Abstract
Creating an optimal microenvironment that mimics the extracellular matrix (ECM) of natural pulp and securing an adequate blood supply for the survival of cell transplants are major hurdles that need to be overcome in dental pulp regeneration. However, many currently available scaffolds fail to mimic essential functions of natural ECM. The present study investigated a novel approach involving the use of scaffold-free microtissue spheroids of dental pulp stem cells (DPSCs) prevascularized by human umbilical vein endothelial cells (HUVECs) in pulp regeneration. In vitro-fabricated microtissue spheroids were inserted into the canal space of tooth-root slices and were implanted subcutaneously into immunodeficient mice. Histological examination revealed that, after four-week implantation, tooth-root slices containing microtissue spheroids resulted in well-vascularized and cellular pulp-like tissues, compared with empty tooth-root slices, which were filled with only subcutaneous fat tissue. Immunohistochemical staining indicated that the tissue found in the tooth-root slices was of human origin, as characterized by the expression of human mitochondria, and contained odontoblast-like cells organized along the dentin, as assessed by immunostaining for nestin and dentin sialoprotein (DSP). Vascular structures formed by HUVECs in vitro were successfully anastomosed with the host vasculature upon transplantation in vivo, as shown by immunostaining for human CD31. Collectively, these findings demonstrate that prevascularized, scaffold-free, microtissue spheroids can successfully regenerate vascular dental pulp-like tissue and also highlight the significance of the microtissue microenvironment as an optimal environment for successful pulp-regeneration strategies.
Keywords: tissue engineering, stem cells, angiogenesis, extracellular matrix, endodontics, regenerative medicine
Introduction
Regeneration of tissues to replace diseased, missing, and traumatized dentin/pulp has emerged from recent progress in stem cell and tissue-engineering research. Dental pulp stem cells (DPSCs) are considered a promising population of cells in regenerative dentistry and have been shown to produce dentin-/pulp-like tissues following implantation in vivo (Gronthos et al., 2000, 2002; Shi et al., 2001). Importantly, DPSCs reside in the microvasculature region of the dental pulp and interact with perivascular cells (Shi and Gronthos, 2003). Therefore, endothelial cells could be a major source of modulators of pulp-dentin development and angiogenesis. We recently showed that co-culture of human umbilical vein endothelial cells (HUVECs) with DPSCs enhances osteo/odontogenic differentiation and angiogenesis in monolayer cultures (Dissanayaka et al., 2012). However, the interactions of these 2 cells in angiogenesis and pulp-like tissue regeneration in vivo remain unknown.
The shaping of functional dentin/pulp complex from monodispersed expanded cell cultures is an ongoing challenge. Tissue engineers have thus relied mostly on biomaterials/scaffolds in which cells can grow and differentiate. Several reports have shown that dental stem cells being seeded onto a matrix scaffold and transplanted in vivo form a new tissue similar to that of the native pulp (Cordeiro et al., 2008; Rosa et al., 2013). However, none of the scaffolds described so far has all the structure and properties of an ideal material, which should most closely resemble the cells’ physiological environment: natural extracellular matrix (ECM) (Galler et al., 2011a). In contrast to the conventional scaffolding approach, self-assembly of monodispersed cells into 3D tissue-mimics permits true physiological interactions between and among different types of cells without any influence from a secondary material. This scaffold-free approach can successfully overcome some of the major challenges related to scaffolding methods, such as failure to mimic natural extracellular matrix, lack of intercellular cross-talk, selective degradation, and remodeling (Kelm and Fussenegger, 2004; Kelm et al., 2006a).
Furthermore, dentin-pulp-complex engineering demands that the particular challenges of this approach, including vascularization, be addressed. When cells are implanted into root canals that have a blood supply only from the apical end, enhanced vascularization is needed to support the vitality of the implanted cells. This can be optimized with the addition of endothelial cells (ECs). However, the placement of 2 or more different cell types inside 3D porous scaffolds is technologically challenging (Desroches et al., 2012). The scaffold-free, self-assembly approach provides an optimum environment for combining DPSCs and ECs to overcome this challenge.
The goal of the present study was to create prevascularized scaffold-free microtissues that mimic the cellular microenvironment of dental pulp cells and to investigate the potential of these spheroids for angiogenesis and pulp-like tissue regeneration in vivo. To the best of our knowledge, this is the first study in which DPSCs and HUVECs are combined in scaffold-free, 3-dimensional, self-assembling microtissue spheroids to regenerate pulp-like tissue.
