Abstract
The periodontal ligament is the key tissue facilitating periodontal regeneration. This study aimed to fabricate decellularized human periodontal ligament cell sheets for subsequent periodontal tissue engineering applications. The decellularization protocol involved the transfer of intact human periodontal ligament cell sheets onto melt electrospun polycaprolactone membranes and subsequent bi-directional perfusion with NH4OH/Triton X-100 and DNase solutions. The protocol was shown to remove 92% of DNA content. The structural integrity of the decellularized cell sheets was confirmed by a collagen quantification assay, immunostaining of human collagen type I and fibronectin, and scanning electron microscopy. ELISA was used to demonstrate the presence of residual basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) in the decellularized cell sheet constructs. The decellularized cell sheets were shown to have the ability to support recellularization by allogenic human periodontal ligament cells. This study describes the fabrication of decellularized periodontal ligament cell sheets that retain an intact extracellular matrix and resident growth factors and can support repopulation by allogenic cells. The decellularized hPDL cell sheet concept has the potential to be utilized in future “off-the-shelf” periodontal tissue engineering strategies.
Keywords: extracellular matrix, fibroblast growth factor, vascular endothelial growth factor, collagen, tissue engineering, polycaprolactone
Introduction
Periodontal regeneration can be considered the ultimate goal of periodontal treatment. However, currently available clinical techniques are unpredictable (Needleman et al., 2006; Esposito et al., 2009). Given that the periodontium is a complex structure involving soft and hard tissues, a tissue engineering approach is inherently suited to periodontal regeneration, since it has the potential to facilitate the correct temporal and spatial arrangement of the multiple components required for periodontal regeneration (Bartold et al., 2000).
The periodontal ligament has long been considered the key tissue required for periodontal regeneration (Nyman et al., 1982). Indeed, periodontal ligament cells have been shown to have superior regenerative properties compared with other cells derived from the periodontium, such as gingival connective tissue and alveolar bone cells (Tsumanuma et al., 2011; Dan et al., 2014). These findings suggest that periodontal ligament cells and the associated extracellular matrix retain important cues that facilitate periodontal regeneration.
Cell-sheet technology allowing for the non-enzymatic harvesting of cultured cells with an intact extracellular matrix provides the opportunity for periodontal ligament cells to be delivered directly to the root surface. This approach has been successfully used to promote periodontal regeneration in a number of small and large animal models (Akizuki et al., 2005; Flores et al., 2008; Ishikawa et al., 2009; Vaquette et al., 2012; Costa et al., 2014).
While tissue-engineered cell-sheet technology is promising, there are underlying limitations in reaching clinical practice, such as the reliance on the patient’s own cells, as well as the need for cell culture facilities and associated technical expertise. The use of decellularized matrices has the potential to overcome these limitations. Multiple studies have shown that decellularized tissues and organs can retain their biological and mechanical properties with no immune response upon transplantation in vivo (Quint et al., 2011; Dijkman et al., 2012; Sadr et al., 2012; Syedain et al., 2013). Further, tissue-engineered decellularized constructs prepared in vitro were shown to retain their structural integrity, maintain their molecular functionality, and enhance tissue regeneration following in vivo transplantation (Sadr et al., 2012).
This study tested the hypothesis that decellularized human periodontal cell sheets can retain their extracellular matrix integrity and support recellularization with allogenic cells. The aim of the study was to utilize a decellularization protocol that was optimized for cell sheets, and to assess the extracellular matrix structural integrity, growth factor retention, and re-cellularization potential of the decellularized periodontal ligament cell sheets.
Materials & Methods
Primary Human Periodontal Ligament Cell Isolation and Culture
Human periodontal ligament cells (hPDLC) were obtained according to an established protocol (Ivanovski et al., 2001). Briefly, after obtaining institutional ethics approval (Griffith University Human Ethics Committee) and informed patient consent, we obtained extracted third molars from patients aged 17 to 30 yr old. Diced periodontal tissues were obtained from the middle thirds of the roots and explanted into 25-cm2 flasks. The cells were subsequently grown and propagated in 175-cm2 flasks with Dulbecco’s Modification of Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin (50 units/mL), and streptomycin (50 µg/mL). Cells between the third and fifth passages were utilized.
