic effect. Additionally, older age is not reported to be related to the therapeutic effect with current peginterferon[30,46], whereas it has been reported that older age has a favorable effect for HBeAg-positive patients[45,52]. Also, advanced fibrosis of the liver was reported to affect treatment response with current peginterferon for chronic hepatitis B[53]. It was reported that the interleukin-28B (IL28B) genotypes affected the HBeAg seroconversion and HBsAg-negative conversion rates[52], although the impact of the IL28B gene on the treatment effect of interferon is controversial.
Interferon has immunostimulatory action, and it is generally necessary to consider the acute exacerbation risk of hepatitis by immunological destruction of HBV infected cells, especially for cirrhotic patients. Therefore, interferon therapy is thought to be contraindicated for HBV-related cirrhosis.
NUCS
NUCs specifically inhibit DNA polymerase that HBV DNA itself produces in the reverse transcription process of HBV replication. NUCs strongly inhibit the synthesis of the plus and minus strand chains in the HBV life cycle. The effect is highly specific and efficient. All NUCs can be administered orally and their use is simple. The short-term adverse events of NUCs are rare and mild, and they are effective for either genotype. Furthermore, unlike interferon, they are easy for cirrhotic patients to use. On the other hand, HBV cannot be completely eliminated because NUCs cannot eliminate mRNA or cccDNA in the host nucleus, which acts as a template for HBV DNA.
Once NUC administration is stopped, HBV DNA starts to reappear or increase, and hepatitis recurs in some patients[54-58]. Additionally, HBeAg that is negatively converted by NUC administration frequently re-appears when NUC administration is stopped (reverse seroconversion)[59,60] after a flare of severe hepatitis[61]. There have been several reports that NUC contributes to HBsAg-negative conversion[62-65]. To improve the long-term prognosis of patients, continuous administration of NUCs for long-term HBV suppression is necessary. Here we focus especially on entecavir and tenofovir disoproxil fumarate (tenofovir), which are now available as first line drugs for chronic hepatitis B in many countries.
Entecavir
Previous reports about entecavir treatment are summarized in Table 4[66-76]. The serum HBV DNA-negative conversion and serum ALT normalization rates of entecavir for 48 to 96 wk were superior to those in response to lamivudine for both HBeAg-positive and HBeAg-negative patients[66,67,70,77]. With entecavir administration for 3 to 5 years, the serum HBV DNA-negative conversion rates were 55% to 88%/1 year, 83% to 93%/2 years, 89% to 95%/3 years, and 91% to 96%/4 years, 94%/5 years; the serum ALT normalization rates were 65% to 84%/1 year, 78% to 88%/2 years, 77% to 90%/3 years, 86%/4 years, and 80%/5 years; and the HBeAg seroconversion rates were 12% to 22%/1 year, 18% to 41%/2 years, 29% to 44%/3 years and 38%/4 years[69,74-76,78]. The resistance mutant emergence rates were reported to be 0.2%/1 year, 0.5%/2 years, and 1.2%/3 to 5 years for the NUCs-treatment-naive patients[69,78].
Table 4.
Treatment efficacy of entecavir in chronic hepatitis B
| Ref. | No. of patients | HBeAg | Therapy regimen | HBeAg loss (%)/seroconversion from HBeAg to anti-HBe (%) | Undetectable of HBV DNA (%) | Normalization of ALT (%) | HBsAg loss (n) |
| 1Chang et al[66] | 354 NUCs - treatment-naive | Positive | ETV 0.5 mg daily for 48 wk | 22/21 | 67 | 68 | 6 |
| 355 NUCs - treatment-naive | Positive | LAM 100 mg daily for 48 wk | 20/18 | 36 | 60 | 4 | |
| 2Gish et al[67] | 243 NUCs - treatment-naive | Positive | ETV 0.5 mg daily for 2 yr | NA/31 | 80 | 87 | 18 |
| 164 NUCs - treatment-naive | Positive | LAM 100 mg daily for 2 yr | NA/26 | 39 | 79 | 10 | |
| 1Lai et al[70] | 296 | Negative | ETV 0.5 mg daily for 48 wk | NA/NA | 90 | 78 | 1 |
| 287 | Negative | LAM 100 mg daily for 48 wk | NA/NA | 72 | 71 | 1 |
Treatment efficacies were assessed at 48 wk1, or 2 years2. ETV: Entecavir; NUCs: Nucleos(t)ide analogues; NA: Not available; LAM: Lamivudine; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; ALT: Alanine aminotransferase; HBsAg: Hepatitis B surface antigen; Anti-HBe: Antibody to HBe antigen.