Materials & Methods
Isolation, Culture, and Characterization of Cells
After obtaining informed consent, we isolated DPSCs from freshly extracted sound third molars from human volunteers aged 18 to 25 yr, as described previously (Gronthos et al., 2000). Briefly, the surfaces of freshly extracted teeth were cleaned and cut at the cemento-enamel junction with a sterile fissure bur used to expose the pulp chamber. The pulp tissue was gently separated from the crown and root and digested in 3 mg/mL collagenase type I (GIBCO-Invitrogen, Carlsbad, CA, USA) with 4 mg/mL dispase (GIBCO-Invitrogen) solution for 1 hr at 37oC. The cells were then passed through a 70-µm strainer (BD Biosciences, Franklin Lakes, NJ, USA) to obtain single-cell suspensions. These cells were seeded in 75-cm2 culture flasks containing α-minimum essential medium (α-MEM) supplemented with 15% fetal bovine serum, L-ascorbic acid-2-phosphate, 100 U/mL penicillin-G, 100 mg/mL streptomycin, and 0.25 mg/mL fungizone (Gemini Bio-Products, Woodland, CA, USA) and cultured under 5% CO2 at 37°C. Human umbilical vein endothelial cells (HUVECs) were obtained commercially (ScienCell Research Laboratories, San Diego, CA, USA) and cultured in endothelial cell medium (ECM) (ScienCell Research Laboratories) at 37°C with 5% CO2. Before DPSCs were used for experiments, the freshly isolated cells were assessed for their ‘‘stemness’’ by flow cytometric analysis for the expression of CD73, CD90, CD105, STRO-1, and CD45. In addition, the multilineage differentiation capacity of DPSCs was confirmed in osteo-/odontogenic, adipogenic, and neurogenic induction media (results not shown). Passages 3 to 6 of each cell type were used in all experiments.
Fabrication of 3D Microtissues
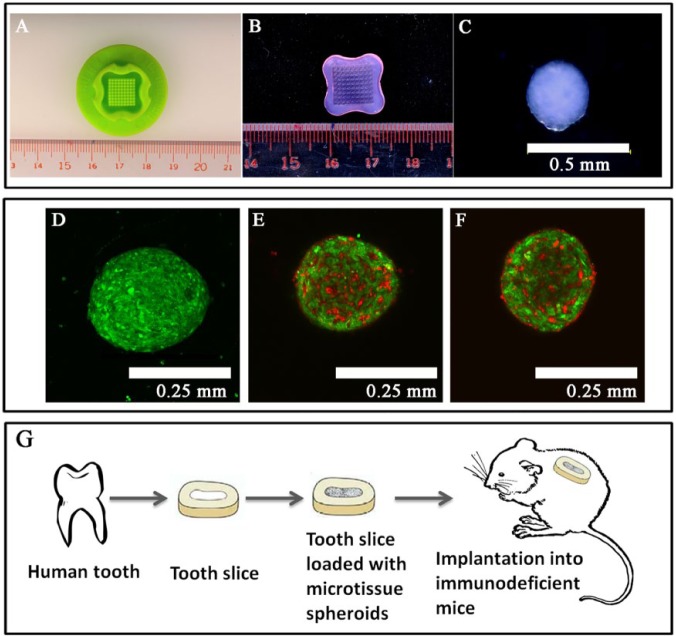
Microtissue spheroids of pure DPSCs or DPSCs with HUVECs in different ratios (3:1 and 1:1) were fabricated in agarose 3D petri dishes (Fig. 1B) made in 12-series micro-molds (Fig. 1A) (MicroTissues, Inc., Sharon, MA, USA) (Desroches et al., 2012). Briefly, the cell suspensions of DPSCs, HUVECs, and DPSC:HUVECs (256,000/190 µL) were added to each 3D petri dish (Fig. 1B) for 10 min to allow the cells to settle into the microwells. After incubation at 37°C in 5% CO2 atmosphere for 3 days, the petri dishes were inverted and centrifuged at 500 rpm for 5 min to harvest microtissue spheroids.
Figure 1.
Fabrication of microtissue spheroids. (A) Micro-mold. (B) 3D petri dish of gelled agarose released from the micro-mold. (C) Magnified view (10x) of a microtissue spheroid (~300 µm in diameter, ~3,375 cells/spheroid); self-assembly of human umbilical vein endothelial cells (HUVECs) within co-cultured microtissues. (D) Green-fluorescent-labeled dental pulp stem cells (DPSCs) organized into a microtissue spheroid (20x). (E, F) Red-fluorescent-labeled HUVECs organized into a vascular network within the co-cultured microtissue spheroids (20x). Formation of the 3D prevascular network within microtissue spheroids was promoted at higher DPSC numbers with lower number of HUVECs. (E) DPSCs:HUVECs 1:1. (F) DPSCs:HUVECs 3:1. (G) Severe combined immunodeficient (SCID) mouse model for subcutaneous transplantation of human tooth slices loaded with microtissue spheroids.
