Abstract
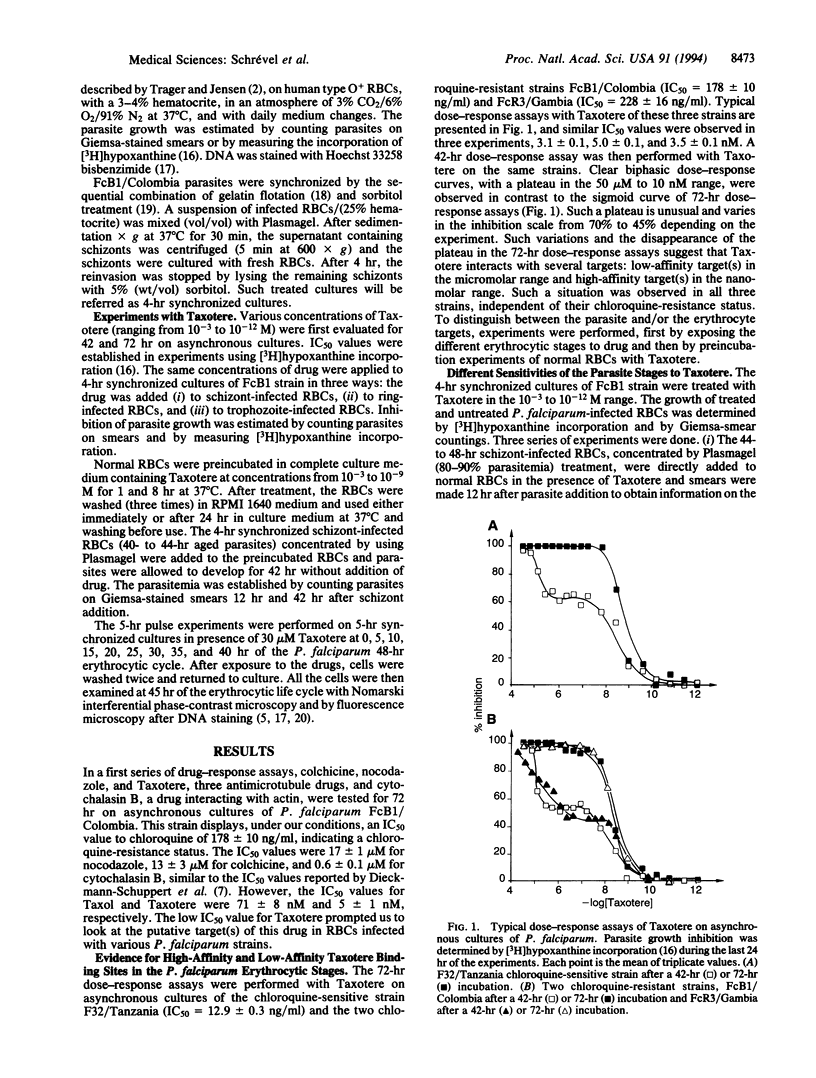
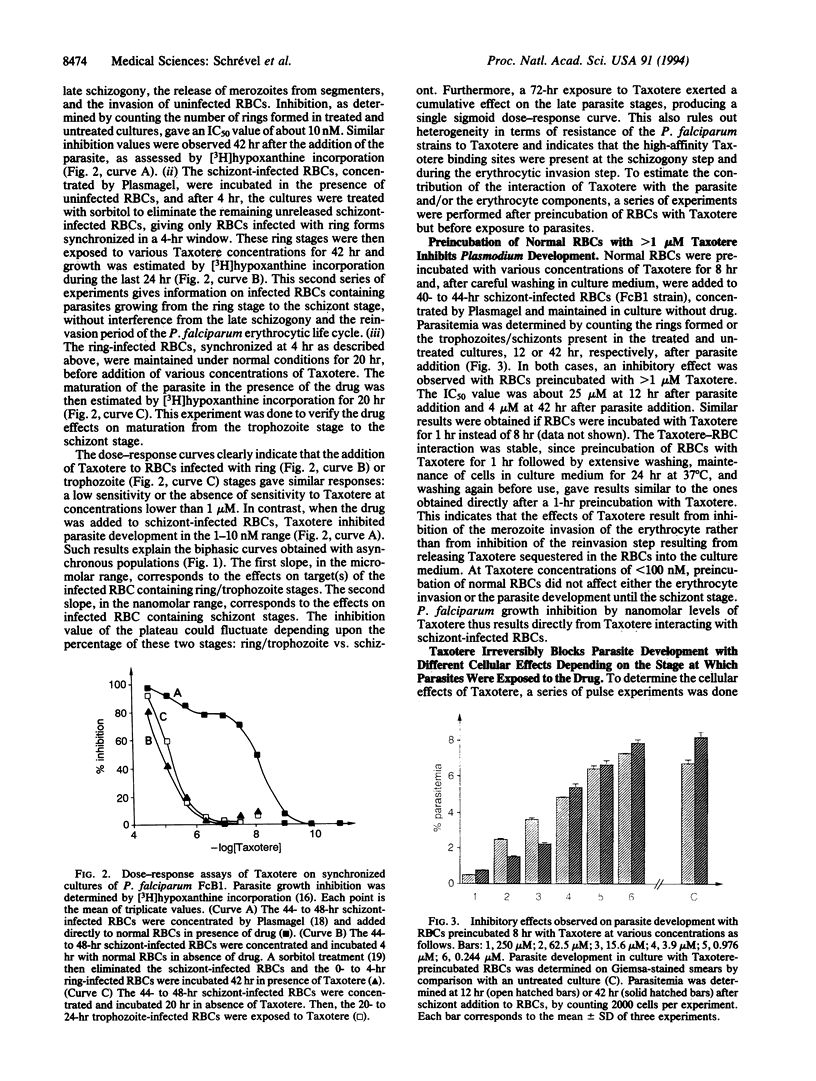
Taxotere (docetaxel) inhibits Plasmodium falciparum erythrocytic development in vitro at nanomolar concentrations, both in chloroquine-sensitive (F32/Tanzania) and chloroquine-resistant (FcB1/Colombia, FcR3/Gambia) strains. The dose-response assays performed on asynchronous cultures during 42 hr showed clear biphasic curves with a plateau from 50 microM to 10 nM and a single sigmoid curve with a concentration inhibiting 50% of growth (IC50) of 3-6 nM observed after a 72-hr incubation. Addition of Taxotere to different stages of FcB1 revealed two types of targets: one type on ring/trophozoite-infected erythrocytes (RBCs), at the micromolar level, and another type on schizont-infected RBCs with Taxotere at micromolar concentrations inhibited the merozoite invasion of erythrocytes and parasite growth. These Taxotere-RBC interactions were stable, at least for 1 day. Pulse experiments of 5 hr with Taxotere efficiently inhibit parasite development regardless of the period of the parasite's erythrocytic life cycle. However, different cellular effects were obtained depending upon periods of drug incubations. The inhibition of P. falciparum development by Taxotere should provide additional strategies to block parasite development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Wittner M., Nadler J. P., Horwitz S. B., Dennis J. E., Schiff P. B., Tanowitz H. B. Taxol, a microtubule stabilizing agent, blocks the replication of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4571–4575. doi: 10.1073/pnas.78.7.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. G., Lassman L. P., Pearce G. W., Walton J. N. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. J Neurol Sci. 1970 Feb;10(2):107–131. doi: 10.1016/0022-510x(70)90013-4. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A., Franklin R. M. Compounds binding to cytoskeletal proteins are active against Plasmodium falciparum in vitro. Cell Biol Int Rep. 1989 May;13(5):411–418. doi: 10.1016/0309-1651(89)90135-5. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990 Apr 20;248(4953):370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Extra J. M., Rousseau F., Bruno R., Clavel M., Le Bail N., Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993 Mar 1;53(5):1037–1042. [PubMed] [Google Scholar]
- Ginsburg H. Some reflections concerning host erythrocyte-malarial parasite interrelationships. Blood Cells. 1990;16(2-3):225–235. [PubMed] [Google Scholar]
