Abstract
AIM: To determine the predictive value of increased prolidase activity that reflects increased collagen turnover in patients with hepatocellular carcinoma (HCC).
METHODS: Sixty-eight patients with HCC (mean age of 69.1 ± 10.1), 31 cirrhosis patients (mean age of 59.3 ± 6.3) and 33 healthy volunteers (mean age of 51.4 ± 12.6) were enrolled in this study. Univariate and multivariate analysis were used to evaluate the association of serum α-fetoprotein (AFP) values with HCC clinicopathological features, such as tumor size, number and presence of vascular and macrovascular invasion. The patients with HCC were divided into groups according to tumor size, number and presence of vascular invasion (diameters; ≤ 3 cm, 3-5 cm and ≥ 5 cm, number; 1, 2 and ≥ 3, macrovascular invasion; yes/no). Barcelona-clinic liver cancer (BCLC) criteria were used to stage HCC patients. Serum samples for measurement of prolidase and alpha-fetoprotein levels were kept at -80 °C until use. Prolidase levels were measured spectrophotometrically and AFP concentrations were determined by a chemiluminescence immunometric commercial diagnostic assay.
RESULTS: In patients with HCC, prolidase and AFP values were evaluated according to tumor size, number, presence of macrovascular invasion and BCLC staging classification. Prolidase values were significantly higher in patients with HCC compared with controls (P < 0.001). Prolidase levels were significantly associated with tumor size and number (P < 0.001, P = 0.002, respectively). Prolidase levels also differed in patients in terms of BCLC staging classification (P < 0.001). Furthermore the prolidase levels in HCC patients showed a significant difference compared with patients with cirrhosis (P < 0.001). In HCC patients grouped according to tumor size, number and BCLC staging classification, AFP values differed separately (P = 0.032, P = 0.038, P = 0.015, respectively). In patients with HCC, there was a significant correlation (r = 0.616; P < 0.001) between prolidase and AFP values in terms of tumor size, number and BCLC staging classification, whereas the presence of macrovascular invasion did not show a positive association with serum prolidase and AFP levels.
CONCLUSION: Considering the levels of both serum prolidase and AFP could contribute to the early diagnosing of hepatocellular carcinoma.
Keywords: Alpha-fetoprotein, Hepatocellular carcinoma, Prolidase, Cirrhosis, Macrovascular invasion
Core tip: Prolidase cleaves dipeptide bonds containing proline, playing a vital role in collagen turnover, matrix remodeling and cell growth. Neoplastic transformation results in deregulation of tissue collagen metabolism, in which metastatic tumor cells produce enhanced amounts of proteases to penetrate basement membranes and the extracellular matrix. Therefore, tumor progression might depend on the breakdown of collagen and other extracellular matrix proteins. The role of prolidase in neoplastic tissues is unknown. Herein, serum prolidase levels in hepatocellular carcinoma (HCC) patients were significantly associated with tumor size and number, Barcelona-clinic liver cancer staging and α-fetoprotein (AFP). Considering the levels of both serum prolidase and AFP could contribute to early diagnosis of HCC.
INTRODUCTION
Despite recent developments in surgery and medical therapy, which have significantly improved the outcome of patients with operable and advanced hepatocellular carcinoma (HCC), HCC remains a major health problem worldwide. The majority of HCC cases occur in patients with chronic liver disease, such as hepatitis B-virus (HBV), hepatitis C-virus (HCV) infection, alcoholic liver diseases and non-alcoholic fatty liver diseases[1,2]. The complex nature of the disease and its high resistance to conventional systemic therapies, results in poor prognosis for advanced HCC patients[1]. Despite regular surveillance to detect small HCCs in these patients, HCC is often diagnosed at an advanced stage, after the symptoms related HCC have appeared, and the 5-year relative survival rate for patients is only 7%[1]. If HCC could be diagnosed at an early stage, potentially curative options, such as resection, ablation, and transplantation may be considered[3]. Early diagnose may serve as a long-term control in patients. Thus, the regular follow-up of patients with risk factors for HCC seems very important.
Surveillance strategies, including ultrasound imaging and serum α-fetoprotein (AFP) concentration measurements, have been recommended to detect HCC at earlier stages, without pathological confirmation. AFP is the most commonly used serological marker worldwide to diagnose hepatocellular carcinoma[4]. HCC differentiation, size and macrovascular invasion are strongly associated with AFP; poor differentiation and HCC size ≥ 10 cm are independent predictors of elevated AFP[5].
