Abstract
Leucine-to-phenylalanine substitution at residue L1014 in the voltage-gated sodium channel, target site of action for dichlorodiphenyltrichloroethane (DDT) and pyrethroids, is the most common knockdown resistance (kdr) mutation reported in several insects conferring resistance against DDT and pyrethroids. Here, we report presence of two coexisting alternative transversions, A>T and A>C, on the third codon position of L1014 residue in malaria vector Anopheles subpictus Grassi (species A) from Jamshedpur (India), both leading to the same amino acid substitution of Leu-to-Phe with allelic frequencies of 19 and 67%, respectively. A single primer-introduced restriction analysis–polymerase chain reaction (PIRA-PCR) was devised for the identification of L1014F-kdr mutation in An. subpictus resulting from either type of point mutation. Genotyping of samples with PIRA-PCR revealed high frequency (82%) of L1014F-kdr mutation in the study area.
Keywords: Anopheles subpictus, knockdown resistance, sodium channel, malaria
Introduction
Anopheles subpictus Grassi sensu lato is a widely distributed species in the Oriental region and in some countries of the Australasian region and has been considered a primary vector of malaria in Australasian zone, Celebes, Portuguese Timor, and South East Asia (Chandra et al. 2010). Natural infection of malaria parasite in this species has been recorded in many states of India (Chandra et al. 2010).
Knockdown resistance (kdr) is one of the mechanisms of resistance against pyrethroids and dichlorodiphenyltrichloroethane, which is conferred by the mutation(s) in the voltage-gated sodium channel (VGSC)—the target site of action for these insecticides. Leucine-to-phenylalanine mutation at residue L1014 is the most common mutation reported in insects including anophelines (Davies and Williamson 2009), mostly because of A>T substitution on the third codon position of residue L1014 (TTA) except in Anopheles sinensis Wiedemann (Kang et al. 2012). Here, we report presence of two coexisting alternative point mutations in An. subpictus on the third codon position of L1014 residue, i.e., A>T and A>C, both leading to the same amino acid substitution of Leu–Phe. Earlier, L1014F mutation in this species has been reported from Sri Lanka arising from A>T substitution (Karunaratne et al. 2007) and from Indonesia, where the type of substitution is not defined (Syafruddin et al. 2010). The substitution A>C has not been reported.
Material and Methods
Mosquito Collection and DNA Isolation
Adult female An. subpictus were collected from two villages, namely, Kasidih and Karandih (situated in close proximity, near longitude 86° 2′ N and latitude 22° 8′ E) of district Jamshedpur (Chhattisgarh, India). The genomic DNA from individual mosquitoes (abdomen containing spermatheca was removed) was isolated according to Livak’s method (1984), eluted in 400 µl of TE buffer and stored at 4°C.
DNA Sequencing
IIS6 transmembrane segment of the VGSC was PCR-amplified from genomic DNA using primers KdrF (5′-GGA CCA YGA TTT GCC AAG ATG-3′) and KdrR (5′-CGA AAT TGG ACA AAA GCA AAG-3′) (Singh et al. 2009), purified using QIAquick PCR purification kit (Qiagen Inc. Valencia, CA, USA) and sequenced using BigDye Terminator v. 3.1 (Applied Biosystems). The DNA sequence chromatograms were analyzed and edited using the Finch TV software.
For the identification of cryptic species of An. subpictus, 24 and 10 mosquitoes were sequenced for D3 domain of 28S rDNA (28S-d3) and ITS2 rDNA, respectively, according to the method described by Singh et al. (2010).
