Abstract
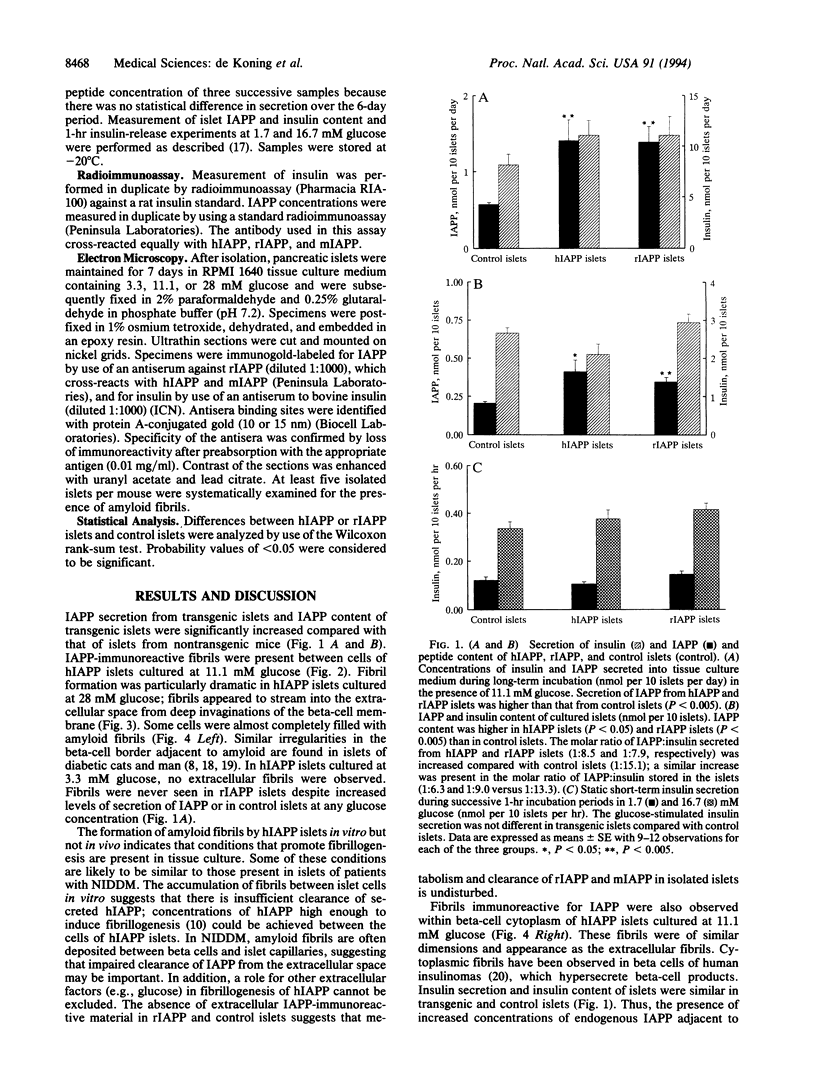
Islet amyloid polypeptide (IAPP) is the constituent peptide of amyloid deposits found in the islets of non-insulin-dependent diabetic patients. Formation of islet amyloid is associated with a progressive destruction of insulin-producing beta cells. Factors responsible for the conversion of IAPP into insoluble amyloid fibrils are unknown. Both the amino acid sequence of human IAPP (hIAPP) and hypersecretion of hIAPP have been implicated as factors for amyloid fibril formation in man. We have generated transgenic mice using rat insulin promoter-hIAPP or rat IAPP (rIAPP) gene constructs. No fibrillar islet amyloid was detectable in vivo in these normoglycemic mice, although small amorphous perivascular accumulations of IAPP were observed in hIAPP mice only. To determine the effects of glucose on IAPP secretion and fibrillogenesis, pancreatic islets from transgenic and control mice were examined in vitro. Islet IAPP secretion and content were increased in transgenic islets compared with control islets. IAPP-immunoreactive fibrils were formed at both intra- and extracellular sites in isolated hIAPP islets cultured with glucose at 11.1 and 28 mM for only 7 days. At 28 mM glucose, fibrils were present in deep invaginations of beta cells as observed in non-insulin-dependent diabetic patients. No fibrils were present at low glucose concentrations in hIAPP islets or at any glucose concentration in rIAPP or control islets. Thus, glucose-induced expression and secretion of hIAPP in transgenic mouse islets can lead to formation of amyloid fibrils similar to that found in non-insulin-dependent diabetes mellitus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Svensson V., Rorsman F., Engström U., Westermark G. T., Wilander E., Johnson K., Westermark P. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989 Aug;183(2):484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Frangione B. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest. 1988 Feb;58(2):122–132. [PubMed] [Google Scholar]
- Clark A., Cooper G. J., Lewis C. E., Morris J. F., Willis A. C., Reid K. B., Turner R. C. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987 Aug 1;2(8553):231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Clark A., Saad M. F., Nezzer T., Uren C., Knowler W. C., Bennett P. H., Turner R. C. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990 May;33(5):285–289. doi: 10.1007/BF00403322. [DOI] [PubMed] [Google Scholar]
- Clark A., Wells C. A., Buley I. D., Cruickshank J. K., Vanhegan R. I., Matthews D. R., Cooper G. J., Holman R. R., Turner R. C. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988 Dec;9(4):151–159. [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppener J. W., Verbeek J. S., de Koning E. J., Oosterwijk C., van Hulst K. L., Visser-Vernooy H. J., Hofhuis F. M., van Gaalen S., Berends M. J., Hackeng W. H. Chronic overproduction of islet amyloid polypeptide/amylin in transgenic mice: lysosomal localization of human islet amyloid polypeptide and lack of marked hyperglycaemia or hyperinsulinaemia. Diabetologia. 