Abstract
The aim of the present study was to compare the effects of a topical anesthetic to a placebo on pain perception during administration of local anesthesia in 2 regions of the oral cavity. A split-mouth, double-blind, randomized clinical trial design was used. Thirty-eight subjects, ages 18–50 years, American Society of Anesthesiologists I and II, received 4 anesthetic injections each in regions corresponding to the posterior superior alveolar nerve (PSA) and greater palatine nerve (GPN), totaling 152 sites analyzed. The side of the mouth where the topical anesthetic (benzocaine 20%) or the placebo was to be applied was chosen by a flip of a coin. The needle used was 27G, and the anesthetic used for administration of local anesthesia was 2% lidocaine with 1:100,000 epinephrine. After receiving the administration of local anesthesia, each patient reported pain perception on a visual analog scale (VAS) of 100-mm length. The results showed that the topical anesthetic and the placebo had similar effects: there was no statistically significant VAS difference between the PSA and the GPN pain ratings. A higher value on the VAS for the anesthesia of the GPN, relative to the PSA, was observed for both groups. Regarding gender, male patients had higher values on the VAS compared with female patients, but these differences were not meaningful. The topical anesthetic and the placebo had similar effects on pain perception for injection of local anesthesia for the PSA and GPN.
Key Words: Topical anesthetic, Pain, Dental, Randomized clinical trial
The discomfort caused by the injection of local anesthetic has been reported as one of the main complaints of dental patients. Topical anesthetics are widely used drugs in dentistry, mainly to control pain associated with the needle penetration in administration of local anesthesia. Topical anesthetics also can be used to relieve discomfort caused by lesions in the mucosa, periodontal treatment, restorative treatments, and biopsy.1
The pharmacologic and the psychological effects on pain control by topical anesthetics have been extensively studied. However, the literature reports are contradictory. While some studies report that topical anesthetics can reduce pain from the needle penetration,2–4 others do not demonstrate any difference in pain using a topical anesthetic compared to a placebo.5–7
Bhalla et al8 related that an application time greater than 2 minutes for topical anesthetics was not necessary because no difference in pain was noted during needle penetration with 2, 5, and 10 minutes of topical anesthetic application. According to Nusstein et al,9 the efficacy of the topical anesthetic depends on the region of the oral cavity to which it is applied. Topical anesthetics proved effective during needle penetration for anesthesia in the anterior region of the maxilla. However, no difference was noted from placebo in the posterior areas of the maxilla or in the inferior alveolar nerve. According to Carr and Horton,10 there was no difference in pain threshold reported by patients when using needles of 25 and 27 gauge for administration of local anesthesia in the oral cavity.
Topical anesthetics must be used carefully because, according to Meechan,11 depending on the type of administration, concentration, and active ingredients used, these drugs can cause adverse effects. In view of the divergence among the research reports regarding the efficacy of topical anesthetics, this study aimed to compare the efficacy of a topical anesthetic with placebo in reducing pain perception during administration of local anesthesia in 2 regions of the oral cavity.
MATERIALS AND METHODS
This experimental study had a randomized clinical trial, double-blind, split-mouth design. Patients attending the integrated clinics of the dentistry course of the Franciscan University Center–UNIFRA–who needed to receive bilateral administration of local anesthesia were invited to participate in the study.
Inclusion and Exclusion Criteria
Inclusion criteria were patients who were older than 18 years, had an American Society of Anesthesiologists (ASA) physical status I or II, and were scheduled to receive bilateral maxillary dental procedures requiring administration of local anesthesia specifically in the areas of the posterior superior alveolar nerves (PSA) and greater palatine nerves (GPN).
Exclusion criteria were patients with reported allergies to the topical or local anesthetics used, previous traumatic experiences related to administration of local anesthesia, smokers, and patients who were pregnant, ASA III or IV, taking analgesics or anti-inflammatory medications chronically, or with signs of inflammation of the oral mucosa.
The Ethics Committee of the Franciscan University Center, Santa Maria, Rio Grande do Sul, Brazil, approved the study protocol. Each participant signed an informed consent form.