Collagen is the main component of connective tissue. Deregulation of tissue collagen metabolism is one of the consequences of neoplastic transformation. Metalloproteinases initiate the breakdown of collagen; however, the final step of collagen degradation is mediated by prolidase[6,7].Prolidase is an important enzyme that cleaves the bonds of dipeptides containing proline (X-Pro), and plays a vital role in collagen turnover, matrix remodeling and cell growth. Metastatic tumor cells produce enhanced amounts of proteases that enable them to penetrate basement membranes and the extracellular matrix (ECM)[8]. Therefore, tumor progression might depend critically on the breakdown of collagen and other ECM proteins[9]. Prolidase seems a rate-limiting factor in the regulation of collagen biosynthesis because of its role in the last step of collagen degradation. The role of prolidase activity in neoplastic tissues is not yet known.
In the present study, we aimed to assess the correlation between the serum prolidase and AFP levels in patients with hepatocellular carcinoma.
MATERIALS AND METHODS
Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in the a priori approval by the Clinical Research Ethics Board of the Bülent Ecevit University. Informed consent was obtained from all individuals.
Patient population
Ninety-four patients with HCC, 54 cirrhosis patients and 33 healthy volunteers, admitted to the gastroenterology clinic of Bulent Ecevit University Medical Faculty between March 2014 and August 2014, were enrolled in this study. Twenty-six patients with HCC and 23 patients with cirrhosis were excluded from the study according to the exclusion criteria. Exclusion criteria were: dilated cardiomyopathy, uncontrolled hypertension, rheumatoid arthritis, ankylosing spondilitis, multiple sclerosis, psoriasis, connective tissue disease, chronic obstructive pulmonary disease, chronic pancreatitis, bipolar disorder and thalassemia major. All patients underwent a baseline evaluation, including a detailed medical history, typical physical examination and blood tests. For all patients in this study, the diagnosing of HCC and cirrhosis was made by histopathology and/or based on typical imaging findings for HCC in three-phase multidedector computed tomography (Activision 16-row scanner computed tomography, Toshiba Medical Systems, Otawara, Japan) or dynamic contrast-enhanced magnetic resonance imaging (Intera Master Gyroscan, Philips Medical Systems, Best, the Netherlands) with increased serum AFP concentrations (> 400 μg/L). Barcelona-Clinic Liver Cancer (BCLC) criteria were used to stage the patients with HCC[10]. The patients were divided into groups according to their tumor size, number and presence of macrovascular invasion (diameters; ≤ 3 cm, 3-5 cm and ≥ 5 cm, number; 1, 2 and 3 ≤, macrovascular invasion; yes/no). Macrovascular invasion was defined as portal vein thrombosis and was demonstrated by any imaging modality. Distinction of malignant portal vein thrombosis from benign thrombosis was made by contrast enhancement pattern at computed tomography or magnetic resonance imaging.
Blood samples
In total, 8-10 cc blood samples were withdrawn from all the participants and the sera were separated by centrifugation at 3000 rpm for 10 min. Sera were stored at -80 °C until the day of measurement.
Prolidase measurement
Serum was diluted 6-fold with 1 mmol/L Mn2+, 50 mmol/L Tris HCl buffer (pH 7.8) and preincubated at 37 °C for 2 h. The reaction mixture containing 94 mmol/L gly-pro, 50 mmol/LTris HCl buffer (pH 7.8) and preincubation serum was incubated at 37 °C for 30 min. The reaction was stopped by adding 1.0 mL of 0.45 mol/L trichloroacetic acid solution. Prolidase activity was measured in the supernatant samples using the method described by Myara et al[11], which is a modification of Chinard’s method and was calculated against proline standards. All reagents were purchased from Sigma. The intra- and inter-assay coefficients of variation were less than 10 %. Prolidase activity was reported as U/L.
AFP measurement
Serum AFP levels were measured using a chemiluminescence immunometric assay in a UniCell DXI 600 (Beckman Coulter, CA, United States) hormone analyzer. The intra-assay and inter-assay coefficients of variation (CV) were both less than < 3.22% (the within run CVs were 3.2%, 2.88%, 2.71% and the between run CVs were 3.22%, 2.04%, 2.07% for the levels of 6.53, 72.1 and 1672.88 ng/mL, respectively) and the measuring range was 0.5-3000 ng/mL. The normal range is less than 10 ng/mL in an adult.
Statistical methods
SPSS 19.0 for Windows was used for statistical analysis. Categorical variables were given with frequency and percent. Numerical variables were shown as the median, with minimum and maximum values. The Shapiro-Wilk test was used for normality tests. For nonparametric variables, the Mann Whitney U test was used for two group comparisons and the Kruskal Wallis test was used for comparisons of three or more groups. Receiver operating characteristic (ROC) curve analysis was performed to determine the predictive value of prolidase and AFP regarding tumor size, number and controls. For all statistical analyses, a P value < 0.05 indicated statistical significance.
Statistical analysis
The statistical analysis of the study was performed by author Dr. Çağatay Büyükuysal, a biostatistician and expert on data analysis. His approval of the methods are documented via his inclusion as a senior author of the manuscript.
