Abstract
The purpose of this study was to investigate the effectiveness of preemptive analgesia with nonsteroidal anti-inflammatory drugs (NSAIDs) in third-molar surgery. A PubMed literature search was conducted for articles restricted to the English language using the following terms (DeCS/MeSH) or combinations: analgesia, third molar, and preemptive. From a total of 704 articles, 6 (n = 420 subjects) were selected. All studies presented a low risk of bias (Cochrane criteria) but exhibited high heterogeneity of methods. Two studies were excluded from the meta-analysis because they did not have adequate numeric values (dichotomous data) for the calculations. Preemptive analgesia showed no significant benefit (n = 298, P = .2227, odds ratio: 2.30, 0.60–8.73) in reducing postoperative pain after removal of lower impacted third molars. However, there was a probable direct relationship between the effectiveness of NSAIDs in preemptive analgesia for removal of third molars and its selectivity for the cyclooxygenase-2 (COX-2). Preemptive analgesia did not have a significant effect in reducing postoperative pain after removal of lower impacted third molars. More homogeneous and well-delineated clinical studies are necessary to determine a possible association between NSAIDs' selectivity for COX-2 and treatment effectiveness.
Key Words: Analgesia, Third molar, Preemptive, Meta-analysis
At the beginning of the last century, Crile1 was one of the first authors to introduce the concept of preemptive analgesia after observation in his studies that if the transmission of pain was blocked before the surgical incision, there was a reduction in postoperative morbidity. Preemptive analgesia is considered a therapy whose goal is to prevent peripheral and central sensitization, thus attenuating (or ideally preventing) the postoperative amplification of the pain sensation.2 The analgesia must provide the patient with analgesia during the surgical procedures and in the beginning of the postoperative period.3 In addition, analgesic efficacy provided by preoperative administration of a local anesthetic is a fundamental requirement for satisfactory clinical outcome of a preemptive treatment.3
To define preemptive analgesia in this context, it is necessary to observe 3 parameters: establishment of the level of postoperative analgesic effectiveness, knowledge about whether the anti-inflammatory mediators can be inhibited in the postoperative period, and assurance that the tissue injury associated with the postoperative inflammation is available to the analgesic.2,3 For Kelly et al,2 when planning to obtain preemptive analgesia, the following must be considered: type of surgery, patient characteristics, pharmacologic options, and clinical evaluation.
Several pharmacologic methods used to obtain preemptive analgesia have been described, such as regional blocks with local anesthetics and/or opioids, administration of intravenous opioids or nonsteroidal anti-inflammatory drugs (NSAIDs), and N-methyl-D-aspartate receptor antagonists.3–6 Woolf and Chong6 described some adverse effects related to the use of these medications such as gastrointestinal bleeding, renal function disturbances, reduction and platelet function, respiratory depression, and profound hypotension. The aim of this study was to conduct a systematized review of the literature about the use of nonsteroidal anti-inflammatory agents as preemptive analgesics administered orally in third-molar surgeries and to evaluate the outcome of their clinical effectiveness by means of meta-analysis.
METHODS
A literature search in the electronic database PubMed was conducted for articles restricted to the English language, using the following terms (DeCS/MeSH) or combinations: analgesia, third molar, and preemptive. There was no restriction regarding to the period of publication. Two reviewers independently evaluated the titles and abstracts of the selected articles in a first round of review. In the second round, all articles that did not fulfill the eligibility criteria were excluded.
Inclusion Criteria
Randomized clinical trials were selected in which NSAIDs were administered orally before the mandibular third-molar surgery procedure performed under local anesthesia, without acute symptomatology, with the aim to obtain preemptive analgesia.
Exclusion Criteria
Excluded from this review were articles that involved the administration of drugs that were not NSAIDs, NSAIDs administered only after surgery, literature reviews, studies that used administration pathways other than the oral route, studies that dealt with the administration of drugs in patients with established acute pain, patients who received general anesthesia for surgery, studies that dealt with methods of analgesia other than medication, and studies that did not disclose the type of NSAID used.
Data Extraction
The following data were collected: gender, age, time of surgical procedure, anesthetic used, protocol instituted for the administration of the NSAIDs, adverse effects of medications, statistical significance of the results, auxiliary analgesic medication(s), and the clinical trial methodology.