Primer-Introduced Restriction Analysis–Polymerase Chain Reaction (PIRA-PCR) Strategy for L1014F-kdr Genotyping
A PIRA-PCR strategy was developed to discriminate L1014F mutation, arising from the A>C or A>T mutations on third position of codon, from the wild type. We designed a forward primer SubF (5′-TCT TAG CTA CGG TAG TAA TAG GAA AaT T-3′) in which a mismatch, T-to-A, was incorporated at the third base from 3′ terminus to create a XapI restriction site (5′-R|AATTY-3′) in the PCR-extension product in the presence of “C” or “T” nucleotide on the third codon position of L1014 residue in the template DNA. The introduced mismatch in the primer has been shown in lowercase in the primer’s sequence mentioned above. The reverse primer, SubR (5′-CAC CTG CAA AAC AAT AAC ATG TTc AAT TC-3′), was designed from the intron region downstream to kdr-locus. Because the intron naturally contained a XapI restriction site, reverse primer was designed from the restriction site and a deliberate mismatch (A-to-C) was introduced in the primer (on the sixth base from the 3′ terminus, indicated above in lowercase) to eliminate this restriction site in the amplified product.
The expected PCR product size formed by the primers is 102 bp and is cleaved when digested with XapI into 74 and 24 bp (each with 4 bp over hang) fragments in presence of “T” or “C” nucleotide on third codon position of L1014 residue (coding Phe). No cleavage occurs when digested with XapI in case of the wild type genotype. Thus, the undigested PCR amplicon (103 bp) will represent L1014 genotype and the cleaved products of 74 and 24 bp will represent 1014F. The 24-bp product will generally be invisible on agarose gel.
Genotyping of L1014F-kdr
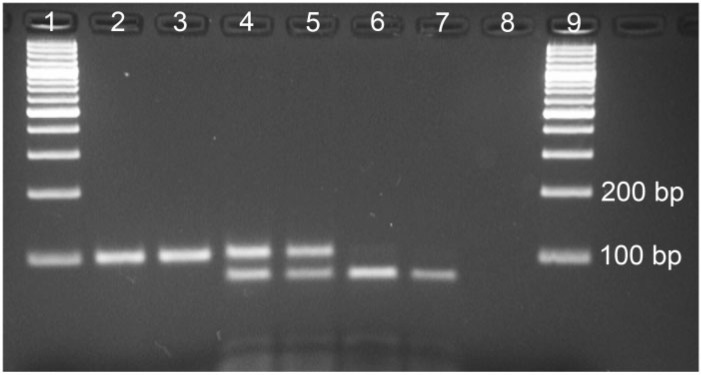
For the identification of kdr mutation, PCR amplification was carried out using primers SubF and SubR in a PCR reaction mixture of 25 μl containing 1× buffer, 1.5 mM MgCl2, 200 μM of each dNTP, 0.5 μM of each primer, and 0.625 unit of Taq DNA polymerase. The PCR conditions were: initial denaturation at 95°C for 5 min, followed by 35 cycles each with denaturation at 95°C for 30 s, annealing at 47°C for 30 s, and extension at 72°C for 40 s, and a final extension of 7 min at 72°C. The PCR product was subjected to restriction fragment analysis with restriction enzyme XapI (Fermentas). The reaction mixture (20 μl) contained 10 μl of the PCR product, 5 units of XapI enzyme, and 1× buffer. The mixture was incubated at 37°C for 4 h or overnight. The products were run on 2.5% agarose gel containing ethidium bromide, and fragments were visualized under ultraviolet illumination (Fig. 1).
Fig. 1.
Gel photograph showing various genotypes of L1014-kdr. Lanes 1 and 9, DNA ladder; lanes 2 and 3, L/L; lanes 4 and 5, L/F; lanes 6 and 7, F/F; lanes 8, negative control.
Statistical Analysis
The Hardy–Weinberg equilibrium was tested by Fisher’s exact test using software Arlequin v. 3.1 (Excoffier et al. 2005).
Results
Identification of Cryptic Species
All the mosquitoes sequenced for ITS2 rDNA (n = 24) had identical sequence and were 100% similar to “Species A” of Sri Lanka (Surendran et al. 2013). In the absence of chromosomal inversion genotype, we consider them as putative species A. Sequencing of 28S-d3 rDNA (n = 10) also did not reveal inter-individual difference and had 100% homology with sequence data of Sri Lankan species A (Singh et al., unpublished), which is suggestive of presence of only one species.