RESULTS
Demographic and clinical data of the patients with hepatocellular carcinoma, cirrhosis patients and the healthy control group are shown in Table 1. Of the 68 patients with HCC, 31 cirrhosis patients and 33 healthy adults, 80 were males (60.6%) and 52 were females (39.4%). Prolidase and AFP values of the patients with HCC were evaluated according to tumor size, number, presence of macrovascular invasion and BCLC staging classification (Table 2). Prolidase values were significantly higher in patients with HCC compared with controls (P < 0.001). Prolidase levels were significantly associated with tumor size and number (P < 0.001, P = 0.002, respectively). Serum prolidase level in tumors ≤ 3 cm was significantly lower than those with tumor size 3-5 cm (P = 0.006) and with tumor size ≥ 5 cm (P < 0.001). In addition, the serum prolidase level in patients with tumor number ≥ 3 cm was significantly higher than those with two tumors (P = 0.008) and with one tumor (P = 0.002). However, prolidase levels showed no positive relation with presence of macrovascular invasion in patients with HCC (P = 0.575). In the BCLC staging classification, prolidase levels at various stages were significantly different in patients with HCC (P < 0.001), with stage B having the highest level of prolidase. However, a significant difference was observed when the prolidase levels in patients with HCC were compared with cirrhosis patients (P < 0.001). There was no significant difference between cirrhotic patients and the control group (P = 0.067) (Table 3).
Table 1.
Demographics and clinical variables of the groups
| Characteristic | Patients with HCC (n = 68) | Cirrhosis patients (n = 31) | Controls(n = 33) |
| Age (yr), mean ± SD | 69.1 ± 10.1 | 59.3 ± 6.3 | 51.4 ± 12.6 |
| Sex% (M/F) | 47 (69.1)/21 (30.9) | 17 (54.8)/14 (45.2) | 16 (48.5)/17 (51.5) |
| Platelet (× 1000/mm3) | 175 (27-413) | 88 (45-120) | 256 (181-396) |
| Hb (g/dL) | 11.92 ± 1.80 | 9.2 ± 1.40 | 12.67 ± 1.63 |
| INR | 1.18 (0.10-2.10) | 1.10 (0,90-1.60) | - |
| Total bilirubin (mg/dL) | 2.0 (0.30-16.90) | 1.17 (0.90-3.00) | 0.80 (0.30-2.00) |
| Albumin (g/dL) | 3.2 (2-4.6) | 3.49 (2.3-4.4) | 4.4 (4-5.3) |
| Prolidase (U/L) median (min-max) | 1179 (1080-1600) | 913 (811-1011) | 880 (816-969) |
| AFP (ng/mL) median (min-max) | 650 (2.5-2300) | 14 (2-40) | 4.2 (1-16) |
| Underlying liver disease | |||
| HBV | 34 | 11 | |
| HCV | 18 | 6 | |
| NASH | 12 | 10 | |
| Alcohol | 4 | 4 | |
HCC: Hepatocellular carcinoma; Hb: Hemoglobin; AFP: α-fetoprotein; HBV: Hepatitis B virus; HCV: Hepatitis C virus; INR: International normalized ratio; NASH: Nonalcoholic steatohepatitis.
Table 2.
Serum prolidase and α-fetoprotein levels of different tumor related factors in patients with hepatocellular carcinoma
| Variables | n = 68 | AFP (ng/L) median (min-max) | P value | Prolidase (U/L) median (min-max) | P value |
| Tumor size (cm) | |||||
| ≤ 3 | 19 | 440 (2.5-2000) | 0.032 | 1120 (1080-1250) | < 0.001 |
| 3-5 | 12 | 548 (45-1740) | 1178 (1123-1260) | ||
| ≥ 5 | 37 | 850 (73-2300) | 1219 (1113-1600) | ||
| Tumor number | |||||
| 1 | 29 | 547 (2.5-2248) | 0.038 | 1150 (1080-1310) | 0.002 |
| 2 | 13 | 550 (9-1430) | 1142 (1110-1265) | ||
| ≥ 3 | 26 | 1160 (84-2300) | 1222 (1120-1600) | ||
| Macrovascular invasion | |||||
| No | 59 | 597 (2.5-2300) | 0.502 | 1174 (1080-1600) | 0.575 |
| Yes | 9 | 770 (110-1750) | 1208 (1120-1276) | ||
| BCLC staging | |||||
| A | 20 | 470 (2.5-2000) | 0.015 | 1128 (1080-1260) | < 0.001 |
| B | 29 | 862 (78-2300) | 1219 (1123-1600) | ||
| C | 8 | 867 (110-1645) | 1174 (1120-1276) | ||
| D | 11 | 615 (73-2050) | 1150 (1113-1286) |
AFP: Alpha fetoprotein; BCLC: The Barcelona-Clinic Liver Cancer Group; HCC: Hepatocellular carcinoma.
Table 3.