Eligible participants took part in both of the 2 test groups: test (topical anesthetic, benzocaine 20%, DFL, Rio de Janeiro, Brazil) and control (placebo, Novaderm, Santa Maria, Rio Grande do Sul, Brazil). The products were contained in packages with distinct and equal wrappings and were identified with labels A and B. One person out of the study wrapped and codified the 2 products. The codes referring to the products were opened only after the statistical analyses of the data. The characteristics relating to color, flavor, and consistency of both tested gels were the same.
Each patient received the product being tested on one side of mouth and the control product on the other side, in the regions related to the nerves being evaluated. Administration of anesthesia followed this sequence: right PSA, right GPN, left PSA, and left GPN. The randomization related to the product to be used on each side of mouth was done, through the toss of a coin, prior to proceeding with the administration of local anesthesia.
Sample Size Calculation
The parameters used to calculate the sample size were based on a clinically relevant difference between the groups of 7.5 mm in the visual analog scale (VAS) scale, with a standard deviation of 16 mm, based in the pilot study corresponding to a clinically significant difference of 25% between the groups. The significance level was 5% and the power of the study was 80. Based on these parameters, it was necessary to have 38 patients in each group.
Experimental Procedures
Prior to submitting to the experimental procedures, the patients responded to questions relating to their current and past conditions of general and oral health and also to behavioral characteristics.
Local anesthesia was administered by only 1 student, in the last semester of the course of dentistry (GCF), who was previously trained in the performance and execution of the procedures. After randomization, the region of needle penetration for administration of local anesthesia was kept dry by isolating that area with gauze and cotton rolls and by using a saliva ejector as needed. The randomized test or control product was positioned in the area with gauze and kept in contact with the mucosa for a period of 2 minutes. The amount of the product used was 2 g for each application, which was measured by removing the products from the packaging with a dosing spoon.
In this way, neither the researcher nor the patient knew which topical product was being used, resulting in a double-blind situation. Next, the local anesthesia was administered. The same anesthetic agent (lidocaine 2% with epinephrine 1:100,000, DFL, Rio de Janeiro, Brazil) and the same needle (27 gauge, double-bevel, Septodont, Barueri, SP, Brazil) were used for every administration of local anesthesia. To anesthetize the PSA and the GPN, the technique advocated by Malamed12 was used.
After injecting 0.9 mL of anesthetic agent into the PSA and then injecting a sufficient quantity of the same to visualize ischemic mucosa, the GPN in the area just anterior to the greater palatine foramen was injected with a volume range between 0.45 and 0.6 mL, and the patients reported their pain. To evaluate pain perception, a VAS was used that had a line of 100 mm, with 0 being no pain marked on the left and 100 being severe pain marked on the right. These represented the extremes of a straight line on which the patient marked a point corresponding to his or her pain.13
Every patient received the 2 anesthetic techniques being evaluated on the same treatment day. Therefore, each patient participated in the 2 test groups and the 2 control groups during the same clinical session.
When the anesthesia and the reporting of pain perception were concluded, the planned clinical procedures were performed; if necessary, additional local anesthesia was administered. The surgical procedures performed included third-molar extraction, periodontal therapy, or restorative procedures.
Statistical Analysis
Normality of the data was tested using the Shapiro-Wilk test. Descriptive analysis was done using means, medians, standard deviation, interquartile intervals, and frequency distribution.
Differences on the VAS between the groups were tested using the Wilcoxon signed ranks test. The divergences between the genders were evaluated using the Mann-Whitney test. The significance level was established at 5%.
RESULTS
Of the 38 patients who participated in the study and completed it, 20 were men. Of these patients, 3 were excluded: 2 were smokers and 1 was pregnant. Regarding the patients included in the study, 8 reported never having received anesthesia for dental treatment. The average age was 27.8 ± 9.97 years, with a range from 18 to 50 years. A total of 152 sites were included: 76 sites of the PSA and 76 sites of the GPN.
The results corresponding to the pain levels reported by the patients on the VAS are shown in the Table.
Table 1.