Meta-analysis
Successful response to the preoperative analgesic therapy promoted by the use of NSAIDs was considered positive, and positive response to the placebo was considered failure. Data were analyzed by means of the DerSimonian-Lair random meta-analysis test, using the statistical BioEstat 5.0 software program.
Because of the absence of data in the study of Kaczmarzyk et al7 about the patient's option of choice regarding preemptive analgesia, the number of individuals who did not make use of rescue with supplementary medication was considered a positive response to preemptive analgesia. In the study of Sisk and Grover8 and Sisk et al,9 all of the different choices of the test drug were considered failures, whereas in the studies of Aznar-Arasa et al10 and Liporaci Junior,11 the calculations were excluded because they did not have suitable data for making the nominal categorical calculations.
For the methodological evaluation of the studies, the following scores proposed by Koes et al12 and modified by Koyyalagunta et al13 were used: (a) homogeneity, (b) comparability of relevant baseline characteristics, (c) appropriate randomization procedure, (d) dropouts described separately for each study group, (e) lost to follow-up, (f) number of patients in the smallest group, (g) interventions included and described in the protocol, (h) pragmatic study, (i) avoided or similar co-interventions, (j) placebo controlled, (k) blinded patients, (l) relevant outcome measures, (m) blinded outcome assessments, (n) adequate follow-up period, (o) intention-to-treat analysis, and (p) frequencies of most important outcomes presented for each treatment group.
RESULTS
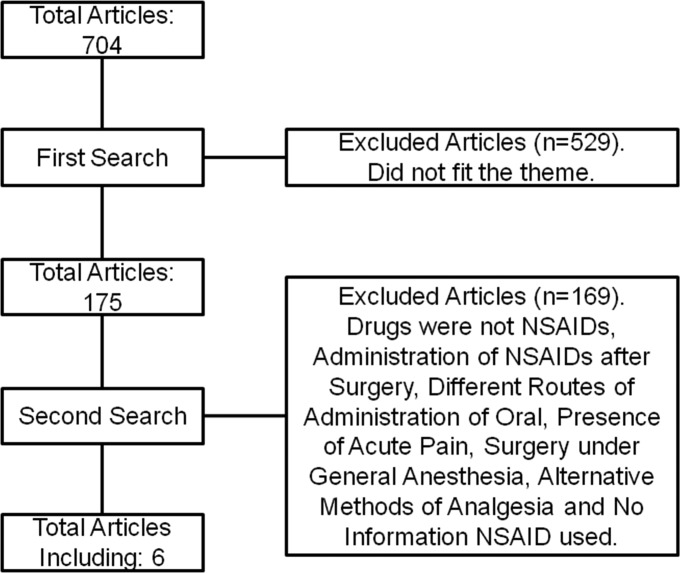
After combining the descriptors adopted in the present study, a total of 704 articles were obtained (Figure 1).
Figure 1.
Flow diagram of the methodology adopted in the present study.
In the first round, the titles and abstracts of the articles were read, and of this total, 529 studies that were not applicable to the theme were excluded. In the second round, 169 articles were excluded because they did not fit the criteria adopted for the present study, as follows: 99 articles that involved the administration of drugs that were not NSAIDs, 18 articles in which NSAIDs were administered only after surgery, 4 articles dealing with literature reviews, 22 studies that used administration pathways other than the oral route, 13 studies that dealt with the administration of drugs in patients with established acute pain, 7 articles in which patients received general anesthesia, 5 studies that dealt with methods of analgesia other than medication, and 1 study that did not inform the NSAID used. Therefore, a total of 6 articles were selected for the systematized review.7,9–11,14,15 All of the studies dealt with randomized, double-blinded, and placebo-controlled clinical trials, and only 2 studies were crossed.9,15
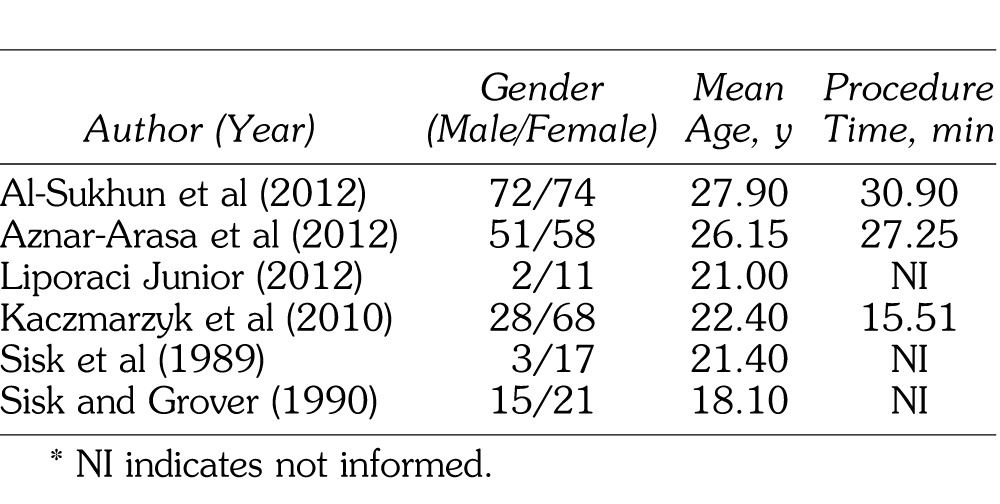
The researched studies were published in the period from 1989 through 2012 (Table 1) and included a total of 420 participants, among them 171 men and 249 women, with a mean age ranging from 18.1 to 27.9 years, showing that all of the participants were young adults. Three authors related the mean time of the surgical procedure (third-molar removal) in minutes: Al-Sukhun et al14 performed the procedure in 30.9 minutes, Aznar-Arasa et al10 in 27.15 minutes, and Kaczmarzyk et al7 in 15.51 minutes.