Today, entecavir is one of the first line NUCs for NUC-treatment-naive patients as well as tenofovir in many countries. However, the serum HBsAg-negative conversion is very rare compared with peginterferon and was reported to be 0 to 5.1%/3 to 5 years and 10%/10 years[69,78].
In general, the patients treated with lamivudine and sustained negative HBV DNA are recommended to switch to entecavir or tenofovir. It has been reported that, if the serum HBV DNA level stays negative and there are no resistance mutants during lamivudine administration, entecavir-resistance mutants rarely emerge even when patients are switched to entecavir[79]. Entecavir resistance easily occurs in lamivudine-resistance mutants (rtL180M plus rtM204V) by adding only one more mutation (rt184G, rtS202I or rtM250V) and these states are thought as a low genetic barrier[80]. On the other hand, tenofovir and adefovir lack cross-resistance to entecavir-resistance (rt184G/S, rtS202G/I, M250V)[81]. Therefore, patients who have viral breakthrough under lamivudine administration could easily have entecavir-resistance mutants if they are switched to entecavir, and adding adefovir to lamivudine is generally recommended. Like lamivudine, entecavir was also reported to have a suppressive effect to HCC development compared with the control, and the reported HCC development rates for entecavir vs control were 3.7%/5 years vs 13.7%/5 years, respectively[10]. The United States Food and Drug Administration (FDA) assigned entecavir to pregnancy category C. Entecavir should not be used for the patients who are co-infected with human immunodeficiency virus (HIV) because of the risk of resistance mutant emergence in HIV.
Tenofovir disoproxil fumarate (tenofovir)
Similar to adefovir, tenofovir is categorized as an acyclic nucleoside phosphonate diester derivative from adenosine monophosphate. Tenofovir has also an antiviral effect against HIV. The usual dosage of tenofovir is 300 mg once a day and higher than that of adefovir 10 mg once a day, owing to the lower nephron-toxicity of tenofovir. The results of tenofovir treatment are shown in Table 5[82-93]. Several reports comparing adefovir 10 mg/d vs tenofovir 300 mg/d for NUC-naïve patients reported that the serum HBV DNA suppression rates (< 400 copies/mL) 48 wk after start of administration were 76% vs 13% for HBeAg-positive patients and 93% vs 63% for HBeAg-negative patients, respectively; also, in general, tenofovir has been superior to adefovir[83]. A study with 144 wk of follow up showed that the serum HBV DNA suppression rates (< 400 copies/mL) were 87% for HBeAg-positive patients and 72% in HBeAg-negative patients at week 144[88]. A 5-year study showed that tenofovir achieved a higher HBsAg-negative conversion rate compared to other NUCs[91].
Table 5.
Treatment efficacy of tenofovir in chronic hepatitis B
| Ref. | No. of patients | HBeAg | Therapy regimen | HBeAg loss (%)/seroconversion from HBeAg to anti-HBe (%) | Undetectable of HBV DNA (%) | Normalization of ALT (%) | HBsAg loss (n) |
| 1Marcellin et al[83] | 176 NUCs - treatment-naive | Positive | TDF 300 mg daily (> 48 wk) | NA/21 | 76 | 68 | 5 |
| 90 | Positive | ADF 10 mg daily (> 48 wk) | NA/18 | 13 | 54 | 0 | |
| 90 | Positive | ADF (48 wk) | NA/18 | 13 | 54 | 0 | |
| 125 | Negative | ADF (48 wk) | NA/NA | 63 | 77 | 0 | |
| 2Heathcote et al[88] | 266 | Positive | TDF (> 144 wk) | 34/26 | 71 | 74 | 20 |
| 365 | Negative | TDF (> 144 wk) | NA/NA | 87 | 81 | 0 | |
| 2Marcellin et al[91] | 266 | Positive | TDF (> 240 wk) | 49/40 | 65 | 73 | 10 |
| 375 | Negative | TDF (> 240 wk) | NA/NA | 83 | 85 | 1 |
The treatment efficacies were assessed at 48 wk1, or 144 wk2. TDF: Tenofovir; ADF: Adefovir; NA: Not available; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; ALT: Alanine aminotransferase; HBsAg: Hepatitis B s antigen; Anti-HBe: Antibody to HBe antigen.