Mean, Median, Standard Deviation, and Percentiles (25–75) of Pain Reporting, Evaluated Using the Visual Analog Scale, After Anesthesia of the Posterior Superior Alveolar and Greater Palatine Nerves (n = 38)
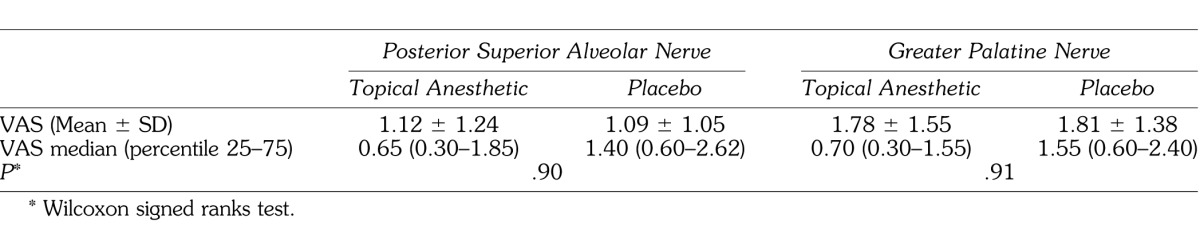
No statistically significant difference was observed between the topical anesthetic and the placebo, neither for the PSA (P = .90) nor for the GPN (P = .91). It was observed that the reported pain perception was higher with the anesthetic procedures on the GPN. The pain perception reported with anesthesia of the PSA with VAS values ≤10 mm occurred in 68.2% and 60.5% for the test and control participants, respectively. The reports of VAS ≤10 mm related to the GPN were 39.5% for both groups, and 84.2% reported VAS ≤30 mm.
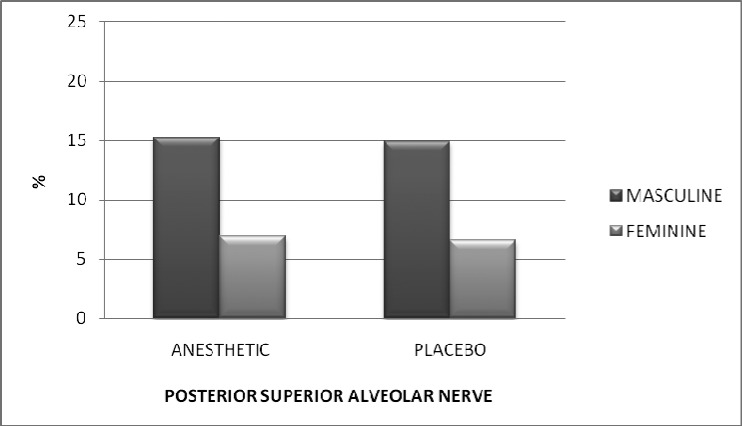
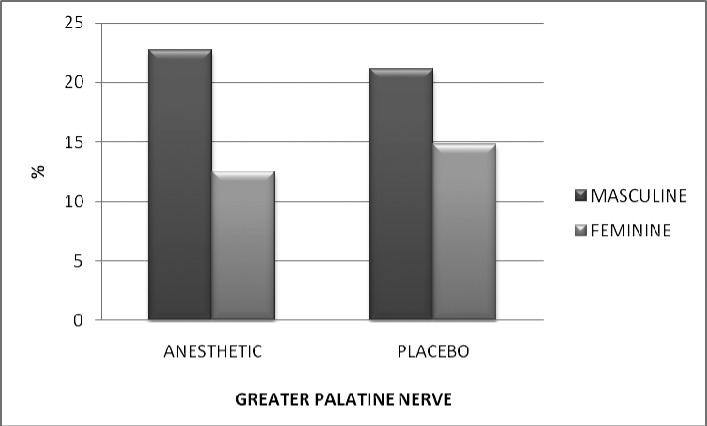
Figures 1 and 2 show the difference in pain, for men and women, for the PSA and the GPN, respectively.
Figure 1.
Pain reported on the VAS (mm), stratified by gender, after anesthesia of the posterior superior alveolar nerve. P = 0.58.
Figure 2.
Pain reported on the VAS (mm), stratified by gender, after anesthesia of the greater palatine nerve. P = 0.09.