Table 1.
Number of Patients Divided According to the Categories Gender, Mean Age, and Mean Time of Surgical Procedure*
Lidocaine (2%) was the basic anesthetic used by 3 authors,9,11,14 and the vasoconstrictor used in the anesthetic solution was epinephrine 1:80,000,14 norepinephrine 1:80,000,11 and epinephrine 1:100,000.9 The authors Aznar-Arasa et al10 and Kaczmarzyk et al7 used 4% articaine with epinephrine 1:100,000 and 1:200,000, respectively; Sisk and Grover8 did not disclose the anesthetic solution used. The NSAIDs used by the authors were celecoxib, ibuprofen, ketoprofen, diflunisal, and naproxen sodium.
Al-Sukhun et al14 used celecoxib 200 mg in 48 patients, ibuprofen 400 mg in 45 patients, and placebo in 53 patients, all 1 hour before the procedure. Aznar-Arasa et al10 used ibuprofen 600 mg 1 hour before, with placebo in the immediate postoperative period in 53 patients and placebo 1 hour before, with ibuprofen 600 mg in the immediate postoperative period in 56 patients. Liporaci-Junior11 conducted a study with 13 patients using the split-mouth type of experimental model. In this study, each patient was submitted to 2 surgical procedures at different times. In one of the procedures, ketoprofen 150 mg was administered for 2 days before the surgical procedure, and on the other side, placebo was administered for 2 days before the surgical procedure. Kaczmarzyk et al7 used ketoprofen 100 mg 1 hour before with placebo 1 hour after surgery in 34 patients, placebo 1 hour before associated with ketoprofen 100 mg 1 hour after surgery in 30 patients and placebo 1 hour before associated with placebo 1 hour after surgery. Sisk and Grover8 used naproxen sodium 550 mg 30 minutes before with placebo 30 min after surgery in 30 patients on one side of the mouth and placebo 30 min before with naproxen sodium 550 mg 30 min after the surgical procedure on the other side of the mouth, in the same patients. Sisk et al9 used sodium diflunisal 1000 mg 30 min before with placebo 30 min after surgery in 20 patients on one side of the mouth and placebo 30 min before with diflunisal 1000 mg 30 min after the procedure on the other side of the mouth, in the same patients.
The NSAIDs were associated with the appearance of adverse effects as reported by Al-Sukhun et al,14 Sisk et al,9 and Sisk and Grover.8 Moreover, a statistically significant relationship could be observed in the studies of Al-Sukhun et al14 and Kaczmarzyk et al,7 while the other authors obtained no significant results. There were authors who prescribed an auxiliary analgesic medication for cases of patients who were unable to obtain comfort with the exclusive use of the instituted protocols. The medications used as analgesics were acetaminophen at doses of 500 mg,7 750 mg,11 1000 mg,14 and metamizol 575 mg.10
To perform the meta-analysis, the studies of Aznar-Arasa et al10 and Liporaci Junior11 were excluded because they did not have data suitable for making the dichotomous test. Furthermore, because the study of Kaczmarzyk et al7 did not present data about the patient's option of choice, the number of individuals who did not make use of rescue with supplementary medication was considered a positive response to preemptive analgesia. In the studies of Sisk and Grover8 and Sisk et al,9 all of the different choices of the test drug were considered failures.