Self-assembly of Microtissues
The self-assembly of DPSCs and HUVECs within microtissues was examined with CellTracker Probes for Long-Term Tracing of Living Cells (Molecular Probes, Inc., Eugene, OR, USA). As suggested by the manufacturer, pre-warmed CellTracker 1 µM dye/serum-free medium solution was added to the attached cells and incubated for 30 min. The dye working solution was then replaced with fresh, pre-warmed medium, and the cells were incubated for another 30 min at 37°C. After being observed under a microscope for fluorescence, the cells were used to fabricate microtissues as described above. DPSCs and HUVECs were labeled with CellTracker Green and CellTracker Orange dyes, respectively. After being harvested, the self-assembled microtissues were analyzed by confocal microscopy to determine the cellular organization of the microspheroids.
Pulp Regeneration in vivo in SCID Mouse Model
All animal experiment protocols were approved by the University of Hong Kong Committee on the Use of Live Animals in Teaching and Research (CULATR). Animal handling procedures were carried out under the guidance and supervision of the Laboratory Animal Unit, University of Hong Kong.
Roots of freshly extracted human teeth were sectioned into segments of 4 mm in length. The root canals were cleaned and were instrumented with the following sequences of files; SX, S1, S2, F1, F2, and F3 (ProTaper and ProFile, Dentsply Tulsa Dental, Tulsa, OK, USA). The root slices were then soaked at room temperature in 17% ethylenediamine tetraacetic acid (EDTA) for 10 min and 19% citric acid for 1 min to remove the smear layer, followed by treatment with Betadine® for 30 min and 5.25% NaOCl for 10 to 15 min for disinfection (Huang et al., 2010). Fragments were then rinsed in sterile PBS, soaked in PBS, and then incubated at 37°C for 3 to 7 days to remove residual disinfection agents and to ensure that there was no microbial contamination.
Four experimental groups with 6 root slices in each group were used: (1) empty root slices; (2) root slices with DPSC-alone microtissues; (3) root slices with 3:1 DPSC:HUVEC microtissues; and (4) root slices with 1:1 DPSC:HUVEC microtissues. Microtissues of mono- or co-cultured DPSCs and HUVECs were loaded into the respective root slices by means of a pipette and incubated overnight for attachment to the dentin surface. Root slices loaded with microtissues were implanted into the subcutaneous space of the dorsum of five- to seven-week-old female severe combined immunodeficient mice (CB.17 SCID) (Fig. 1 III). Two implants were placed bilaterally in each mouse. After 4 wk, the implants were retrieved, fixed in 4% formaldehyde for 24 hr, demineralized with EDTA until the dentin offered no resistance to being cut with a blade (4 wk), and then processed for histology and immunohistochemistry.
Histology and Immunohistochemistry
Hematoxylin and eosin (H&E) staining was performed to examine the morphology and structure of the regenerated pulp-like tissues. Immunohistochemistry for human specific mitochondria [Abcam (Hong Kong) Ltd. HKSP, N.T. Hong Kong] and human specific CD31 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was carried out to confirm the contributions of the transplanted DPSCs and HUVECs, respectively, in regenerated tissues. Human specific anti-nestin antibody (Abcam) and anti-dentin sialoprotein (DSP) antibody (Santa Cruz Biotechnology, Inc.) were used to detect the odontoblast-like cells in newly formed pulp-like tissues. Immunohistochemical detection was performed with an ImmunoCruz mouse ABC staining system (Santa Cruz Biotechnology, Inc.). In each immunohistochemical experiment, a negative control was included with tissue sections incubated without the addition of the primary antibody. The percentages of cells with positive human mitochondria staining were determined independently by two examiners. Quotients (positive cells/total counted cells) were calculated as percentage and rounded to the nearest integer. Values are given as mean value ± standard deviation.
Quantification of Blood Vessels
For quantification of blood vessels, 3 sections per root-slice stained with human specific anti-CD31 antibody and counterstained with hematoxylin were analyzed. Microscopic images were taken at 20- fold magnification of 3 randomized areas per section. Endothelial cell-lined lumens were counted as total vascular lumens. To determine the microvascular density of human vessels (mean number of capillaries per mm2), we manually counted the number of structures with lumen surrounded by human CD31+ cells. To determine the microvascular density of mouse blood vessels, we counted the number of lumenized structures with surrounding H&E-stained, but human, CD31- cells. Endothelial cell-lined vessels with intraluminal red blood cells were quantified as perfused lumens, while lumens without red blood cells were considered as non-perfused lumens. Values are given as mean value ± standard deviation.