A recent report with 288 wk of follow up suggested that no apparent resistance mutations were observed[94]. For decompensated cirrhosis, the combination of tenofovir with emtricitabine was reported to achieve positive results[89]. With tenofovir treatment, the serum HBV DNA-negative conversion rate for patients without adefovir-resistance was 100%, but the rate was down to 52% for patients with adefovir-resistance[89]. An important feature of tenofovir is that tenofovir alone or with emtricitabine exerts an anti-viral effect to lamivudine, adefovir or entecavir resistance mutants[85,86,93,95,96]. For example, an article reported that for patients who achieved an insufficient effect by lamivudine, adefovir or the combination of these two drugs, tenofovir resulted in a serum HBV DNA-negative conversion rate of 79%, HBeAg-negative conversion rate of 24% and HBsAg-negative conversion rate of 3% of all patients (the median time from administration to HBsAg-negative conversion was 23 mo)[86]. For patients who achieved insufficient effect by lamivudine and adefovir, tenofovir alone or with lamivudine achieved a 64% serum HBV DNA-negative conversion rate 96 wk after changing therapy[93]. They also reported that they did not observe an obvious resistance mutant[93].
It has been reported that the long-term administration of NUCs improves liver fibrosis. Tenofovir treatment resulted in improvement of the histological findings in 87% of all patients and improvement of liver fibrosis in 51% of all patients[91]. They also reported that 10% of HBeAg-positive patients achieved HBsAg-negative conversion, and most of them were genotype A or D[91]. Tenofovir is only classified as pregnancy category B by the United States FDA.
Stoppage of entecavir or tenofovir
Apart from interferon, with the use of NUCs such as entecavir and tenofovir, it is always possible for resistant mutants to emerge[97-101]. Suzuki et al[101] reported that 51-year-old Japanese women with chronic hepatitis B and cirrhosis have virological breakthrough during combination therapy with tenofovir and entecavir against entecavir-resistant virus. Even long-term therapy with tenofovir against the entecavir-resistant virus has the potential to induce virological breakthrough and resistance. We also reported that virological breakthrough during NUC therapies is also dependent on the adherence to medication[99,100]. In treatment with stronger NUCs, such as entecavir, viral breakthrough associated with poor adherence could be a more important issue[102]. Although we do not know whether durable control of HBV is observed after NUCs are discontinued, NUCs could possibly be stopped in selected patients without causing advanced liver fibrosis.
Adefovir or tenofovir-related Fanconi syndrome is a severe adverse event that results from proximal renal tubular toxicity, which leads to impaired re-absorption of amino acids, uric acid, bicarbonate, glucose and phosphate associated with the increased urinary excretion of these solutes[103-106]. Some cases associated with Fanconi syndrome induced by NUCs-treatment were fully recovered following tenofovir withdrawal[106]. Mitochondrial DNA depletion results in mitochondrial dysfunction in the lamivudine/telbivudine-associated neuromyopathy[107]. During treatment with NUCs, attention should be paid to these adverse events.
FUTURE TREATMENT FOR HBV
Tenofovir alafenamide
Compared with tenofovir, tenofovir alafenamide (GS-7340) is a new tenofovir prodrug, which has demonstrated more potent antiviral activity and lower tenofovir exposures. These might lead to lower nephrotoxicity. Further clinical study will be needed[108-110] (Table 6).
Table 6.
Summary of current and future treatments for hepatitis B virus infection
| Current treatments for HBV |
| Peginterferon therapy |
| NUCs |
| Entecavir |
| Tenofovir disoproxil fumarate (tenofovir) |
| Future treatments for HBV |
| NUCs |
| Tenofovir alafenamide |
| Treatments for HBV cccDNA |
| Entry inhibitors targeting of sodium taurocholate cotransporting polypeptide |
NUCs: Nucleos(t)ide analogues; HBV: Hepatitis B virus; cccDNA: Covalently closed circular DNA.
