Abstract
This article presents details of fabrication, biological activity (i.e., anti–matrix metalloproteinase [anti-MMP] inhibition), cytocompatibility, and bonding characteristics to dentin of a unique doxycycline (DOX)–encapsulated halloysite nanotube (HNT)–modified adhesive. We tested the hypothesis that the release of DOX from the DOX-encapsulated nanotube-modified adhesive can effectively inhibit MMP activity. We incorporated nanotubes, encapsulated or not with DOX, into the adhesive resin of a commercially available bonding system (Scotchbond Multi-Purpose [SBMP]). The following groups were tested: unmodified SBMP (control), SBMP with nanotubes (HNT), and DOX-encapsulated nanotube-modified adhesive (HNT+DOX). Changes in degree of conversion (DC) and microtensile bond strength were evaluated. Cytotoxicity was examined on human dental pulp stem cells (hDPSCs). To prove the successful encapsulation of DOX within the adhesives—but, more important, to support the hypothesis that the HNT+DOX adhesive would release DOX at subantimicrobial levels—we tested the antimicrobial activity of synthesized adhesives and the DOX-containing eluates against Streptococcus mutans through agar diffusion assays. Anti-MMP properties were assessed via β-casein cleavage assays. Increasing curing times (10, 20, 40 sec) led to increased DC values. There were no statistically significant differences (p > .05) in DC within each increasing curing time between the modified adhesives compared to SBMP. No statistically significant differences in microtensile bond strength were noted. None of the adhesives eluates were cytotoxic to the human dental pulp stem cells. A significant growth inhibition of S. mutans by direct contact illustrates successful encapsulation of DOX into the experimental adhesive. More important, DOX-containing eluates promoted inhibition of MMP-1 activity when compared to the control. Collectively, our findings provide a solid background for further testing of encapsulated MMP inhibitors into the synthesis of therapeutic adhesives that may enhance the longevity of hybrid layers and the overall clinical performance of adhesively bonded resin composite restorations.
Keywords: bonding, matrix metalloproteinase, stem cells, drug delivery systems, collagen, biocompatible materials
Introduction
Dental caries continues and will likely remain a global health issue for many years to come, affecting nearly every individual (Ferreira-Zandona et al., 2012). Regrettably, no single material for direct tooth restorations has totally replaced the use of amalgam, even with concerns over mercury toxicity. Meanwhile, with the increased demand for more aesthetically pleasing restorations, light-cured resin composites have been used, even though they do not invariably last as long (Rekow et al., 2013). Accordingly, one high-priority research focus of the National Institute of Dental and Craniofacial Research (2014) is on the innovations in materials for long-lasting and nontoxic direct tooth restorations. Despite remarkable advances in adhesive science and dentin bonding knowledge, research data continue to report a significant decrease in resin-dentin bond strength over time (Breschi et al., 2008; Liu et al., 2011; Pashley et al., 2011; Tjäderhane et al., 2013). Simply speaking, the failure of resin restorations is believed to be due in part to collagen fibril degradation in the dentin at the adhesive resin-dentin interface (i.e., hybrid layer) by matrix metalloproteinases (MMPs; Mazzoni et al., 2011, 2013).
In recent years, several approaches have been proposed to prolong the durability and overall clinical success of adhesively bonded restorations. For instance, the use of MMP inhibitors, such as the application of chlorhexidine (Carrilho et al., 2007; Liu et al., 2011; Mazzoni et al., 2011) and galardin (Breschi et al., 2010), after acid etching has been investigated. Meanwhile, doxycycline (DOX), a tetracycline derivative, at subantimicrobial dosages has displayed a valuable therapeutic role of MMP inhibition in periodontal disease (Choi et al., 2004). Through a materials-based strategy, our group recently reported (Bottino et al., 2013a) on the potential benefits of modifying adhesive resins using biocompatible, hydrophilic, and high mechanical strength aluminosilicate clay nanotubes (i.e., halloysite nanotubes [HNTs]; Lvov et al., 2013). Notably, several recent studies have highlighted the potential of using HNT as safe reservoirs for drug encapsulation and sustained release (Qi et al., 2010, 2013). Accordingly, the synthesis of DOX-encapsulated nanotube-modified dentin adhesive could serve as a carrier for the sustained release of DOX at the adhesive layer for MMP inhibition and overall improvement of dentin bond durability. We tested the hypothesis that the release of DOX from the DOX-encapsulated nanotube-modified adhesive can inhibit MMP activity. Furthermore, changes in degree of conversion (DC) and microtensile bond strength (µTBS) to dentin were evaluated to ensure that modification with HNTs or DOX-encapsulated HNTs would not compromise these characteristics of a commercial reference adhesive.