Statistical Analysis
Number of vessels was expressed as mean ± standard deviation and statistically analyzed by one-way analysis of variance. p < .05 was considered statistically significant.
Results
3D Microtissues
DPSCs seeded with or without endothelial cells were self-assembled into microtissue spheroids within the microwells of an agarose mold (Figs. 1A-1F). Endothelial cells alone in monocultures could not survive for more than 12 hr and did not arrange into microtissue spheroids. Macroscopic and microscopic examination revealed the successfully fabricated 300-µm-diameter microtissues of DPSCs and DPSC:HUVECs (Figs. 1C-1F).
Fluorescently labeled cells, which were allowed to self-assemble into microtissue spheroids, showed that HUVECs organized into a vascular-like tube network within the co-cultured microtissue spheroids (Figs. 1D-1F). The organization of HUVECs into a network was prominent at greater DPSC:HUVEC ratios (e.g., 3:1 DPSC:HUVEC ratio) (Fig. 1E).
Pulp-like Tissue in vivo
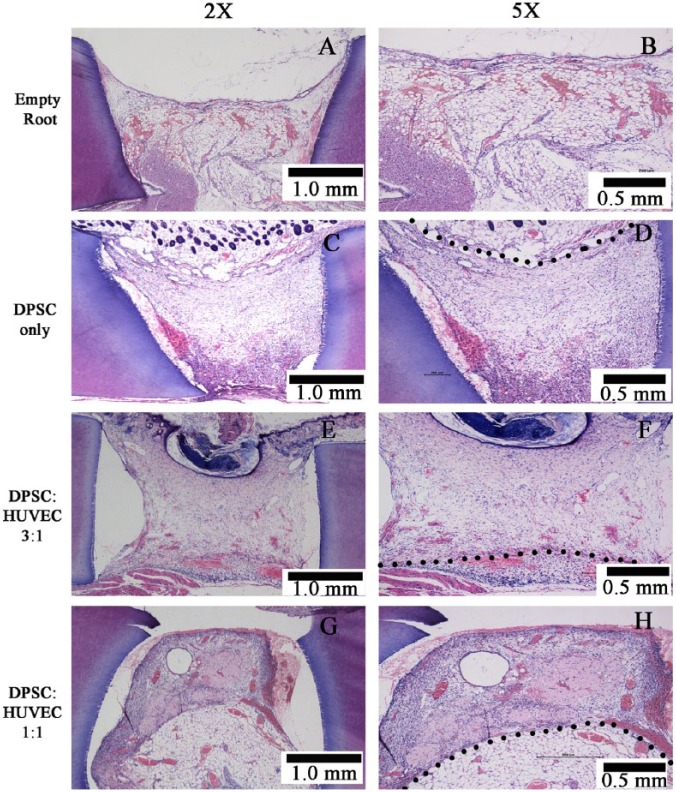
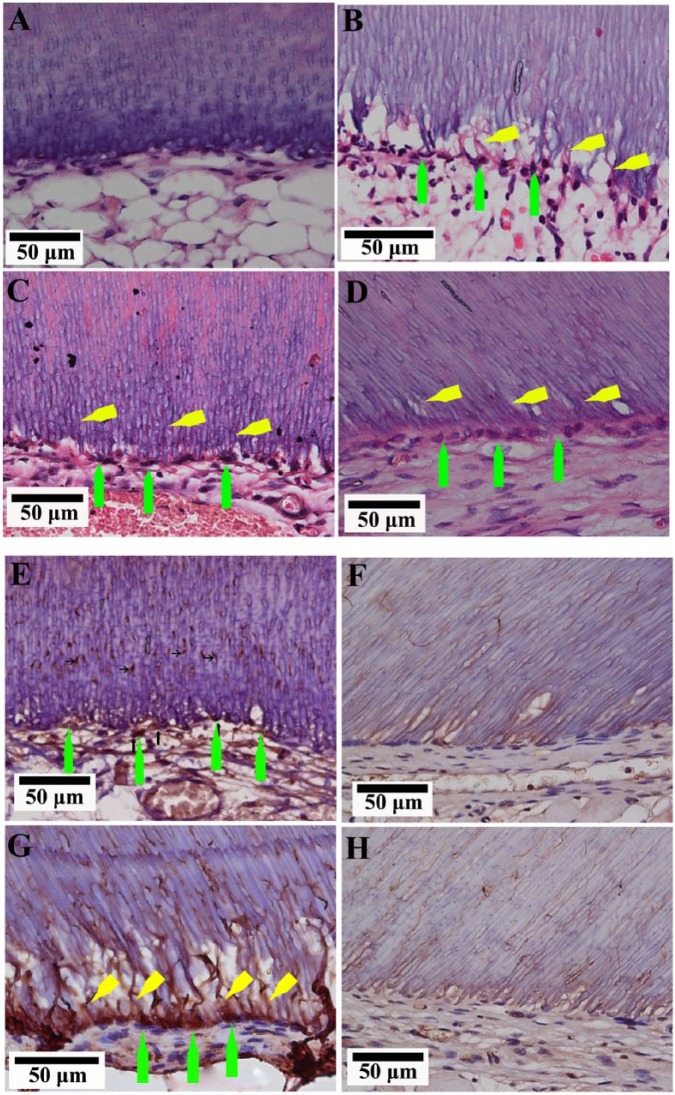
The successful integration of the in vitro-fabricated microtissues to the mouse host tissues and vasculature was observed for all 3 groups (DPSC-alone, DPSC:HUVEC 3:1, and DPSC:HUVEC 1:1) (Fig. 2). All 3 transplantation groups resulted in pulp-like tissue regeneration (Figs. 2C-2H) compared with empty tooth-root fragments transplanted as control groups, which showed only ingrowth of mouse subcutaneous fat tissue (Figs. 2A, 2B). The center region of the tooth-root canal space of microtissue transplanted groups was filled with a vascular connective tissue, while the periphery was connected with the host adipose tissue. An odontoblast-like cell layer with their processes projecting into