DNA Sequence Analysis of VGSC
We sequenced domain IIS6 of VGSC targeting L1014 residue from 53 An. subpictus individuals. The DNA sequences of 52 individuals with read length >200 bp are deposited in the GenBank (accession numbers KF023519–KF023570). All sequences had an intronic region of 67 bp. DNA sequence analysis revealed presence of two nonsynonymous mutations, i.e., A>C and A>T on the third codon position of Leu1014 residue both leading to Leu (TTA)-to-Phe (TTT or TTC) amino acid substitution. The number and proportions of different L1014 genotypes, as revealed by DNA sequencing, are presented in Table 1. The allelic frequencies of alleles TTA, TTT, and TTC were 0.14, 0.19, and 0.67, respectively. Thus, the combined allelic frequency of mutant alleles coding the Phe amino acid (TTT and TTC) was 0.86. The different genotypes were in Hardy–Weinberg equilibrium (He = 0.5004; Ho = 0.4717; P = 0.656). No other mutations were recorded except for a single substitution in intron (c.3045 + 48A>G) in one sample, which was heterozygote for A/G.
Table 1.
Genotyping of An. subpictus populations through DNA sequencing
| Village and district | Nucleotide genotypes |
||||||
|---|---|---|---|---|---|---|---|
| A/A | A/T | A/C | T/C | T/T | C/C | Total | |
| Kasidih, Jamshedpur | 2 | 1 | 7 | 10 | 2 | 14 | 36 |
| Karandih, Jamshedpur | 0 | 1 | 2 | 4 | 0 | 10 | 17 |
| Total | 2 (3.8%) | 2 (3.8%) | 9 (17.0%) | 14 (26.4%) | 2 (3.8%) | 24 (45.3%) | 53 |
Genotyping of An. subpictus Population With PIRA-PCR
In total, 101 samples were subjected to genotyping through PIRA-PCR, of which 2 samples failed to amplify. The genotyping results of the remaining 99 samples are shown in Table 2. The allelic frequency of 1014F was 0.82.
Table 2.
Genotyping of An. subpictus populations through PIRA-PCR assay
| Village and district | Amino acid genotypes |
|||
|---|---|---|---|---|
| Leu/Leu | Leu/Phe | Phe/Phe | Total | |
| Kasidih, Jamshedpur | 5 | 14 | 35 | 54 |
| Karandih, Jamshedpur | 0 | 12 | 33 | 45 |
| Total (%) | 5 (5.05) | 26 (26.26) | 68 (68.69) | 99 |
Specificity of PIRA-PCR
The specificity of PIRA-PCR was evaluated by comparing genotyping results of the 53 samples that were sequenced for domain IIS6. The two samples genotyped as homozygous wild (nt genotype TTA/TTA) through DNA sequencing were genotyped as Leu/Leu by PIRA-PCR. All TTA/TTC (n = 9) and TTA/TTT (n = 2) were genotyped as Leu/Phe. All TTT/TTT (n = 2), TTC/TTC (n = 24), and TTC/TTT (n = 14) were genotyped as Phe/Phe. Thus, the PIRA-PCR results were in agreement with sequencing data.