Treatment for HBV cccDNA
In the HBV-infected liver, free HBV DNA and its products are causally related to the activity of liver disease, but the persistence of HBV infection is maintained by the nuclear cccDNA, which serves as a transcription template for HBV mRNA[111,112]. Although there are several opposing views[113], it was reported that HBV cccDNA is noncytolytically degraded by agents that up-regulate apolipoprotein B mRNA editing enzyme and catalytic polypeptide-like (APOBEC) 3A and 3B[23]. In the near future, new therapeutic options to control HBV cccDNA are needed[114-117].
Sodium taurocholate cotransporting polypeptide
Sodium taurocholate cotransporting polypeptide (NTCP) membrane transporter was reported as an HBV entry receptor[118,119]. Iwamoto et al[120], Watashi et al[121] and Tsukuda et al[122] reported that cyclosporine A and its analogs blocked HBV entry through inhibiting the interaction between NTCP and the HBV large surface protein. HBV entry inhibitors might also be useful for controlling HBV infection in the near future.
CONCLUSION
The development of therapies aimed at HBsAg loss, which is the final goal of hepatitis B, is a goal for future research. Further improvements in the therapeutic options for HBV cccDNA are needed.
Footnotes
P- Reviewer: Devanarayana NM, Frider B, Poovorawan Y, Waisberg J, Zhu F S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: Akinobu Tawada has no conflict of interest to declare. Tatsuo Kanda reports receiving lecture fees from Chugai Pharmaceutical, MSD, Tanabe-Mitsubishi, Daiichi-Sankyo, and Bristol-Myers Squibb, and Osamu Yokosuka reports receiving grant support from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, Tanabe-Mitsubishi, and Bristol-Myers Squibb.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 10, 2015
First decision: March 6, 2015
Article in press: April 30, 2015
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381–396. doi: 10.1016/j.cld.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 7.Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19–26. doi: 10.1053/jhep.2003.50036. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49:S72–S84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Lampertico P, Del Ninno E, Viganò M, Romeo R, Donato MF, Sablon E, Morabito A, Colombo M. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology. 2003;37:756–763. doi: 10.1053/jhep.2003.50148. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara K, Yokosuka O, Ehata T, Chuang WL, Imazeki F, Saisho H, Omata M. The two different states of hepatitis B virus DNA in asymptomatic carriers: HBe-antigen-positive versus anti-HBe-positive asymptomatic carriers. Dig Dis Sci. 1998;43:368–376. doi: 10.1023/a:1018870709286. [DOI] [PubMed] [Google Scholar]
- 14.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149.e3; quiz e13-14. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis B Virus Infection. Hepatol Res. 2014;44 Suppl S1:1–58. doi: 10.1111/hepr.12269. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, Williams J, Livingston SE. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 18.Castéra L, Bernard PH, Le Bail B, Foucher J, Trimoulet P, Merrouche W, Couzigou P, de Lédinghen V. Transient elastography and biomarkers for liver fibrosis assessment and follow-up of inactive hepatitis B carriers. Aliment Pharmacol Ther. 2011;33:455–465. doi: 10.1111/j.1365-2036.2010.04547.x. [DOI] [PubMed] [Google Scholar]
- 19.Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151–155. doi: 10.1055/s-0029-1245244. [DOI] [PubMed] [Google Scholar]
- 20.Kim SU, Lee JH, Kim do Y, Ahn SH, Jung KS, Choi EH, Park YN, Han KH, Chon CY, Park JY. Prediction of liver-related events using fibroscan in chronic hepatitis B patients showing advanced liver fibrosis. PLoS One. 2012;7:e36676. doi: 10.1371/journal.pone.0036676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg HB, Pollard RB, Lutwick LI, Gregory PB, Robinson WS, Merigan TC. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976;295:517–522. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- 25.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong DK, Cheung AM, O’Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]
