Abstract
AIM: To investigate spleen status in psoriasis and its relationship with hepatic steatosis, Psoriasis Area and Severity Index, and insulin resistance.
METHODS: Seventy-nine psoriatic patients who were not suffering from any chronic inflammatory disease were retrospectively selected for inclusion in this study, and their complete medical records were accessed. An age- and sex-matched group of 80 non-psoriatic, obese patients was included as a control. The following relevant data were collected: age, sex, weight, height, body mass index, waist circumference, blood pressure, insulin resistance status, age at psoriasis onset, and severity of psoriasis. Abdominal ultrasonography was performed to determine spleen longitudinal diameter (SLD), and hepatic steatosis grade.
RESULTS: The SLD of control obese patients was greater than that of psoriatic subjects (P = 0.013), but body mass index predicted the size of the spleen in psoriatic patients (P < 0.001). The SLD of psoriatic patients with normal weight was significantly reduced with respect to the overweight/obese psoriatic patients (P = 0.002). A multiple regression analysis revealed that body mass index was a unique predictor of the spleen size (P < 0.001). Finally, the disease duration predicted the spleen size in psoriatic subjects (P = 0.038).
CONCLUSION: This study shows a correlation between the SLD and the duration of psoriasis.
Keywords: Hepatic steatosis, Inflammation, Nonalcoholic fatty liver disease, Psoriasis, Spleen size
Core tip: The specific role of the spleen in psoriatics could help in more comprehensively understanding the inflammatory mechanism underlying this illness; psoriasis would be the most superficial manifestation of a chronic inflammatory process involving various organs and systems. The increased diameter of the spleen found in psoriatic patients with long-term illness may be the expression of the immune system’s response to the state of chronic inflammation.
INTRODUCTION
Psoriasis is a chronic, relapsing, inflammatory skin disease that affects about 2% of the Caucasian population, causing a significant impairment of quality of life, particularly if it is diffuse and recalcitrant to treatments[1-3]. Much attention has been drawn towards upgrading psoriasis from a skin condition to a systemic disease, as serum biomarkers for inflammation [interleukin (IL)-1β, IL-6, IL-10, C-reactive protein, intracellular adhesion molecule-1, E-selectin, and tumour necrosis factor-α] are raised. Psoriatic patients could therefore have a higher risk of developing systemic comorbidities, including psoriatic arthritis, inflammatory bowel diseases (Crohn’s disease and ulcerative colitis), or cardio-metabolic disorders (such as myocardial infarction hypertension, obesity, diabetes, dyslipidemia, fatty liver disease, and hyperuricemia)[4-7]. Recently, hospital-based observational studies suggested that patients with psoriasis are 1.5- to 3-fold more likely to have nonalcoholic fatty liver disease (NAFLD). This increased risk of NAFLD and subsequent risk of liver damage was explained by an increased prevalence of NAFLD risk factors such as obesity, diabetes mellitus, and alcohol consumption among patients with psoriasis[8]. Moreover, among the various pathways that contribute to the development of hepatic steatosis (HS), circulating concentrations of inflammatory cytokines are considered to be the most important factor in causing and maintaining insulin resistance (IR). Low-grade chronic inflammation, of which IL-6 is the main involved cytokine, is fundamental in the progression of NAFLD toward higher-risk cirrhotic states, via nonalcoholic steatohepatitis (NASH)[9]. NASH is a progressive liver disease characterized by Kupffer cell dysfunction, which contributes to its pathogenesis. Noteworthy, the reticular-endothelial system also plays a key role in the spleen. Indeed, Tsushima et al[10] found an association between NAFLD and enlarged spleen volume measured by computed tomography. Moreover, as it is now known, obesity and IR are strongly associated with systemic markers of inflammation[11]. Studies attempting to find a noninvasive method that could likely assess the presence of NASH and using histology as a gold standard to diagnose NAFLD, revealed that the NASH subjects had a higher spleen longitudinal diameter (SLD) and significantly higher IL-6 and vascular endothelial growth factor concentrations than healthy controls and patients with fatty liver[12]. The aim of this retrospective study was to establish if psoriatic patients presented a larger spleen volume, as an index of low-grade chronic inflammation, and to what extent HS was present. Further the relationships between Psoriasis Area and Severity Index (PASI), the most used score to evaluate the clinical severity, and anthropometric data, IR, grade of HS, and spleen volume were analyzed.