Decellularization, Immunostaining, and Confocal Imaging of Cultured Cell Monolayers
To characterize the structure of the cell monolayer extracellular matrix, we seeded the cells at a density of 2 x 104 cells on 13-mm-diameter Thermanox coverslips (Thermo Scientific NuncTM, Scoresby, Vic, Australia) and grown for 9 days in culture medium supplemented with ascorbic acid (100 µg/mL). At the end of the culture period, a cell monolayer was formed and decellularized according to a protocol originally developed by Beacham et al. (2007) and modified by our group. Briefly, 800 µL of NH4OH (20 mM) and Triton X-100 (0.5%) were added to the coverslips for 20 min and kept at 37oC. The coverslips were then rinsed twice with 1 mL of sterile water and kept in phosphate-buffered saline (PBS) at 4oC.
Fresh and decellularized cell monolayers were imaged by confocal laser microscopy. Antibodies against human collagen I and fibronectin (Life Technologies, Invitrogen, Carlsbad, CA, USA) were used to visualize the extracellular matrix. 4′, 6-diamidino-2-phenylindole (DAPI, 5 µg/mL) and phalloidin–tetramethylrhodamine B isothiocyanate conjugate (Phalloidin-TRITC, 0.8 U/mL; Life Technologies, Invitrogen) were utilized to stain the nuclei and the actin fibers, respectively. A detailed protocol is included in the online Appendix.
Melt-electrospun Carrier Membrane Fabrication
Polycaprolactone (PCL, CAPA®6400, Perstorp, Warrington, UK) was utilized for fabricating the carrier membranes, utilizing a house-built melt electrospinner on a static flat collector. The PCL granules were loaded into a 2-mL syringe and melt-electrospun at 6 kV, 95oC, at a feed rate of 20 µL/hr with a spinneret-collector distance of 4 cm. A biopsy punch was used to produce 5-mm-diameter membranes (Fig. 1A). To enhance the scaffold hydrophilicity, we performed a 2M NaOH treatment at 37°C for 30 min, followed by 5 rinses in ultrapure water. The membranes were sterilized by immersion in ethanol for 30 min, followed by a 30-minute UV irradiation. The membranes were used to support the cell sheet during the handling and decellularization process.
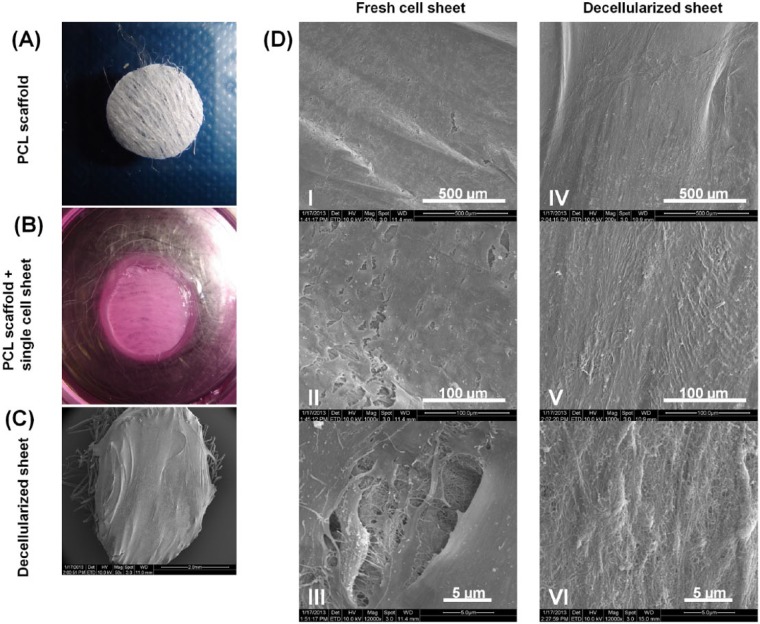
Figure 1.