- 27.Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 28.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 29.Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, Chan FK, Hung LC, Lee YT, Tam JS, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005;142:240–250. doi: 10.7326/0003-4819-142-4-200502150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, Tan DM, Chen XY, Gane E, Piratvisuth T, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 31.Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, So TM, Feinman SV, Mach T, Akarca US, Schutten M, Tielemans W, van Vuuren AJ, Hansen BE, Janssen HL. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–467. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Wong VW, Wong GL, Yan KK, Chim AM, Chan HY, Tse CH, Choi PC, Chan AW, Sung JJ, Chan HL. Durability of peginterferon alfa-2b treatment at 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:1945–1953. doi: 10.1002/hep.23568. [DOI] [PubMed] [Google Scholar]
- 33.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos VP, Chrysagis DN, Protopapas AN, Goulis IG, Dimitriadis GT, Mimidis KP. Peginterferon alfa-2b as monotherapy or in combination with lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomised study. Med Sci Monit. 2009;15:CR56–CR61. [PubMed] [Google Scholar]
- 35.Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169–2179.e1-4. doi: 10.1053/j.gastro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Li WC, Wang MR, Kong LB, Ren WG, Zhang YG, Nan YM. Peginterferon alpha-based therapy for chronic hepatitis B focusing on HBsAg clearance or seroconversion: a meta-analysis of controlled clinical trials. BMC Infect Dis. 2011;11:165. doi: 10.1186/1471-2334-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lampertico P, Viganò M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, Boninsegna S, Farci P, Fargion S, Giuberti T, et al. Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62:290–298. doi: 10.1136/gutjnl-2011-301430. [DOI] [PubMed] [Google Scholar]
- 38.Chan HL, Wong VW, Chim AM, Chan HY, Wong GL, Sung JJ. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther. 2010;32:1323–1331. doi: 10.1111/j.1365-2036.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 39.Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010;52:1251–1257. doi: 10.1002/hep.23844. [DOI] [PubMed] [Google Scholar]
- 40.Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425–1430. doi: 10.1053/jhep.2002.37139. [DOI] [PubMed] [Google Scholar]
- 41.Erhardt A, Blondin D, Hauck K, Sagir A, Kohnle T, Heintges T, Häussinger D. Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut. 2005;54:1009–1013. doi: 10.1136/gut.2004.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998–1002. doi: 10.1016/s0168-8278(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki F, Arase Y, Akuta N, Tsubota A, Suzuki Y, Sezaki H, Hosaka T, Someya T, Kobayashi M, Saitoh S, et al. Efficacy of 6-month interferon therapy in chronic hepatitis B virus infection in Japan. J Gastroenterol. 2004;39:969–974. doi: 10.1007/s00535-004-1430-x. [DOI] [PubMed] [Google Scholar]
- 44.Shindo M, Hamada K, Nishioji K, Muramatsu A, Oda Y, Okuno T. The predictive value of liver fibrosis in determining the effectiveness of interferon and lamivudine therapies for chronic hepatitis B. J Gastroenterol. 2004;39:260–267. doi: 10.1007/s00535-003-1293-6. [DOI] [PubMed] [Google Scholar]
- 45.Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 46.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 47.Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, et al. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699–705. doi: 10.1136/gut.2005.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 49.Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R, Verhey E, Van Vuuren AJ, Boucher CA, ter Borg MJ, Janssen HL. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–461. doi: 10.1002/hep.23722. [DOI] [PubMed] [Google Scholar]
- 50.Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25:1498–1506. doi: 10.1111/j.1440-1746.2010.06282.x. [DOI] [PubMed] [Google Scholar]
- 51.Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–1150. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- 52.Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL, et al. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology. 2012;142:513–520.e1. doi: 10.1053/j.gastro.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Buster EH, Hansen BE, Buti M, Delwaide J, Niederau C, Michielsen PP, Flisiak R, Zondervan PE, Schalm SW, Janssen HL. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology. 2007;46:388–394. doi: 10.1002/hep.21723. [DOI] [PubMed] [Google Scholar]
- 54.van Nunen AB, Hansen BE, Suh DJ, Löhr HF, Chemello L, Fontaine H, Heathcote J, Song BC, Janssen HL, de Man RA, et al. Durability of HBeAg seroconversion following antiviral therapy for chronic hepatitis B: relation to type of therapy and pretreatment serum hepatitis B virus DNA and alanine aminotransferase. Gut. 2003;52:420–424. doi: 10.1136/gut.52.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 56.Ito K, Tanaka Y, Orito E, Hirashima N, Ide T, Hino T, Kumashiro R, Kato A, Nukaya H, Sakakibara K, et al. Predicting relapse after cessation of Lamivudine monotherapy for chronic hepatitis B virus infection. Clin Infect Dis. 2004;38:490–495. doi: 10.1086/380965. [DOI] [PubMed] [Google Scholar]