MATERIALS AND METHODS
Seventy-nine consecutive psoriatic patients who attended the Dermatology Clinic of the University of Naples Federico II between July 2012 and October 2013, and who were not suffering from any chronic inflammatory diseases, viral or bacterial infections, or cancer were retrospectively included in this study, and their complete medical records were accessed. A similar group of 80 obese patients with body mass index (BMI) > 30 attending the outpatient obesity clinic, without psoriasis, and were well matched for age (max tolerance: three years of difference) and sex was compared as a control. The source population for cases and controls was the same.
Patients aged ≥ 18 years with moderate or severe plaque psoriasis were included in the study. Psoriasis was diagnosed according to clinical criteria. Severity was assessed according to PASI, body surface area (BSA) measurement, and static Physician’s Global Assessment. Disease severity was classified as ≥ 10 according to the PASI. Disease severity was scored as moderate when PASI was 10-20 and severe when PASI score was > 20. Chronic plaque psoriasis was considered localized or disseminated when it covered less or more than 10% of the BSA, respectively. No patient suffering from psoriasis was on beta-blockers, lithium, or anti-malarials.
Patients receiving any systemic treatment for psoriasis including acitretin, ciclosporin, methotrexate, phototherapy, or biologics for ≥ 6 mo before enrollment were not included in the study. After signed informed consent was obtained, all subjects were visited by a dermatologist who registered demographic, biometric, and other relevant data on a case-report form. Relevant data collected included age, sex, weight, height, BMI, waist circumference, blood pressure, smoking habit, age at psoriasis onset, type and severity of psoriasis, concomitant medications, and abdominal US. Obesity was determined by measuring BMI and was corrected for abdominal adiposity. Measurements of height and body weight were taken by a trained research staff for each patient. Height was measured to the nearest 1 mm and weight was measured to the nearest 0.1 kg. The BMI was calculated as weight in kg divided by height in m2 and was categorized into three groups: normal weight was defined as BMI < 25 kg⁄m2; overweight was defined as 25 kg⁄m2 < BMI > 29.99 kg⁄m2; obesity was defined as BMI > 30 kg⁄m2. Visceral obesity was identified by measuring waist circumference at the midpoint between the lower border of the rib cage and the iliac crest. Hip circumference was measured around the widest part of the buttocks, with the tape parallel to the floor, and the waist to hip ratio was calculated. IR status was determined by the homeostatic metabolic assessment (HOMA), which was assessed by the formula: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405. Moreover, as the repeated HOMA measurements presented high within-person variability in obese patients, HOMA values were averaged on the basis of at least five determinations to avoid misclassification. Patients with psoriatic arthritis, inflammatory bowel diseases, rheumatoid arthritis, or other autoimmune disease such as lupus erythematosus and primary biliary cirrhosis were excluded.
Ultrasound evaluation
Ultrasonographic measurements were performed using an Esaote system (Genoa, Italy). SLD as an index of low-grade chronic inflammation was chosen to evaluate spleen volume and was carried out by postero-lateral scanning[12]. Maximum length (the optically greatest overall longitudinal dimension obtained from one of the two poles) and cranio-caudal length (the optically maximal transversal dimension intercepting one of the two poles) were measured; the resulting values were then averaged, as the two measurements do not always coincide. The classification of HS (commonly defined as “bright liver”) was based on the following scale of hyper-echogenicity at ultrasound (US): grade 0 = absent, grade 1 = light, grade 2 = moderate, grade 3 = severe, pointing out the difference between the densities of the liver and the right kidney[13]. Technically, echo intensity can be influenced by many factors, particularly by gain intensity. To avoid confounding factors that could modify echo intensity and thus bias comparisons, mean brightness levels of both liver and right kidney cortex were obtained on the same longitudinal sonographic plane.