Materials & Methods
Fabrication of DOX-Encapsulated Nanotube-Modified Adhesive
DOX (Sigma, St. Louis, MO, USA)—a Food and Drug Administration–approved MMP inhibitor (Emingil et al., 2004)—was dissolved at 10% (w/v) in phosphate buffered saline (PBS; pH 7.2, Fisher Scientific, Pittsburgh, PA, USA) at 50°C for 2 hr under stirring conditions (Fisher Thermix, model 310T, Fisher Scientific). The encapsulation process was modified from a previously reported procedure (Qi et al., 2010). Briefly, aluminosilicate (Al2Si2O5(OH)4·nH2O) clay nanotubes (HNTs; Dragonite 1415JM, Applied Minerals Inc., New York, NY, USA) were sieved (45-µm sieve), mixed with the DOX solution (1 g/10 mL), and sonicated for 2 hr to enhance dispersion. Vacuum (25 inHg) was applied and maintained for 1 hr to remove any air between and within the HNTs (Qi et al., 2010). The solution was then stirred for 1 hr, and vacuum was reapplied. Finally, the HNT+DOX solution was dried in an incubator at 37°C for 5 d. On the basis of our findings that HNT can be incorporated up to 20% (w/v) without jeopardizing physicochemical and bonding properties of the adhesive (Bottino et al., 2013a), we fabricated adhesives incorporated with 15% (w/v) of HNT, encapsulated or not with DOX. The dried HNT+DOX powder was sieved before incorporation (150 mg of DOX-encapsulated nanotubes per 1 mL of adhesive) into the adhesive resin of a commercial bonding system (Adper Scotchbond Multi-Purpose [SBMP]; 3M ESPE, St. Paul, MN, USA) by mixing with a stirrer and a conical micropestle followed by sonication to further enhance HNT dispersion (Bottino et al., 2013a). It is important to note that the experimental adhesives were fabricated with foil-covered light-proof (amber) microcentrifuge tubes, because of DOX photosensitivity (Smith and Leyden, 2005). All specimens were fabricated in a constant-temperature room (23°C) with a filtered lighting system to minimize unintentional polymerization.
Degree of Conversion
DC was used to determine whether adhesive polymerization could be affected by DOX incorporation. Disc-shaped specimens (7 × 0.24 mm, n = 5) were fabricated and light cured for 10 sec (manufacturer’s recommendation), 20 sec, and 40 sec. A light-emitting diode curing system (DEMI LED, Kerr, Orange, CA, USA) with an output irradiance of circa 1,300 mW/cm2 was used and periodically monitored (Managing Accurate Resin Curing, MARC-RC Calibrator, BlueLight analytics Inc., Halifax, Nova Scotia, Canada).
Prepared specimens were stored for 24 hr in the dark under dry conditions. DC was evaluated with Fourier transform infrared spectroscopy (FTIR; Jasco 4100, Jasco Corp., Tokyo, Japan) in attenuated total reflection mode. The area under 2 absorbance bands at 1,637 cm-1 (methacrylate group C=C) and 1,715 cm-1 (ester group C=O) was used in the following equation to calculate the DC (Zhang and Wang, 2012; Bottino et al., 2013a). The scans were run twice per specimen (n = 5) and the average used for analysis (Zhang and Wang, 2012):
Dentin Bonding
Thirty-six caries-free human third molars were used within 6 mo of extraction under an approved (NS1004-03) local Institutional Review Board protocol (Indiana University). The occlusal third of each tooth was removed with a low-speed diamond disk (Buehler Ltd., Lake Bluff, IL, USA) mounted on a cutting machine (Isomet 1000, Buehler Ltd.). Following that, a flat midcoronal dentin surface was obtained with SiC papers (240-600 grit) to standardize the smear layer (Bottino et al., 2013a). Teeth were then randomly assigned into 3 adhesive groups (n = 12): unmodified adhesive (SBMP, control), SBMP with HNTs (HNT), and SBMP with DOX-encapsulated HNTs (HNT+DOX). Prior to resin bonding, dentin surfaces were etched with 37% phosphoric acid, primed, bonded (according to the manufacturer’s recommendations), polymerized (DEMI LED, Kerr, Orange, CA, USA), and restored with composite (~1-2 mm thick/increment; Z100, 3M ESPE). All specimens were stored in 37°C deionized water for 24 hr. Specimens were then cut into beams, 0.8 × 0.8 mm (Carrilho et al., 2007). The beams were kept in 37°C artificial saliva for 24 hr and tested at a crosshead speed of 1 mm/min with a universal testing machine (MTS Sintech Renew 1123, Eden Prairie, MN, USA; Raposo et al., 2012). Beams (n = 152) were tested for bond strength. Fracture surfaces were examined under a light microscope (40×) of 121 recovered beams (80% recovery). Failure type was classified as cohesive failure in dentin, cohesive failure in resin composite, adhesive failure, or mixed failure. Randomly selected fractured specimens were sputter coated with gold and imaged with a scanning electron microscope (SEM; JSM-5310LV, JEOL, Tokyo, Japan).