Comparison of the groups for serum prolidase levels
| Prolidase (U/L) median (min-max) | P value | |
| Patients with HCC(n = 68) | 1179 (1080-1600) | < 0.001 |
| Cirrhosis patients(n = 31) | 913 (811-1011) | 0.067 |
| Controls (n = 33) | 880 (816-969) | 0.067 |
HCC: Hepatocellular carcinoma.
In terms of tumor size and number, a significant relation was found among AFP values (P = 0.032, P = 0.038, respectively). The AFP value in tumors ≥ 5cm was significantly higher (P = 0.006) than in tumors ≤ 3 cm (P = 0.013), whereas there was no significant difference between patients with tumor ≥ 5 cm and with tumors of 3-5 cm (P = 0.171). In addition, the AFP level in patients with tumor number ≥ 3 was significantly higher than those with one tumor (P = 0.028) and with two tumors (P = 0.030). However there was no positive relation between AFP and presence of macrovascular invasion in patients with HCC (P = 0.502). AFP values also significantly differed in patients at various stages of BCLC staging classification (P = 0.015): tumors at stage B had the highest levels of AFP. AFP values were significantly higher in the A stage of BCLC compared with stage B and C (P = 0.002, P = 0.028, respectively), whereas, there was no significant relation between stage A and D (P = 0.113). In terms of serum AFP values, there was a significant difference between HCC patients and cirrhotic patients (P < 0.001). AFP values of both HCC and cirrhotic patients were significantly higher than the healthy control group (P < 0.001, P < 0.001, respectively).
In patients with HCC, there was a significant correlation (r = 0.616; P < 0.001) between prolidase and AFP values regarding tumor size, number and BCLC staging classification (Figure 1), whereas the presence of macrovascular invasion [yes (r = 0.276; P = 0.472)/no (r = 0.646; P < 0.001)] did not show a positive relation with serum prolidase and AFP levels (Figure 2). The correlation between prolidase and AFP regarding tumor size [diameters; ≤ 3 cm (r = 0.746; P < 0.001), 3-5 cm (r = 0.119; P = 0.712 ) and ≥5 cm (r = 0.683; P < 0.001)], number [1 (r = 0.503; P = 0.005), 2 (r = 0.694; P = 0.008) and 3 ≤ (r = 0.662; P < 0.001)] and BCLC staging classification [stage A (r = 0.600; P = 0.005), B (r = 0.668; P < 0.001), C (r = 0.419; P = 0.301) and D (r = 0.492; P = 0.124)] are shown in Figure 2.
Figure 1.
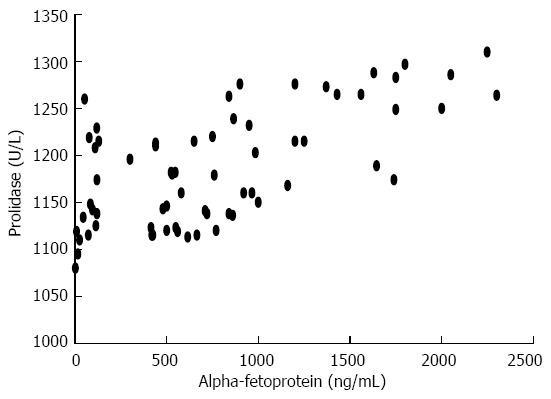
Relationship between serum prolidase and alpha fetoprotein levels in patients with hepatocellular carcinoma (r = 0.616; P < 0.01).
Figure 2.
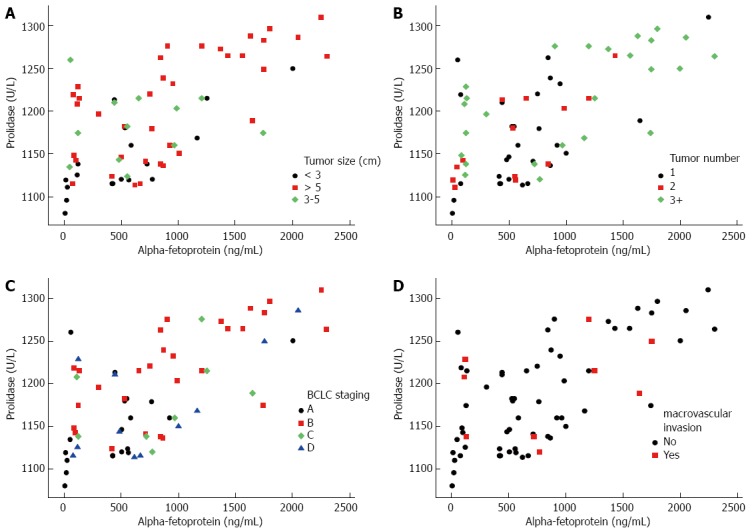
Relationship between serum prolidase and alpha fetoprotein levels in patients with hepatocellular carcinoma with regard to tumor size (A), number (B), barcelona-clinic liver cancer staging (C) and macrovascular invasion (D).