- 57.Nevens F, Main J, Honkoop P, Tyrrell DL, Barber J, Sullivan MT, Fevery J, De Man RA, Thomas HC. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 58.Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32:300–306. doi: 10.1016/s0168-8278(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 59.Lee CM, Ong GY, Lu SN, Wang JH, Liao CA, Tung HD, Chen TM, Changchien CS. Durability of lamivudine-induced HBeAg seroconversion for chronic hepatitis B patients with acute exacerbation. J Hepatol. 2002;37:669–674. doi: 10.1016/s0168-8278(02)00267-2. [DOI] [PubMed] [Google Scholar]
- 60.Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32:803–806. doi: 10.1053/jhep.2000.16665. [DOI] [PubMed] [Google Scholar]
- 61.Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635–639. doi: 10.1053/jhep.2000.16333. [DOI] [PubMed] [Google Scholar]
- 62.Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–683. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: new trick of old dog. J Gastroenterol. 2013;48:13–21. doi: 10.1007/s00535-012-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi M, Hosaka T, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Kawamura Y, Kobayashi M, Saitoh S, Arase Y, et al. Seroclearance rate of hepatitis B surface antigen in 2,112 patients with chronic hepatitis in Japan during long-term follow-up. J Gastroenterol. 2014;49:538–546. doi: 10.1007/s00535-013-0821-2. [DOI] [PubMed] [Google Scholar]
- 65.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Clearance of hepatitis B surface antigen during long-term nucleot(s)ide analog treatment in chronic hepatitis B: results from a nine-year longitudinal study. J Gastroenterol. 2013;48:930–941. doi: 10.1007/s00535-012-0688-7. [DOI] [PubMed] [Google Scholar]
- 66.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 67.Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, Han KH, Chao YC, Lee SD, Harris M, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M, et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72–79. doi: 10.1002/hep.22658. [DOI] [PubMed] [Google Scholar]
- 69.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 70.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 71.Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, Pastore G, Bacon BR, Poynard T, Joshi S, et al. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198–1209. doi: 10.1053/j.gastro.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 72.Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–2049. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Reijnders JG, Deterding K, Petersen J, Zoulim F, Santantonio T, Buti M, van Bömmel F, Hansen BE, Wedemeyer H, Janssen HL. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J Hepatol. 2010;52:493–500. doi: 10.1016/j.jhep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Yokosuka O, Takaguchi K, Fujioka S, Shindo M, Chayama K, Kobashi H, Hayashi N, Sato C, Kiyosawa K, Tanikawa K, et al. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol. 2010;52:791–799. doi: 10.1016/j.jhep.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 75.Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264–1271. doi: 10.1038/ajg.2011.45. [DOI] [PubMed] [Google Scholar]
- 76.Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitou S, Arase Y, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57:508–514. doi: 10.1016/j.jhep.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 77.Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, Walsh A, Fang J, Hsu M, Mazzucco C, et al. Entecavir resistance is rare in nucleoside naïve patients with hepatitis B. Hepatology. 2006;44:1656–1665. doi: 10.1002/hep.21422. [DOI] [PubMed] [Google Scholar]
- 78.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki F, Akuta N, Suzuki Y, Yatsuji H, Sezaki H, Arase Y, Hirakawa M, Kawamura Y, Hosaka T, Kobayashi M, et al. Efficacy of switching to entecavir monotherapy in Japanese lamivudine-pretreated patients. J Gastroenterol Hepatol. 2010;25:892–898. doi: 10.1111/j.1440-1746.2009.06161.x. [DOI] [PubMed] [Google Scholar]