In Figure 1, we can see that men reported twice the pain on the VAS for both the topical anesthetic and placebo. Figure 2 shows the level of pain reported for the GPN injection, where we can see a difference in reported pain by men in relation to women of 7.25 mm for the topical anesthetic and 6.38 mm higher for the placebo. There was no statistically significant difference observed between the genders, however.
After the experimental procedures, all of the dentistry procedures were performed. In 9 patients, it was necessary to augment with additional local anesthesia. No adverse effect was observed either in relation to the topical anesthetic and the placebo or in relation to the injected anesthetic.
DISCUSSION
This study demonstrated that the topical anesthetic benzocaine 20% produced no statistically significant difference when compared to placebo in reducing pain perception during administration of local anesthesia of the PSA and the GPN.
The randomized, blinded experimental design of this study provides a high level of evidence for comparing these therapeutic procedures by attempting to eliminate several biases that could confound the observed results.14–16 In the present study, the patients were blinded in relation to the medications applied prior to administration of local anesthesia, as was the researcher who administered the anesthetic. The split-mouth design allows control of the differences associated with interindividual variability. This methodology reinforces the internal validity of the results observed. According to Seymour,13 the method used to measure the pain in a randomized clinical trial can directly affect the outcomes obtained in a study. In the present study, we used the VAS, which is an instrument with good clinical relevance; it is frequently used because it has good validity for determining the perceived intensity of pain, it is easily understood by patients, and it is a reliable method for representing pain.17,18
The application time for both the topical anesthetic and the placebo was 2 minutes. One study, by Bhalla et al,8 evaluated the time of application of topical anesthetic and concluded that the period of 2 minutes is sufficient for proper action of the products on the tissues. The needles used in this study for administration of local anesthesia were 27 gauge. A comparison between the 25G and 27G gauges needles was done by Carr and Horton,10 who reported no difference in pain perception by patients for administration of local anesthesia in the oral cavity.
The data obtained in this study showed that, between the PSA and the GPN, there was no statistically significant difference in pain perception between the topical anesthetic and the placebo groups during administration of local anesthesia. The values reported using the VAS showed that the report of pain perception had more elevated mean values associated with anesthesia of the GPN. The greater density of the palatine fibromucosa, as well as the lesser possibility of dispersion of anesthetic during infiltration, can be associated with these results.12 Keller,6 Martin et al,7 and Bhalla et al8 found similar results in their studies, showing that the topical anesthetic benzocaine 20% is no more effective than placebo in reducing pain during injection of local anesthesia in the oral cavity. However, Bhalla et al8 found that topical anesthetic had better action than placebo in reducing the pain reported by patients to needle penetration, without injecting anesthetic in tissues, when used for an application period of 2 minutes.
In the present study, the measurement of the VAS was done after an injection of anesthetic that was enough to allow the clinical procedures that would be performed. The low mean values measured on the VAS can be related to the technical care related to the anesthetic and allows evaluation of pain perception observed by the patients during these procedures. Studies by Rosivack et al3 and Alqareer et al2 reported a statistically significant difference in injection pain comparing topical anesthetic with placebo in pain perception of patients based on the VAS. They showed that the topical anesthetic was better than placebo in reducing pain from needle penetration, without infiltration of any anesthetic solution into the tissues, in the canine region of the maxilla. The difference in the results obtained in this study can be explained by the use of another region of the oral cavity for analysis. However, we believe that the main factor for the divergence of outcomes is that in those studies, penetration was done without the application of anesthetic in tissues.
The topical anesthetic as well as the placebo may have had a psychological effect on the pain report of patients, which is extremely important to point out and can be associated with the similarity of observed outcomes. Koshi and Short19 reported that the placebo effect can have a powerful effect on the expectation of pain, reducing anxiety and consequently reducing the pain perception of the administration of local anesthesia. Thus, the topical anesthetic as well as the placebo could have produced a psychological effect on the patients, which may have reduced the pain perception reported on the VAS. The psychological effects were not evaluated and may have affected the results of this study to some degree.