Statistical analysis
Alanine and aspartate aminotransferase level ratio, SLD, and HOMA were not normally distributed when analyzed by the Shapiro-Wilk (S-W) test (P < 0.05), and were expressed as median (interquartile range). Age and PASI were derived from a normally distributed population, and were articulated as mean ± SD. Grade of HS was an ordinal variable and analyzed by a nonparametric method. Appropriate tests for matched case-control studies included the paired t test or the nonparametric Wilcoxon test. McNemar’s test for frequencies was used instead of the independent t and χ2 tests because the former take into account the dependent nature of the case and control subjects[14]. When dealing with subgroup analyses, i.e., psoriatic patient with normal weight or overweight/obese, the Man-Whitney U test was used. For univariate analysis, to assess the independent effect of a quantitative variable on the prediction of another one, the linear regression analysis (least squares) was used, evaluating the coefficient with its standard error and the t (t-stat). A t > 1.96 with a significance < 0.05 indicates that the independent variable is a significant predictor of the dependent variable within and beyond the sample. To evaluate the association between spleen size and the grade of HS in psoriatics, the Spearman’s coefficient of rank correlation (ρ) was calculated. To establish what the best combination of independent variables would be to predict the dependent variable, a multiple regression (enter method) was adopted. BMI was used as dependent variable, while the independent ones were BMI, HOMA, and PASI. To avoid multi-collinearity, i.e., situations in which the predictors are correlated with each other to some degree, the variance inflation factor and tolerance were set at > 10 and < 0.1, respectively. Similarly, to get the sense of which variables contribute more or less to the regression equation, the magnitude of standardized coefficient beta (β) was calculated.
RESULTS
Seventy-nine patients with psoriasis (51 male and 28 female; age: 46.55 ± 15.56 years, range: 18-76 years), and 80 age- and sex-matched controls (52 male and 28 female; age: 44.3 ± 12.87 years, range: 19-73 years) participated in the study. No statistically significant difference was noted in age between the groups. The mean duration of psoriasis was 17.9 years (range: 1-63 years). PASI score ranged from 6.4 to 59.0 (14.75 ± 12.78), and 55/79 (69.62%) had moderate to severe psoriasis (PASI > 10). BSA ranged from 2% to 85%, (15.23% ± 11.09%), while 43/79 (54.43%) patients had involved BSA > 10%. SLD of obese patients was greater than that of psoriasis group [11.1 (10.2-12.4) vs 10.4 (9.4-11.9), P = 0.013] (Figure 1A). BMI predicted the size of the spleen, evaluated as SLD in psoriatic patients (coefficient: 2.56, t = 5.57; P < 0.001) (Figure 1B). The SLD of psoriatic patients with normal weight was significantly reduced when compared to the SLD of the overweight/obese psoriatic patients [9.6 (9.2-10.4) vs 11 (9.9-12.3), P < 0.01] (Figure 1C). There was no difference in frequency of HS presence in the two groups; 65/79 psoriatic patients and 73/80 obese subjects showed HS (McNemar test; P = 0.070). The median grade of HS was significantly different between patients and obese controls [2.0 (1.0-3.0) vs 2.0 (2.0-3.0), P = 0.006] (Figure 1D).
Figure 1.
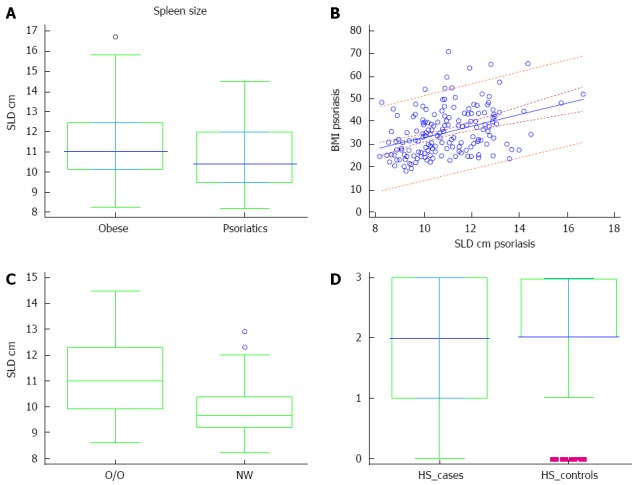
Spleen size, body mass index, and hepatic steatosis. A: Spleen longitudinal diameter (SLD) in the psoriatic patients and obese controls; B: Prediction of body mass index (BMI) on SLD of patients with psoriasis (dotted lines near the regression line are the 95% confidence curves, the far ones are the 95% prediction curves); C: SLD in overweight/obese psoriatic patients (O/O) and normal weight (NW) psoriatic patients; D: Hepatic steatosis (HS) grade in patients (cases) and controls: grade 0 = absent; 1 = mild; 2 = moderate; 3 = severe. Boxes in A, C and D indicate the interquartile ranges and the transverse lines represent the median.