Harvesting of fresh human periodontal ligament (HPDL) cell sheet and scanning electron microscopy (SEM) showing fresh and decellularized PDL sheet. (A) PCL scaffold 5 mm in diameter, after NH4OH treatment. (B) Fresh HPDL cell sheet attached to sterile PCL scaffold. (C) SEM image of decellularized PDL sheet on top of a PCL scaffold. (D) Different SEM magnification of fresh (I-III) and decellularized (IV-VI) sheets. This figure is available in color online at http://jdr.sagepub.com.
Cell-sheet Harvesting
hPDL cells were seeded in 24-well cell-culture wells at a seeding density of 5 x 104 cells/well in media supplemented with ascorbic acid (1,000 µg/mL). For the first 48 hr, the ascorbic acid concentration was ten-fold greater than the standard concentration, to enhance early extracellular matrix formation. The cells were then grown for 19 days in media supplemented with ascorbic acid (100 µg/mL); media were changed every 48 hr.
At the end of the 21-day culture period, the borders of the cell sheet were gently detached from the base of the well and pulled toward the edges of the PCL membranes by means of sterile fine-curved tweezers. The samples were further incubated in culture media for 24 hr.
For comparison with the PCL-single cell sheet construct, a multi-layered construct consisting of 4 cell sheets was also prepared.
Decellularization of Cell Sheets
A bi-directional perfusion system developed by our group was utilized to decellularize the cell sheets. It was composed of a pump, a 30-mL plastic syringe, a 3-mm-diameter silicone tube, and 2 x 15 mL falcon tubes stacked on each other. Rapidly prototyped polylactic acid (PLA) porous constructs (lay-down pattern 0/90) were used as separators to divide the falcon tubes into compartments and ensure the appropriate positioning and stability of the PCL scaffolds within the falcon tubes. We used sterile tweezers to place 3 scaffolds in a sandwich pattern between 2 PLA constructs, with a maximum of 9 scaffolds decellularized at a time. The decellularization solution, consisting of 30 mL of 20 mM NH4OH solution with 0.5% Triton X-100, was bi-directionally perfused though the scaffold for 60 min at a rate of 1,000 mL/hr, with a flow inversion every 50 sec. This was followed by perfusion in a DNase I solution (100 U/mL, Invitrogen) at 37oC in CaCl2 (0.9 mM) and MgCl2 (0.5 mM) in sterile PBS for 60 min. The PCL membrane-cell sheet constructs were finally perfused with sterile water at 37oC for another 60 min.
Confocal Imaging of Cell Sheets
The PCL membrane-cell sheet constructs were immunostained for human collagen I and human fibronectin before and after decellularization for assessment of sheet integrity, by the same technique described above.
DNA Content
DNA content was measured for both fresh cell and decellularized samples by means of a Quant – iT PicoGreen kit after matrix digestion in proteinase K as described in a previous study (Vaquette et al., 2013). For each group, 6 biological replicates were utilized, and each measurement was performed in triplicate. A detailed protocol can be found in the online Appendix.
Scanning Electron Microscopy (SEM) of Cell Sheets
SEM imaging of fresh and decellularized cell sheets was performed with a FEI Quanta 200 microscope. A detailed protocol can be found in the online Appendix.
Growth Factor ELISA Assay
ELISA assays were used to detect basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) levels in both fresh and decellularized samples. Growth factor extraction was performed by the addition of 300 µL of NaCl (2M) in 20 mM HEPES with EDTA protease inhibitor cocktail (Roche complete mini; Roche Applied Science, Indianapolis, IN, USA) to each sample and incubated for 60 min at room temperature with gentle shaking on an orbital shaker. Samples were collected into 1.5-mL Eppendorf tubes and centrifuged at 2,000 rpm for 5 min. Growth factor quantification was carried out by a Bioplex assay (Bio-Plex Pro; Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
Collagen Quantification
Collagen content in the cell sheets was measured in both fresh and decellularized samples, by means of a hydroxyproline assay kit (Chondrex, Inc., Redmond, WA, USA; catalog #6017) according to the manufacturer’s instructions. A detailed protocol can be found in the online Appendix.
Recellularization of Decellularized Sheets
We assessed decellularized sheets for their recellularization potential by seeding allogenic hPDL cells on the top of the decellularized constructs at a seeding density of 5,000 cells/scaffold. Recellularization was assessed over 3, 7, and 21 days with confocal imaging, SEM imaging, and DNA quantification by the PicoGreen assay, according to the same methods as described earlier. For comparison, the cells were also loaded onto a commercially available non-cross-linked collagen membrane (Bio-Gide®, Geistlich Pharma AG, Wolhusen, Switzerland).