the dentinal tubules was observed adjacent to the existing dentin in all 3 microtissue transplanted groups (Figs. 3B-3D), whereas the empty roots transplanted did not show such cells (Fig. 3A). Positive immunohistochemical staining of these cells and their processes to odontoblast markers, nestin (Figs. 3E, 3F- negative control) and DSP (Figs. 3G, 3H- negative control), suggested their odontoblastic lineage.
Figure 2.
Pulp-like tissue regenerated in different transplantation groups. Black dotted line indicates the border between the transplanted microtissues and the host tissue.
Figure 3.
Regenerated pulp-like tissue with odontoblast-like cells. (A) Empty root slice filled with host adipose tissue did not show an odontoblast-like cell lining. (B) DPSC-alone. (C) DPSC:HUVEC 3:1. (D) DPSC:HUVEC 1:1 microtissue transplanted groups showed odontoblast-like cells (green arrows) along the dentin, projecting their processes (yellow arrows) into the dentinal tubules. Immunohistochemistry for nestin (E, F - negative control) and DSP (G, H - negative control). Green arrows indicate the positively immunostained odontoblast-like cells, and yellow arrows indicate their processes.
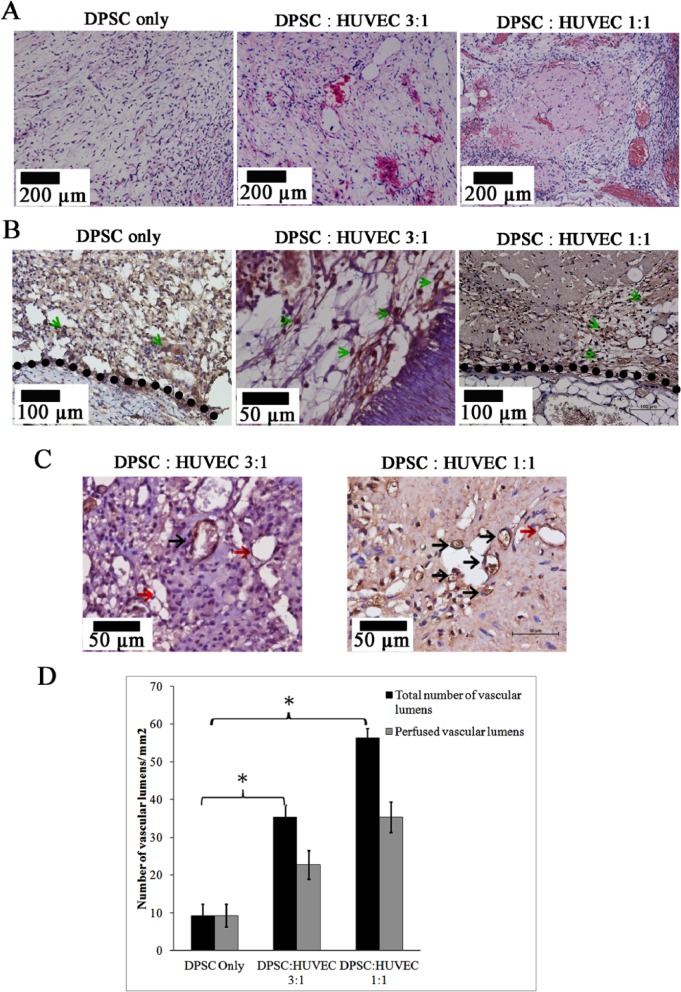
Positive staining for antibodies against human mitochondria confirmed the contributions of transplanted macrotissues of human cells in regenerated pulp-like tissue (Fig. 4B). Of the cells, 49% (± 8), 57% (± 3), and 59% (± 8) were positive for anti-human mitochondria staining in the DPSC-alone, DPSC:HUVEC 3:1, and DPSC:HUVEC 1:1 groups, respectively. We delineated the border between the transplanted microtissue-derived pulp-like tissue and the host tissue by examining the images of H&E-stained and immuno-stained sections for human mitochondria.