Additionally, prolidase values were significantly higher in HCC patients (n = 9) with low AFP levels (less than 80 ng/mL) compared with both cirrhotic patients and controls (P < 0.001, P < 0.001, respectively). Unfortunately, the small number of patients that had low AFP levels was not enough to perform powerful analysis for the value of prolidase.
Receiver operating characteristic (ROC) curve analysis was performed to determine the predictive value of prolidase in terms of tumor size (diameters; ≤ 3 cm and > 3 cm) and number (number; ≤ 1 and ≥ 2). The cut off values of prolidase for tumor size and tumor number were 1138 U/L, 1189 U/L, respectively. For prolidase, the areas under the curve (AUC) regarding tumor size and tumor number [AUC: 0.810 (sensitivity 83.6%; specificity 68.4%), AUC: 0.678 (sensitivity 57.8%; specificity 72.4%), respectively] are shown in Figure 3. The cut off values of AFP for tumor size (diameters; ≤ 3 cm and > 3 cm) is 580 ng/mL. For AFP, the areas under the curve (AUC) regarding tumor size [AUC: 0.680 (sensitivity 62.5%; specificity 73.6%)], are shown in Figure 3A. However, The AFP level was not discriminative in terms of tumor number (number; ≤ 1 and ≥ 2) by performing ROC curve analysis (Figure 3B). The AUC for prolidase was higher than the AUC for AFP regarding tumor size (diameters; ≤ 3 cm and > 3 cm).
Figure 3.
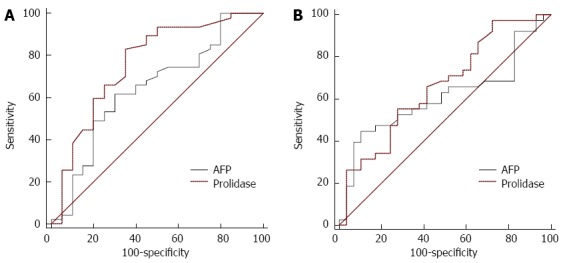
Receiver operating characteristic curves of prolidase and alpha fetoprotein levels. A: Predictive value of prolidase and α-fetoprotein (AFP) regarding tumor size (diameters; ≤ 3 cm and > 3 cm ); B: Predictive value of prolidase and AFP regarding tumor number (number; ≤ 1 and ≥ 2 ).
DISCUSSION
Prolidase enzyme is a cytosolic exopeptidase that cleaves imidodi- and imidotripeptides with C terminal proline or hydroxyproline. Prolidase plays an important role in collagen metabolism, matrix remodeling and cell growth[12]. By releasing proline or hydroxyproline, prolidase helps make them available for collagen resynthesis. Although extracellular collagenases initiate the breakdown of collagen, the final step of collagen degradation is catalyzed by intracellular prolidase. Moreover, it has been suggested that prolidase activity may be a rate-limiting factor in the regulation of collagen biosynthesis[7]. Collagen is the major component of the extracellular matrix (ECM), which represents a major barrier against invasion by neoplastic cells. Tumor cells can produce proteolytic enzymes that catalyze the breakdown of tissue barriers, which enables them to penetrate basement membranes and the ECM[8,13]. Therefore, tumor progression depends critically on the degradation of collagen and other ECM proteins[14]. The final step of collagen degradation is catalyzed by intracellular prolidase; therefore, it may be associated with neoplastic transformation. Increased prolidase activities have been observed in some of cancers, such as lung cancer[15], breast cancer[16], endometrial cancer[17], stomach cancer[18], renal cell cancer[19] and ovarian cancer[20]. On the other hand, Palka et al[6] showed reduced levels of prolidase in pancreatic cancer.
Myara et al[21] used an experimental animal model that demonstrated hepatic damage in rats by chronic CCl4 intoxication. Consequently, they observed a relationship between elevated prolidase values and hepatic fibrosis. In another study, Myara et al[11] demonstrated increased prolidase activity in chronic liver disease. Elevated prolidase levels in some common conditions, such as hepatitis B infection, hepatitis C infection, nonalcoholic steatohepatitis and alcoholic liver disease, which cause hepatic damage and hepatic fibrosis, have also been reported previously[22-25]. Although numerous non-invasive markers have been described that predict the severity of hepatic fibrosis[26], liver biopsy remains a gold standard method for assessing the severity of liver fibrosis and cirrhosis[27].