We saw a non–statistically significant higher value of pain reported on the VAS by men in relation to women, equally for the PSA and the GPN. The data from Martin et al7 showed that men have higher values on the VAS than women do, which corresponds to our outcomes. The differences related to pain perception seem to be related to sociocultural, psychological, and biological factors.20,21
Ester topical anesthetics can present adverse effects such as allergy, methemoglobinemia, and even plasmatic alterations. The amount of topical anesthetic used must be considered, principally in children.11
Within the limitations of this study, it can be concluded that use of the topical anesthetic benzocaine 20% did not differ from placebo in reducing pain from the administration of local anesthesia in the region of the PSA and the GPN.
REFERENCES
- 1.Meechan JG. Intra-oral topical anaesthetics: a review. J Dent. 2000;28:3–14. doi: 10.1016/s0300-5712(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 2.Alqareer A, Alyahya A, Andersson L. The effect of clove and benzocaine versus placebo as topical anesthetics. J Dent. 2006;34:747–750. doi: 10.1016/j.jdent.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Rosivack RG, Koenigsberg SR, Maxwell KC. An analysis of the effectiveness of two topical anesthetics. Anesth Prog. 1990;37:290–292. [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers ER, Punnia-Moorthy A. A clinical evaluation of three topical anaesthetic agents. Aust Dent J. 1992;37:267–270. [PubMed] [Google Scholar]
- 5.Gill CJ, Orr DL., II A double-blind crossover comparison of topical anesthetics. J Am Dent Assoc. 1979;98:213–214. doi: 10.14219/jada.archive.1979.0476. [DOI] [PubMed] [Google Scholar]
- 6.Keller BJ. Comparison of the effectiveness of two topical anesthetics and a placebo in reducing injection pain. Hawaii Dent J. 1985;16:10–11. [PubMed] [Google Scholar]
- 7.Martin MD, Ramsay DS, Whitney C, Fiset L, Weinstein P. Topical anesthesia: differentiating the pharmacological and psychological contributions to efficacy. Anesth Prog. 1994;41:40–47. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhalla J, Meechan JG, Lawrence HP, Grad HA, Haas DA. Effect of time on clinical efficacy of topical anesthesia. Anesth Prog. 2009;56:36–41. doi: 10.2344/0003-3006-56.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nusstein JM, Beck M. Effectiveness of 20% benzocaine as a topical anesthetic for intraoral injections. Anesth Prog. 2003;50:159–163. [PMC free article] [PubMed] [Google Scholar]
- 10.Carr MP, Horton JE. Evaluation of a transoral delivery system for topical anesthesia. J Am Dent Assoc. 2001;132:1714–1719. doi: 10.14219/jada.archive.2001.0127. [DOI] [PubMed] [Google Scholar]
- 11.Meechan JG. Intraoral topical anesthesia. Periodontol 2000. 2008;46:56–79. doi: 10.1111/j.1600-0757.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 12.Malamed SF. Handbook of Local Anesthesia. 5th ed. St Louis, Mo: Mosby;; 2004. [Google Scholar]
- 13.Seymour RA. The use of pain scales in assessing the efficacy of analgesics in post-operative dental pain. Eur J Clin Pharmacol. 1982;23:441–444. doi: 10.1007/BF00605995. [DOI] [PubMed] [Google Scholar]
- 14.Grimes DA, Schulz KF. An overview of clinical research: the lay of the land. Lancet. 2002;359:57–61. doi: 10.1016/S0140-6736(02)07283-5. [DOI] [PubMed] [Google Scholar]
- 15.Stanley K. Design of randomized controlled trials. Circulation. 2007;115:1164–1169. doi: 10.1161/CIRCULATIONAHA.105.594945. [DOI] [PubMed] [Google Scholar]
- 16.Montenegro R, Needleman I, Moles D, Tonetti M. Quality of RCTs in periodontology—a systematic review. J Dent Res. 2002;81:866–870. doi: 10.1177/154405910208101214. [DOI] [PubMed] [Google Scholar]
- 17.Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients' anesthetic concerns. Anesth Analg. 2000;90:706–712. doi: 10.1097/00000539-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Tiplady B, Jackson SH, Maskrey VM, Swift CG. Validity and sensitivity of visual analogue scales in young and older healthy subjects. Age Ageing. 1998;27:63–66. doi: 10.1093/ageing/27.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Pract. 2007;7:4–20. doi: 10.1111/j.1533-2500.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 20.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]