- Gordon-Weeks P. R., Mansfield S. G., Curran I. Direct visualisation of the soluble pool of tubulin in the neuronal growth cone: immunofluorescence studies following taxol polymerisation. Brain Res Dev Brain Res. 1989 Oct 1;49(2):305–310. doi: 10.1016/0165-3806(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Grellier P., Rigomier D., Clavey V., Fruchart J. C., Schrevel J. Lipid traffic between high density lipoproteins and Plasmodium falciparum-infected red blood cells. J Cell Biol. 1991 Jan;112(2):267–277. doi: 10.1083/jcb.112.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellier P., Rigomier D., Schrével J. Induction in vitro de la schizogonie de Plasmodium falciparum par les lipoprotéines humaines de haute densité (HDL). C R Acad Sci III. 1990;311(10):361–367. [PubMed] [Google Scholar]
- Hommel M., Semoff S. Expression and function of erythrocyte-associated surface antigens in malaria. Biol Cell. 1988;64(2):183–203. doi: 10.1016/0248-4900(88)90078-0. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Battye F. L., Mitchell G. F. Plasmodium-infected blood cells analyzed and sorted by flow fluorimetry with the deoxyribonucleic acid binding dye 33258 Hoechst. J Histochem Cytochem. 1979 Apr;27(4):803–813. doi: 10.1177/27.4.87413. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Banyal H. S. Plasmodium falciparum: synchronization of asexual development with aphidicolin, a DNA synthesis inhibitor. Exp Parasitol. 1984 Feb;57(1):48–54. doi: 10.1016/0014-4894(84)90061-4. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Komiya Y., Kurokawa M. Preferential blockade of the tubulin transport by colchicine. Brain Res. 1980 May 26;190(2):505–516. doi: 10.1016/0006-8993(80)90292-9. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lataste H., Senilh V., Wright M., Guénard D., Potier P. Relationships between the structures of taxol and baccatine III derivatives and their in vitro action on the disassembly of mammalian brain and Physarum amoebal microtubules. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4090–4094. doi: 10.1073/pnas.81.13.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis J. F., Verhave J. P., Jap P. H., Meuwissen J. H. Transformation of sporozoites of Plasmodium berghei into exoerythrocytic forms in the liver of its mammalian host. Cell Tissue Res. 1985;241(2):353–360. doi: 10.1007/BF00217180. [DOI] [PubMed] [Google Scholar]
- Read M., Sherwin T., Holloway S. P., Gull K., Hyde J. E. Microtubular organization visualized by immunofluorescence microscopy during erythrocytic schizogony in Plasmodium falciparum and investigation of post-translational modifications of parasite tubulin. Parasitology. 1993 Apr;106(Pt 3):223–232. doi: 10.1017/s0031182000075041. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrével J., Asfaux-Foucher G., Bafort J. M. Etude ultrastructurale des mitoses multiples au cours de la sporogonie du Plasmodium b. berghei. J Ultrastruct Res. 1977 Jun;59(3):332–350. doi: 10.1016/s0022-5320(77)90043-0. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Usanga E. A., O'Brien E., Luzzato L. Mitotic inhibitors arrest the growth of Plasmodium falciparum. FEBS Lett. 1986 Dec 1;209(1):23–27. doi: 10.1016/0014-5793(86)81077-8. [DOI] [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]