Cell Culture and Cytotoxicity Test
Cytotoxicity of the nanotube-modified adhesives with and without DOX, as well as the SBMP (control), was evaluated on human dental pulp stem cells (see Appendix; Zhang et al., 2013a, 2013b).
Biological Activity
To prove the successful encapsulation of DOX within the adhesives but, more important, to further support our hypothesis that the HNT+DOX adhesive would release DOX at subantimicrobial levels, we tested first the antimicrobial activity of synthesized adhesives and the DOX-containing eluates against Streptococcus mutans through agar diffusion assays (Bottino et al., 2013b). Eight disk-shaped adhesive specimens (5 × 2 mm) per group were fabricated with a Teflon mold, followed by light curing for 10 sec. Specimens were kept at 37°C for 24 hr, disinfected by ultraviolet light exposure (30 min/side), and then assigned to 2 distinct assays. Four specimens (n = 4 per group) were tested by directly placing the disks on blood agar plates (BAPs; Fisher Scientific) containing a freshly prepared lawn of S. mutans (UA159). S. mutans was cultured aerobically in tryptic soy broth (Difco Laboratories, Detroit, MI, USA) for 24 hr in 5% CO2 at 37°C. Then, 100 µL of the bacterial suspension was swabbed onto BAPs to create the lawn (Gregson et al., 2012; Huang and Gregory, 2012). In a different assay, the remaining specimens were used to obtain aliquots containing the eluates (i.e., the leachable compounds) from the adhesives. Subsequently, specimens were individually incubated in 10 mL (37°C) of PBS (pH 7.2), and 1-mL aliquots were removed after 1, 7, and 14 d. An equal amount of sterile PBS was added to replace the removed volume. Aliquots were stored at –20°C until tested. Each BAP containing a freshly prepared lawn of S. mutans was divided into zones; the adhesive disks or 10 µL of the obtained aliquots were placed into the center of each zone; and the plates were incubated in 5% CO2 at 37°C. The inhibition zones (mm) were measured after 96 hr. Chlorhexidine (0.12%) and sterile water served as positive and negative controls, respectively (Bottino et al., 2013b).
MMP-mediated casein cleavage assays were performed to evaluate the anti-MMP activity of the synthesized DOX-encapsulated nanotube-modified adhesive. Disk-shaped specimens (5 × 2 mm, n = 3) were placed into 12-well plates and incubated in 2 mL of 50 mM Tris with 0.2 M NaCl, 10 mM CaCl2, and 1 µM ZnCl2 (pH 7.4). On days 1, 7, and 14, aliquots (500 μL) containing the eluates from the adhesive groups (i.e., HNT and HNT+DOX) were collected. An equal volume of the solution was added back to the wells to keep the volume constant. Aliquots were stored at –20°C until tested. MMP-1 purified from human gingival fibroblast-conditioned media by anticollagenase affinity chromatography was incubated with β-casein at 37°C (Windsor et al., 1994, 1997) with or without the aliquots (200 µL, n = 3) in a total volume of 400 µL. Samples were periodically (0-120 min) removed, and the reaction was terminated by addition of 1,10-phenanthroline to a final concentration of 10 mM; then, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added (1:1). Samples were resolved in 10% SDS-PAGE, stained with Coomassie blue, and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Data were analyzed with 1-way analysis of variance—except the microtensile dentin bond strength data, in which a mixed-model analysis of variance was used. Tukey’s test was used for post hoc comparisons. The level of significance was set at 0.05.