Homa IR predicted the severity of psoriasis evaluated by the means of PASI (coefficient: 0.051, t = 2.35; P = 0.020) (Figure 2A). The grade of HS was predicted by PASI (coefficient 0.05, t = 2.9; P = 0.005) (Figure 2B). The spleen size strongly predicted the grade of HS (coefficient: 0.47, t = 3.6; P < 0.001) (Figure 2C). However, SLD did not predict PASI (coefficient: 0.03, t = 54). A multiple regression analysis including the anthropometric and disease severity measures showed that BMI was the unique predictor of spleen size (β = 0.39; P < 0.001). Finally, when evaluating the impact of disease age in psoriatic patients, the duration of psoriasis (counted as years of disease) predicted the SLD (coefficient: 1.8, t = 2.11; P = 0.038) (Figure 2D). Interestingly, the values of transaminases of this population fell in the upper normal range: alanine transaminase, 40 (34-46) U/L; aspartate transaminase, 34 (30-43) U/L. Finally, the association between spleen size and the severity of HS was significant (ρ = 0.415; P < 0.001).
Figure 2.
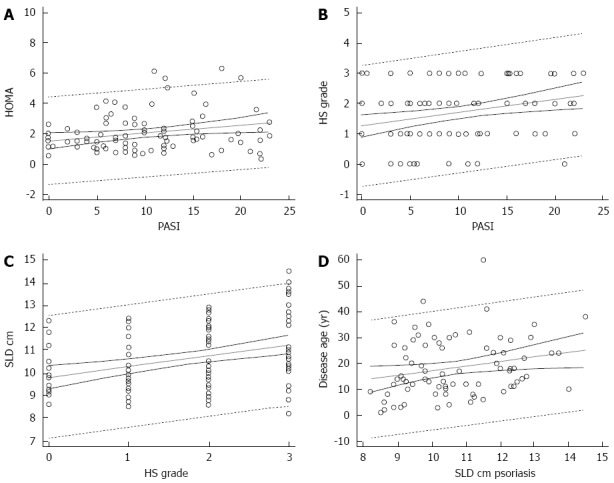
Insulin resistance, hepatic steatosis, spleen size, severity of psoriasis, and disease age of psoriatic patients. A: Prediction of insulin resistance on severity of psoriasis; B: Prediction of hepatic steatosis (HS) on severity of psoriasis; C: Prediction of SLD on severity of psoriasis; D: Prediction of disease age of psoriatic patients on SLD. Dotted lines near the regression line are the 95% confidence curves, the far ones are the 95% prediction curves. HOMA: Homeostatic metabolic assessment; PASI: Psoriasis area and severity index; SLD: Spleen longitudinal diameter.
DISCUSSION
The key findings of this research can be summarized as follows. Firstly, robust links were found among HS, further expression of the metabolic syndrome, and PASI, as well as between IR and PASI. Secondly, a strict relationship between disease duration and spleen size was observed. However, few studies published during the last decade investigated the link between spleen and psoriasis. On the other hand, many studies worldwide have shown that people with psoriasis have comorbidities such as diabetes, hypertension, and lipid abnormalities[15-17] .
With regard to possible mechanisms to explain these findings, we hypothesize that this association to the psoriatic march, the process by which inflammatory mediators released in the course of the psoriatic autoimmune reaction, causes IR, which is correlated to an increased prevalence of metabolic syndrome[18]. Earlier studies have shown that IR is common in patients with psoriasis and that the PASI is the major determinant of IR[19]. The data presented here confirm this association. These observations support the concept of synergistic effects from the chronic state of inflammation caused by obesity and the chronic systemic Th1 lymphocyte-mediated inflammation characteristic of psoriasis, which has recently been put forward by Hamminga et al[20]. In the literature, there is no data about the importance of the spleen as an indicator of systemic inflammation in patients with psoriasis. Although the data reported here do not reveal a correlation between PASI and SLD, there is a correlation between the duration of the disease and the SLD.