To date, prolidase levels have not been evaluated in HCC. In the present study, we assessed serum prolidase levels in patients with HCC and asked whether prolidase activity might show a correlation with AFP; thus contributing to early diagnosis of HCC while following up patients. We observed that serum prolidase levels were higher in HCC than in healthy volunteers, and elevated prolidase levels showed a significant relationship between size and number of HCCs. This might be a consequence of tumor pathogenesis, which might reflect progression of HCC. Moreover, there are few studies that have revealed a correlation between the serum prolidase levels and stage of liver fibrosis according to the results of liver biopsy[24,28-30]. In contrast, Duygu et al[22,23] did not observe a relationship between the degree of liver fibrosis and serum prolidase levels in patients with chronic hepatitis B and C. In this study, we observed a significant difference when the prolidase levels in cirrhosis patients were compared with patients with HCC. This might indicate enhanced turnover of collagen synthesis in patients with HCC, which can be affected by the degree of neoplastic transformation during the development of HCC, on the basis of the cirrhotic process. By contrast, we did not observe a significant difference between cirrhosis patients and healthy volunteers. Hence, our study needs to be validated by further large population studies to illustrate the changing prolidase levels among patients with liver diseases.
However, the diagnostic value of is being questioned because of poor sensitivity and specificity. The diagnosis of HCC without pathological confirmation is achieved by analyzing serum AFP levels combined with imaging techniques, including ultrasonography, magnetic resonance imaging and computerized tomography[5]. The relationship between serum AFP levels and tumor characteristics, such as tumor size, tumor number or macrovascular invasion, has been evaluated in many studies[31-34]. Liu et al[5] showed a relationship between AFP levels and tumor size, however, AFP concentrations were not correlated with tumor number in their study. Wang et al[35] and Kasahara et al[36] have also reported a significant relationship between tumor size and serum AFP levels. Furihata et al[37] observed a significant correlation between serum AFP levels and both size and number of HCCs. Furthermore, AFP elevation in HCC was associated with macrovascular invasion in some studies[38,39]. In the present study, serum AFP levels were correlated with tumor size, which is consistent with the results of previous studies[5,35,36]. There was also a relationship between AFP levels and the tumor number, similar to the other studies[37,40]. Moreover, macrovascular invasion was not associated with high AFP levels, contrary to previous studies[38,39]. There were only nine patients (13.2%) with macrovascular invasion, which was insufficient to produce powerful statistical results.
In the present study, we observed elevated levels of serum prolidase and AFP in patients with HCC, although the absence of an increase in these markers does not exclude the diagnosis of HCC. Notably, a similar relationship between AFP concentrations and the size and number of tumors was observed for serum prolidase levels. Furthermore, in terms of tumor size (diameters; ≤ 3 cm and > 3 cm) and number (number; ≤ 1 and ≥ 2), serum prolidase activity exhibited higher sensitivity and specificity than AFP values in HCC patients. We believe that tumor burden and aggressiveness are the main characteristics that may explain the elevated prolidase levels in HCC patients.
The findings of this study suggested that increased serum prolidase levels might reflect increased collagen turnover associated with the tumor burden in HCC patients. Increased prolidase activity may, in part, play a role in the pathogenesis of HCC. Therefore, consideration of the levels of both serum prolidase and AFP would contribute to the early diagnosis of HCC. Our study has several limitations, such as the low number of patients and no long-term outcomes of HCC patients regarding prolidase levels. Nevertheless, future comprehensive studies covering larger populations are needed to determine the value of prolidase activity during follow-up of the patients with chronic liver diseases.
ACKNOWLEDGMENTS
The authors would like to thank the patients and the healthy subjects who willingly participated in the study.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer death. Despite recent developments in surgery and medical therapy that have significantly improved the outcome of patients with operable and advanced HCC, HCC remains a major health problem worldwide. Screening strategies including ultrasound imaging and serum α-fetoprotein (AFP) concentration are useful to detect the early stage of HCC development. Via neoplastic transformation, tumor cells acquire the ability to penetrate basement membranes and the extracellular matrix (ECM), involving breakdown of collagen and other ECM proteins. Prolidase is a matrix metalloproteinase that cleaves the bonds of dipeptides containing proline (X-Pro) during collagen metabolism. Several investigators have reported enhanced serum levels of prolidase in certain of cancers, such as lung cancer, breast cancer, endometrial cancer, stomach cancer, renal cell cancer and ovarian cancer.
Research frontiers
Prolidase is an important enzyme that plays a vital role in collagen turnover, matrix remodeling and cell growth. It is thought that the growth, invasion and spread of tumor cells depend on the breakdown of collagen and other ECM proteins. This behavior of tumor cells suggests that prolidase activity may reflect the tumor burden and aggressiveness. AFP is the most commonly used serological marker worldwide with radiological imaging for diagnosing hepatocellular carcinoma. Measuring the prolidase activity as complementary test is worth exploring for possible use in detecting HCC at earlier stages. In conclusion, a comprehensive study with a larger samples size is needed to elucidate the value of prolidase activity during follow-up of the patients with chronic liver diseases.
Innovations and breakthroughs
The authors assessed the correlation between serum prolidase and AFP levels with regard to the features of HCC in patients. The present study had a large enough sample size such statistically relevant results could be obtained. For clinical practice, this article may be beneficial for physicians by permitting detection of HCC at earlier stages, as long as the serum prolidase results are verified by other diagnosis techniques.