Results
Increasing curing times led to a general increase in DC values: 60.7%-64.1% (10 sec), 63.7%-69.7% (20 sec), and 70.8%-72.6% (40 sec). There were no statistically significant differences (p > .05) in DC within each curing time between the modified adhesives compared to SBMP (Fig. 1). Mean µTBSs (Appendix Table) were 44.6 ± 15.2 MPa (HNT), 51.7 ± 23.3 MPa (SBMP), and 54.3 ± 19.1 MPa (HNT+DOX). Statistical analysis revealed no significant differences in µTBS among the groups (p = .07). Optical microscopy of the fractured surfaces revealed predominantly mixed failures (52.78%-60.98%; Appendix Table). Representative SEM micrographs of the fractured surfaces are shown in Figure 2. At high magnification (Fig. 2D, 2F), rodlike structures suggest HNT presence (Fig. 2A, 2B).
Figure 1.
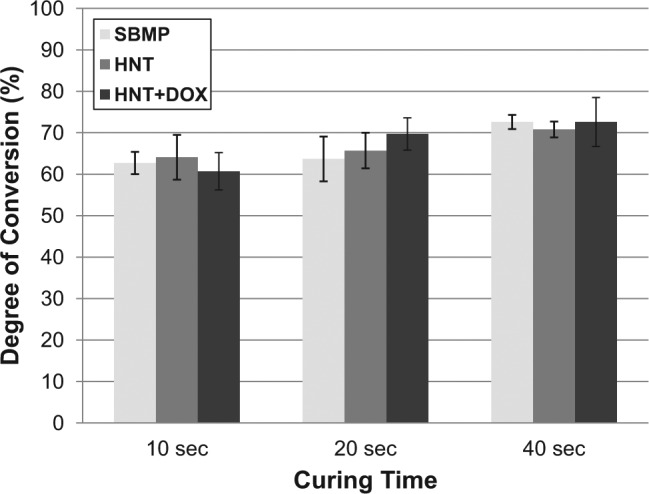
Graphic depicting the degree of conversion (DC) for control (SBMP) and experimental adhesives that were polymerized for 10, 20, and 40 sec. Increasing curing times led to a general increase in DC values: 60.7%-64.1% (10 sec), 63.7%-69.7% (20 sec), and 70.8%-72.6% (40 sec). There were no statistically significant differences (p > .05) in DC within each curing time between the modified adhesives compared to SBMP. DOX, doxycycline; HNT, halloysite nanotube; SBMP, Scotchbond Multi-Purpose.
Figure 2.
Micrographs of halloysite nanotubes (HNTs; Dragonite 1415JM, Applied Minerals Inc., New York, NY, USA): (A) scanning electron microscope (25,000×; bar, 1 µm) and (B) transmission electron microscope (98,000×; bar, 100 nm). Representative scanning electron microscope micrographs of fractured surfaces: (C, D) SBMP with HNTs (100×, 5,000×) and (E, F) SBMP with doxycycline-encapsulated HNTs (100×, 5,000×). Arrows show rodlike structures approximately 1 to 2 µm in length representing the HNTs incorporated into the experimental adhesive. SBMP, Scotchbond Multi-Purpose.
Growth inhibition of S. mutans (12.0 ± 0.0 mm; Fig. 3) supported the successful encapsulation and release of DOX from the experimental adhesive (HNT+DOX). No growth inhibition was seen for DOX-free groups (i.e., SBMP and HNT). Eluates from HNT+DOX adhesive specimens, incubated up to 14 d in PBS, did not inhibit bacterial growth (data not shown). Figure 3E shows cytocompatibility assay results on human dental pulp stem cells viability in response to different eluate concentrations from SBMP, HNT, and HNT+DOX groups. Human DPSC viability ranged from 92.0% to 107.8% (Fig. 3E). No statistically significant differences were detected among all the adhesive groups and dilutions tested when compared to the control (p > .05). MMP-mediated casein cleavage assays (Fig. 4) demonstrated that 14-d eluates from HNT+DOX adhesive inhibited 13.4% of the MMP-1 activity, compared to 48% by 0.1% DOX (positive control). Eluates from days 1 and 7 (HNT+DOX) demonstrated 0% inhibition, whereas eluates from HNT adhesive (negative control) ranged from 0% to 1.8% inhibition.