Statistical Analysis
Results were expressed as means ± standard deviation, and Student’s t test was used to analyze the data. The significance level of the statistical analysis was set at p < .05.
Results
Scanning Electron Microscopy
The incorporation of ascorbic acid into the media along with in vitro cell culture resulted in the deposition of a well-developed collagenous network, and hence a mature cell sheet was formed. The sheets were thick enough after 3 wk of culture to be mechanically harvested with fine-curved tweezers. This permitted the harvesting and placement of the cell sheet onto a PCL melt electrospun scaffold (Fig. 1B). Attachment of the cell sheet to the PCL scaffold was rapid, provided that the scaffolds were surface-treated with sodium hydroxide to increase their hydrophilicity. It was found that a 24-hour period was sufficient for the cell sheet to adhere firmly to the scaffold and withstand the subsequent fluid perfusion decellularization process. The SEM images revealed that both the fresh and the decellularized cell sheet remained intact and well-attached to the PCL scaffold (Figs. 1C, 1D). Higher magnification images of the decellularized samples demonstrated the presence of a fine network of extracellular matrix fibers with a morphology and structural integrity similar to that observed in the fresh cell sheet (Figs. 1D-III, 1D-VI). The SEM images of the multi-layered (4) cell-sheet construct are shown in Appendix Fig. 2. The construct had a thickness of approximately 100 µm, and although some porosity could be demonstrated (Appendix Fig. 3C), the decellularized four-layered construct did not appear to have the same degree of porosity as the decellularized construct consisting of a single sheet (Fig. 1D-VI).
Extracellular Matrix Characterization
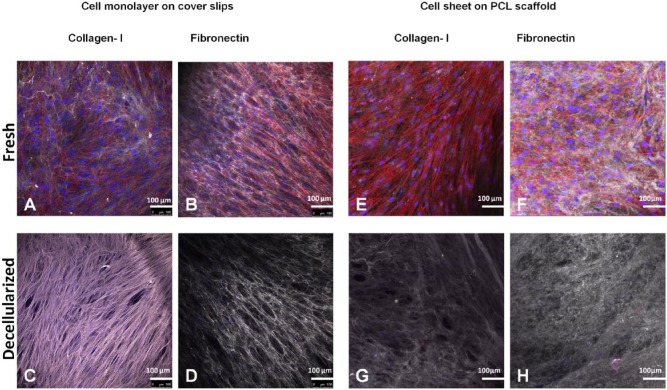
Figs. 2A and 2B display representative immunostaining of hPDLC monolayers cultured on a coverslip, showing a well-developed network of fibronectin and collagen fibers. Upon decellularization, the components of the extracellular matrix formed by the monolayers were well-preserved (Figs. 2C, 2D), with no apparent alteration in their structural integrity when compared with the fresh matrices.
Figure 2.
Immunostaining of human collagen type I and fibronectin. (A-D) Staining of cell monolayers on coverslips. (E-H) Staining of mature cell sheet – polycaprolactone constructs. Nuclei (DAPI) in blue, actin filaments (phalloidin) in red, human collagen type I and human fibronectin in gray. This figure is available in color online at http://jdr.sagepub.com.
Similarly, in the case of the mature cell sheets placed on the PCL membranes, the decellularization protocol resulted in preservation of the quality and integrity of the extracellular matrix components (Figs. 1G, 1H). Negligible traces of DNA remnants (in blue) and actin filaments (in red) were detected in the decellularized sheets, indicating efficient removal of cellular contents by this decellularization protocol.
DNA Quantification
DNA quantification confirmed the efficacy of the decellularization protocol in removing the cellular components, with 92% of DNA successfully eliminated from the hPDLC sheets (Fig. 3A).
Figure 3.