1993 Dec;36(12):1258–1265. doi: 10.1007/BF00400803. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Westermark P. Newly identified pancreatic protein islet amyloid polypeptide. What is its relationship to diabetes? Diabetes. 1991 Mar;40(3):310–314. doi: 10.2337/diab.40.3.310. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., D'Alessio D. A., Schwartz M. W., Fujimoto W. Y., Ensinck J. W., Taborsky G. J., Jr, Porte D., Jr Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990 May;39(5):634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Röcken C., Linke R. P., Saeger W. Immunohistology of islet amyloid polypeptide in diabetes mellitus: semi-quantitative studies in a post-mortem series. Virchows Arch A Pathol Anat Histopathol. 1992;421(4):339–344. doi: 10.1007/BF01660981. [DOI] [PubMed] [Google Scholar]
- Schneider H. M., Störkel S., Will W. Das Amyloid der Langerhansschen Inseln und seine Beziehung zum Diabetes mellitus. Dtsch Med Wochenschr. 1980 Aug 15;105(33):1143–1147. doi: 10.1055/s-2008-1070828. [DOI] [PubMed] [Google Scholar]
- Silvestre R. A., Peiró E., Dégano P., Miralles P., Marco J. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Regul Pept. 1990 Oct 29;31(1):23–31. doi: 10.1016/0167-0115(90)90192-y. [DOI] [PubMed] [Google Scholar]
- Svensson C., Hellerström C. Long-term effects of a high glucose concentration in vitro on the oxidative metabolism and insulin production of isolated rat pancreatic islets. Metabolism. 1991 May;40(5):513–518. doi: 10.1016/0026-0495(91)90233-m. [DOI] [PubMed] [Google Scholar]
- Westermark P., Engström U., Johnson K. H., Westermark G. T., Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973 Mar 20;359(1):1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano B. L., Hayden D. W., Johnson K. H. Feline insular amyloid. Ultrastructural evidence for intracellular formation by nonendocrine cells. Lab Invest. 1981 Aug;45(2):149–156. [PubMed] [Google Scholar]
- Young I. D., Ailles L., Narindrasorasak S., Tan R., Kisilevsky R. Localization of the basement membrane heparan sulfate proteoglycan in islet amyloid deposits in type II diabetes mellitus. Arch Pathol Lab Med. 1992 Sep;116(9):951–954. [PubMed] [Google Scholar]
- Zucker-Franklin D., Warfel A., Grusky G., Frangione B., Teitel D. Novel monocyte-like properties of microglial/astroglial cells. Constitutive secretion of lysozyme and cystatin-C. Lab Invest. 1987 Aug;57(2):176–185. [PubMed] [Google Scholar]
- de Koning E. J., Bodkin N. L., Hansen B. C., Clark A. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia. 1993 May;36(5):378–384. doi: 10.1007/BF00402271. [DOI] [PubMed] [Google Scholar]
- de Koning E. J., Höppener J. W., Verbeek J. S., Oosterwijk C., van Hulst K. L., Baker C. A., Lips C. J., Morris J. F., Clark A. Human islet amyloid polypeptide accumulates at similar sites in islets of transgenic mice and humans. Diabetes. 1994 May;43(5):640–644. doi: 10.2337/diab.43.5.640. [DOI] [PubMed] [Google Scholar]