Abstract
We experienced a case of life-threatening hypotension and bronchoconstriction associated with edema in a patient undergoing resection of a tumor of the right mandible following intravenous midazolam for induction of general anesthesia. We decided to postpone surgery for further examination of a possible drug-induced allergic reaction, and we rescheduled surgery for 1 week later. After administering H1 and H2 histamine antagonists, we administered a slow induction with sevoflurane in nitrous oxide and oxygen plus intravenous atropine sulfate after performing a test dose injection. We safely induced and maintained anesthesia with nitrous oxide, oxygen, and sevoflurane.
Key Words: Midazolam, Anaphylactoid reaction
There is potential risk of an allergic reaction with latex and all drugs including muscle relaxants, antibiotics, local anesthetics, induction drugs, and opioids administered during anesthesia.1,2 Although midazolam is considered a relatively safe drug, it has been reported that midazolam may cause a life-threatening anaphylactoid reaction and hypersensitivity, such as hypotension, edema, and bronchoconstriction, during anesthesia.3–9 We obtained the patient's consent for publication of protected health information of this case.
CASE DESCRIPTION
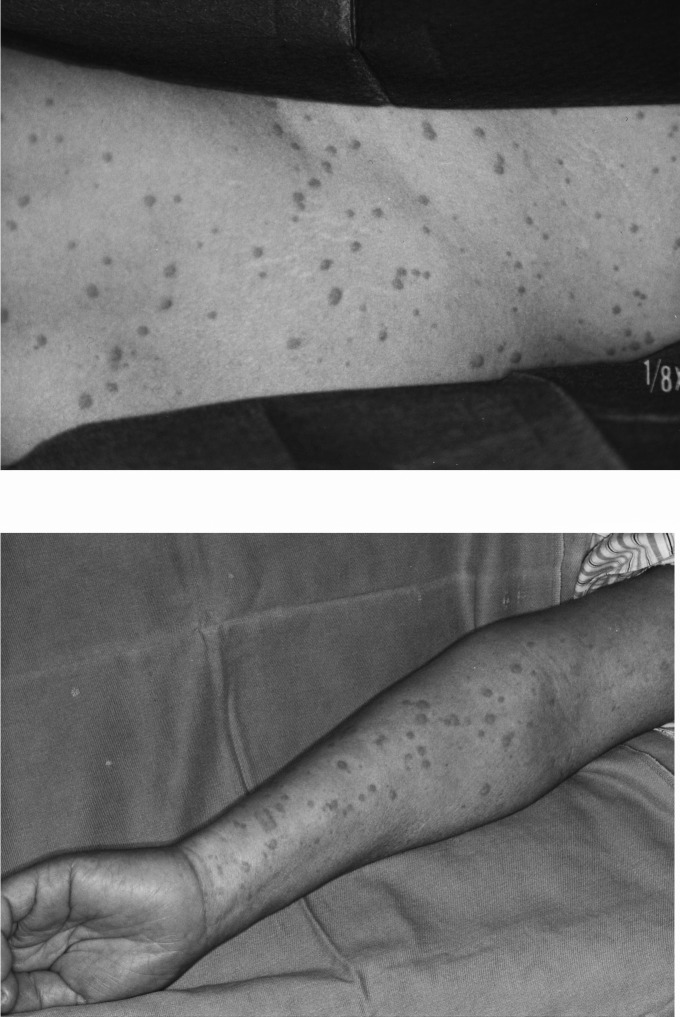
We experienced a case of life-threatening hypotension, bronchoconstriction, and associated edema in a 59-year-old woman (152 cm height, 56 kg body weight) undergoing resection of a tumor of the right mandible after she received midazolam intravenously for induction of general anesthesia. Preoperatively there was a history of skin reactions to several antibiotics and contrast medium during a CT scan. Because preoperative questioning suggested allergy to several drugs, we decided with the consent of the patient to not use thiopental or other histamine-releasing anesthetic drugs. In addition, we planned to initially use a small test dose of the short-acting benzodiazepine midazolam prior to induction. When anesthesia was induced with midazolam 5 mg (0.1 mg/kg) after the test dose of 0.01 mg/kg, the patient suddenly developed severe hypotension with a systolic blood pressure of 65 mm Hg and an associated onset of wheezing. There was a widespread flush on the body, including the neck, and edema of the eyelids (Figure). With a suspected diagnosis of anaphylactoid reaction, epinephrine 50 μg and methyl-prednisolone 1000 mg were injected intravenously. Because of sustained hypotension and ischemic ST depression in lead II, dopamine and nitroglycerine were continuously infused for 30 minutes. After the patient stabilized, we decided to postpone surgery until she was fully recovered from shock and for further examination of a possible allergic drug reaction. Even though several allergy tests, including skin tests, were completed, we did not obtain clear evidence for an allergic drug reaction.