The spleen is the largest lymphoid organ in the body and plays an important role in host immune function and blood filtration via the removal and destruction of aged or damaged erythrocytes and other blood cells[21]. Splenic gene expression of proinflammatory cytokines, such as tumor necrosis factor-α and IL-6, is decreased in the setting of obesity[22]. In contrast, IL-10, which is synthesized within multiple organs, including the spleen, is a potent anti-inflammatory cytokine that inhibits the synthesis of proinflammatory cytokines. Large amounts of IL-10 are produced from activated B-cells that mature in the marginal zone of the spleen. Recent studies suggest that IL-10-producing B-cells play a regulatory role in suppressing harmful immune responses[23]. Gotoh et al[24] have supported the hypothesis that obesity suppresses the splenic synthesis of the anti-inflammatory cytokine, IL-10, thereby resulting in chronic inflammation. IL-10 may contribute to disease susceptibility in psoriasis, and it has been reported that IL-10 deficiency is a feature of psoriasis[25]. The increased diameter of the spleen found in our psoriatic patients with long-term illness may be the expression of the immune system’s response to the state of chronic inflammation. It should be emphasized that the increased spleen volume represents a clinical finding, not a comorbidity, such as obesity, linked to the low-grade chronic inflammatory status.
The results of this study show a clear link between psoriasis and HS as well as the spleen. The specific role of the spleen in psoriatics could help us to more comprehensively understand the inflammatory mechanism underlying this illness: psoriasis would be the most superficial manifestation of a chronic inflammatory process involving various organs and systems. Evaluation of the SLD could help to ameliorate the approach of psoriasis, helping clinicians to identify psoriatic patients who need early attention via modification of their lifestyle and alimentary habits. However, further studies are needed to better understand the potential involvement of the spleen in psoriasis inflammatory context.
Limitations
A limitation of the present study is the lack of liver biopsies to better define the HS, even though the US determination of moderate- to high-grade HS is quite reliable[26]. Furthermore, detection of the levels of serum inflammatory markers, clues of both psoriasis and NAFLD, would have strengthened the impact of these results. However, there is a large body of evidence that confirms the main role of C-reactive protein and IL-6 as main mechanisms of psoriasis and NAFLD, evidenced by high serum levels of this acute-phase reactant and cytokine, respectively[11,12].
COMMENTS
Background
Psoriasis is a chronic, relapsing, inflammatory skin disease causing a significant impairment of quality of life, particularly if it is diffuse and recalcitrant to treatments.
Research frontiers
Much attention has been drawn towards upgrading psoriasis from a skin condition to a systemic disease, as serum biomarkers for inflammation [interleukin (IL)-1β, IL-6, IL-10, C-reactive protein, intracellular adhesion molecule-1, E-selectin, and tumor necrosis factor-α] are raised.
Innovations and breakthroughs
Summarizing the advances from other research, this study shows a clear link between psoriasis and the spleen, showing a correlation between the duration of the disease and spleen size.
Applications
The actual application values of this research lie in a better comprehension of psoriasis mechanisms, especially those related to hepatic steatosis, strictly linked to spleen involvement.