Figure 4.
Regenerated pulp-like tissue in different transplantation groups. (A) Different groups of microtissues after implantation for 4 wk in vivo. Co-cultured microtissues showed higher numbers of vascular lumens and extracellular matrix deposition compared with that of DPSC-only microtissues. (B) Immunohistochemistry for human mitochondria in different transplantation groups. Green arrows indicate the positively stained cells. Black dotted line indicates the border between the transplanted microtissue and the host tissues. (C) Immunohistochemistry for human CD31 in transplanted microtissues. Black arrows: CD31+ perfused vessel lumens (lumens with red blood cells). Red arrows: CD31+ non-perfused vessels. Magnification 50x. (D) Quantification of vascular lumens in regenerated pulp-like tissue of different transplantation groups (*p < .05).
DPSC:HUVEC co-cultured groups showed enhanced extracellular matrix formation compared with the DPSC-alone group, which showed loosely arranged tissue with less cellularity and matrix (Fig. 4A).
Analysis of Neovascularization
Enhanced vasculature was evident in the co-cultured groups (DPSC:HUVEC 3:1 and 1:1) compared with that of the DPSC-only group (Fig. 4A). In addition to perfused vessels, non-perfused, endothelial-lined lumens were found in co-cultured groups, while only perfused vessels were found in the DPSC-only group (Fig. 4A). Quantification of the number of vascular lumens showed significantly higher (p < .05) total numbers of vascular lumens and numbers of perfused vessels in co-cultured groups compared with those in the DPSC-alone group (Fig. 4D).
Immunohistochemistry for human CD31 detected HUVEC-lined vascular lumens in co-cultured groups, and, as expected, none of those was detected in the DPSC-only group (Fig. 4C). Some of the positively stained lumens were filled with red blood cells, suggesting integration of in vitro-formed HUVEC prevascular structures into the host vasculature (Fig. 4C). Co-cultured groups showed both human CD31+ and CD31− endothelial lined vascular lumens showing the co-existence of mouse and human vessels (Fig. 4C) in the regenerated tissue.
Discussion
Recent studies have demonstrated that DPSCs have the potential to regenerate dentin-pulp complex after being encapsulated in a scaffold and transplanted in vivo (Gronthos et al., 2000; Huang et al., 2010). However, the identification of an optimal scaffold that mimics the extracellular matrix of natural pulp, and securing a good blood supply to ensure the survival of transplanted DPSCs in vivo are 2 major hurdles that still need to be overcome. The current study demonstrates the potential utility of a scaffold-free, self-assembling microtissue approach for dental pulp tissue engineering. In this study, when the microtissue spheroids of DPSCs and HUVECs were inserted into human tooth-root slices and transplanted in vivo, the dental pulp-like tissue that was regenerated was vascular and containedodontoblast-like cells along the dentin surface, as seen by nestin and DSP expression.
Scaffold-free approaches range from layering of cell sheets formed on temperature-sensitive polymer surfaces to rotational cultures, gravity-enforced assembly in hanging drops, and non-adhesive surface culture systems for the generation of spherical microtissues (Desroches et al., 2012). A recent study reported a scaffoldless cell sheet technique whichcan be used in regenerative endodontic therapy (Syed-Picard et al., 2014). The current study presents a scaffold-free approach based on fabrication of microtissue spheroids on non-adhesive agarose hydrogels containing concave recesses. Compared with the cell-sheet technique, the current approach enables the production of hundreds of uniform-sized self-assembled microtissues, allows for fine control of microtissue size and media exchange, and is suitable for high-quality imaging.