There was a widespread flush on the body and neck and edema of the eyelids.
One week later, she underwent a resection of her tumor of the right mandible and reconstruction of the tissue defect. After administering H1 and H2 histamine antagonists, we performed a slow induction with sevoflurane plus nitrous oxide and oxygen with atropine sulfate after first performing a test dose injection. We safely induced anesthesia and completed a nasotracheal intubation. We maintained anesthesia with sevoflurane in nitrous oxide and oxygen for the next 18 hours and 42 minutes until all the surgical procedures were completed. Over the following 15 years, she had several surgical procedures, including gynecologic surgery and plastic reconstructive surgery, with the same sevoflurane anesthesia technique in a different hospital without apparent difficulty.
DISCUSSION
Midazolam hydrochloride is a short-acting benzodiazepine that is used for preoperative sedation, induction of anesthesia, and conscious sedation. However, several clinical reports have suggested the occurrence of severe adverse reactions, such as hypotension, edema, and bronchoconstriction, associated with the drug. Uchimura et al8 reported that a 26-year-old female patient had facial edema and pruritus after intravenous injection of 2 mg of midazolam. Fujita et al3 also published a case of anaphylactoid reaction associated with hypotension and a widespread flush on the body and neck. Most recently, Shrivastava9 stated that even though a midazolam-provoked hypersensitivity may occur only rarely, the clinician should nevertheless use this drug carefully. In our case, because of poor storage conditions of the blood samples collected during the allergic reaction, we could not obtain any direct evidence of a midazolam-induced anaphylactoid reaction. We have used midazolam and atropine sulfate for induction of anesthesia in many cases, and cannot deny the possibility of an atropine sulfate allergic reaction. However, because we administered a slow induction with sevoflurane plus nitrous oxide and oxygen and an intravenous test dose, followed by a therapeutic dose, of atropine sulfate after first administering H1 and H2 histamine antagonists in several subsequent anesthetics, atropine sulfate was judged as not likely to have caused the reaction. In addition, because of the timing of the injection, midazolam was considered to be the most likely cause of the anaphylactoid reaction. This case illustrates the potential for anaphylactoid reactions with midazolam in patients with a history of allergy to multiple drugs.
REFERENCES
- 1.Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381–1395. doi: 10.1213/01.ANE.0000082993.84883.7D. [DOI] [PubMed] [Google Scholar]
- 2.Holdcroft A. UK drug analysis prints and anaesthetic adverse drug reactions. Pharmacoepidemiol Drug Saf. 2007;16:316–328. doi: 10.1002/pds.1261. [DOI] [PubMed] [Google Scholar]
- 3.Fujita Y, Ishikawa H, Yokota K. Anaphylactoid reaction to midazolam. Anesth Analg. 1994;79:811–812. doi: 10.1213/00000539-199410000-00043. [DOI] [PubMed] [Google Scholar]
- 4.Yakel DL, Jr, Whittaker SE, Elstad MR. Midazolam-induced angioedema and bronchoconstriction. Crit Care Med. 1992;20:307–308. doi: 10.1097/00003246-199202000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Engstrom RH, Cohen SE. A complication associated with the use of midazolam. Anesthesiology. 1989;70:719. doi: 10.1097/00000542-198904000-00036. [DOI] [PubMed] [Google Scholar]
- 6.McIlwain M, Primosch R, Bimstein E. Allergic reaction to intranasal midazolam HCl: a case report. Pediatr Dent. 2004;26:359–361. [PubMed] [Google Scholar]
- 7.Davis DP, Hamilton RS, Webster TH. Reversal of midazolam-induced laryngospasm with flumazenil. Ann Emerg Med. 1998;32:263–265. doi: 10.1016/s0196-0644(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 8.Uchimura A, Yogo H, Kudoh I, Ohmura A. Facial edema and pruritus after intravenous injection of midazolam [in Japanese] Masui. 2006;55:76–78. [PubMed] [Google Scholar]
- 9.Shrivastava S. An experience with midazolam anaphylactoid reaction. J Anesth. 2012;26:642–643. doi: 10.1007/s00540-012-1386-6. [DOI] [PubMed] [Google Scholar]