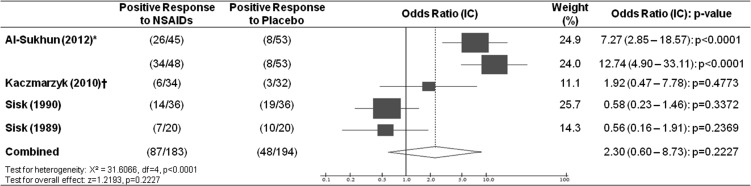
The data from the surveyed articles showed heterogeneity (P < .0001), and there was no statistically significant difference regarding analgesic response promoted by the preoperative use of NSAIDs (P = .2227), with a combined odds ratio of 2.30 (0.60–8.73; Figure 2).
Figure 2.
Response to use of nonsteroidal analgesics. *Ibuprofen and celecoxib, respectively, were considered analgesics separately. †There was no information available about the choice of patient by better response to NSAID or placebo used. Data represent the number of patients who did not make use of supplementary rescue analgesia.
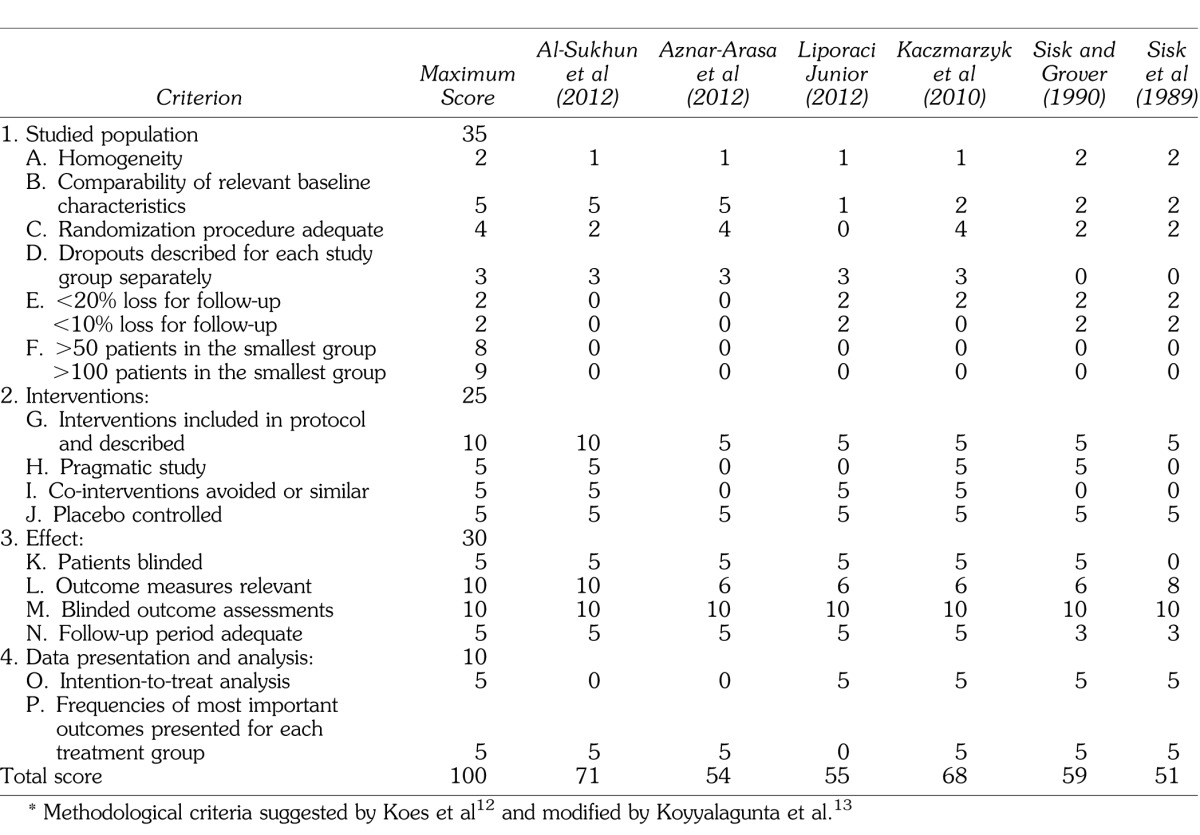
A methodological quality assessment of the studies that met inclusion criteria was carried out for 6 studies using the Cochrane review criteria.12,13 Various methodological deficiencies were found, as shown in Table 2, with the evaluated studies attaining a minimum score of 51 and maximum of 71 on a scale ranging from 0 to 100 (mean ± SD of 59.7 ± 8.1). The minimum Cochrane score for article inclusion is 50 points. The evaluated studies showed minimal quality for inclusion in a systematic review. Research showing the lowest score demonstrated lack of information or data omission, which was primarily responsible for the reduction in the mean score. In addition, there were no studies reporting more than 50 or 100 patients per group, a criterion that considerably increases the score of the study.