- 80.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, Plym M, Pokornowski K, Yu CF, Angus P, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498–3507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghany M, Liang TJ. Drug targets and molecular mechanisms of drug resistance in chronic hepatitis B. Gastroenterology. 2007;132:1574–1585. doi: 10.1053/j.gastro.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 82.Benhamou Y, Fleury H, Trimoulet P, Pellegrin I, Urbinelli R, Katlama C, Rozenbaum W, Le Teuff G, Trylesinski A, Piketty C. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 83.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 84.Matthews GV, Avihingsanon A, Lewin SR, Amin J, Rerknimitr R, Petcharapirat P, Marks P, Sasadeusz J, Cooper DA, Bowden S, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naïve individuals in Thailand. Hepatology. 2008;48:1062–1069. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 85.Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D, et al. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207–1217. doi: 10.1053/j.gastro.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 86.van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 87.de Vries-Sluijs TE, Reijnders JG, Hansen BE, Zaaijer HL, Prins JM, Pas SD, Schutten M, Hoepelman AI, Richter C, Mulder JW, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010;139:1934–1941. doi: 10.1053/j.gastro.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 88.Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, Horban A, Wang C, Kwan P, Buti M, Prieto M, Berg T, Kitrinos K, Peschell K, Mondou E, Frederick D, Rousseau F, Schiff ER. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 90.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, Sievert W, Wong D, Lovegren M, Cohen D, Llamoso C. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology. 2012;143:619–28.e1. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 91.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 92.Berg T, Zoulim F, Moeller B, Trinh H, Marcellin P, Chan S, Kitrinos KM, Dinh P, Flaherty JF, McHutchison JG, et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014;60:715–722. doi: 10.1016/j.jhep.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 93.Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247–254. doi: 10.1136/gut.2010.223206. [DOI] [PubMed] [Google Scholar]
- 94.Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, Borroto-Esoda K, Miller MD. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442. doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 95.van Bömmel F, Zöllner B, Sarrazin C, Spengler U, Hüppe D, Möller B, Feucht HH, Wiedenmann B, Berg T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318–325. doi: 10.1002/hep.21253. [DOI] [PubMed] [Google Scholar]
- 96.Tan J, Degertekin B, Wong SN, Husain M, Oberhelman K, Lok AS. Tenofovir monotherapy is effective in hepatitis B patients with antiviral treatment failure to adefovir in the absence of adefovir-resistant mutations. J Hepatol. 2008;48:391–398. doi: 10.1016/j.jhep.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 97.Seta T, Yokosuka O, Imazeki F, Tagawa M, Saisho H. Emergence of YMDD motif mutants of hepatitis B virus during lamivudine treatment of immunocompetent type B hepatitis patients. J Med Virol. 2000;60:8–16. doi: 10.1002/(sici)1096-9071(200001)60:1<8::aid-jmv2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 98.Wu S, Fukai K, Imazeki F, Arai M, Kanda T, Yonemitsu Y, Yokosuka O. Initial virological response and viral mutation with adefovir dipivoxil added to ongoing Lamivudine therapy in Lamivudine-resistant chronic hepatitis B. Dig Dis Sci. 2011;56:1207–1214. doi: 10.1007/s10620-010-1423-y. [DOI] [PubMed] [Google Scholar]
- 99.Kamezaki H, Kanda T, Wu S, Nakamoto S, Arai M, Maruyama H, Fujiwara K, Imazeki F, Yokosuka O. Emergence of entecavir-resistant mutations in nucleos(t)ide-naive Japanese patients infected with hepatitis B virus: virological breakthrough is also dependent on adherence to medication. Scand J Gastroenterol. 2011;46:1111–1117. doi: 10.3109/00365521.2011.584898. [DOI] [PubMed] [Google Scholar]
- 100.Kamezaki H, Kanda T, Arai M, Wu S, Nakamoto S, Chiba T, Maruyama H, Fujiwara K, Kanai F, Imazeki F, et al. Adherence to medication is a more important contributor to viral breakthrough in chronic hepatitis B patients treated with entecavir than in those with Lamivudine. Int J Med Sci. 2013;10:567–574. doi: 10.7150/ijms.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki F, Sezaki H, Akuta N, Suzuki Y, Kawamura Y, Hosaka T, Kobayashi M, Saitoh S, Arase Y, Ikeda K, et al. Virologic breakthrough in a patient with chronic hepatitis B by combination treatment with tenofovir disoproxil fumarate and entecavir. Drug Des Devel Ther. 2014;8:869–873. doi: 10.2147/DDDT.S65349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyauchi T, Kanda T, Shinozaki M, Kamezaki H, Wu S, Nakamoto S, Kato K, Arai M, Mikami S, Sugiura N, et al. Efficacy of lamivudine or entecavir against virological rebound after achieving HBV DNA negativity in chronic hepatitis B patients. Int J Med Sci. 2013;10:647–652. doi: 10.7150/ijms.