Discussion
Amongst several mutations reported in the VGSC of insects, L1014F is the most common mutation reported in a wide array of insects of agricultural and health importance unless codon constraint exists. In all anophelines reported so far, except in An. sinensis (Kang et al. 2012), the L1014 is coded by TTA, where the L1014F-kdr substitution is known to be conferred by A>T mutation on third codon position. The other possible alternative nucleotide substitution leading to L1014F mutation is A>C, which is very uncommon in insects and has not been reported earlier in any anopheline. In this study, we report the presence of A>C substitution coexisting with A>T substitution. Such co-occurrence is rare and is reported in Culex quinquefasciatus (Say) in Sri Lanka (Wondji et al. 2008). A>C mutation in this species also is predominant over A>T, as we found in An. subpictus. Besides the fact that there is no evident difference in mutation rate between A-to-T and A-to-C transversions, the latter is very rare for L1014-kdr loci in insects. However, occurrence of A>C mutation in other anopheline can not be ruled out and it is possible that such mutation if present in anopheline in low frequency (in heterozygous condition) is being overlooked by PCR-based methods that are developed while keeping in mind only A>T substitution for L1014 mutation. The presence of such mutation should therefore be examined by sequencing some of the representative samples from different populations beside PCR genotyping. In addition, diagnostic assays should be designed to detect both the possible nucleotide substitutions causing L1014F mutation (TTT/TTC).
Presence of two different type of mutations in a population may be because of two independent origin of mutations in an interbreeding population or in two different inbreeding populations. We ruled out latter situation because of insignificant difference in expected and observed heterozygosities (pHWE = 0.656; Fisher’s exact test) for kdr alleles and low inbreeding coefficient (0.048). Absence of variation in rDNA and lack of polymorphism in VGSC intron downstream of the L1014 locus also explain presence of random mating species. The intron-sequence downstream of the L1014 locus is highly variable in house flies and several other insects (Rinkevich et al. 2012), which was exploited to study the origin of kdr-type mutations, but the same was found highly conserved in An. subpictus.
Anopheles subpictus s.l. has been recognized as a complex of four sibling species, A through D, identified on the basis of arrangement of two inversions present on polytene chromosome X (Suguna 1982, Suguna et al. 1994) and also on the morphological basis (Reuben and Suguna 1983, Suguna et al. 1994). However, doubt has been raised on the validity of such morphological characteristics (Subbarao et al. 1988; Surendran et al. 2013). The sequencing of ITS2-rDNA and 28S rDNA of randomly selected samples of An. subpictus from this study revealed their 100% homology with species A of Sri Lanka. However, we consider them as putative species A until validation of rDNA sequence data of chromosomally identified specimens.
For routine monitoring of kdr mutations, a reliable and simple diagnostic method is needed. Several PCR-based methods are available for kdr detection that has been reviewed by Bass et al (2007). The PIRA-PCR assay developed here, unlike other assays, can detect L1014F mutation resulting from all possible alternative point mutations. Further, the PIRA-PCR is superior over conventional ASPCR or other PCR-based assays in being specific. The specificity of the PCR or hybridization assays based on a single single-nucleotide polymorphism (SNP) requires high stringency as a single SNP often does not prevent extension or hybridization, whereas in PIRA-PCR, there is no such contemplation.
Here, we recorded a high frequency of L1014F-kdr mutation (0.82) through PIRA-PCR in an An. subpictus population, which is almost near fixation. In India, such a high frequency of kdr mutations has not been reported earlier in any other anopheline where kdr mutations are reported (Singh et al. 2009, 2010, 2011).
Acknowledgments
We are thankful to Mrs. Alka Kapoor for the genotyping of samples. The financial support to C.L.D. was provided by Defence Research Laboratory, Gwalior and National Institute of Health grant U19AI099676.