Peer-review
There are studies showing a higher prevalence of metabolic syndrome in patients with psoriasis. In this paper, the authors wish to say that spleen longitudinal diameter of obese patients is greater in psoriatic patient and normal in non-obese individuals. This is an interesting study reflecting the role of body mass index in psoriasis.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 9, 2014
First decision: August 6, 2014
Article in press: December 16, 2014
P- Reviewer: Duryee MJ, Mascitelli L, Nagarajan P S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Wang CH
References
- 1.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64 Suppl 2:ii18–i23; discussion ii24-25. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2012;26:787–801. doi: 10.3122/jabfm.2013.06.130055. [DOI] [PubMed] [Google Scholar]
- 3.Patruno C, Ayala F, Megna M, Napolitano M, Balato N. Patient-physician relationship in patients with psoriasis. Indian J Dermatol Venereol Leprol. 2013;78:228. doi: 10.4103/0378-6323.93657. [DOI] [PubMed] [Google Scholar]
- 4.Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 5.Balato A, Di Caprio R, Canta L, Mattii M, Lembo S, Raimondo A, Schiattarella M, Balato N, Ayala F. IL-33 is regulated by TNF-α in normal and psoriatic skin. Arch Dermatol Res. 2014;306:299–304. doi: 10.1007/s00403-014-1447-9. [DOI] [PubMed] [Google Scholar]
- 6.Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7:284–289. doi: 10.7150/ijms.7.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, van de Kerkhof P, Ståhle M, Nestle FO, Girolomoni G, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 8.van der Voort EA, Koehler EM, Dowlatshahi EA, Hofman A, Stricker BH, Janssen HL, Schouten JN, Nijsten T. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: Results from a population-based study. J Am Acad Dermatol. 2014;70:517–524. doi: 10.1016/j.jaad.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773–4783. doi: 10.3748/wjg.v16.i38.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsushima Y, Endo K. Spleen enlargement in patients with nonalcoholic fatty liver: correlation between degree of fatty infiltration in liver and size of spleen. Dig Dis Sci. 2000;45:196–200. doi: 10.1023/a:1005446418589. [DOI] [PubMed] [Google Scholar]
- 11.Tarantino G, Colicchio P, Conca P, Finelli C, Di Minno MN, Tarantino M, Capone D, Pasanisi F. Young adult obese subjects with and without insulin resistance: what is the role of chronic inflammation and how to weigh it non-invasively? J Inflamm (Lond) 2009;6:6. doi: 10.1186/1476-9255-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarantino G, Conca P, Pasanisi F, Ariello M, Mastrolia M, Arena A, Tarantino M, Scopacasa F, Vecchione R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol. 2009;21:504–511. doi: 10.1097/MEG.0b013e3283229b40. [DOI] [PubMed] [Google Scholar]
- 13.Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909–914. doi: 10.2214/AJR.07.4016. [DOI] [PubMed] [Google Scholar]
- 14.Breslow NE, Day NE. Classical Methods of Analysis of Matched Data. In: Breslow NE, Day NE, editors. Statistical methods in cancer research. Volume 1-The analysis of case-control studies. Lyon, France: International Agency for Research on Cancer; 1980. pp. 162–192. [Google Scholar]
- 15.Christophers E. Comorbidities in psoriasis. J Eur Acad Dermatol Venereol. 2006;20:52–55. doi: 10.1111/j.1468-3083.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 16.Balato N, Balato A, Gallo L, Napolitano M, Patruno C, Ayala F. Psoriasis and osteoporosis: data from a Southern Italian population. Arch Osteoporos. 2012;7:321–323. doi: 10.1007/s11657-012-0112-1. [DOI] [PubMed] [Google Scholar]
- 17.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014–1024. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Boehncke WH, Boehncke S, Schön MP. Managing comorbid disease in patients with psoriasis. BMJ. 2010;340:b5666. doi: 10.1136/bmj.b5666. [DOI] [PubMed] [Google Scholar]
- 19.Savastano S, Balato N, Gaudiello F, Di Somma C, Brancato V, Colao A, Ayala F, Tarantino G. Insulin-like growth factor-1, psoriasis, and inflammation: a ménage à trios? European J of Inflammation. 2011;9:277–284. [Google Scholar]
- 20.Hamminga EA, van der Lely AJ, Neumann HA, Thio HB. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768–773. doi: 10.1016/j.mehy.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Dameshek W. Hypersplenism. Bull N Y Acad Med. 1955;31:113–136. [PMC free article] [PubMed] [Google Scholar]
- 22.Lamas O, Martínez JA, Marti A. Decreased splenic mRNA expression levels of TNF-alpha and IL-6 in diet-induced obese animals. J Physiol Biochem. 2004;60:279–283. doi: 10.1007/BF03167074. [DOI] [PubMed] [Google Scholar]
- 23.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh K, Inoue M, Masaki T, Chiba S, Shimasaki T, Ando H, Fujiwara K, Katsuragi I, Kakuma T, Seike M, et al. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity-induced inflammation in white adipose tissue and liver. Diabetes. 2012;61:1994–2003. doi: 10.2337/db11-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Döcke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, Torella R, Persico M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]