The results of the present study showed that DPSCs secreted ECM while self-assembling into microtissues and pulp-like tissue in vivo. Furthermore, HUVEC-DPSC interactions have attenuated ECM deposition, resulting in a more stable microenvironment in co-cultures, as observed in vivo. These results indicate that self-assembly of cells into microtissues can promote development of a ECM-containing microenvironment without a secondary artificial material, as shown with other cell types (Anderer and Libera, 2002; Kelm et al., 2004, 2006a,b).
The results further showed anodontoblast-like cell lining, adjacent to the dentin, which was positive for the odontoblastic markers nestin and DSP. Cellular processes extending into the dentinal tubules and positivity for DSP indicated that these cells are active odontoblast-like cells that secrete dentin (Quispe-Salcedo et al., 2012). Although H&E-stained sections did not reveal a distinct newly secreted dentin layer, tubular dentin formation cannot be ruled out, since tetracycline staining was not performed in the current study (Rosa et al., 2013).
The dentin pre-treatment procedure followed in the current study was similar to that in the study conducted by Huang et al., which showed successful regeneration of de novo pulp with a continuous dentin layer (Huang et al., 2010). However, a more recent study has shown that pre-treatment of dentin with NaOCl prior to transplantation results in unfavorable effects at the dentin-cell interface, such as a rugged dentin borderline with multinucleated cells that create resorption lacunae (Galler et al., 2011). The results of the current study, despite the use of NaOCl to treat the dentin, did not show such adverse effects. In contrast, the results showed a close association of cells with the dentin matrix with cellular processes extended into the tubules. Furthermore, these cells were positively stained for DSP and nestin antibodies, suggesting the odontoblastic differentiation. The contrasting results between the Huang et al. and current studies may be due to the concurrent use of EDTA and prolonged (3-7 days) washing with phosphate-buffered saline following NaOCl treatment.
In vitro incorporation of endothelial cells is a potential solution to the challenge of achieving rapid connection with the host vasculature following transplantation in vivo (Verseijden et al., 2010). However, this is even more challenging with the conventional scaffolding methods, since endothelial cells become apoptotic when embedded within most scaffolds (Vogel and Baneyx, 2003). The results of the present study showed that although endothelial cells could not survive on their own as microtissue spheroids, when co-cultured with DPSCs in a microtissue environment, HUVECs not only survived but also organized into a tubular network. It was shown that DPSCs secrete pro-angiogenic factors which promote survival and function of HUVECs in microtissue spheroids (Tran-Hung et al., 2006). In addition, direct cell-cell contact, physical environment (e.g., oxygen stress), and mechanical properties such as shear stress might also play a role in this context (Hurley et al., 2010).
Vascular structures formed by endothelial cells in vitro have been shown to be able to connect to the host vasculature upon transplantation in vivo (Rouwkema et al., 2006). In the animal studies described herein, it was observed that the human CD31+-stained lumina contained erythrocytes, demonstrating a connection between the HUVEC-formed vascular structures and the mouse vasculature. Furthermore, there were higher numbers of vascular lumens in the co-cultured microtissue transplants compared with the DPSC-alone transplants. The higher number of vascular lumens in the co-cultured groups is likely to be due to the presence of HUVEC-formed prevascular structures as well as the enhanced ingrowth of mouse vasculature to the transplant (Keating, 2012).
The animal model used in this study, in which tooth-root slices loaded with microtissues were transplanted into the subcutaneous space of the severe combined immunodeficient mice, is a well-established and widely used approach in investigating dental pulp regeneration (Cordeiro et al., 2008; Rosa et al., 2013). However, analysis of emerging data suggests that stem/progenitor cells, in addition to cell replacement, act as paracrine sources to mobilize endogenous stem cells to the target site of regeneration (Keating, 2012). Therefore, the xenogenic nature and ectopic site of the transplantation raise the need for a better animal model which can mimic the in vivo milieu of the dental pulp more accurately. Autologous transplantation in clinically simulated pulp spaces of teeth in a bigger animal model, such as the mini-pig or dog, may provide an improved approach to the examination of microtissue spheroids in pulp regeneration.
Although HUVECs are a readily available source and were used in this study, because of immune incompatibility, they will not be a practical option in the clinical setting. Potential alternatives are microvascular endothelial cells and endothelial progenitor cells (Rouwkema et al., 2009), which have already been investigated in vasculogenic studies. It would also be interesting to investigate the effects of microvascular ECs on DPSCs, since few previous studies have suggested different activity of the microvascular EC matrix on MSCs compared with that of HUVEC matrix.