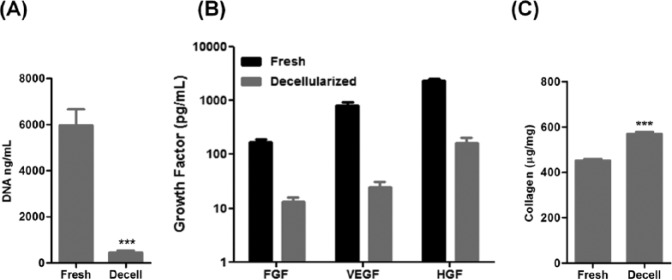
Comparison of DNA amounts, growth factor concentrations, and collagen contents of fresh and decellularized periodontal ligament cell-sheet constructs. (A) DNA content before and after decellularization. (B) Growth factors retained in fresh and decellularized sheets, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF). (C) Collagen quantification showing preservation of collagen content after the decellularization process.
Growth Factor ELISA and Collagen Quantification
bFGF, VEGF, and HGF were found to be retained in the decellularized sheets. As shown in Fig. 3B, approximately 10% of the initial growth factor content in the fresh cell sheet remained after decellularization. Collagen quantification revealed increased collagen content in the decellularized cell sheets, indicating that this decellularization method did not negatively affect the amount of retained collagen (Fig. 3C).
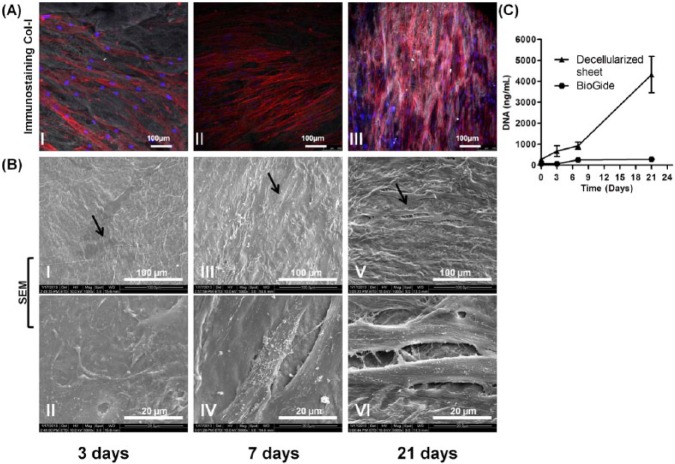
Cell Growth on Decellularized Sheets
The decellularized matrices were re-seeded with allogenic hPDL cells and cultured in vitro over 21 days. The recellularized constructs showed a gradual and significant increase in DNA content, indicating that the matrices were capable of supporting cell adhesion and proliferation (Fig. 4C). The cells adopted a spindle-like morphology (black arrows) as seen by confocal laser microscopy (Fig. 4A) and SEM (Fig. 4B). Newly formed extracellular matrix was observed at the later time points (Figs. 4A-III, and 4B-VI), indicating excellent cyto-compatibility of the decellularized substrate. While the cells were also demonstrated to populate the Bio-Gide® membrane (Appendix Fig. 2), the proliferation rate was significantly lower compared with that of the decellularized construct, with proliferation noted over the first 6 days and then remaining constant over the following two-week period, up to 21 days (Fig. 4C).
Figure 4.
Recellularization potential of the decellularized sheet after being seeded with allogenic hPDL cells after 3, 7, and 21 days. (A) Confocal imaging and immunostaining of human collagen type I (gray), nuclei (blue), and actin filaments (red). (B) SEM showing hPDL cells at different time points. (C) DNA quantification showing cell proliferation on decellularized constructs and Bio-Gide® membrane over 21 days. This figure is available in color online at http://jdr.sagepub.com.
Discussion
Decellularization is an emerging technology in regenerative medicine which is showing considerable potential in providing “off-the-shelf” tissue-engineered constructs for clinical use (Weber et al., 2013). Most of the attention in this field has focused on the decellularization of whole organs and tissues, with fewer reports on the decellularization of in vitro tissue-engineered constructs. Notably, commercially available products based on decellularization approaches, such as Alloderm®, have been used in dental applications, namely, mucogingival surgery (Nevins et al., 2011). The existing literature would suggest that decellularized organs, tissues, and constructs are viable options for tissue-engineering applications, with considerable clinical potential (Tapias and Ott, 2014).