Table 2.
Methodological Analysis of the Articles of the Systematized Review*
DISCUSSION
NSAIDs are medications used frequently all over the world. NSAIDs may adequately control postoperative symptoms after the removal of impacted third molars. Effective analgesia provides the patient with a better quality of life in the postoperative period, allowing faster recovery and earlier return to their daily activities.10,11,14,16
According to Bridgman et al17 and Liporaci-Junior et al,11 one of the mechanisms that helps in postoperative pain control is the effect of the local anesthetic used, and the NSAIDs are not the main factors responsible for the preemptive analgesic effect. The present systematized review of the literature showed that 2 local anesthetics were used: lidocaine (2%) and articaine (4%). This is an important factor for evaluating the efficacy and duration of analgesia, considering that they have different onsets of action and potency. Another factor to be taken into consideration is the concentration of the vasoconstrictors, which is related to the degree of absorption of the anesthetic solution and degree of local vasoconstriction. The authors listed in this study used both different concentrations and types of vasoconstrictors.
Common forms of postoperative pain prevention include the preoperative administration of NSAIDs, corticosteroids, and/or long-lasting local anesthetics.8 The degree of nociception is related in part to the concentration of histamine, kinines, and prostaglandins at the site of inflammation. The maximum concentration of prostaglandins in acute tissue injuries occurs simultaneously with the peak of postoperative pain intensity (3 to 4 hours after injury). NSAIDs are capable of limiting peripheral sensitization by reducing prostaglandin synthesis at the site of surgery.7,9,15
The NSAIDs used by the authors selected in this systematized review were ibuprofen, celecoxib, ketoprofen, diflunisal, and naproxen sodium. The authors Sisk et al9 and Sisk and Grover8 related the use of intravenous midazolam together with the NSAID in some patients in the preoperative period, but in their studies, they did not specify which patients used midazolam. This is why these studies were included in the present review. Ibuprofen was used at doses of 400 mg and 600 mg, both 1 hour before surgery, and showed no statistical significance. The preoperative administration of ibuprofen did not reduce pain, facial edema, or trismus when compared with postoperative administration.10
Celecoxib was used at a dose of 200 mg and was shown to be statistically more effective than ibuprofen in the study of Al-Sukhun et al.14 When celecoxib is administered in low doses (200 mg or lower), there was good onset of action, duration, and greater pain relief than ibuprofen without any adverse effect.14
Ketoprofen was used at concentrations of 100 mg and 150 mg, 1 hour before and for 2 days before surgery, respectively, in 2 distinct studies; however, there was no significant difference between the concentrations when it was administered in the preoperative period. Only in the study of Kaczmarzyk et al7 was there a significant difference with regard to postoperative pain between the group that received placebo 1 hour before, associated with ketoprofen 1 hour after surgery, and the group that did not receive ketoprofen. Ketoprofen has good analgesic potency and few adverse effects,11 and according to Kaczmarzyk et al,7 this medication was most effective when administered in the postoperative period.