Applications
The present study assessed the predictive relationship between prolidase activity and HCC progression, which may represent a promising approach to detect HCC at earlier stages, allowing physicians to make appropriate clinical decisions.
Terminology
Prolidase is an important enzyme that cleaves the bonds of dipeptides containing proline (X-Pro) and plays a vital role in collagen turnover, matrix remodeling and cell growth. Metalloproteinases initiate the breakdown of collagen; however, the final step of collagen degradation is mediated by prolidase (E.C.3.4.13.9). AFP is a glycoprotein and is mainly expressed in the fetal yolk sac and liver, but not in normal adult tissues. Serum AFP elevation is accompanies by some kinds of cancer, such as gastric cancer, lung cancer, pancreatic cancer, testicular carcinoma and, particularly, HCC.
Peer-review
This preliminary study, having a large enough sample, concerns the possible value of prolidase activity to detect HCC at earlier stages, as long as the serum prolidase results are verified by other diagnosis techniques. It carries a good message for the reader and may represent a valuable contribution to the literature.
Footnotes
Ethics approval: The study was reviewed and approved by the Clinical Research Ethics Board of the Bülent Ecevit University.
Clinical trial registration: The study was reviewed and approved by the Clinical Research Ethics Board of the Bülent Ecevit University.
Informed consent: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest: There are no conflicts of interest to report for any study authors.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at aysesemra@hotmail.com. The presented data cannot be linked to individuals and risk of personal identification is minimal as such.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 27, 2014
First decision: November 14, 2014
Article in press: February 13, 2015
P- Reviewer: Li Z, Troncoso MF, Xu JJ S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN
References
- 1.Shin JW, Chung YH. Molecular targeted therapy for hepatocellular carcinoma: current and future. World J Gastroenterol. 2013;19:6144–6155. doi: 10.3748/wjg.v19.i37.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802–1806. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811–1819. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palka J, Surazynski A, Karna E, Orlowski K, Puchalski Z, Pruszynski K, Laszkiewicz J, Dzienis H. Prolidase activity disregulation in chronic pancreatitis and pancreatic cancer. Hepatogastroenterology. 2002;49:1699–1703. [PubMed] [Google Scholar]
- 7.Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase-dependent regulation of collagen biosynthesis. Amino Acids. 2008;35:731–738. doi: 10.1007/s00726-008-0051-8. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson GL, Poste G. Tumor cell diversity and host responses in cancer metastasis--part II--host immune responses and therapy of metastases. Curr Probl Cancer. 1983;7:1–42. doi: 10.1016/s0147-0272(83)80005-1. [DOI] [PubMed] [Google Scholar]
- 9.Bolon I, Gouyer V, Devouassoux M, Vandenbunder B, Wernert N, Moro D, Brambilla C, Brambilla E. Expression of c-ets-1, collagenase 1, and urokinase-type plasminogen activator genes in lung carcinomas. Am J Pathol. 1995;147:1298–1310. [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 11.Myara I, Myara A, Mangeot M, Fabre M, Charpentier C, Lemonnier A. Plasma prolidase activity: a possible index of collagen catabolism in chronic liver disease. Clin Chem. 1984;30:211–215. [PubMed] [Google Scholar]
- 12.Jackson SH, Dennis AW, Greenberg M. Iminodipeptiduria: a genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can Med Assoc J. 1975;113:759, 762–763. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WT. Membrane proteases: roles in tissue remodeling and tumour invasion. Curr Opin Cell Biol. 1992;4:802–809. doi: 10.1016/0955-0674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43 Suppl:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 15.Karna E, Surazynski A, Palka J. Collagen metabolism disturbances are accompanied by an increase in prolidase activity in lung carcinoma planoepitheliale. Int J Exp Pathol. 2000;81:341–347. doi: 10.1111/j.1365-2613.2000.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cechowska-Pasko M, Pałka J, Wojtukiewicz MZ. Enhanced prolidase activity and decreased collagen content in breast cancer tissue. Int J Exp Pathol. 2006;87:289–296. doi: 10.1111/j.1365-2613.2006.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arioz DT, Camuzcuoglu H, Toy H, Kurt S, Celik H, Aksoy N. Serum prolidase activity and oxidative status in patients with stage I endometrial cancer. Int J Gynecol Cancer. 2009;19:1244–1247. doi: 10.1111/IGC.0b013e3181af711e. [DOI] [PubMed] [Google Scholar]