Scaffold-based approaches provide structural templates/surfaces for the cells, whereas scaffold-free approaches promote and depend on cellular self-assembly and organization in the absence of external cues. While interactions between cells and external cues dominate in scaffold-based cultures, cell-cell interactions dominate in scaffold-free 3-D models, in which the cells themselves create specific environments, including ECM. Therefore, scaffold-free approaches provide an optimum in vivo microenvironment for tissue engineering compared withscaffold-based approaches, which often fail to do so. However, the scaffold-free microtissue approach has its own limitations. A major limitation would be the large number of cells required to fabricate a substantial quantity of tissue. In addition, thescaffold-free approach lacks the external cues received from a scaffold, which might be useful in pulp regeneration of a full-length root canal. Therefore, a combinatory approach that amalgamates scaffold-based and scaffold-free techniques could be an ideal approach for future studies in regenerative endodontics.
In conclusion, the present study showed that HUVEC-incorporated scaffold-free microtissue spheroids of DPSCs, when transplanted in vivo, can give rise to vascularized dental-pulp-like tissue. Findings of the current study highlight the significance of developing a 3D microenvironment that supports cell-cell interactions which can in turn contribute toward an optimal atmosphere for successful pulp-regeneration strategies.
Acknowledgments
The authors thank Edith H.Y. Tong and Raymond Tong for their excellent technical support.
Footnotes
This work was supported by a General Research Fund (GRF) grant (Project code: HKU784912M) from the Research Grants Council, Hong Kong SAR. The funding sources had no role in study design, in the conduct, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Anderer U, Libera J. (2002). In vitro engineering of human autogenous cartilage. J Bone Miner Res 17:1420-1429. [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969. [DOI] [PubMed] [Google Scholar]
- Desroches BR, Zhang P, Choi BR, King ME, Maldonado AE, Li W, et al. (2012). Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells. Am J Physiol Heart Circ Physiol 302:H2031-H2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka WL, Zhan X, Zhang C, Hargreaves KM, Jin L, Tong EH. (2012). Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J Endod 38:454-463. [DOI] [PubMed] [Google Scholar]
- Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. (2011). Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod 37:1536-1541. [DOI] [PubMed] [Google Scholar]
- Galler KM, D’Souza RN, Hartgerink JD, Schmalz G. (2011). Scaffolds for dental pulp tissue engineering. Adv Dent Res 23:333-339. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625-13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. (2002). Stem cell properties of human dental pulp stem cells. J Dent Res 81:531-535. [DOI] [PubMed] [Google Scholar]
- Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. (2010). Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16:605-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JR, Balaji S, Narmoneva DA. (2010). Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol 299:C444-C453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709-716. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Fussenegger M. (2004). Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol 22:195-202. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Ehler E, Nielsen LK, Schlatter S, Perriard JC, Fussenegger M. (2004). Design of artificial myocardial microtissues. Tissue Eng 10:201-214. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP, et al. (2006a). Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng 12:2151-2160. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Ittner LM, Born W, Djonov V, Fussenegger M. (2006b). Self-assembly of sensory neurons into ganglia-like microtissues. J Biotechnol 121:86-101. [DOI] [PubMed] [Google Scholar]
- Quispe-Salcedo A, Ida-Yonemochi H, Nakatomi M, Ohshima H. (2012). Expression patterns of nestin and dentin sialoprotein during dentinogenesis in mice. Biomed Res 33:119-132. [DOI] [PubMed] [Google Scholar]
- Rosa V, Zhang Z, Grande RH, Nör JE. (2013). Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwkema J, de Boer J, Van Blitterswijk CA. (2006). Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 12:2685-2693. [DOI] [PubMed] [Google Scholar]
- Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. (2009). The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A 15:2015-2027. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696-704. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. (2001). Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29:532-539. [DOI] [PubMed] [Google Scholar]
- Syed-Picard FN, Ray HL, Jr, Kumta PN, Sfeir C. (2014). Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J Dent Res 93:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Hung L, Mathieu S, About I. (2006). Role of human pulp fibroblasts in angiogenesis. J Dent Res 85:819-823. [DOI] [PubMed] [Google Scholar]
- Verseijden F, Posthumus-van Sluijs SJ, Farrell E, van Neck JW, Hovius SE, Hofer SO, et al. (2010). Prevascular structures promote vascularization in engineered human adipose tissue constructs upon implantation. Cell Transplant 19:1007-1020. [DOI] [PubMed] [Google Scholar]
- Vogel V, Baneyx G. (2003). The tissue engineering puzzle: a molecular perspective. Annu Rev Biomed Eng 5:441-463. [DOI] [PubMed] [Google Scholar]