In the study of Sisk et al,9 diflunisal was used at a dose of 1000 mg 30 minutes before surgery and showed no significant difference in comparison with the control group in which the placebo was used. Differently from aspirin and many NSAIDs, it is believed that diflunisal has no significant effects on platelet function. Diflunisal (1000 mg) has been reported to be an efficient analgesic.9
In the study of Sisk and Grover,8 sodium naproxen was used at a concentration of 550 mg 30 minutes before surgery and showed no significant difference in comparison with the control group in which the placebo was used. Sodium naproxen has an onset of action of 1 hour, and plasma levels are attained in 2 to 4 hours; it is considered more effective than aspirin 650 mg and may have a similar analgesic capacity as meperidine at a concentration between 100 mg and 150 mg.8
According to Al-Sukhun et al,14 and Aznar-Arasa et al,10 NSAIDs may present some adverse effects such as ulcers, gastrointestinal bleeding, and increase in transoperative bleeding. Some authors have reported adverse effects related to the administration of NSAIDs such as headache, nausea, vomiting, sleepiness, and dizziness. However, the 2 last effects were related by the authors Sisk et al9 and Sisk and Grover,8 who affirmed that midazolam was used in some patients, which may explain these symptoms.
Moreover, some authors opted for prescribing auxiliary analgesics to provide greater comfort to those patients who did not obtain satisfactory postoperative analgesia. Acetaminophen 500 mg, 750 mg, 1000 mg, and metamizol 575 mg were used. There was a significant difference only in the study of Al-Sukhun et al.14 The group in which celecoxib was administered before the surgical procedure consumed fewer rescue analgesics than the group in which ibuprofen and placebo were administered to provide preemptive analgesia.
Although some authors found that preemptive analgesia was not an efficient therapeutic modality when used for third-molar extractions, likely because of the continuing presence of inflammatory mediators at the site of the tissue injury leading to persistent pain perception, it would be interesting to conduct more detailed studies.17
Although the meta-analysis was unable to demonstrate a reduction in postoperative pain after the use of preemptive analgesia with NSAIDs from the statistical point of view, the exclusion of the studies of Aznar-Arasa et al10 and Liporaci Junior11 from the calculations (because of a lack of numerical contingency data) should be taken into consideration.
Liporaci Junior showed no significant difference in his results using ketoprofen as the drug for preemptive analgesia.11 Ketoprofen was the same medication studied by Kaczmarzyk et al,7 who also found no significant odds ratio in favor of its use (Figure 2). Thus, the inclusion of Liporaci Junior11 in the statistical analysis would probably not change the meta-analysis.
Nevertheless, Aznar-Arasa et al,10 evaluating preoperative ibuprofen, showed a reduction in pain in the first 4 hours, partial reduction of trismus, and reduction of the amount of rescue medication used, denoting that ibuprofen demonstrated a significant positive effect when used for preemptive analgesia. Ibuprofen was the same drug as that studied by Al-Sukhun et al.14 Aznar-Arasa et al10 observed a significant positive odds ratio. If these authors have presented satisfactory dichotomous data to be included in the present meta-analysis, maybe these data could directly influence the preemptive analgesia outcome.
This hypothesis can be justified by a possible relationship between the efficacy of preemptive analgesia in third-molar surgery and the potency of the drug used to inhibit cyclooxygenase (Cox)–2.17–19 Celecoxib, a Cox-2–selective NSAID, and ibuprofen, a drug with only slightly more Cox-1 versus Cox-2 activity, used by Al-Shukun et al14 and Aznar-Arasa et al,10 showed a greater potency to inhibit Cox-2 in comparison with ketoprofen, a predominantly Cox-1 drug, used by Kaczmarzyk et al7 and Liporaci-Junior,11 who showed no significant beneficial effect, and naproxen, a slightly more Cox-1–active drug than ibuprofen, used by Sisk and Grover,8 who also showed no difference between the use and the absence of use of the therapeutic modality.
However, it is impossible to infer this relationship, especially because of a very great variability in the methodologies (treatment protocols and methods of evaluation) used in the analyzed clinical trials. This heterogeneity of scientific methods also interferes in the evaluation of the quality of these studies (Table 2), making it difficult to perform a more precise comparative analysis and thus limiting this type of conclusion from the set of data presented by us.
CONCLUSION
Preemptive analgesia continues to be a very controversial topic. Our analysis did not find a basis for preemptive analgesia with various NSAIDs in the third-molar surgical model. However, this may have been due to the many differences between the experimental models, including different methods of administration, combinations of drugs, evaluation of time of postoperative pain, types of surgery, concomitant use of NSAIDs, use of sedation, and different local anesthetics and vasoconstrictors, which hamper comparison among the effects. Thus, further careful clinical trials are necessary to enable analysis of the real effect and clinical applicability of preemptive analgesia with NSAIDs in dental applications.