- 18.Guszczyn T, Sobolewski K. Deregulation of collagen metabolism in human stomach cancer. Pathobiology. 2004;71:308–313. doi: 10.1159/000081726. [DOI] [PubMed] [Google Scholar]
- 19.Pirinççi N, Kaba M, Geçit I, Günes M, Yüksel MB, Tanik S, Arslan A, Demir H. Serum prolidase activity, oxidative stress, and antioxidant enzyme levels in patients with renal cell carcinoma. Toxicol Ind Health. 2013:Epub ahead of print. doi: 10.1177/0748233713498924. [DOI] [PubMed] [Google Scholar]
- 20.Camuzcuoglu H, Arioz DT, Toy H, Kurt S, Celik H, Aksoy N. Assessment of preoperative serum prolidase activity in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2009;147:97–100. doi: 10.1016/j.ejogrb.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Myara I, Miech G, Fabre M, Mangeot M, Lemonnier A. Changes in prolinase and prolidase activity during CCl4 administration inducing liver cytolysis and fibrosis in rat. Br J Exp Pathol. 1987;68:7–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Duygu F, Aksoy N, Cicek AC, Butun I, Unlu S. Does prolidase indicate worsening of hepatitis B infection? J Clin Lab Anal. 2013;27:398–401. doi: 10.1002/jcla.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duygu F, Koruk ST, Karsen H, Aksoy N, Taskin A, Hamidanoglu M. Prolidase and oxidative stress in chronic hepatitis C. J Clin Lab Anal. 2012;26:232–237. doi: 10.1002/jcla.21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayadibi H, Gültepe M, Yasar B, Ince AT, Ozcan O, Ipcioglu OM, Kurdas OO, Bolat B, Benek YZ, Guveli H, et al. Diagnostic value of serum prolidase enzyme activity to predict the liver histological lesions in non-alcoholic fatty liver disease: a surrogate marker to distinguish steatohepatitis from simple steatosis. Dig Dis Sci. 2009;54:1764–1771. doi: 10.1007/s10620-008-0535-0. [DOI] [PubMed] [Google Scholar]
- 25.Brosset B, Myara I, Fabre M, Lemonnier A. Plasma prolidase and prolinase activity in alcoholic liver disease. Clin Chim Acta. 1988;175:291–295. doi: 10.1016/0009-8981(88)90105-2. [DOI] [PubMed] [Google Scholar]
- 26.Afdhal NH. Biopsy or biomarkers: is there a gold standard for diagnosis of liver fibrosis? Clin Chem. 2004;50:1299–1300. doi: 10.1373/clinchem.2004.035899. [DOI] [PubMed] [Google Scholar]
- 27.Gabrielli GB, Capra F, Casaril M, Squarzoni S, Tognella P, Dagradi R, De Maria E, Colombari R, Corrocher R, De Sandre G. Serum laminin and type III procollagen in chronic hepatitis C. Diagnostic value in the assessment of disease activity and fibrosis. Clin Chim Acta. 1997;265:21–31. doi: 10.1016/s0009-8981(97)00103-4. [DOI] [PubMed] [Google Scholar]
- 28.Horoz M, Aslan M, Bolukbas FF, Bolukbas C, Nazligul Y, Celik H, Aksoy N. Serum prolidase enzyme activity and its relation to histopathological findings in patients with non-alcoholic steatohepatitis. J Clin Lab Anal. 2010;24:207–211. doi: 10.1002/jcla.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Büyükhatipoğlu H, Etkar İ, Eren MA, Demir M, Taşkın A, Aksoy N. The relationship between prolidase enzyme activity and ultrasonographic grading in hepatosteatosis. J Harran University Med Facul. 2010;7:54–57. [Google Scholar]
- 30.Tarçin O, Gedik N, Karakoyun B, Tahan V, Sood G, Celikel C, Tözün N. Serum prolidase and IGF-1 as non-invasive markers of hepatic fibrosis during four different periods after bile-duct ligation in rats. Dig Dis Sci. 2008;53:1938–1945. doi: 10.1007/s10620-007-0073-1. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 32.Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, Shimamoto F, Asahara T. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311–316. doi: 10.1002/jso.20661. [DOI] [PubMed] [Google Scholar]
- 33.Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:900–905. doi: 10.1016/j.ejso.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Lu XY, Xi T, Lau WY, Dong H, Xian ZH, Yu H, Zhu Z, Shen F, Wu MC, Cong WM. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol. 2011;137:567–575. doi: 10.1007/s00432-010-0909-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, Lin TJ, Liao LY. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6115–6119. doi: 10.3748/wjg.v11.i39.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasahara A, Hayashi N, Fusamoto H, Kawada Y, Imai Y, Yamamoto H, Hayashi E, Ogihara T, Kamada T. Clinical evaluation of plasma des-gamma-carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci. 1993;38:2170–2176. doi: 10.1007/BF01299891. [DOI] [PubMed] [Google Scholar]
- 37.Furihata T, Sawada T, Kita J, Iso Y, Kato M, Rokkaku K, Shimoda M, Kubota K. Serum alpha-fetoprotein level per tumor volume reflects prognosis in patients with hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology. 2008;55:1705–1709. [PubMed] [Google Scholar]
- 38.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 39.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 40.Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–782. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]