Figure 3.
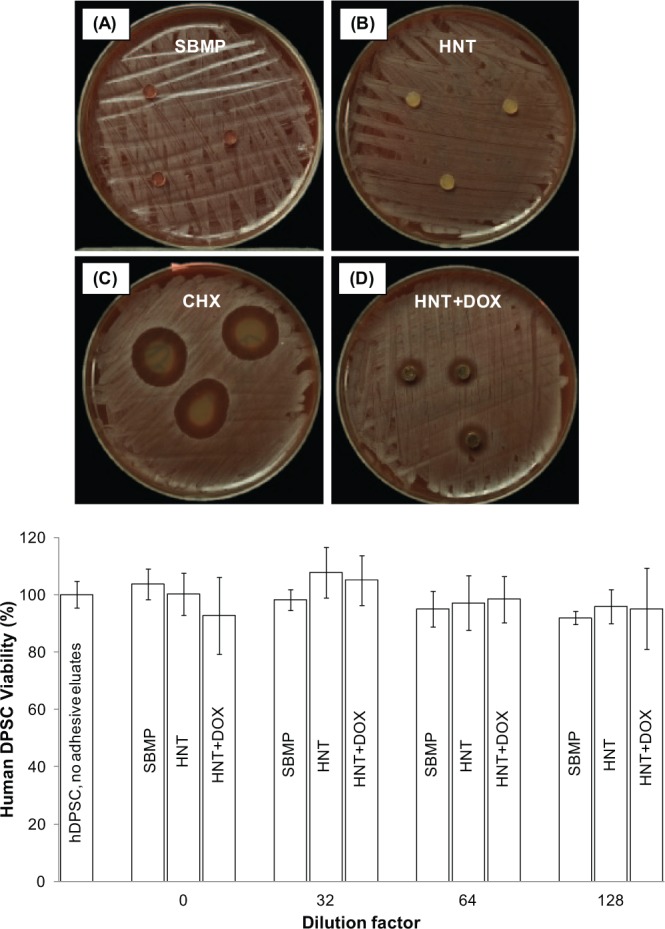
Representative macrophotographs of agar diffusion test against Streptococcus mutans at 96 hr of incubation: (A) SBMP (Scotchbond Multi-Purpose), (B) HNT (SBMP with halloysite nanotubes), (C) CHX (0.12% chlorhexidine; positive control), and (D) HNT+DOX (SBMP with doxycycline-encapsulated HNTs). Mean inhibition zone of 0.12% chlorhexidine and HNT+DOX was 21.0 ± 1.0 mm and 12.0 ± 0.0 mm, respectively. No inhibition zone was observed from SBMP and HNT. (E) Cytotoxicity of adhesive resin eluates released from specimens tested on human dental pulp stem cells. No significant differences (p = .225) were found comparing all groups of study and dilutions tested.
Figure 4.
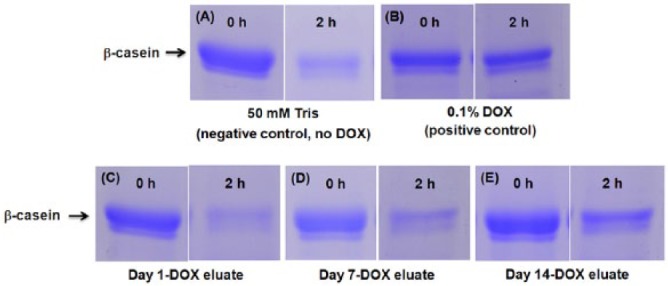
Representative images of Coomassie blue–stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels of β-casein cleavage assays. (A) Negative control: 50 mM Tris with 0.2 M NaCl, 10 mM CaCl2, and 1 µM ZnCl2 (pH 7.4). (B) Positive control: 0.1% doxycycline (DOX) solution (Sigma). DOX eluates: (C) day 1, (D) day 7, and (E) day 14. As depicted, more β-casein is present in panel E compared to the negative control (50 mM Tris) at 2 hr, which is not seen in day 1 and 7 aliquots.