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamori A, Enomoto M, Kobayashi S, Iwai S, Morikawa H, Sakaguchi H, Habu D, Shiomi S, Imanishi Y, Kawada N. Add-on combination therapy with adefovir dipivoxil induces renal impairment in patients with lamivudine-refractory hepatitis B virus. J Viral Hepat. 2010;17:123–129. doi: 10.1111/j.1365-2893.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka M, Suzuki F, Seko Y, Hara T, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, et al. Renal dysfunction and hypophosphatemia during long-term lamivudine plus adefovir dipivoxil therapy in patients with chronic hepatitis B. J Gastroenterol. 2014;49:470–480. doi: 10.1007/s00535-013-0779-0. [DOI] [PubMed] [Google Scholar]
- 105.Gracey DM, Snelling P, McKenzie P, Strasser SI. Tenofovir-associated Fanconi syndrome in patients with chronic hepatitis B monoinfection. Antivir Ther. 2013;18:945–948. doi: 10.3851/IMP2649. [DOI] [PubMed] [Google Scholar]
- 106.Viganò M, Brocchieri A, Spinetti A, Zaltron S, Mangia G, Facchetti F, Fugazza A, Castelli F, Colombo M, Lampertico P. Tenofovir-induced Fanconi syndrome in chronic hepatitis B monoinfected patients that reverted after tenofovir withdrawal. J Clin Virol. 2014;61:600–603. doi: 10.1016/j.jcv.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 107.Xu H, Wang Z, Zheng L, Zhang W, Lv H, Jin S, Yuan Y. Lamivudine/telbivudine-associated neuromyopathy: neurogenic damage, mitochondrial dysfunction and mitochondrial DNA depletion. J Clin Pathol. 2014;67:999–1005. doi: 10.1136/jclinpath-2013-202069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Clercq E. A Cutting-Edge View on the Current State of Antiviral Drug Development. Med Res Rev. 2013:Epub ahead of print. doi: 10.1002/med.21281. [DOI] [PubMed] [Google Scholar]
- 109.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, Zhong L, Ramanathan S, Rhee MS, Fordyce MW, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 110.De Clercq E. Dancing with chemical formulae of antivirals: a personal account. Biochem Pharmacol. 2013;86:711–725. doi: 10.1016/j.bcp.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 111.Yokosuka O, Omata M, Imazeki F, Ito Y, Okuda K. Hepatitis B virus RNA transcripts and DNA in chronic liver disease. N Engl J Med. 1986;315:1187–1192. doi: 10.1056/NEJM198611063151903. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Mao R, Yan R, Cai D, Zhang Y, Zhu H, Kang Y, Liu H, Wang J, Qin Y, et al. Transcription of hepatitis B virus covalently closed circular DNA is regulated by CpG methylation during chronic infection. PLoS One. 2014;9:e110442. doi: 10.1371/journal.pone.0110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chisari FV, Mason WS, Seeger C. Virology. Comment on “Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA”. Science. 2014;344:1237. doi: 10.1126/science.1254082. [DOI] [PubMed] [Google Scholar]
- 114.Huang Q, Huang R, Wei L, Chen Y, Lv S, Liang C, Zhang X, Yin F, Li H, Zhuo L, et al. Antiviral activity of methyl helicterate isolated from Helicteres angustifolia (Sterculiaceae) against hepatitis B virus. Antiviral Res. 2013;100:373–381. doi: 10.1016/j.antiviral.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 115.Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 2013;97:195–197. doi: 10.1016/j.antiviral.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 116.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 117.Seeger C, Sohn JA. Targeting Hepatitis B Virus With CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2014;3 doi: 10.7554/eLife.00049. [DOI] [PubMed] [Google Scholar]
- 119.Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, Gao Z, Jing Z, Song M, Xu G, et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol. 2013;87:7977–7991. doi: 10.1128/JVI.03540-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iwamoto M, Watashi K, Tsukuda S, Aly HH, Fukasawa M, Fujimoto A, Suzuki R, Aizaki H, Ito T, Koiwai O, et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun. 2014;443:808–813. doi: 10.1016/j.bbrc.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 121.Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP) Hepatology. 2014;59:1726–1737. doi: 10.1002/hep.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsukuda S, Watashi K, Iwamoto M, Suzuki R, Aizaki H, Okada M, Sugiyama M, Kojima S, Tanaka Y, Mizokami M, et al. Dysregulation of retinoic acid receptor diminishes hepatocyte permissiveness to hepatitis B virus infection through modulation of sodium taurocholate cotransporting polypeptide (NTCP) expression. J Biol Chem. 2015;290:5673–5684. doi: 10.1074/jbc.M114.602540. [DOI] [PMC free article] [PubMed] [Google Scholar]