REFERENCES
- 1.Crile GW. The kinetic theory of shock and its prevention through anoci-association. Lancet. 1913;182(4688):7–16. [Google Scholar]
- 2.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth. 2001;48:1000–1010. doi: 10.1007/BF03016591. [DOI] [PubMed] [Google Scholar]
- 3.Kissin I. Preemptive analgesia. Anesthesiol. 2000;93:1138–1143. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 4.Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull. 2004;13:13–27. doi: 10.1093/bmb/ldh030. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia II: recent advances and current trends. Can J Anaesth. 2001;48:1091–1101. doi: 10.1007/BF03020375. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 7.Kaczmarzyk T, Wichlinski J, Stypulkowska J, et al. Preemptive effect of ketoprofen on postoperative pain following thrid molar surgery: a propective, randomized, double-blinded clinical trial. Int J Oral Maxillofac Surg. 2010;39:647–652. doi: 10.1016/j.ijom.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Sisk AL, Grover BJ. A comparison of preoperative and postoperative naproxen sodium for suppression of postoperative pain. J Oral Maxillofac Surg. 1990;48:674–678. doi: 10.1016/0278-2391(90)90048-7. [DOI] [PubMed] [Google Scholar]
- 9.Sisk AL, Mosley RO, Martin RP. Comparison of preoperative and postoperative diflunisal for suppression of postoperative pain. J Oral Maxillofac Surg. 1989;47:464–468. doi: 10.1016/0278-2391(89)90278-4. [DOI] [PubMed] [Google Scholar]
- 10.Aznar-Arasa L, Harutunian K, Figueiredo R, et al. Effect of preoperative ibuprofen on pain and swelling after lower third molar removal: a randomized controlled trial. Int J Oral Maxillofac Surg. 2012;41:1005–1009. doi: 10.1016/j.ijom.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Liporaci Junior JLJ. Assessment of preemptive analgesia efficacy in surgical extraction or third molars. Rev Bras Anestesiol. 2012;62:502–510. doi: 10.1016/S0034-7094(12)70148-4. [DOI] [PubMed] [Google Scholar]
- 12.Koes BW, Scholten RJ, Mens JM, et al. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63:279–288. doi: 10.1016/0304-3959(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 13.Koyyalagunta D, Bruera B, Solanki D, et al. Systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician. 2012;15(3):es39–es58. [PubMed] [Google Scholar]
- 14.Al-Sukhun J, Al-Sukhun S, Penttilä H, et al. Preemptive analgesic effect of low doses of celecoxib is superior to low doses of traditional nonsteroidal anti-inflammatory drugs. J Craniofac Surg. 2012;23:526–529. doi: 10.1097/SCS.0b013e31824cd4fb. [DOI] [PubMed] [Google Scholar]
- 15.Scott R, Ellis E, III, Upton LG, et al. Double-blind evaluation of etodolac (200mg, 400mg) compared with zomepirac (100mg) and placebo on third molar extraction pain. Oral Surg Oral Med Oral Pathol. 1986;62:638–642. doi: 10.1016/0030-4220(86)90255-0. [DOI] [PubMed] [Google Scholar]
- 16.Ouellet M, Falgueyret J-P, Percival DM. Detergents profoundly affect inhibitor potencies against both cyclo-oxygenase isoforms. Biochem J. 2004;377:675–684. doi: 10.1042/BJ20030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bridgman JB, Gillgrass TG, Zacharias M. The absence of any pre-emptive analgesic effect for non-steroidal anti-inflammatory drugs. Br J Oral Maxillofac Surg. 1996;34:428–431. doi: 10.1016/s0266-4356(96)90101-1. [DOI] [PubMed] [Google Scholar]
- 18.Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isiordia-Espinoza MA, Sánchez-Prieto M. Tobías-Azúa, et al. Pre-emptive analgesic effectiveness of meloxicam versus tramadol after mandibular third molar surgery: a pilot study. J Oral Maxillofac Surg. 2012;70:31–36. doi: 10.1016/j.joms.2011.03.039. [DOI] [PubMed] [Google Scholar]