Discussion
Over the past few years, several approaches have been proposed to inhibit MMP activity within the hybrid layer, such as a local single application of chlorhexidine or galardin onto demineralized dentin (Carrilho et al., 2007; Breschi et al., 2010; Stanislawczuk et al., 2011). Our long-term goal is to be able to inhibit the degradation of collagen fibrils within the hybrid layer by taking advantage of the unique and novel strategy of using HNT as a reservoir for MMP inhibitors and cysteine cathepsins. Here we take the first step toward our long-term goal by proving the concept that MMP inhibitors such as DOX can indeed be incorporated into dentin bonding agents through encapsulation within HNTs.
To the best of our knowledge, this is the first report that associates nanotubes with an MMP inhibitor (i.e., DOX) aiming to promote MMP inhibition through its release from the adhesive resin matrix. Our data clearly show that the amount of drug encapsulated into the nanotubes and consequently incorporated within the resin was high enough to inhibit the growth of S. mutans when in direct contact, as shown by the agar diffusion test. As anticipated, through the collection of eluates over a 14-d period, it was confirmed that the DOX concentration in those aliquots was not adequate to inhibit the growth of S. mutans. Importantly, at day 14, the subantimicrobial concentration was sufficient to inhibit MMP-1 activity. Taken together, the data led us to accept our hypothesis that DOX-encapsulated adhesives can, in fact, inhibit MMP activity through a gradual release of subantimicrobial DOX dosages.
Knowing that monomer conversion in dental adhesives plays a significant role in the attainment of stable and, more important, durable resin-dentin bonds (Reis et al., 2004), not only did we decide to evaluate the DC using the 10-sec curing time per the manufacturer’s instructions, but we also tested the effects of 20- and 40-sec exposures. Overall, the DC data showed no statistically significant difference between the modified adhesives when compared to SBMP (control) when tested with the same curing time. Nonetheless, it is important to point out that even though DOX incorporation led to a slightly darker adhesive, it did not compromise resin monomer conversion. Therefore, from a clinical point of view, these modified adhesives could be potentially handled and used in the same fashion as the commercial one.
Regarding the bonding characteristics, although DOX-encapsulated nanotubes did not enhance resin-dentin bond strength, it also did not jeopardize it, as no differences were seen among all groups (p = .07). SEM images (Fig. 3D, 3F) confirmed the presence of HNT within the adhesive layer. One might speculate over the potential difficulty associated with ensuring an effective infiltration of these aluminosilicate clay nanotubes (halloysite) into the collagen-rich layer of the demineralized dentin to be able to perform the intended task—that is, release of MMP inhibitors such as DOX. Although prior research has shown the inability of spherical colloidal silica nanoparticles to infiltrate into the hybrid layer (Tay et al., 1999), it is important to note that the general characteristics of the HNT crystals include dimensions of 50 to 200 nm in diameter, 0.5 to 1.5 µm in length, and an aspect ratio (L/D) of 20 (Lvov et al., 2013), which could enhance their infiltration ability. Transmission electron microscopy studies need to be conducted to show and/or confirm the distribution of the nanotubes across the adhesive interface. Importantly, our group recently reported on the presence of HNT within resin tags according to cross-section SEM imaging of dentin/HNT-modified adhesive specimens (Bottino et al., 2013a). Nonetheless, as previously highlighted, the presence of HNT within the adhesive layer might serve as a reservoir for the sustained release and subsequent diffusion of DOX and play a key role in prolonging the bond durability. To that end, the other half of the beams collected in this study are being aged (6 mo) in artificial saliva at 37°C. We anticipate, on the basis of the observed MMP inhibition capability of the DOX-modified adhesive, to see a significantly higher µTBS associated with the HNT+DOX group when compared with SBMP (control).
Results from the agar diffusion assays confirmed the successful incorporation of DOX into the nanotube-modified adhesive. HNT+DOX disks that contacted the BAP inhibited S. mutans growth. Conversely, the eluates obtained after incubation in PBS of the DOX-encapsulated adhesives for 2 wk exhibited no inhibition. This led to our assumption that the concentration of DOX released from the specimens did not reach the minimum inhibitory concentration (250-500 µg/mL; Al-Ahmad et al., 2014). In other words, the eluates obtained from DOX-encapsulated specimens might contain subantimicrobial level of DOX.