The harvesting of a mature cell sheet with an intact extracellular matrix is a key requirement for the decellularization protocol. Periodontal ligament cell sheets have been shown to support periodontal regeneration (Ishikawa et al., 2009), although a significant issue in the outcome is the ability to deliver and maintain an intact cell sheet on the tooth surface (Tsumanuma et al., 2011). To this end, we have previously utilized melt-electrospun PCL membranes to successfully deliver and stabilize the cell sheets in experimental periodontal defects (Dan et al., 2014). Therefore, in this study, prior to decellularization, the cell sheets were transferred to PCL membranes, which provided support during the decellularization process and allowed for ease of handling of the decellularized construct. The PCL material was chosen since it is a clinical-grade product, which would facilitate future translation of the decellularized construct to the clinic.
We adopted a decellularization protocol that was originally used by Beacham et al. (2007). Changes to the protocol included the addition of DNase digestion as well as the bi-directional perfusion steps, which aimed to enhance the removal of cellular contents from the decellularized sheet, thus minimizing the possibility of eliciting an immune response upon future in vivo transplantation (Brown et al., 2009). In the present study, we were successful in removing more than 92% of DNA content, which is comparable with the findings of Sadr et al. (2012), who showed 94% DNA removal following decellularization in a perfusion bioreactor and no adverse effects following in vivo implantation. Studies have demonstrated DNA removal above 99% with the use of sodium dodecyl sulfate (SDS) as the decellularization agent, but this comes at the expense of extracellular matrix integrity (Syedain et al., 2013).
A key requirement of the decellularization process is that the integrity of the extracellular matrix is maintained so that it retains the biological cues that would lead to tissue-specific cell differentiation of native cells upon in vivo transplantation. Type I collagen and fibronectin are 2 of the predominant proteins present in the native periodontal ligament tissue (Nanci and Bosshardt, 2006), and we were able to demonstrate their presence and structural integrity following decellularization of both hPDL cell monolayers and the mature cell sheet–PCL membrane constructs. Further, collagen quantification showed increased collagen content after decellularization. This suggests that most if not all of the original collagen was retained in the decellularized matrices, with the observation of increased concentration values consistent with other reports in the literature (Quint et al., 2011), and likely to be due to the removal of the cellular contents from the sheets. The results were consistent with those of other studies that have demonstrated intact extracellular matrix following the decellularization of tissue-engineered constructs (Quint et al., 2011).
It was also demonstrated that growth factors could be retained in the decellularized cell sheets, at levels which were approximately 10% of those detected in fresh cell sheets. These results were consistent with the findings of Reichert et al. (2010), who also identified growth factors in their decellularized matrices formed by primary human osteoblasts on a polymeric substrate. Notably, the growth factor concentrations obtained in our study were approximately 10 times higher than those reported by Reichert et al. (2010). This may be due to differences in methodology, since Reichert et al. (2010) used a different cell type and culture method, and did not use perfusion or DNAse. The amounts of growth factors remaining in the cell sheet (range, 10-100 pg/mL for FGF-2 and VEGF, and 100-1,000 pg/mL for HGF) are likely to be of physiological relevance, since they are consistent with the reported serum concentrations of these growth factors (Veselý et al., 2004; Etto et al., 2008).
The allogenic hPDL cells were able to attach to the decellularized constructs, as demonstrated by confocal imaging and SEM. Furthermore, the allogenic cells continued to proliferate and deposit extracellular matrix for the full course of the recellularization experiment, which ran for 21 days. This finding is consistent with that from another report where decellularized tissue-engineered heart valves could be subsequently recellularized with allogenic mesenchymal stem cells (Syedain et al., 2013). The decellularized constructs showed superior ability to support cell proliferation compared with a commercially available non-cross-linked membrane. This is likely to be the result of a more porous structure and the presence of residual growth factors in the decellularized construct.
In conclusion, this study describes the fabrication of decellularized periodontal cell-sheet constructs with extracellular matrix integrity and the ability to support allogenic cell re-population. These constructs introduce the possibility of utilizing the instructive biological signals that are constituent to periodontal ligament cells and the associated extracellular matrices as an “off-the-shelf” tissue-engineering application.