References Cited
- Bass C., Nikou D., Donnelly M. J., Williamson M. S., Ranson H., Ball A., Vontas J., Field L. M. 2007. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar. J. 6: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G., Bhattacharjee I., Chatterjee S. 2010. A review on Anopheles subpictus Grassi–a biological vector. Acta Trop. 115: 142–154. [DOI] [PubMed] [Google Scholar]
- Davies T.G.E., Williamson M. S. 2009. Interactions of pyrethroids with the voltage-gated sodium channel. Bayer Crop Sci. J. 62: 159–178. [Google Scholar]
- Excoffier L., Laval G., Schneider S. 2005. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- Kang S., Jung J., Lee S., Hwang H., Kim W. 2012. The polymorphism and the geographical distribution of the knockdown resistance (kdr) of Anopheles sinensis in the Republic of Korea. Malar. J. 11: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaratne S.H.P.P., Hawkes N. J., Perera M.D.B., Ranson H., Hemingway J. 2007. Mutated sodium channel genes and elevated monooxygenases are found in pyrethroid resistant populations of Sri Lankan malaria vectors. Pest Biochem. Physiol. 88: 108–113. [Google Scholar]
- Livak K. J. 1984. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107: 611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R., Suguna S. G. 1983. Morphological differences between sibling species of the taxon Anopheles subpictus Grassi in India, with notes on relationship with known forms. Mosq. Syst. 15: 117–126. [Google Scholar]
- Singh O. P., Nanda N., Dev V., Bali P., Sohail M., Mehrunnisa A., Adak T., Dash A. P. 2010. Molecular evidence of misidentification of Anopheles minimus as Anopheles fluviatilis in Assam (India). Acta Trop. 113: 241–244. [DOI] [PubMed] [Google Scholar]
- Singh O. P., Bali P., Hemingway. J., Subbarao S. K., Das A. P., Adak T. 2009. PCR-based methods for the detection of L1014 kdr mutation in Anopheles culicifacies sensu lato. Malar. J. 8: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. P., Dykes C. L., Das M. K., Pradhan S., Bhatt R. M., Agrawal O. P., Adak T. 2010. Presence of two alternative kdr-like mutations, L1014F and L1014S, and a novel mutation, V1010L, in the voltage gated Na+ channel of Anopheles culicifacies from Orissa, India. Malar. J. 9: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. P., Dykes C. L., Lather M., Agrawal O. P., Adak T. 2011. Knock-down resistance (kdr)-like mutations in the voltage gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection. Malar. J. 10: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich F. D., Hedtke S. M., Leichter C. A., Harris S. A., Su C., Brady S. G., Taskin V., Qiu X., Scott J. G. 2012. Multiple origins of kdr-type resistance in the house fly, Musca domestica. PLoS ONE, 7: e52761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao S. K., Vasantha K., Sharma V. P. 1988. Cyto-taxonomy of certain malaria vectors in India, pp. 25–37. In Service M. W. (ed.), Biosystematics of haematophagous insects. Systematics Association Special vol. 37 Oxford Clarendon Press, Oxford, UK. [Google Scholar]
- Suguna S. G. 1982. Cytological and morphological evidences for sibling species in Anopheles subpictus Grassi L. J. Com. Dis. 14, 1–8. [PubMed] [Google Scholar]
- Suguna S. G., Gopala Rathinam K., Rajavel A. R., Dhanda V. 1994. Morphological and chromosomal descriptions of new species in the Anopheles subpictus complex, Med. Vet. Entomol. 8: 88–94. [DOI] [PubMed] [Google Scholar]
- Surendran S. N., Singh O. P., Jude P. J., Ramasamy R. 2010. Genetic evidence for malaria vectors of the Anopheles sundaicus complex in Sri Lanka with morphological characteristics attributed to Anopheles subpictus species B. Malar. J. 9: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S. N., Sarma D. K., Jude P. J., Kemppainen P., Kanthakumaran N., Gajapathy K., Peiris L. B., Ramasamy R., Walton C. 2013. Molecular characterization and identification of members of the Anopheles subpictus complex in Sri Lanka. Malar. J. 12: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafruddin D., Hidayati A. P., Asih P. B., Hawley W. A., Sukowati S., Lobo N. F. 2010. Detection of 1014F kdr mutation in four major Anopheline malaria vectors in Indonesia. Malar. J. 9: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondji C. S., Priyanka De Silva W. A., Hemingway J., Ranson H., Parakrama Karunaratne S. H. 2008. Characterization of knockdown resistance in DDT- and pyrethroid-resistant Culex quinquefasciatus populations from Sri Lanka. Trop. Med. Int. Health 13: 548–555. [DOI] [PubMed] [Google Scholar]