To prove the concept of subantimicrobial levels of DOX, we further tested the DOX-containing adhesive eluates on the activity of MMP-1 using casein cleavage assays. According to the literature, only MMP-1, MMP-13, and MMP-8 are true collagenases that can cleave native type I collagen effectively. One should note that, along with MMP-2 and MMP-9, MMP-1 has been found in dentin, although at a lower concentration (van Strijp et al., 2003; Wang et al., 2012). Although MMP-2 and MT1-MMP can cleave native type I collagen, they are not as efficient as MMP-1 in digesting type I collagen (Song et al., 2006). The casein cleavage assay can examine inhibition of DOX-encapsulated modified dentin adhesive eluates on MMP-1 activity. The 14-d sample demonstrated 13.4% inhibition of MMP-1 activity, suggesting a gradual release of DOX from the nanotube-modified adhesives. Notably, the DOX mechanism of inhibition through the chelation of zinc present in the catalytic domain is the same for all MMPs. Therefore, if DOX inhibits MMP-1 as demonstrated in this work, it will certainly have a high chance of inhibiting other MMPs as well. Nonetheless, it is essential to expand the time frame over which eluates are collected and to obtain the exact amount of DOX released over time through analytic instrumentation (e.g., high-performance liquid chromatography).
Taken together, these findings led to our conclusion that DOX-containing eluates indeed had subantimicrobial properties and the ability to inhibit MMPs. Although DOX has been successfully used to control the progression of periodontal disease by systemic delivery at a subantimicrobial level (i.e., Periostat, 20 mg, orally, 2×/d) and localized delivery at antimicrobial levels (Atridox, 10% DOX hyclate, Atrix Laboratories, Fort Collins, CO, USA; Choi et al., 2004), this is the first report in adhesive dentistry focused on the strategy of locally delivering a subantimicrobial level of DOX through its encapsulation within nanotubes to inhibit MMP.
In conclusion, our results establish the successful encapsulation and release of DOX from the nanotube-modified dentin adhesive. Furthermore, the results from the cell viability assays further support the use of DOX-encapsulated nanotube-modified dentin adhesive in the clinical setting. Collectively, our findings provide a solid background for further testing of encapsulated MMP inhibitors into the synthesis of therapeutic adhesives that may enhance the longevity of hybrid layers and the overall clinical performance of adhesively bonded resin composite restorations.
Supplementary Material
Acknowledgments
We thank Mr. George J. Eckert (IU School of Medicine) for his assistance with statistical analyses and Dr. Afnan AlZain for experimental help. We are grateful to Mr. Andre Zeitoun (Applied Minerals Inc., New York, NY, USA) for the kind donation of the halloysite nanotubes employed in this project. There is a pending patent request under the name of Indiana University Purdue University Indianapolis that refers to the proposed innovation.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
M.C.B. acknowledges start-up funds from the Indiana University School of Dentistry.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Al-Ahmad A, Ameen H, Pelz K, Karygianni L, Wittmer A, Anderson AC, et al. (2014). Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic infections associated with root-filled teeth. J Endod 40:223-230. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Batarseh G, Palasuk J, Alkatheeri MS, Windsor LJ, Platt JA. (2013a). Nanotube-modified dentin adhesive-physicochemical and dentin bonding characterizations. Dent Mater 29:1158-1165. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, et al. (2013b). Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res 92:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. (2008). Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90-101. [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjaderhane L, et al. (2010). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjaderhane L, et al. (2007). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533. [DOI] [PubMed] [Google Scholar]
- Choi DH, Moon IS, Choi BK, Paik JW, Kim YS, Choi SH, et al. (2004). Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res 39:20-26. [DOI] [PubMed] [Google Scholar]
- Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. (2004). The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol 75:106-115. [DOI] [PubMed] [Google Scholar]
- Ferreira-Zandoná A, Santiago E, Eckert GJ, Katz BP, Pereira de Oliveira S, Capin OR, et al. (2012). The natural history of dental caries lesions: a 4-year observational study. J Dent Res 91:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson KS, Shih H, Gregory RL. (2012). The impact of three strains of oral bacteria on the surface and mechanical properties of a dental resin material. Clin Oral Investig 16:1095-1103. [DOI] [PubMed] [Google Scholar]
- Huang R, Li M, Gregory RL. (2012). Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci 120:319-325. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lvov Y, Aerov A, Fakhrullin R. (2013). Clay nanotube encapsulation for functional biocomposites. Adv Colloid Interface Sci 207:189-198. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Carrilho M, Papa V, Tjäderhane L, Gobbi P, Nucci C, et al. (2011). MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. J Dent 39:470-477. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Scaffa P, Carrilho M, Tjaderhane L, Di Lenarda R, Polimeni A, et al. (2013). Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J Dent Res 92:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research (2014). Increasing the service life of dental resin composites. URL accessed on 8/13/2014 at: http://www.nidcr.nih.gov/grantsandfunding/See_Funding_Opportunities_Sorted_By/ConceptClearance/CurrentCC/DentalResinComposites.htm.
- Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. (2011). State of the art etch-and-rinse adhesives. Dent Mater 27:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, Guo R, Shen M, Cao X, Zhang L, Xu J, et al. (2010). Electrospun poly(lactic-co-glycolic acid)/halloysite nanotube composite nanofibers for drug encapsulation and sustained release. J Mater Chem 20:10622-10629. [Google Scholar]
- Qi R, Guo R, Zheng F, Liu H, Yu J, Shi X. (2013). Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surf B Biointerfaces 110:148-155. [DOI] [PubMed] [Google Scholar]
- Raposo LH, Armstrong SR, Maia RR, Qian F, Geraldeli S, Soares CJ. (2012). Effect of specimen gripping device, geometry and fixation method on microtensile bond strength, failure mode and stress distribution: laboratory and finite element analyses. Dent Mater 28:e50-e62. [DOI] [PubMed] [Google Scholar]
- Reis AF, Arrais CAG, Novaes PD, Carvalho RM, De Goes MF, Giannini M. (2004). Ultramorphological analysis of resin-dentin interfaces produced with water-based single-step and two-step adhesives: nanoleakage expression. J Biomed Mater Res B Appl Biomater 71:90-98. [DOI] [PubMed] [Google Scholar]
- Rekow ED, Fox CH, Petersen PE, Watson T. (2013). Innovations in materials for direct restorations: why do we need innovations? Why is it so hard to capitalize on them? J Dent Res 92:945-947. [DOI] [PubMed] [Google Scholar]
- Smith K, Leyden JJ. (2005). Safety of doxycycline and minocycline: a systematic review. Clin Ther 27:1329-1342. [DOI] [PubMed] [Google Scholar]
- Song F, Wisithphrom K, Zhou J, Windsor LJ. (2006). Matrix metalloproteinase dependent and independent collagen degradation. Front Biosci 11:3100-3120. [DOI] [PubMed] [Google Scholar]
- Stanislawczuk R, Reis A, Loguercio AD. (2011). A 2-year in vitro evaluation of a chlorhexidine-containing acid on the durability of resin-dentin interfaces. J Dent 39:40-47. [DOI] [PubMed] [Google Scholar]
- Tay FR, Moulding KM, Pashley DH. (1999). Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent 1:103-117. [PubMed] [Google Scholar]
- Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. (2013). Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater 29:999-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. (2003). Host-derived proteinases and degradation of dentine collagen in situ. Caries Res 37:58-65. [DOI] [PubMed] [Google Scholar]
- Wang DY, Zhang L, Fan J, Li F, Ma KQ, Wang P, et al. (2012). Matrix metalloproteinases in human sclerotic dentine of attrited molars. Arch Oral Biol 57:1307-1312. [DOI] [PubMed] [Google Scholar]
- Windsor LJ, Bodden MK, Birkedal-Hansen B, Engler JA, Birkedal-Hansen H. (1994). Mutational analysis of residues in and around the active site of human fibroblast-type collagenase. J Biol Chem 269:26201-26207. [PubMed] [Google Scholar]
- Windsor LJ, Steele DL, LeBlanc SB, Taylor KB. (1997). Catalytic domain comparisons of human fibroblast-type collagenase, stromelysin-1, and matrilysin. Biochim Biophys Acta 1334:261-272. [DOI] [PubMed] [Google Scholar]
- Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, et al. (2013a). Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J Dent 41:464-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li F, Imazato S, Cheng L, Liu H, Arola DD, et al. (2013b). Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater 101:929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y. (2012). The effect of hydroxyapatite presence on the degree of conversion and polymerization rate in a model self-etching adhesive. Dent Mater 28:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