Supplementary Material
Footnotes
The study was supported by a grant from the National Health and Medical Research Council of Australia.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, et al. (2005). Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res 40:245-251. [DOI] [PubMed] [Google Scholar]
- Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. (2000). Tissue engineering: a new paradigm for periodontal regeneration. Periodontol 2000 24: 253-269. [DOI] [PubMed] [Google Scholar]
- Beacham DA, Amatangelo MD, Cukierman E. (2007). Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol Chapter 10:Unit 10.9. [DOI] [PubMed] [Google Scholar]
- Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. (2009). Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30:1482-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW.(2014). Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Perio 41:283-294. [DOI] [PubMed] [Google Scholar]
- Dan H, Vaquette C, Fisher A, Hamlet SM, Xiao Y, Hutmacher DW, et al. (2014). The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 35:113-122. [DOI] [PubMed] [Google Scholar]
- Dijkman PE, Driessen-Mol A, Frese L, Hoerstrup SP, Baaijens FP. (2012). Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 33:4545-4554. [DOI] [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV. (2009). Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst Rev 4:CD003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etto L, Lacerda E, Baiocchi O, Silva V, Dalboni M, Alves A, et al. (2008). Clinical correlations and prognostic relevance of HGF, VEGF and FGF expression in Brazilian patients with non-Hodgkin lymphoma. Leuk Lymphoma 49:257-264. [DOI] [PubMed] [Google Scholar]
- Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I. (2008). Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol 35:1066-1072. [DOI] [PubMed] [Google Scholar]
- Ishikawa I, Iwata T, Washio K, Okano T, Nagasawa T, Iwasaki K, et al. (2009). Cell sheet engineering and other novel cell-based approaches to periodontal regeneration. Periodontol 2000 51: 220-238. [DOI] [PubMed] [Google Scholar]
- Ivanovski S, Haase HR, Bartold PM. (2001). Expression of bone matrix mRNAs by primary and cloned cultures of the regenerative phenotype of human periodontal fibroblasts. J Dent Res 80:1665-1671. [DOI] [PubMed] [Google Scholar]
- Nanci A, Bosshardt DD. (2006). Structure of periodontal tissues in health and disease. Periodontol 2000 40: 11-28. [DOI] [PubMed] [Google Scholar]
- Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. (2006). Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev 2:CD001724. [DOI] [PubMed] [Google Scholar]
- Nevins M, Nevins ML, Kim SW, Schüpbach P, Kim DM. (2011). The use of mucograft collagen matrix to augment the zone of keratinized tissue around teeth: a pilot study. Int J Periodontics Restorative Dent 31:367-373. [PubMed] [Google Scholar]
- Nyman S, Gottlow J, Karring T, Lindhe J. (1982). The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol 9:257-265. [DOI] [PubMed] [Google Scholar]
- Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. (2011). Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA 108:9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JC, Quent VM, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW. (2010). Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials 31:7928-7936. [DOI] [PubMed] [Google Scholar]
- Sadr N, Pippenger BE, Scherberich A, Wendt D, Mantero S, Martin I, et al. (2012). Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials 33:5085-5093. [DOI] [PubMed] [Google Scholar]
- Syedain ZH, Bradee AR, Kren S, Taylor DA, Tranquillo RT. (2013). Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A 19:759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias LF, Ott HC. (2014). Decellularized scaffolds as a platform for bioengineered organs. Curr Opin Organ Transplant 19:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, et al. (2011). Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 32:5819-5825. [DOI] [PubMed] [Google Scholar]
- Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. (2012). A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 33:5560-5573. [DOI] [PubMed] [Google Scholar]
- Vaquette C, Ivanovski S, Hamlet SM, Hutmacher DW. (2013). Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 34:5538-5551. [DOI] [PubMed] [Google Scholar]
- Veselý D, Astl J, Lastůvka P, Matucha P, Sterzl I, Betka J. (2004). Serum levels of IGF-I, HGF, TGFbeta1, bFGF and VEGF in thyroid gland tumors. Physiol Res 53:83-89. [PubMed] [Google Scholar]
- Weber B, Dijkman PE, Scherman J, Sanders B, Emmert MY, Grünenfelder J, et al. (2013). Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 34:7269-7280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.